Paper - The developmental alterations in the vascular system of the brain of the human embryo (1921)
| Embryology - 30 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Streeter GL. The developmental alterations in the vascular system of the brain of the human embryo. (1921) Contrib. Embryol., Carnegie Inst. Wash. 8:7-38.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Developmental Alterations in the Vascular System of the Brain of the Human Embryo
By George L. Streeter with five plates and twelve text-figures (1921).
Introduction
One of the most striking features in the development of the blood-vessels of the head is the clear way in which they demonstrate the embryological principle of what may be termed integrative development. It is quite evident that the vascular apparatus does not independently and by itself "unfold" into the adult pattern. On the contrary, it reacts continuously in a most sensitive way to the factors of its environment, the pattern in the adult being the result of the sum of the environmental influences that have played upon it throughout the embryonic period. We thus find that this apparatus is continuously adequate and complete for the structures as they exist at any particular stage; as the environmental structures progressively change, the vascular apparatus also changes and thereby is always adapted to the newer conditions. Furthermore, there are no apparent ulterior preparations at any time for the supply and drainage of other structures which have not yet made their appearance. For each stage it is an efficient and complete going-mechanism, apparently uninfluenced by the nature of its subsequent morphology.
With these factors in mind, one can better understand the architectural arrangements of the vascular system of the head that appear in different periods of development. In the primordial or precirculatory period the vessels that then exist are engaged principally in growth and in the elaboration of a plexus, and their form then is little influenced by conditions that would favor the circulation of the contained fluid. As the circulatory flow of the blood becomes established we find that the vascular plexus responds by conforming to the hydrodynamic requirements, becoming adequately adapted to the form of the neural tube as then existing, with favorably situated aortic feeders and simple and direct drainage channels. As the brain becomes more complicated, and as the skull-membranes form, there occur, step by step, the necessary adaptations on the part of the blood vessels. Finally, when the permanent form is attained, the vessels lose their transitory character and develop permanent and more highly differentiated walls, properly suited to the adult functional requirements.
It is possible to subdivide the development of the blood-vessels of the brain into five successive periods, each showing special adaptations to their changing environmental conditions. To facilitate the description of this process an arbitrary order of that kind will be adopted in this paper. During the first of these five periods there are established the primordial endothelial blood-containing channels, which are neithei arteries nor veins, but constitute the source from which all the arteries, veins, and capillaries of the brain are derived. These primordial blood vessels lie bilaterally close along the brain-wall, at first either in the form of a single, slender, longitudinal tube, as seen along the hindbrain, or a plexiform space as seen near the forebrain and midbrain. Soon after the primordial vessels are established, endothelial buds sprout from their walls and in conjuurtion with them form an irregular endothelial vascular meshwork which tends to spread over the surface of the brain-wall, especially in the region of the forebrain and midbrain. There is thus formed a plexiform system which constitutes a germinal bed of endothelium rather than a circulatory apparatus.
During the second developmental period the primordial blood-vessel plexus of the head slowly resolves itself into veins, arteries, and capillaries, and becomes architecturally suited to the circulatory flow of the blood. That portion of the plexus which lies against the brain spreads out as a flattened capillary sheet, conforming everywhere to the shape of the brain-wall and its attached ganglia and sense-organs. The more superficial part of the plexus develops a coarser mesh and forms larger channels, which tend to unite into continuous trunks and gradually, by virtue of their communications, can he recognized as definite arteries and veins. The intermediate loojis of the plexus maintain the anastomosis between the deep capillary sheet and the more superficial trunks, forming tributaries of the veins and branches of the arteries. The second period thus establishes the primary type of the circulation of the head, in which there is a capillary bed, fed by arterial branches from the aortic system and drained bilaterally by a continuous venous trunk which extends back to the venous end of the heart.
The third period is inaugurated by the differentiation of the membranous skull, the Template:Duramater, and the arachnoid-pial membranes. As a result of this stratification of the tissues of the head, the more ventral of the anastomosing channels that connect the deep capillary plexus with the superficial vessels become closed off and there is a general separation or cleavage of the vessels immediately surrounding the brain-wall from those belonging to the membranous skull and its coverings. This process begins at the base of the skull and extends bilaterally upward toward the middle line of the vault, in which region the communications between the deeper and more superficial systems are to some extent maintained. In this way the cerebral vessels are gradually separated from the dural vessels and in a similar manner the superficial vessels of the head become isolated by the laying down of the primordium of the membranous skull, after which their course of development is quite independent of that of the dural and cerebral systems.
By the time the third period is well under way it is overlapped by the fourth period, under which we include the remarkable series of adjustments in the arrangement of the blood-vessels, in adaptation to the developmental alterations in the form, size, and condition of the structures of the head region. The brain is one of the chief factors in this process. The marked change in its form, and especially the prolonged relative growth of the cerebral hemispheres, render necessary a continuous series of alterations in the blood-channels that extend far into the late fetal stages. In the earlier stages a fundamental change results from the growth of the labyrinth and its cartilaginous capsule, whereby a mechanical obstruction is introduced that results in the obliteration of a considerable part of the largest vein of the head. This is compensated for by a new channel which takes a more dorsal course, and which eventually forms the sigmoid portion of the lateral sinus.
Finally, under the fifth period we would include the late histological changes in the walls of the vessels that convert them into the adult arteries, veins, and the various types of sinuses. These histological factors, however, are not considered in the present paper and are merely mentioned to complete the sequence, as they have no determining influence on the phenomena of the preceding four periods.
The observations that are reported are chiefly concerned with the third and fourth developmental periods; that is, after the primary circulation of the head is established and during the cleavage and adjustmental stages. A review, however, will be made of the history of the vascular system of the head previous to that time, which will be based in large part on the important observations of Evans (1909, 1912) and Pabin (1915, 1917a, 1917b). The study of the development of the vascular system of the head of the human embryo was initiated in this laboratory by Professor Mall (1905). Later I continued the same investigation and reported (Streeter, 1915) some of the features of the adaptive metamorphosis of the dural veins. In the present paper I shall include much of the same subject-matter and incorporate with it further observations made on additional material, thus making it possible to treat the general subject more completely and to demonstrate the relations of the dural veins to the principal arteries of the head.
Establishment of Primordial Vascular System of Head
A distinct advance in our knowledge of the origin of the vascular system has recently been made by Professor Sabin through the study of living preparations of the growing chick. By that means it was possible to observe directly the principal steps in blood-vessel formation. Certain steps in the process that were still under dispute have thus become established, as well as others which are new observations. According to her description (8abin, 1917b), the vaso-formative cells or angioblasts are differentiated from the mesoderm, and on proliferation they form small, dense, syncytial masses which join one another by means of tim- processes of cytoplasm. In this way there are formed plexuses of solid angioblastic cords, the growth of which is maintained partly by proliferation of their constituent cells and partly by the further addition of new angioblasts which differentiate from the adjacent mesoderm.
During the formation of these angioblastic plexuses a liquefaction of their cytoplasm occurs in such a manner as to convert the solid angioblastic cords into vessels filled with clear fluid. This is brought about by a process of vacuolization and the formation of an "intracellular" lumen. Vacuoles appear near the nuclei and rai)idly enlarge, so that in from one to two hours there occurs a complete destruction of the cytoplasm and nuclei of the central part of the angioblastic cords, resulting in the formation of a clear plasma. the periphery of the angioblastic mass being preserved as an endothelial boundary. The liciuefaction of cytoplasm occurs both in the loops of the angioblastic plexuses and in masses of angioblasts that are still isolated, in the latter case resulting in small vesicles that subsequently join the main plexus. These observations were made for the most part in the area pellucida of the yolk-sac, but the same phenomena were seen also within the body of the embryo. The dorsal aorta in the trunk region and a portion of it within the head was seen to differentiate in situ in the above manner.
The angioblasts are not all converted into endothelium and blood-plasma; some of them take part in the formation of red blood-corpuscles. During the lumen formation in the angioblastic strands, small clumps of the original angioblasts become partially separated by the liquefaction of the cytoplasm around them. Such masses soon show the presence of hemoglobin and constitute bloodislands which eventually break apart as free red blood-cells and float away in the blood-plasma. Blood-islands can also be seen to be derived secondarily from the endothelium.
Concerning the distribution of the earliest blood-vessels and concerning the form and development of the primordial vascular system of the head, I have followed the descriptions published by Evans (1912) and Sabin (1917a), both of whom studied injected specimens of the chick and pig. On account of the difficulty of making observations of this region in living preparations, the details in the growth of this endothelial meshwork have not been actually seen. The plexiform transformation of the aortic arches has been demonstrated only partly and it is not known how much of the first endothelial system of the head is derived from angioblasts that are differentiated locally and how much to the proliferation of the cells of the aortic arches. It is possible, even after the laying-out of the main parts of the primordial system, that angioblasts continue to differentiate in the new territory around its margins and become incorporated with it. The essential features, however, in the development of this system are admirably shown by injected material, as can be seen in figures 398 (duck, 13 somites) and 393 (chick, 15 somites) of Evans (1912), and plate 1, figure 3 (chick, 9 somites), and plate 2, figure 1 (chick, 14 somites) and figure 2 (chick, 16 somites) of Sabin (1917a).
At about the time that the dorsal aortas become established in the head region, it is seen that the first pair of aortic arches connecting them with the heart are plexiform in character. Sprouting from this plexiform area an endothelial meshwork arises and extends dorsalward and caudalward toward the forebrain and midbrain, to become the primary head-plexus. Very soon afterwards, a slender longitudinal channel is formed bilaterally along the ventro-lateral margins of the hindbrain which communicates with the primary head-plexus in front and caudally with the anterior cardinal vein by way of the transverse veins of the first two interspaces. A few slender communications are established very early between it and the dorsal aorta of its respective side. This channel has been designated by Sabin (1917a) as the "vasa primitiva rhombencephali." It seems jirobable from her observations that this channel is not a derivative from sprout.s from the dorsal aorta or from the primary head-plexus, but is differentiated in situ, and its communications with them are secondary. This .slender channel, together with the primary head-plexus, constitutes the primordial system from which all the blood vessels of the brain and its membranes are derived. Sabin has suggested a greater restriction in the use of the terms "artery" and "vein" and has warned against making a too early identification of the adult vessels in the embryo. The pnmordial vascular system of the brain as seen in the chick of 15 somites consists morphologically of a sprouting meshwork rather than a set of definite supply and drainage channels. It is to be considered as a bed of proliferating endothelium rather than as a circulatory apparatus. Even after the blood begins to move through its meshes it is some Uttle time before the circulatory function becomes the dominating influence in the determination of its architectural features, and thus, in these early stages of the vascular system, we meet arrangements which, as regards their form, size, and communications, are distinctly ineflacient as to the circulatory flow of the contained blood, but are quite characteristic of the germinal period of an endothelial meshwork. Any attempt to distinguish arteries from veins in this primordial system results only in hopeless confusion.
Differentiation of Primordial System into Arteries, Veins and Capillaries
Our information regarding the angioblastic period in which the primordial blood-vessels are laid down has been derived mainly from study of chick embryos. The transition into the second developmental period in which the primordial system gradually undergoes resolution into arteries, veins, and capillaries has been demonstrated in manmalian material (pig embryos), and toward the end of the period, as the primary circulation becomes established, the chief features have been described in human embryos. As illustrating these transitions, the reader is referred to figures 394, 395, and 400 of Evans (1912), and figure 1, plate 4, of Sabin (1917a). From the time when the primordial blood-vessel system of the head is first laid down, its endothelial walls undergo active proliferation and a sprouting meshwork extends from it, invading the interval between the ectoderm and the brain-wall. This spreading of the endothelial plexus is more active in some directions than in others. In general it tends to spread toward and over the surface of the neural tube and- the nerve structures connected with it. It extends early over the midbrain and forebrain and soon encircles the optic stalk. Along the hindbrain the plexus formation is somewhat slower. In fact, in the chick the primordial vessel in this region persists as a simple channel until the embryo has acquired about 29 somites, by which time the growth in the more cephalic region is quite extensive. Persisting so long unchanged, as it does in the chick, it was named the "vena capitis media," in contradistinction to the more laterally situated channel that develops later, the so-called "vena capitis lateralis." It was shown, however, by Sabin (1917a) that what exists here in the chick at this time is the persisting primordial channel and not a true vein.
In its earlier stages the proliferating meshwork shows considerable irregularity in the form and size of its constituent channels. Gradually it can be seen that the more superficial loops of the mesh are taking the form of larger and more directly continuous channels. The deeper loops of the mesh flatten out in a more uniform capillary sheet that lies in direct apposition to the brain-wall and its attached structures. Tho intermediate portions of the mesh maintain the communications between the deep capillary sheet and the superficial main channels. The few communications of the aortic system with the intermediate and deeper part of the plexus are maintained and vmite with selected loops of the plexus to form slender continuous channels which are eventually lost in the plexus. In this way the irregular plexus of proliferating endothelial tubes is resolved into a definite circulatory system, consisting of a capillary mesh that is fed by deep branches from the aortic system and drained by superficial tributaries into a main channel that extends backward to empty into the common vitello-umbilical vein. By the time that this is accomplished the contained blood is already slowly circulating through this primitive system.
The main drainage-channel which thus becomes established on each side of the head was originally called the anterior cardinal vein until it was recognized that only the caudal portion of it properly belonged to the cardinal system. Evans (1912, p. 67G) suggested, as more appropriate for it, the name "primitive headvein," and the same term was utilized by Sabin (1917«)- This primary head-vein develops essentially in the same manner both in the chick and the pig — that is, through the elaboration of a simpler and more direct channel through the superficial part of the proliferating plexus.
The difference, however, in the anatomy of chick and pig embryos is associated with some difference in the details of the development of this primary head-vein. The pre-trigeminal portion of it is identical in both forms and consists of a superficial main channel, receiving tributaries from the deeper part of the plexus. It forms in the interval between the thalamus and the optic bulb and leads backward, median and ventral, to the trigeminal ganglion, where it temporarily connects with the primordial system. The middle portion of the primary head- vein, the portion in the interval between the trigeminal and vagus nerves, is formed later than the pre-trigeminal portion, and is formed relatively later in the chick than in the pig. In the chick, it has been shown by Sabin (1917a) that the primortlial channel running along the ventro-lateral margin of the hindbrain persists, in embryos of 29 somites, as a single large channel communicating in front with the elaborate plexus of the midbrain region and the already formed pre-trigeminal portion of the main drainage channel. Caudally it communicates through the transverse vein of the first interspace with the anterior cardinal vein, the latter forming the caudal portion of the primary head-vein. Thus, in the chick the hindbrain portion of the priniordial blood-channel (the vasa primitiva rhombencephali of Sabin) serves temiwrarily as a part of the circulatory apparatus before its proliferative function has been completed, but in the stage to which we arc referring proliferating loops have begun to spread from the main channel over the wall of the hindbrain and its attached ganglia. Derived partly from these and partly from the plexuses of the gill-arches, there is formed a series of superficial loops which link themselves together into a slender longitudinal channel communicating in front with the pre-trigeminal portion of the main drainage-channel and extending backward lateral to the otic vesicle, to eter into the anterior cardinal \ciii. This constitutes the middle portion and completes the formation of the main drainage-channel of the head, the primary head-vein. In the pig, the primordial blood-channel along the margin of the hindbrain, instead of enlarging as a simple temporary channel as seen in the chick, becomes resolved almost at once into a proliferating plexus, and from its superficial loops, and perhaps also from some of the loops of the adjacent branchial plexuses, there is evolved the middle portion of the primary head-vein, which is completed nearly as soon as the pre-trigeminal portion and before there is any considerable circulation of the blood. The caudal part of the primary head- vein is made up of the anterior cardinal vein, which, as we have seen, is originally continuous with the primordial channel, joining it (in the pig) in front of the occipital myotomes instead of through the transverse vein of the first interspace, as seen in the chick. As the more superficial loops, sprouting from the primordial system, become established lateral to the otocyst in the formation of the middle portion of the primary head-vein, their communication with the anterior cardinal becomes larger, whereas the original communication of the anterior cardinal with the primordial channel becomes more restricted and breaks up into a plexus; thus, by the linking-up of these three parts, a continuous superficial channel is formed which extends the whole length of the head and provides for the adequate and efficient drainage of all its structures.
It has been noted that, from the beginning, communications exist between the aortic system and the more dorsally placed primordial vascular system of the head. The largest and most constant are the paired trunks that connect the first aortic arches with the deeper loops of the ventral part of the forebrain plexus. What was originally a plexiform communication is later resolved into a single trunk that eventually forms part of the internal carotid artery of its respective side. Along the hindbrain region are other irregularly placed, slender communications, and on reaching the myotome region there are the segmental dorsal branches of the dorsal aorta?, which anastomose with the proliferating plexus of the neural tube. The primordial blood-channel along each side of the hindbrain proliferates in the form of a plexus, and as the plexuses of the two sides spread on the surface of the brain-wall they gradually establish an anastomosis along the mid-ventral line. At the same time a series of the more superficial loops of the plexus, in association with the communications from the dorsal aortae, become elaborated into a slender bilateral longitudinal channel which is continued caudally into the spinal region, and orally it connects with similar loops which are associated with the embryonic internal carotid artery. There is thus established, on each side along the ventral surface of the neural tube, a continuous arterial channel which is connected dorsally by many loops with the neural capillary plexus and ventrally by a few branches with the aortic system. From these simple channels are derived later the main arteries of the brain and spinal cord.
With the establishment of the primary head-vein, we may regard the first type of circulation of the head as completed. It consists essentially of a series of arterial feeders from the aortic system, which lose themselves in the sheet of capillaries that invests the neural tube, which capillaries in turn are drained by many anastomosing loops into the more superficially placed primary head-vein, and thereby are connected with the duct of Cuvier and the venous end of the heart. It is this primary arrangement that exists in human embryos 4 mm. long, and this is the earliest stage that was examined in connection with the present study. In figures 22 and 23, plate 2, are shown the left and right profiles of a model made by the Born construction method of such an embryo (Carnegie Collection, No. 588, 4 mm. long). This is slightly younger than the stage shown by Mall (1905) in his figure 3, although the conditions in the two are very similar. It is distinctly younger than both the Ingalls (1907) and the Elze (1907) specimens, although they also show the same primary type of circulation. In figures 22 and 23 the neural tube, eye-stalk, trigeminal ganglion, and ear vesicle are shown in gray and the reconstruction was planned so as to show the relation to these structures of the main arteries and veins, the latter being colored red and blue respectively. On examining these figures it is to be remembered that only the definite supply and drainage channels are shown. In order to complete the system one must imagine the neural tube as almost completely ensheathed by a capillary sheet into which the arterial feeders open and from which the small venous tributaries arise. In figure 23 fragments of this capillary sheet showing this relation to the drainage vessels are indicated in the hindbrain region.
The morphological details of the models will perhaps be more readily understood by the study of these two figures, in which the attempt has been made to indicate the form and relations as clearly as possible, than by the following of a descriptive text. Attention, however, may be directed to a few of the general features. In the first place, it seems to be the rule that, throughout the whole body of the embryo, the source of blood-supply has a central position and its flow to the tissue is in a peripheral direction, whereas the return drainage system lies more superficially and the flow is consequently in a central direction. As for the drainage of the head, there is provided a simple and adequate channel, the primary head-vein. Its only deflections from a perfectly direct course are those rendered necessary by the structure of the parts. Its position is affected by the trigeminal, facial, glossopharyngeal, and vagus nerves, due to their respective placodal relations to the ectoderm. It could not pass lateral to the trigeminal mass, and so is deflected inward. The facial and glossopharyngeal ganglia lie in positions sufficiently ventral so as not to interfere with its superficial position; the former, however, deflects it upward slightly. The ganglion nodosum of the Angus lies directly in its course and, as in the case of the trigeminal, the channel is thereby forced to take a median course, since the preferred superficial course is ruled out by the placodal attachment. We thus meet with a median deflection at that point, so that the primary head-vein curves caudally around the vagus trunk.
Just as the main trunk of the primary head-vein follows the most simple and direct cour.se possible, so its plexiform tributaries are favorably situated for draining the various areas which are present at that time. Those from the capillary sheet of the brain-tube join it for the most part on its dorsal and medial border. In accordance with the topography of the region, these tributaries are arranged in three groups: (1) an anterior group from the forebrain and midbrain, leading into the pre-trigeminal portion of the primary head- vein; (2) a middle group from the cerebellar region, emptying into its otic or middle segment, that portion between the trigeminal and glossopharyngeal nerves; and (3) a posterior or occipital group which accompanies the vagus rootlets and empties near the junction of its middle and cardinal portions. This posterior group usually empties by a common trunk. This trunk corresponds to the original communication between the anterior cardinal vein and the primordial blood-channel of the hindbrain, which now has proliferated into the meshwork forming the capillary sheet that invests the brain-wall. Before the completion of the middle or otic portion of the primary head-vein, this trunk from the occipital group of tributaries was the only communication between the anterior cardinal and the vessels of the more oral region of the head. Especial attention is directed to these three tributary groups, as their arrangement is significant for the later stages, as will presently be seen. In addition to these tributaries from the brain, the primary head- vein receives ventral tributaries from the eye region, from the nerve-ganglion masses, and from the region of the first and second gill-arches, which communicate with the plexuses derived from the proliferating elements of the first two aortic arches.
The arterial supply to the head region at this time is primarily through the internal carotid arteries, which form relatively slender though direct channels. On each side they are made up of the trunk that connected the primary headplexus with the first aortic arch and the portion of the dorsal aorta corresponding to the first two arches. It will be noted that the first and second vascular arches are more or less incomplete, having broken up into irregular plexuses ramifying in the tissues of their respective gill-bars. A part of the plexus of the second arch apparently becomes incorporated in the external carotid artery, although the trunk of this artery is probably represented by the short stem seen in figures 22 and 23, projecting from the oral border of the third vascular arch. The capillaries and venous drainage plexuses of the first and second arches are shown only at their point of entrance into the primary head-vein above. The internal carotid artery extends forward to the root of the optic stalk, where it bifurcates into its terminal branches, which soon become lost by anastomosis with the capillary sheet of the brain- wall. The continuation of this channel backward along the ventral wall of the brain could not be satisfactorily modeled, though this channel and its anastomosis with the basilar and vertebral arteries doubtless already exist. It is shown in the reconstruction by Ingalls (1907) in a 4.9 mm. embryo.
The cut ends of communications such as originally connected the dorsal aorta with the primordial vascular system of the brain are indicated. As long as they persist, these must be regarded as arterial feeders through the basilar arteries to the capillary sheet of the brain. One of these may doubtless be looked upon as the stapedial artery (for example, the one between the first and second arches). The vertebral arteries apparently were established in this specimen, but they could not be satisfactorily outhned and so were omitted. They must, however, be included as part of the source of blood-supply. With this manner of blood-supply, and with this manner of drainage, as illustrated in figures 22 and 23, one must feel that the provision for the blood-circulation is well designed and is perfectly adequate for the structures as then existing, and were there no further alterations in the structures themselves no further change in their blood-vessels would be necessary. On the other hand, there are no superfluous channels present and no evident vascular provision for structures not yet developed, that is, disregarding the potential proliferative power which exists in such an endothelial system. The establishment of this primary type of the circulation of the head completes our second period in the growth of the cranial vascular system.
Cleavage of Blood Vessels of Head into Separate Systems
The differentiation of the dura mater and the formation of the arachnoid mesh begins in the region of the base of the skull, and from there the process spreads slowly upward toward the vertex of the head. As these structures form it can be seen that the anastomosing channels connecting the capillary sheet of the brain with the more superficial drainage channels are gradually closed off, the more ventral ones first and the more dorsal ones later. In this way the dura forms a partition that results in a general separation or cleavage of the superficial vessels (consisting mainly of the primary head-vein and its tributaries) from the deeper vessels in intimate contact with the brain-wall, including the capillary sheet and the vessels su]>plying and draining it. The latter or deeper system, however, continues to drain into the former at certain restricted places. As this cleavage occurs, we can distinguish between dural vessels which are chiefly veins and cerebral or pial vessels which include arteries as well as veins. Soon after, coincident with the formation of the membranous skull, the dural system becomes more or less completely separated from the vessels of the integument and its subjacent soft parts. We then have three different main strata of blood-vessels — the external, the dural, and the cerebral. The most conspicuous of the early external vessels are those belonging to the integument. They make their appearance around the base of the skull in embryos between 12 mm. and 20 mm. long and spread upward toward the vault. In spreading upward they exhibit a characteristic growing edge consisting of anastomosing loops of the mesh which can be seen with the naked eye as an advancing narrow line marking off the non-vascularized area above from the vascularized part below, as has been clearly pointod out In- Ilochstetter (1916) and as indicated in our figure 10.
The first steps in this cleavage are well under way in embryos 14 nun. long. An embryo of this stage (Carnegie Collection, No. 940) is shown in figure 1. The veins of the head were distended with a natural blood injection and at the same time the surrounding tissues were quite transparent; as a result, it was possible to determine their arrangement from a surface examination with considerable detail. A photograph was made of the specimen, and in it were added the details that could be seen with the aid of a binocular microscope. It was in this way that figure 1 was obtained. What is seen there is the primary head-vein and its tributaries. To see the deeper structures it was found necessary to make a plastic model of the region. Such a model i.s shown in figures 24 and 25, plate 3, being a wax-plate reconstruction made from an embryo 11.5 mm. long (Carnegie Collection, No. 544). Mall (1905) has pictured about the same stage in his figure 9, and this stage is also pictured by Alarkowski (1911) in his figure 1.
Figure 1. Drawing of the primary head-vein and its main tributaries in a human embryo 13.8 mm long (Carnegie Collection, No. 940). The tributaries are arranged in the form of three plexuses — the anterior, middle, and posterior dural plexuses. The trunk of the posterior plexus passes through the foramen jugulare in order to empty into the primary head-vein, and this point marks the junction of the intrinsic head portion of the primary head-vein with the neck portion or anterior cardinal vein. The latter eventually becomes the internal jugular vein.
In their main points all of these embryos correspond rather closely, and apparently the vascular system at this time does not show any great variation. A large venous channel is formed in the region lateral to the diencephalon and passes backward median to the trigeminal nerve and lateral to the otic capsule through the region of the future middle ear, where it bends sharply downward in the neck region to finally empty into the duct of Cuvier. All the veins of the cranial region drain into this main channel. This constitutes the primary head-vein with which we are already familiar. This vein at 14 mm. differs from its condition at 4 mm. only in being more deflected in its course by the structures through which it threads its way. It still forms a fairly direct and efficient drainage-channel. It was this primary head-vein that was described by different writers as the anterior cardinal vein until Grosser (1907) showed that only the caudal portion of it — the part that is found in the region of the somites and later forms the internal jugular vein — could be properly spoken of as the anterior cardinal. 8alzer (1895) designated the portion in the presegmental region in the guinea-pig as vena capitis medialis and vena capitis lateralis, depending on whether it was found median or lateral to the cranial nerve-trunks. The more cephalic portion, in the trigeminal region, is always found median to the nerve and hence is always vena capitis medialis. Caudal to the trigeminal nerve, Salzer describes it as at first coursing medial to the facial, glossopharyngeal, and vagus nerves, and subsequently, by a process of "island formation," migrating lateral to these same nerves — that is, changing from vena capitis mediaUs to vena capitis lateralis. These terms were advocated on the basis of an homology with similar veins in the lower vertebrates, and were used in the recent paper by Shindo (1915). We now know, however, from the work of Sabin (1917a) that what Salzer called the vena capitis medialis is the primordial channel of the hindbrain, whose purpose is primarily the proliferation of endothelium, and hence is not to be regarded as a pure drainage-channel. The primary head- vein is the first true drainage-channel in this region. Its composite origin has already been pointed out. It has been shown that it belongs in part to the trunk (the anterior cardinal vein) and in part is intrinsic to the head. As we shall presently see, it is the trunk portion or anterior cardinal that forms the internal jugular vein, whereas the intrinsic head portion in its more anterior segment becomes the cavernous sinus, the otic or more posterior portion (the so-called vena capitis lateralis) disappearing entirely and being replaced by a more dorsally situated channel.
The tributaries draining into the primary head- vein join it mainly along its dorsal margin, though there are also ventral tributaries which are especially large and numerous in the neighborhood of the optic stalk and the trigeminal ganglion, as seen in figures 24 and 25. A large plexiform sheet lies median to the maxillary trunk of the trigemmal nerve, draining the structures of the maxillary arch. It is continuous with the plexus that envelops and penetrates into the substance of the trigeminal ganglion. It is a modification of this plexus that forms the infraorbital vein and the venous plexus in the region of the pterygoid fossa. The ophthalmic vein corresponds to the ventral tributaries just in front of this and median to the first division of the trigeminal nerve. This is contrary to the view of Mabkowski (1911, p. 600), who thought that it was the more caudal and larger of these tributaries, the one draining the maxillary process, that becomes the ophthalmic vein; whereas the more anterior tributaries, arising from the orbital fossa, he described as undergoing retrogression and disappearing. That it does not disappear, but forms the main portion of the oiihthalmic vein, we shall be able to see in older stages.
The tributaries draining dorsally into the primary head-vein are arranged in three plexiform groups, as was pointed out by Mall (1905), the first group emptying into the main channel in front of the semilunar ganglion, the second group between the semilunar and the acustico-facial ganglia, and the third group caudal to the otic capsule. These were designated respectively the anterior, middle, and posterior cerebral veins. The last one empties into the main channel through a single trunk, but the other two groups tend to maintain the character of the original plexus and usually have multiple openings into the primary head-vein. Furthermore, due to the cleavage effect of the dura, which tends to .separate them from the deeper vessels, the veins forming the-se three groups belong chiefly to the dura mater and the tissues forming the membranous cranium. There is, therefore, an advantage in adopting for the temporary description of this period of development a terminology- something like that of Markowski (1911). In doing so, a distinction between the lateral and mesial portions will not be made, but the three groups as given by Mall will be retained. We shall thus speak of the anterior, middle, and posterior dural plexuses, or (more formally) plexus durae matris anterior, plexus durae matris medialis, and plexus durae matris posterior, as they are indicated in figures 1, 24, and 25. In these figures only the larger channels of the plexus are shown and it is to be understood that an intervening smaller venous mesh connects them more or less completely.
These three plexuses are not exactly uniform as regards their pattern, but from the very first they exhibit an individuality that seems to correspond to the difference in structure of the areas which they drain. The anterior plexus is modified along its oral margin in adaptation to the form of the bulging hemisphere. The middle plexus has two larger trunks, one of which drains the capillaries on the oral surface of the cerebellar plate; the other drains the anterior part of the roof of the fourth ventricle. It is this latter one that was pictured by His (1904) and incorrectly described as the sinus transversus (p. 121). In the posterior plexus there can be recognized usually a single channel that is larger than the others and that tends to cross thedorso-median line to anastomose with the plexus of the opposite side, the main channel of the other side being correspondingly smaller, thus giving rise to a bilateral asymmetry. Another place at which a larger channel is frequently seen crossing dorsally over the median line in an asymmetrical manner is at the junction of the midbrain and hindbrain. A third favorable place of this kind is over the diencephalon along the caudal margin of the cerebral hemisphere. Here is formed the beginning of the transverse sinus, which consequently shows an asymmetrical relation to the superior sagittal sinus, as will be pointed out later.
In the ventral portions the dural plexuses are more or less completely separated from the deeper-lying plexus or capillary sheet that closely invests the wall of the neural tube, in which are developed the cerebral veins and main arterial supply. In tracing the plexus dorsalward toward the median line, we find an increasing frequency of communication between the two, and near the median line they are so intimately connected that it is impossible to distinguish between them; in other words, in this region the cleavage between these two layers is not yet established. The manner in which the tips of the dural plexuses communicate with the capillary sheet of the brain-wall is indicated in figures 24 and 25, where a portion of the sheet is drawn in over the occipital pole of the cerebral hemisphere. It is to be remembered that a capillary meshwork of this kind invests the central nervous system everywhere, the pattern of the mesh varying somewhat according to the region.
The main arterial trunks are well established at this stage and afford a more abundant supply of blood to the brain than existed in the 4 mm. embryo. Whereas the aortic system in the latter conformed to the branchial arches, it now presents a definite aortic arch derived from the truncus arteriosus and the fourth branchial arch of the left side. The innominate artery is formed by the fourth arch of the right side. The third arch has been taken up on each side in the formation of the common carotid and its bifurcating portion, including the plexiform external carotid. The internal carotid, basilar and vertebral arteries are present in practically the adult form. It was noticed in studying the left vertebral artery in this specimen that its communication with the aorta was more caudal than one would expect, judging by its position in the adult. It is probable, however, that the arrangement is a temporary one, and that one of the communications above this, too slender to model, is destined to become the final trunk of the vertebral artery, thus affording an instance of spontaneous migration of a blood-channel.
Figure 2. Profile reconstruction of the dural venous system in a human embryo 15 mm. long (Carnegie Collection, No. 144). The primary head-vein is still intact: a more dorsal channel, however, is forming through the meshes of the middle dural plexus, coursing backward into the posterior plexus. This new channel becomes the permanent sinus tranavensus by which the sreater part of the brain is finally drained through the foramen jugulare. A course completely intercranial (sinus transversus) thus replaces one that was in part extracranial (primary head-vein). Median to the second division of the trigeminal nerve can he seen the plexiform maxillary vein, which drain the structure of the maxillary process.
Similarly, as was seen in the 4 mm. embryo, the distribution of the arterial supply and the arrangement of the venous drainage are here (in the 14 mm. embryo) efficiently laid out from the standpoint of the structures of the head as th<Mi existing. The primary head-vein is already somewhat bcnl out of its course by the car-vesicle and nerve-trunks, and would necessarily become much more so by the growth that rapidly follows were it not that a new provision is established to take care of this, as we shall now see.
Adjustments of Blood-channels due to Growth and Change in form of the Brain and Ear
Before the cleavage between the dural and cerebral vascular .systems is completed, certain alterations in their pattern, associated with the changes in their environment, rapidly follow one another. If one examines a number of series of about the same age as that just described, and a little older, it is seen that the primary head-vein maintains the same general course and relations, but the pattern of the dural plexuses is constantly changing, which in the end results in a change in the primary head-vein itself. In embryos about 18 mm. long an important change occurs by which the blood from the middle dural plexus, which heretofore had drained into the primary head-vein, in the interval between the trigeminal and the acustico-facial ganglia, now drains caudalward into the posterior dural plexus through anastomosing loops that exist between these two plexuses, passing dorsalward to the otic capsule and just lateral to the endolymphatic sac.
Figure 3. Profile reconstruction of the dural venous system in a human embryo 21 mm long (Carnegie Collection, No. 460). The sinus transi-ersus is now clearly established and there is left of the primary head-vein only that portion which persists as the sinus cavernosus. There is a remnant of its otic portion (marked x) that originally connected the cavernous region with the internal jugular vein. A more complete reconstruction of the blood-vessels of the right side of this same embryo is shown on plate 4.
This can be seen in figure 2, which shows a graphic reconstruction of a human embryo 18 mm. long (No. 144, Carnegie Collection, CR length 18 mm. in formalin, 14 mm. on slide). This is the same embryo shown in Mall's figure 11 and is about the same age as the embryo pictured in figure 2 of Markowski. In some respects the reconstruction referred to differs from both of these. From Mall it differs in that the greater part of the midbrain and forebrain is still drained by the primary head- vein. From Markowski it differsin that in our specimen the single large channel passsing backward from the anterior and middle dural plexuses is not yet established, but instead the region occupied by the anterior and middle plexuses still shows an extensive anastomosing network not differing much from the pattern we have already seen in figures 1, 24, and 25. The basis for this new channel
from the middle to the posterior plexus, dorsal to the otic capsule, already existed
in slightly younger stages (fig. 25) in the form of a venous plexus extending across
this region. We are not to conclude, however, that this plexus was placed there
for this particular purpose; it is only such a plexus as tends to form everywhere throughout the dural system.
An interesting feature in connection with the establishment of the new channel just described is the fact that the trunks that originally drained the middle dural plexus into the primary head-vein nearly disappear, owing to the fact that the blood that they heretofore carried — i. e., from the cerebellar region and the posterior part of the midbrain — adopts the new channel that is formed dorsal to the otic capsule and is thus drained into the posterior dural plexus. As a result of this the original trunks that connected the middle plexus with the primary head-vein
become relatively small and partially break up into a small plexus. We shall see later, however, that with the next change in the head-vein a trunk will open up here again as an important channel.
In taking up the question of terminology for figure 2, it is found that most of the terms used in figures 1, 24, and 25 are still applicable. There are the three dural
plexuses draining into the primary head-vein and also the ophthalmic and maxillary
veins. The anterior dural plexus, however, can be seen to be reshaping itself so
as to come into a more free anastomosis with the middle dural plexus. The middle
dural plexus, by draining as it does over the otic capsule, presents the first stage in
the formation of the transverse sinus — that is, the sigmoid portion of it. The
posterior dural plexus shows less change in its form and connections than any other
group of the head-veins, and this is true also in the later stages. There are some
minor alterations in its pattern, but otherwise it simply extends to become the
occipital sinus of the adult. The primary head-vein can be subdivided into the
trigeminal portion that is to form the cavernous sinus and the otic portion which
passes lateral to the otic capsule accompanying the seventh nerve, and lastly, the
cervical portion or internal jugular vein, the boundary of which is indicated in
figure 2 by the label for. jug. The otic portion already shows a diminution in
volume as a result of the establishment of the new drainage-channel dorsal to
the otic capsule. Dorsal to the otic capsule there is sufficient free space for the
development of a vascular channel, whereas the region ventro-lateral to it becomes crowded by the development of the cochlea and the structures of the middle ear
This constitutes a mechanical factor that doubtless has a determining influence
upon the change in the course of this blood-channel.
In embryos about 20 mm. long the veins of the head have an arrangement that
is intermediate between the embryonic type and the adult type. The veins in the
basal portion of the skull closely resemble those of the adult, while the dorsal veins
still have many embryonic features. In figures 3 and 26 are shown reconstructions
of the head of such an embryo (Carnegie Collection. Xo. 460. 21 mm. long). Figure
3, showing left side of head, is a profile reconstruction, and figure 26, showing right
side of head, is from a wax-plate reconstruction. The reconstruction of the blood vessels in this case was greatly facilitated by the work already done on the head of
this embryo by Professor Lewis, who kindly put all of his tracings and photographs
at my disposal. The study was further facilitated through the fact that the blood vessels had been injected through the umbilical vein with India ink b\- Professor
Sabin while the heart was still beating, so that there is a beautiful injection of the
entire vascular system. Before the embryo was cut, sketches and photographs
of the vessels that could be seen from the surface were made by Professor Evans.
For the sake of comparison another embryo slightly older (Carnegie Collection,
No. 632, 24 mm.) was studied, and a profile reconstruction of it is shown in figure 4.
On examining figure 3. showing left side of the head, it is seen that the primary
head-vein is now separated into its adult parts. In the trigeminal-nerve region we
can speak of it as the cavernous sinus, receiving as tributaries the ophthalmic and
maxillary veins and a large cerebral vein draining the lateral wall of the diencephalon. This latter vein belongs to the cerebral- vein system, eventually becoming
the middle cerebral vein, and runs for the greater part of its course through the
pia-arachnoid membranes. It penetrates the dura and runs a short dural course
before joining the cavernous sinus. It may be regarded as one of the diminishing
number of channels that drain the cerebral venous system into the dural system.
There are also smaller tributaries from a network in the region of the semilunar
ganglion. Xo tributaries were detected flowing into the cavernous sinus from the
caudal pole of the cerebral hemisphere, such as were found up to this time; all of
this blood now flows in the opposite direction, caudalward through the middle
dural plexus and the developing transverse sinus. On the right side of this same
embryo (see fig. 26) a communication still exists between the cavernous sinus and
the anterior dural plexus, though it is thinning out. In this respect, then, figure 26 is just before figure 3, and a comparison of the two shows just how this interesting reversal of the blood-current takes place.
Tracing the cavernous sinus backward, it can be seen that the interruption between it and the internal jugular vein is complete, though there is still a remnant of that connection which extends as a blind channel a short way along the facial nerve. It is interesting to note that there is occasionally found in the adult skull a persistent foramen, the foramen jugular e spurium of Luschka, which corresponds to the exit of this decadent channel. The vein itself, however, has never been described as persisting, although it exists normally in lower forms as a drainage for the anterior part of the brain, passing through this extracranial course to empty into the internal jugular vein. In the stage we are studying the drainage of the
cavernous sinus is upward over the semilunar ganglion into what may now be
recognized as the transverse sinus. This communication is through a short channel
that approximately^ represents the original trunk of the middle dural plexus and
constitutes the superior petrosal sinus. This channel is designated by Markowski
(1911) as the vena prootica, and he gives a different origin for the superior petrosal
sinus. According to him (p. 599), it takes its origin from a small cerebral vein
derived from the basal surface of the hindbrain which empties into the vena prootica.
In the further development, the opening of the vein migrates by anastomosis along
the vena prootica toward the sinus transversus and empties either into that sinus
or into the vena prootica near it. According to Markowski, the superior petrosal
sinus has little connection with the cavernous sinus and morphologically represents
a metencephalic vein. Regarding the eventual fate of the vena prootica, he has
apparently made no observations, though he pictures it as a large channel in an
embryo 46.5 mm. long. From the specimens I have examined I can not confirm
Markowski's description of the superior petrosal sinus, and I feel convinced that his
vena prootica and the superior petrosal sinus are one and the same thing, and that
which he regards as the superior petrosal sinus is, instead, one of its tributaries.
Mall ( 1905, p. 17) also described the superior petrosal sinus as the adult form of the
"vena cerebralis media," which, it will be remembered, is the same as the trunk
of our middle dural plexus.
With the alterations in the primary head-vein the anterior, middle, and posterior dural plexuses are drained by means of the new dorsal channel which empties
through the jugular foramen into the internal jugular vein. This channel can be
at once recognized as the transverse sinus, and the sigmoid portion of it presents
relations that are much the same as are found in the adult. The three dural plexuses
are still of the embryonic type. The posterior or occipital plexus is practically
the same as was seen in 18 mm. embryos. Only its coarser meshes are shown in
figure 3. It is more completely shown in figure 26. It will be noted that it is
rather of a different character from the now combined anterior and middle dural
plexuses and (as we shall later see) it is to take less part in the further metamorphosis of these vessels.
The whole dural area lying between the cerebral hemis])heres and the margin of the cerebellum constitutes the tentorium cerebelh. It is very broad dorsally and is more constricted ventrally; thus in profile it is wedge-shaped. In the loose tissue composing it are found the meshes of the dural plexus, the combined anterior
and middle plexuses. As this region becomes more compressed, consequent union
the growth of the cerebrum and cerebellum, there is a contiiuial adjustment of the
contained venous channels with repeated alterations in the pattern of the meshes.
In general we find the larger channels radiating upward toward the midbrain region,
and as we approach the median line the plexus becomes finer and there is an intimate anastomosis with the subjacent plexus lielonging to the brain-wall.
On comparing embryos 21 mm long with those 18 mm. long two characteristic changes are observed in the pattern of the anterior dural plexus at this time (Compare figs. 3 and 26 with fig. 2). In the first place, the anterior dural plexus
annexes itself to the middle dural plexus and drains backward through this into the
newly established channel dorsal to the otic capsule. We will therefore, from now
on, refer to the combined anterior and middle dural plexuses as the tentorial plexus,
on the basis of its distribution, which is probably a more satisfactory terminology
than to group both of them under the designation anterior dural plexus, as was
done in the former paper (Streeter 1915). In the second place, there is differentiated along the margin of the cerebrum and between the hemispheres a subdivision of this plexus that is eventually to constitute the superior sagittal sinus
(marked plexus sagittalis in figs. 3, 4, and 26).
Figure 4. Profile reconstruction of the main dural veins in a human embryo 24 mm. long (Carnegie Collection. No. 632). The sigmoid portion of the transverse sinus can be identified as that part between the point of entry of the superior petrosal sinus and the jugular foramen (marked x). Enlarged about 5 diameters.
Examination of photographs and sketches of embryos of about this age shows
that there is a tendency to the formation of a larger channel along the anterior
margin of the tentorial plexus— that is, along the caudal margin of the cerebrum.
This was designated by Markowski (1911) as the anterior marginal vein (vordere Grenzvene); the large tributary, draining the lateral surface of the cerebrum, that
empties into it, he calls the lateral telencephalic vein, of which there may be several.
IMarkowski describes the anterior marginal veins of the two sides as extending
forward and toward the median line and uniting in the formation of a plexus out
of which is to be derived eventually the superior sagittal sinus. From examination
of figures 2, 3. and 4 it can be seen that there is no sharp line between the sagittal
plexus as described by us and the more ventral loops of what was the anterior
dural plexus of which it is a part. The anterior marginal vein of Markowski is a
part of both of them, as can be plainly seen in figure 26. The discussion regarding
the formation of the superior sagittal sinus will be reserved for a subsequent part
of this paper. We may, however, point out at this time that the anterior marginal
vein of Markowski is apparently not a definite vein, but rather a constantly changing
channel. What we find is that the more anterior loops of the tentorial plexus are
constantly dropping out and are replaced by the development of the more caudal
channels. By comparing figures 3 and 4 we can see this change occurring. Our
interpretation of the condition found in figure 4 is that what had been a larger
channel along the cerebral margin of the tentorial plexus is now dwindling into a
small mesh, whereas the main blood-stream forms for itself a new course in a more
caudal loop of the plexus.
| Figure 5. Camera-lucida sketch of a portion of the right transverse sinus in a human embryo 27.5 mm long (Carnegie Collection, No. 1458). It illustrates the manner of channel-migration in the loops of the tentorial plexus, whereby the main channel follows a course in adaptation to the caudal growth of the cerebral hemisphere. While this process is under way there are found along the cerebra! margin the slender remnants of discarded channels. | 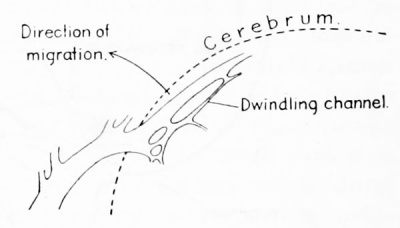
|
In this connection it may be pointed out that "migration of veins" may occur
in at least two ways. There may be a passive change in position or direction of
the endothelial tube itself, due to mechanical causes arising from alterations in its
environment; this is illustrated by the sigmoid portion of the transverse sinus
and its change in form in the later stages (embryos more than 20 mm. long) . On the other hand, a vein may change its jiosition by forming or adopting a new
endothelial channel and at the same time reliiufuishing its original endothelial channel. The embryonic plexiform character of the veins in the region of the tentorium
is especially favorable for this procedure, and we find this type of alteration in
the blood-channels repeatedly illustrated in this region. In other words, under
migration of veins we are to distinguish between passive migration (where there is a
change in position due to some flexion or traction on the vein-wall itself) and spontaneous ))ii<jrati(»i (where there is a change in position of the blood-stream only), and
where, by a process of what might l)e called circumfluent anastomosis or anastomotic progression, the blood-stream develops a new channel in the adjacent loops
of the plexus, with a corresponding dwindling of the previously used loop, as is
illustrated in figure 5. From the observations of Evans (1912) on the ventral branches of the aorta, it is apparently possible to obtain a spontaneous migration
without the aid of collateral loops. Here the result is obtained by unequal growth
of the endothelial walls. As a subhead under spontaneous migration we might
include replacement channels. In this process there is the formation of a new channel
and the obliteration of an old one. A replacement channel differs from other
spontaneously migrating channels in that it is not a gradual and progressive change
in position, but an abrupt and immediately complete one. Furthermore, the new
channel lacks the morphological characteristics of the old one. An illustration of a
replacement channel is the channel dorsal to the otic capsule (transverse sinus),
which supplants the otic portion of the primary head- vein.
The lateral telencephalic veins of INIarkowski apparently correspond to the inferior cerebral veins of the adult, so we will label them in that way. Though emptying into the dural system, they develop their course through the intradural
membranes and become typical cerebral veins. It is interesting to note that in the 21 mm. embryo certain definite topographical points in the transverse sinus are already determined, namely, the jugular foramen, the location of the endolymphatic sac, the points of entry of the superior petrosal sinus and of the inferior cerebral veins. Thus we see that more than half of the sinus is already established and that it is the terminal or jugular portion that is established first. The remainder of the sinus is relatively late in assuming a permanent form, which is doubtless the result of the prolonged, period of growth of the cerebrum, making a continued
adjustment of the tentorial plexus necessary. Even in embryos 50 mm. long, which we are about to examine, the proximal end of this sinus is still in the formative stage. Before leaving this stage, and once more comparing figures 3, 4, and 26, it should be pointed out that the great drainage-channels of the head are efficiently adapted to the drainage of its parts as then existing. We are not to think of them as busily engaged in building the transverse and sagittal sinuses, but as carrying on their functional activity in the best manner possible for the moment and with regard to the available space and the amount of given work. The completed transverse and sagittal sinuses will come in good time, as determined by later conditions.
To cover the period of embryos about 50 mm. long, the writer examined four series belonging to the Carnegie Collection: No. 886, 42 mm. coronal; No. 84, 50 mm. transverse; No. 96, 50 mm. sagittal; and No. 448, 52 mm. sagittal, injected. There was also an embryo of about the same age (No. 458, 54 mm.) that had been injected with India ink, the head of which was removed and partly dissected, and then cleared after the Spalteholz method. This gave excellent total views of the blood vessels. The profile reconstruction shown in figure 6 is based on series No. 96 and was made by preparing tracings on transparent paper which were then superimposed and a composite tracing made of the whole series. This is about the same stage that is shown by Markowski in his figure 4. The reconstruction shown in figure 27 is of a younger embryo and was made after the Born-Lewis method.
At this period the arterial supply and the venous drainage of the head are established along channels that correspond fairly well to those found in the adult.
It is clearly subdivided into three separate systems: (1) the superficial system
belonging to the integument and soft parts, (2) the dural system lying between the
dura and bone, and (3) the cerebral system. All three are originally outgrowths
of the same capillary plexus. The separation of the dural veins and the cerebral
veins we have traced through step by step. The superficial vessels in embryos
20 mm. long are already separated off from the dural system by the membranous
and cartilaginous cranium. They appear first in the lower parts of the head,
where, in consequence of the earlier maturation of this region, they are originally
separated off from the deep system and are in the form of a plexus that gradually
sjMeads upward over the vault. They maintain a few anastomoses with the dural
.system, which constitute the so-called emissary veins. One of these is shown in
figures 6 and 27. Aside from the channel maintained through the orbit, the chief
drainage from the superficial system is through the external jugular vein, which is
pictured by Salzer (1895) as already present in guinea-pig embryos 20 mm. long.
On examining the dura in embryos 50 mm. long it will be seen that for the greater part it closely invests the interior of the developing cranium and is relatively poor in blood-vessels. This is true especially in those portions where the cartilaginous and bony cranium is more advanced in its differentiation, as in the base of the skull and in the frontal, temporal, and lower occipital regions. In other regions the dura projects within the cranial cavity, being separated from the future bony skull by a layer of areolar tissue, in the meshes of which are found the large blood channels and their tributaries. The largest area of this kind is situated over the midbrain, extending from the caudal margin of the cerebral hemispheres to the cerebellum. This area extends laterally down to the base of the skull, narrowing as it does so. It constitutes what is known later as the tentorium cerebelli, and in it is included the greater part of the dural venous system. A basal extension of the tentorium widens out in the region of the semilunar ganglion and in its meshes is formed the cavernous sinus. A thinner area of the same tissue extends caudalward from the cavernous sinus, median to the otic capsule, to join the jugular region.
The slender plexus of veins extending through this constitutes the inferior pc^trosal sinus. Along all the sinuses we find this same areolar meshwork. It is not to be confused with the developing arachnoid tissue, from which it is every where separated by the dura. Blood-vessels supplying and draining the brain are also found in the arachnoid at this time and in some regions they are quite numerous, such as the region of the Sylvian fissure and along the more ventral parts of the midbrain and hindbrain. These cerebral vessels are everywhere separated and distinct from the dural blood-channels, with the exception of the few points where they
empty into the big dural channels, as occurs in the adult. The connection between
the dural system and the cerebral system is no longer ?? a multii)I<> anastomosis of small vessels, but instead by isolated larger veins.
Examination of figures 6 and 27 shows that we have in fetuses at this time an
arrangement of the dural venous system that in most respects follows the adult
arrangement. The cavernous sinus still has a simpler character than is found in
the adult. It is situated median and ventral to the semilunar ganglion and has the large ophthalmic and maxillary tributaries in front. In figure 27 it receives a
large terminal trunk lateral to the infundibulum made up of tributaries coming
from the region of the Sylvian fissure; this corresponds to the middle cerebral
vein of the adult. Caudally the cavernous sinus communicates with the main
blood-stream by means of the superior and inferior petrosal sinuses. The superior
petrosal sinus passes over the cochlear part of the otic capsule and empties above into the transverse sinus. The inferior petrosal sinus consists of a plexus of veins
that passes median to the otic capsule to empty at the point of origin of the internal
jugular vein. It is shown in figure 6 but not in figure 27.
Figure 6. Profile reconstruction of the dural veins in a human embryo 50 mm long (Carnegie Collection No. {{CE96))). The outlines of the veins of the falx cerebri can be seen through the cerebral hemisphere. That part of the transverse sinus situated caudal to the superior petrosal sinus (sigmoid) shows practically the adult relations. The more anterior part is still in the form of a plexus embedded in the embryonic tissue of the tentorium.
As regards the transverse sinus, it has been pointed out that the. terminal or jugular portion of it is established first. In both figures 5 and 27 it can be seen that it consists of a single large channel from the point of entry of the superior
petrosal sinus to the jugular fossa (in other words, the sigmoid portion) and has the same tributaries and the same general relations that are found in the adult. The remainder or proximal portion of the transverse sinus is less well established, and the large capillary meshwork found along its dorsal margin shows that the blood channels here are still in the formative stage and must still be spoken of as the temporary tentorial plexus. The main channel is forming along the anterior margin of this plexus, into which the inferior cerebral vein empties. It can be seen how this portion of the transverse sinus undergoes spontaneous migration backward
in adjustment to the growth of the hemisphere and thus comes to assume a more and more horizontal course. This change in direction, together with an increase in length and diameter of the main channel at the expense of the formative meshwork, remains to be completed before the adult condition can be considered as established. The variations found in the adult in the region of the confluens sinuum can be readily understood as variations in channel selection through this tentorial meshwork.
In the region of the forebrain a fold of dura is interposed between the two
hemispheres and is compressed into a flattened sheet which is to constitute the falx
cerebri. This and the vascular meshwork belonging to it are directly continuous
with the tentorium. Like the tentorium, it passes through a prolonged adjustmental period. In embryos 50 mm. long two of its permanent channels, which are
to belong to the dural sinus system, can be readily recognized; these are the superior
sagittal sinus and the straight sinus. In figure 6 the superior sagittal sinus is
quite irregular in outline, which is a result of shrinkage of the specimen. In the
normal state, as seen in other embryos, it passes evenly along the margin of the
cerebrum. Certain details regarding the vessels belonging to the falx cerebri and
the drainage of the chorioidal masses will now be taken up in connection with the
formation of the superior sagittal sinus.
Development of Sinus Sagittalis Superior
Under the description of embryos 21 mm. long mention was made of the formation of a plexus sagittalis as a subdivision of the anterior dural plexus. At that stage the plexus is clearly differentiated from the remainder of the anterior dural plexus, as can be seen in the dorsal view of an embryo of about that age shown in figure S and in the reconstruction shown in figure 26. Earlier than this, in embryos about 14 mm. long (fig. 7), the plexus can be recognized, though here it is not so clearly separated from the general plexus. In such embryos it can be seen that the larger tributaries of the anterior and middle dural plexuses stop short of the median line, with the exception of anteriorly, where they merge into a longitudinal plexus that dips in between the developinghemispheres. It is in the meshes of this plexus that we find the begnming of the superior sagittal sinus; and the principal steps in its transformation can be seen by comparing figures 7, 8, and 9. Sketches like these necessarily have to be simplified, and on examining them it should be remembered that only the larger cliamicls arc .shown and that in between there is everywhere a fine anastomosing network. Also, the channels do not lie all in the same plane. Furthermore, it is to be noted that there exists in embryos of the same age a considerable variation in the pattern formed by these channels. The three specimens selected, however, may be regarded as illustrating fairly definite stages in this transformation.
In figure 7 is shown a dorsal view of the head of the same embryo previously shown in figure 1 (Carnegie Collection, No. 940, 13.8 mm. long). It is about the
same age as the embryos shown in figures 24 and 25. Here we find the sagittal plexus represented in its simplest form. It will be noted that it possesses two
characteristic features: In the first place, there is a tendency to an enlargement of certain portions of the plexus, irrespective of a continuous channel. We thus have
a series of small lakelets connected by narrow channels. A definite single superior sagittal sinus can not yet be said to exist. In the second place, the plexus is distinctly asymmetrical and shows a tendency to drain more freely to one side than the other, in this case to the right.
Figs. 7, 8. and 9. Three stages in the formation of the sagittal plexus, showing its asymmetrical character and its conversion into the superior sagittal sinus. Draining into it from below is the drainage-channel from the chorioidal bodies that is later known as the straight sinus. The channels marked x are interpreted as undergoing retrogression, being replaced by more caudal channels. Large dural channels do not persist in the areas covering the cerebral hemispheres; they are found only in the loose embryonic tissue filling the spaces and fissures that lie between the different subdivsions of the brain. Figure 7 is a vertex view of a human embryo 13.8 mm. long (Carnegie Collection. No. 940). Figure 8 is a vertex view of an embryo 20 mm. long (Carnegie Collection, No. 349). Figure 9 is a drawing of an injected and cleared specimen 54 mm. long (Carnegie Collection, No. 458) reversed.
A more definite and simpler channel system is found in 20 mm. embryos, an example of which is shown in figure 8 (Carnegie Collection, No. 349). Here one might possibly speak of a superior sagittal sinus. The channels, however, are still in the form of a plexus, and hence the term plexus sagittalis is retained. This view regarding the early identity of the superior sagittal sinus differs from that given by Evans, who pictures the primitive capillary plexus creeping up on each side of the forebrain in 8 mm. pig embryos. A portion of the dorsal margin of this plexus he labels as the primitive superior sagittal sinus (Evans, 1909, fig. 156; Evans, 1912, figs. 399 and 400). According to him it is thus originally paired and bilaterally symmetrical. According to the present writer, it is not until later that we can speak of a superior sagittal sinus. It is not until the plexuses, described by Evans, have anastomosed across the median line and have formed a longitudinal network in the meshes of which an asymmetrical channel is finally established, that we can speak of a superior sagittal sinus. In some cases two or more larger longitudinal channels are formed in the mesh, as is shown in figure 10; but in such cases they are not strictly bilaterally symmetrical.
Owing to the growth of the cerebral hemispheres in 20 mm. embryos, there is formed a well-marked cerebral longitudinal fissure which is occupied by embryonic tissue. This rapidly takes the form of the adult falx cerebri. It is in the dorsal part of this loose dural tissue that the meshes of the sagittal plexus are found.
Figure 10. Camera-lucida sketch of the vertex of the head of a human embryo 27.5 mm. long (Carnegie Collection, No. 1458), in which the blood-vessels were conspicuous through a natural injection. It is the exception where two channels are found so nearly the same size. From a single specimen, like this, one might incorrectly gain the impression that the superior sagittal sinus is at first bilaterally symmetrical. Even here, however, it can be seen that both of the larger channels drain caudally to the right side. The minute anastomosing loops that connect the two main channels with each other and with the channels in the more ventral parts of the falx are not shown. On each side can be seen the serrated upper margin of the advancing capillary plexus of the skin. Above this margin the skin is as yet nonvascular. On account of the dilatation of these vessels along this advancing edge the margin is conspicuous.
At this time it can be seen that one or more larger channels are opening along the dorsal mid-line, which will form the superior sagittal sinus, and connected with them by anastomosing loops is a more ventrally situated large channel that constitutes the sinus rectus. This latter extends forward and drains the lower part of the falx. It has two converging limbs in front that drain the choroidal masses of the hemispheres. In figure 20 a portion of the right hemisjihere is removed to expose the region of the falx cerebri. Here the straight sinus can be seen as differentiated out from the sagittal plexus, with which it anastomoses freely at its caudal end in a plexiform manner. Anteriorly the straight sinus bifurcates, enters the choroidal fissure on each side, and terminates in the sinus-like choroidal bodies. These, on the other hand, are fed from the caudal end by the choroidal arteries.
On coming to embryos 50 mm long, figure 9 (Carnegie Collection, No. 458, 54 mm.), we find that here the superior sagittal sinus is established, at least in part.
In its cephalic portion there is a large characteristic channel, lacking only the dural connective-tissue investment to make it an adult type. In its more caudal portion
it still exhibits a plexiform character that indicates its transitional state. Upon
comparison of a number of series, the writer is led to interpret the formation of a
single channel as the outcome of more than one process; in some segments there
seems to be the selection of a favorable loop of the plexus which enlarges and becomes the main channel, and in other segments there is apparently an enlargement
of two or more collateral loops which subsequently fuse into a more or less common
channel. Both processes are apparently represented in figure 9. It is to be
expected that we will find a considerable variation in this respect in different
brains. In figure 10 is shown a specimen which is about the same age as that
shown in figure 8. In this case two collateral channels of about equal size have formed, both draining, however, to the same side.
| Fig. 11. Section showing the sagittal plexus in a human embryo 14 mm long (Carnegie Collection, No. 940 (slide 15 row 3. section 1) The section shows the falciform area, the hemispheres being retracted from its lateral margins. It will be noted that there are two main plexiform vascular sheets — a superficial one near the skin and a deeper one directly against the brain-wall, the latter draining into the former by anastomosing loops. The superior sagittal sinus develops in the meshes of the superficial plexus. and the straight sinus develops in the meshes of the deep plexus over the area corresponding to the third ventricle. | 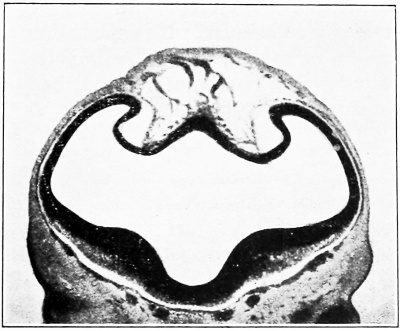
|
| Flg. 12. Section showing the sagittal plexus in a human embryo 21 mm. long (Carnegie Collection, No. 460, slide 11, row 3, section 4), injected with India ink. The arrangements are similar, but more advanced than those shown in figure 11. The straight sinus can be recognized. The superior sagittal sinus still has the form of a bilaterally asymmetrical plexus from which a finer meshwork extends into the loose falciform tissue intervening between the two hemispheres and anastomoses with the straight sinus. On the left side a characteristic communication can be seen connecting the deep cerebral plexus with the coarser sagittal plexus above. | 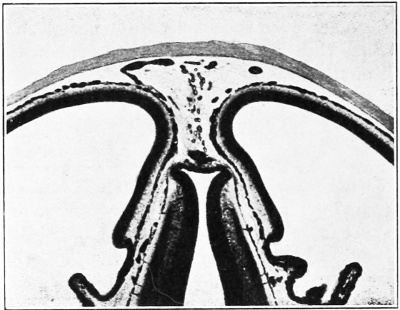
|
No attempt was made to study the histological changes that occur in the completion of the superior sagittal sinus, or of the cavernous sinus. These involve details with which the present paper is not concerned. The caudalward growth, however, of the superior sagittal sinus in adjustment to the corresponding growth of the hemispheres is of interest in our general problem. By comparing figures 7, 8, and 9 it can at once be seen that this caudal development is accomplished at the expense of the meshes of the tentorial plexus, in which process the transverse and straight sinuses also take part. These channels gradually obtain a more caudal course by what we have already described as spontaneous migration. The channel repeatedly shifts into a more caudal loop of the plexus, the new loop enlarging and the old loop dwindling. The veins marked X in figures 8 and 9 may thus be interpreted as discarded channels. The eventual confluens sinuum (torcular Herophili) represents the point at which this caudal development reaches its completion — or, in other words, is a remnant of the embryonic tentorial plexus and usually retains a trace of the plexiform character that is found throughout the embryonic stages. It is interesting to note that the asymmetry of the superior sagittal sinus expresses itself in the embryo as well as in the adult by a tendency to drain more to one side of the head than to the other. This becomes established by the time the embryo is 20 mm. long. The drainage is preponderantly toward the right side. It happens that in figure 9 the main drainage was in reality toward the left side. In reproducing the sketch the figure was reversed right for left, in order to facilitate its comparison with figures 7 and 8. In the accompanying table is given a list of embryos which were examined as to this point, and it will be seen that of 18 specimens all but 2 drained predominantly toward the right side, that is, about 89 per cent. In order that account should be taken of the artificial element introduced in those specimens where the vascular system had been injected with coloring matter, such specimens are indicated in the table by an asterisk. No explanation has thus far been reached to explain this interesting asymmetry. The drainage of the straight sinus could not be determined as well in the younger stages, and there were not enough of the older stages upon which to base an average. A similar asymmetry might be expected here.
Summary
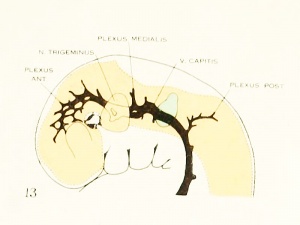
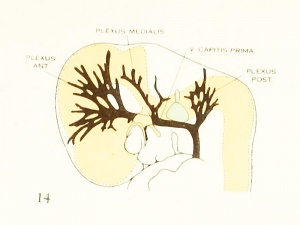
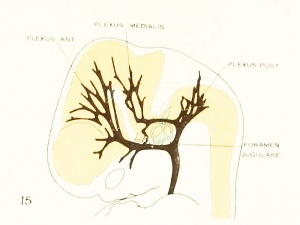
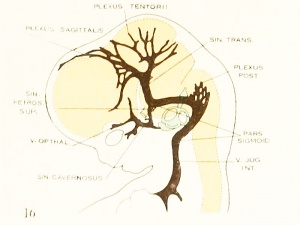
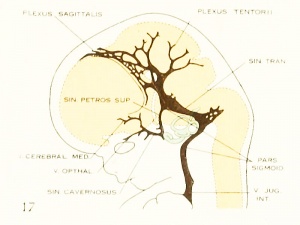
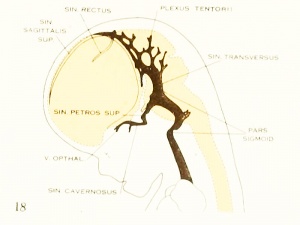
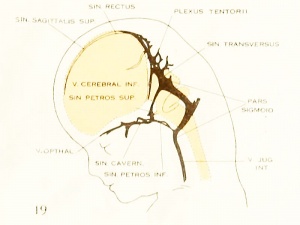
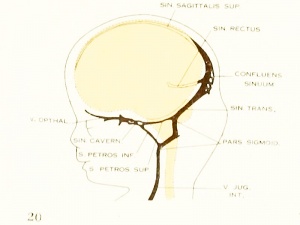
In describing the development of the blood-vessels of the drain the process has been subdivided into the following five arbitrary periods:
- angioblastic period, in which the primordial vascular system becomes established;
- resolution of the primordial system into arteries, veins, and capillaries, and the establishment of the primary type of circulation;
- cleavage of the vascular system of the head into the external, dural, and cerebral layers;
- adjustments of vascular channels, due chiefly to growth and change in form of the otic capsule and the brain; and
- completion of histological differentiation of the walls of the vessels.
Aside from adding perhaps more emphasis to the morphological aspects of the pre-circulatory type of the vascular system, the contributions of this paper are concerned only with the third and fourth developmental periods, and even there they are more or less restricted to the main drainage-channels and their gradual metamorphosis into the adult dural veins and sinuses. It has been possible to present a rather complete series of stages from which the essential factors in this process can be clearly deduced. In order to facilitate a review of the successive steps in this interesting metamorphosis, there have been assembled on plate 1, figures 13 to 21, a series of simplified sketches, and through the aid of these it is hoped that the steps that are outlined in the following summary can be readily identified.
In the primary type of circulation the arrangement for the drainage of the capillaries of the head (figs. 13, 14) consists bilaterally of one main channel, the "primary head-vein," that starts in the region of the midbrain, runs caudalward alongside of the brain-tube, and terminates at the duct of Cuvier. The primary head-vein is composite in origin. That portion of it rostral to the vagus nerve is an intrinsic vein of the head; the remaining caudal portion is in reality a neck- vein and constitutes the anterior cardinal vein — eventually the internal jugular vein. Together these portions form a continuous channel, the primary head-vein, into
which the blood from the capillary sheet immediately investing the brain-tube is
drained by means of anastomosing venous loops. These loops are arranged more
or less in the form of three plexuses — the anterior dural plexus, the middle
dural plexus, and the posterior dural plexus. Other small tributaries which are
not all shown in the figures empty into the primary head-vein, thereby draining
the structures ventral and lateral to the brain-tube, such as the nerve-ganglion masses and the maxillary and mandibular gill-bars. A large one comes from the eye region and eventually is modified into the ophthalmic vein.
From this simple group of drainage channels are eventualy derived all the
adult venous sinuses. The metamorphosis which they undergo is based on a series
of circulatory adjustments that are made necessary by certain changes in their
environment, the two most conspicuous being the changes in the region of the
cartilaginous capsule of the labryinth and the still greater changes involved in the
growth and marked alteration in the form of the brain. Among the factors involved
in these circulatory adjustments may be mentioned the reduction of plexuses into
simple channels, the conversion of channels into plexuses, the total obliteration
of established channels, and the change in position of channels. Under this latter
phenomenon there is to be recognized a " passive migration," where there is a change
in the position of the vein-wall itself, due to the movement of its environment,
which exerts a flexion or traction force upon it. We also recognize a "spontaneous
migration," where there is a change in position of the blood-stream only, where in a
circumfluent manner the blood-stream develops a new channel in the adjacent loops
of the plexus, with a corresponding dwindling of the previously used channel.
The "replacement channel" might be mentioned as another type of spontaneous
migration, in which the venous channels are changed in position and direction in
this process of adjustment. In the replacement channel there is the formation of
a new channel and the obliteration of an old one, as in other types of spontaneous migration. It, however, differs from them in that it is not a gradual and
progressive change in position, but an abrupt and immediately complete one.
Furthermore, the new channel lacks the morphological characteristics of the
old one. With these various factors in mind one can readily follow the steps by
which the primary head-vein and its tributaries gradually merge into adult dural
sinuses.
While the three head-plexuses are spreading upward (figs. 14 and 15), the outlines of the dura mater and the arachnoid spaces make their appearance, and first of all in the ventral parts. This results in a general separation or cleavage of the more superficial primary head-vein and its throe tributary plexuses from the subjacent vessels that arise from and drain the capillary sheet directly investing the brain-tube. This deeper system, however, continues to drain into the former at certain places, notably in the more dorsal parts. The primary head-vein and its three tributary plexuses thus become established as a true dural system as distinguished from the deeper "cerebral veins" belonging to the arachnoid-pial membrane. The diploic veins are a later subdivision of the dural system. The superficial vessels of the head belonging to the integument and soft parts are separated off in the more ventral regions and from there sj^read ui)ward over the head independently of the dural system. We then have for the head three separate systems: (1) the superficial layer belonging to the integument and soft parts;
(2) the middle layer belonging to the dura and diploe; (3) the deep layer of cerebral vessels belonging to the brain. It is the middle layer, or dural system, that is exclusively concerned in the formation of the dural sinuses and whose changes in form and position we are now following.
In the region of the cartilaginous capsule of the labyrinth adaptive changes
in the dural channels occur early (figs. 14, 15, and 16). Owing to the marked
elaboration of these structures in this region, the course of the primary head-vein,
ventro-lateral to the otic capsule, becomes an unfavorable one. If it persisted it
would be tortuous and remote from the area drained; instead, this part of it becomes
obliterated, and during this obliterating process an adjustment is made in two ways
(figs. 14, 15, and 16): first, a channel is established in the venous plexus above
the otic capsule, and through this the middle dural plexus thereafter drains caudally
into the loops of the posterior dural plexus; second, the anterior dural plexus,
which originally drained into the primary head- vein, completely reverses its direction of flow and drains through anastomosing loops into the middle dural plexus and
through the newly established channel dorsal lo the otic capsule.
In this way a complete trunk for the drainage of the head becomes established
which is everywhere dorsal to the primary head-vein as far as the jugular foramen,
where it is continuous with the internal jugular vein. Of the primary head-vein
there is left, in addition to the cardinal portion of it or internal jugular vein, only
that part in the region of the trigeminal nerve. This may now be spoken of as the
"cavernous sinus." Into it drain a vein from the base of the brain and the veins
from the orbital and maxillary regions: whereas it, in turn, drains upward through
the original trunk of the head plexxus, which is now the superior jictros.'il sinus.
into the newly established dorsal channel. By comparing with later stages (figs.
17 to 21) it will be seen at once that this dorsal channel is the transverse sinus, of
which that part between the superior petrosal sinus and the jugular foramen forms
its sigmoid portion. Thus in the 21 mm. embryo the dural channels in the region
of the tempotral bone have aquired essentially all their permanent connections, with
the exception of the inferior petrosal sinus, which appears a little later fig. 19).
Otherwise there remains to complete the adult condition only a certain amount of passive migration in accommodation to the changes in the adjacent parts.
The adjustment in the dural channels rendered necessary by the protracted growth of the hemispheres extend much later in fetal life. A large part of this adjustment is accomplished by spontaneous migration of the principal channels, and for this reason a venous plexus is essential. We thus find in the neighborhood of the advancing occipital pole of the hemispheres a continuous persistence of the transitory or embryonic dural plexus from which are evoh-ed all the veins of the falx cerebri and of the tentorium cerebelli.
An anterior subdivision of the plexus extends forward in the median line as the plexus sagittalis, being interposed as a vertical curtain between the hemispheres.
Among its dorsal meshes is developed an asymmetrical longitudinal channel which we know as the "superior sagittal sinus." In its early stages this channel is made
up of several collateral anastomosing veins. The eventual single channel is formed in the anterior portions by the selection and enlargement of the most, favorable
vein with a corresponding disappearance of the others. In the posterior portions there is apparently some coalescence of adjacent veins. The anterior part of the
sinus is completed first. As the hemispheres extend backward the sinus correspondingly elongates itself by incorporating the more caudal loops of the plexus.
Transverse sections through this part of the sinus in older fetuses thus usually reveal incomplete coalescence of the separate loops. The sagittal plexus very early
exhibits a tendency to drain more to one side of the head than to the other and usually toward the right side. As the superior sagittal sinus becomes established we thus find that caudalward it is usually continuous with the ventral main channel of the right anterior plexus (or tentorial plexus as it is better called in the late stages),
which eventually forms part of the right transverse sinus. The straight sinus is formed in the ventral part of the sagittal plexus and its caudal adjustment is
essentially like that of the superior sagittal sinus. It may drain chiefly toward the right or left plexus or equally toward both.
In embryos between 35 and 50 mm. long (figs. 18 and 19) we can recognize a
main channel of the tentorial plexus that is to become the transverse sinus.
If we disregard the sigmoid portion of it, it forms a fairly straight line with the
internal jugular vein. In the interval between the 50 mm. embryo and the adult
the transverse sinus bends backward until it comes to lie at an angle of 90° with
the internal jugular. This marked change in position is accomplished in large
part by spontaneous migration, by the repeated shifting back of the main blood current into more caudal loops of the plexus, with subsequent dwindling of the
discarded anterior loops. As the sinus becomes more definitely established the
tentorial plexus becomes relatively smaller (fig. 20) and the final change in position
is completed by passive migration, that is, actual traction on the vein-wall by its
environment. In this change in position of the transverse sinus the superior
sagittal sinus and the straight sinus participate and we find in the adult, at the point
where they meet, an anastomosis, the confluens sinuum which is usually plexiform in character and represents the last trace of the embryonic tentorial plexus.
References Cited
Elze, C. 1907. Beschreiliuiig eiiip.s menschlichen Embryo von za. 7 mm. grosstcr Lange. Anat. Hefto. No. 106, vol. Abth. 1.
Evans HM. On the development of the aortae, cardinal and umbilical veins, and the other blood vessels of vertebrate embryos from capillaries. (1909) Anat. Rec. 3: 498-518.
Evans HM. The development of the vascular system. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp570-708.
Grosser, O. 1907. Die Elemente des Kopfvenensyatem der Wirbeltiere. Verh. Anat. Gesell., Anat. Anz., Bd. ,S0,
Erriinz. H/t. und E. Brezin.\. 1895. I'eber die Entwicklung der \'eiien des Kopfes und Halses bei Reptilien. MoHJh. Jahrbuch, Bd. 23.
His, W. 1904. Die Entwickelung des menschlichen Gehirns. Leipzig.
Hochstetter. K. 1916. Ucber die Vaskularisation der Haut des Schadeldaches menschlicher Embryonen. K. .\kad. d. Wias. Wien, Math.-Naturwiss. Kl., Bd. 93.
Ingalls NW. Description of a human embryo of 4.9 mm (Beschreibung eines menschlichen Embryos von 4.9 mm). (1907) Arch. f. mik. Anat., 70: 506-576.
Mall FP. On the development of the blood-vessels of the brain in the human embryo. (1905) Amer. J Anat. 4(1): 1–18.
Mabkowski, J. 1911. Ueber die Entwicklung der Sinus durae matris und der Hirnvenen bei menschlichen Embryonen. Bull. d. I'Acad. d. .Sci. de Cracovje. ("I. d. ,Scl, math. -Nat., .sSerie B.
Sabin FR. On the fate of the posterior cardinal veins and their relation to the development of the vena cava and azygos in the embryo pig. (1915) Pub. No. 223 Contrib. Embryol., Carnegie Inst. Wash. 3(7): 5-32. PDF
Sabin FR. Origin and development of the primitive vessels of the chick and of the pig. (1917) Contrib. Embryol., Carnegie Inst. Wash. 6: 61–124.
Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma, and red blood-cells as seen in the living chick. (1917) Anat. Rec. 13: 199-204
Salzer, H. 1895. Ucber die Entwicklung der Kopfvenen des Meerschweinchens. Morph. Jahrbuch, Bd. 23.
Shindo, T. 1915. Ueber die Bedeutung des Sinus cavernosus der Sauger mit vergleichend anatomischer Beriicksichtigung anderer Kopfvenen. Anat. Heft«, Abth. 1, Heft 157, Bd. 52.
Explanation of Plates
Plate 1
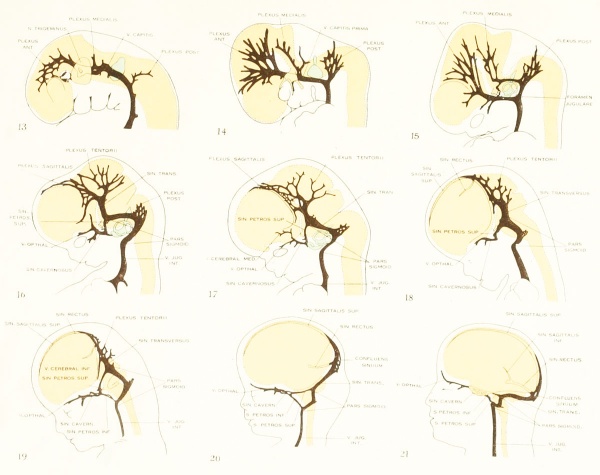
|
Simplified profile drawings of the dural veins, showing the manner in which they adapt themselves to the growth and change in form of the brain in human embryos from 4 mm to birth.
|
Fig. 13, embryo No. 588, 4 mm
Fig. 14, embryo No. 940, 14 mm
Fig. 15, embryo No. 144, 18 mm
Fig. 16, embryo No. 460, 21 mm
Fig. 17, embryo No. 632, 24 mm
Fig. 18, embryo No. 199, 35 mm
Fig. 19, embryo No. 96, 50 mm CR length
Fig. 20, embryo No. 234a, 80 mm CR length
Plate 2
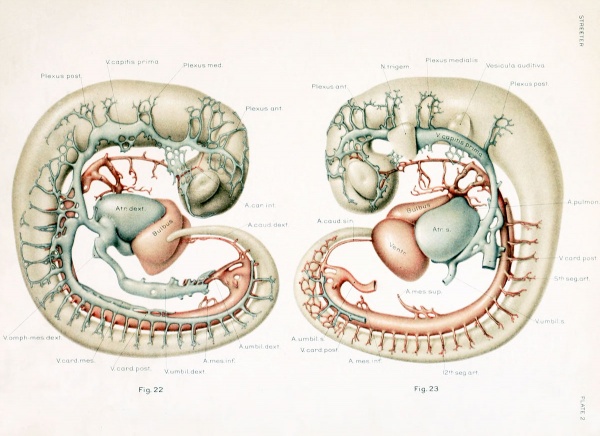
|
Right and left profile views of a wax-plate reconstruction of the main arteries and veins in a human embryo 4 mm. long
(Carnegie Collection, No. 588). Enlarged about 40 diameters. (original print version)
|
Plate 3
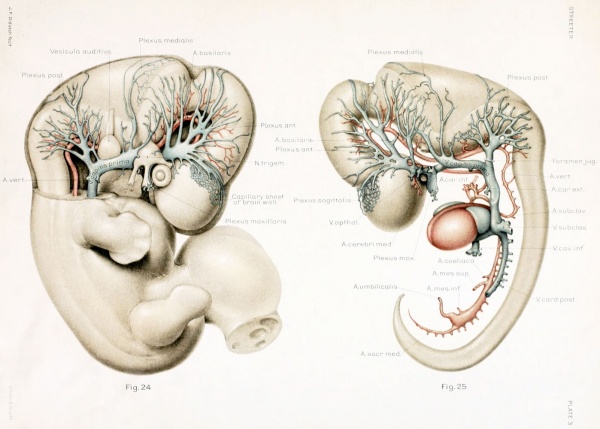
|
Right and left profile views of a wax-plate reconstruction of the blood-vessels of the brain in a human embryo 11.5 mm long.
Carnegie Collection Embryo No. 544 Enlarged about 14 diameters. (original print version)
|
Plate 4
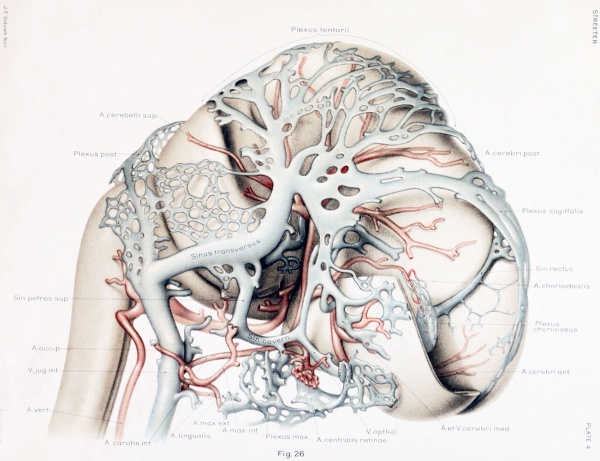
|
Left lateral view of a wax-plate reconstruction of the larger blood-vessels of the brain in a human embryo 21 mm long (Carnegie Collection, No. 460). Enlarged 16.4 diameters. (original print version)
|
Plate 5
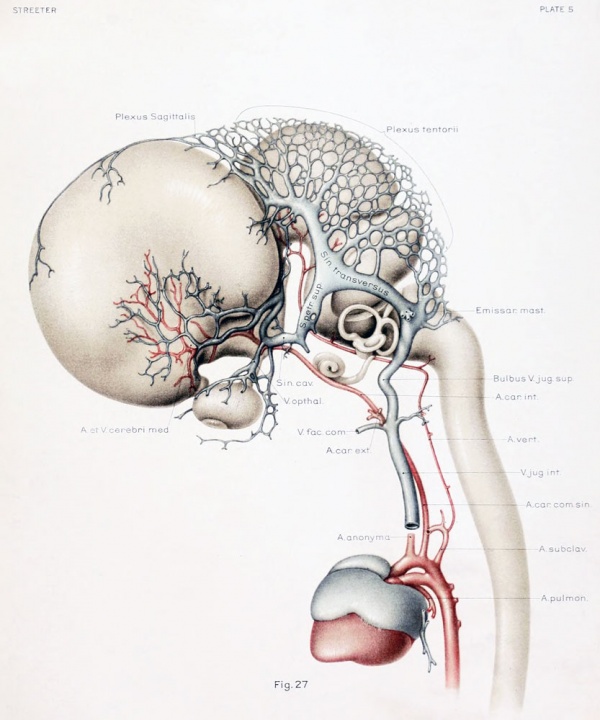
|
Left profile view of a wax-plate reconstruction of the blood-vessels of the brain in a human embryo 43 mm long (Carnegie Collection, No. 886). Enlarged 8 diameters. (original print version)
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 30) Embryology Paper - The developmental alterations in the vascular system of the brain of the human embryo (1921). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_developmental_alterations_in_the_vascular_system_of_the_brain_of_the_human_embryo_(1921)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
- Neural
- Cardiovascular
- Head
- Historic Embryology
- 1920's
- Human
- George Streeter
- Carnegie Embryo 84
- Carnegie Embryo 96
- Carnegie Embryo 144
- Carnegie Embryo 199
- Carnegie Embryo 234a
- Carnegie Embryo 349
- Carnegie Embryo 458
- Carnegie Embryo 460
- Carnegie Embryo 544
- Carnegie Embryo 588
- Carnegie Embryo 632
- Carnegie Embryo 940
- Carnegie Embryo 1458


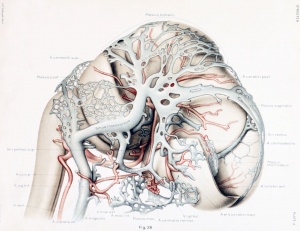
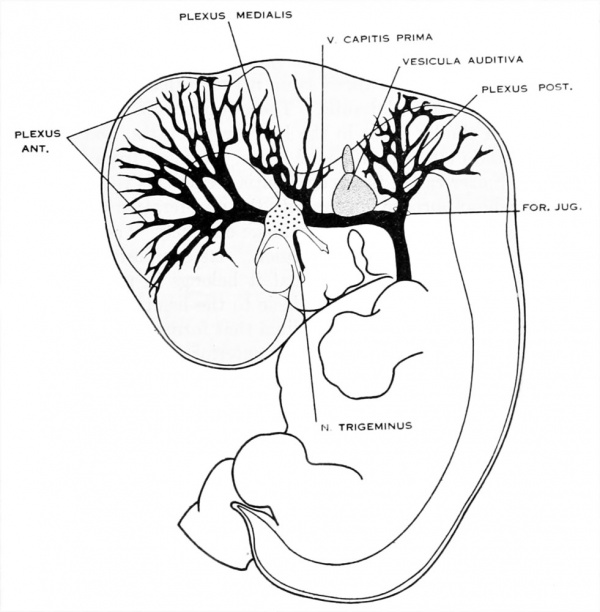
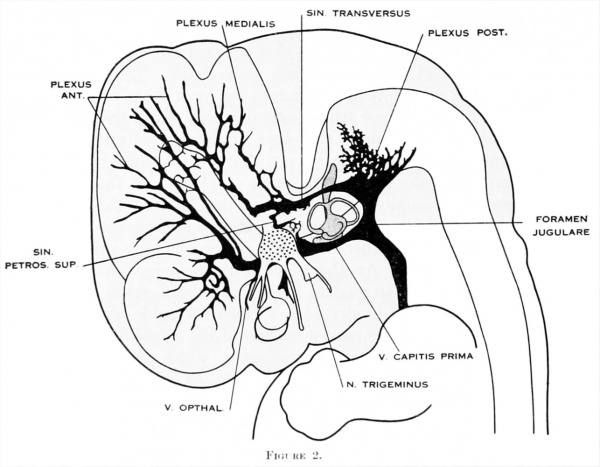
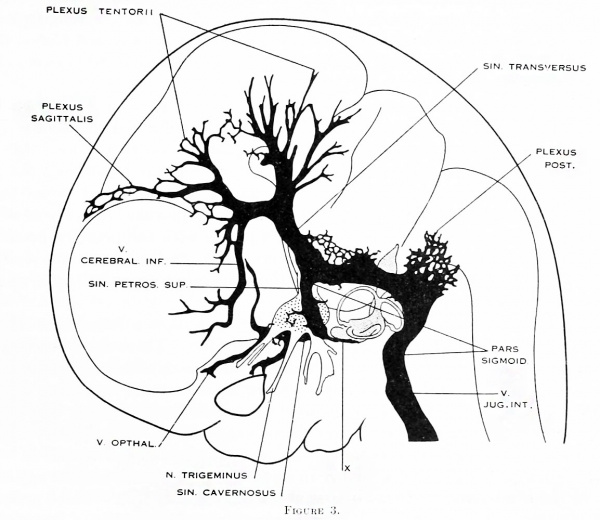
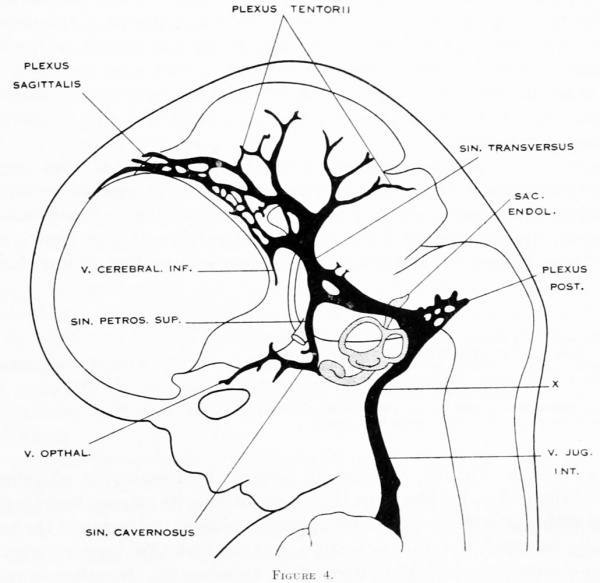
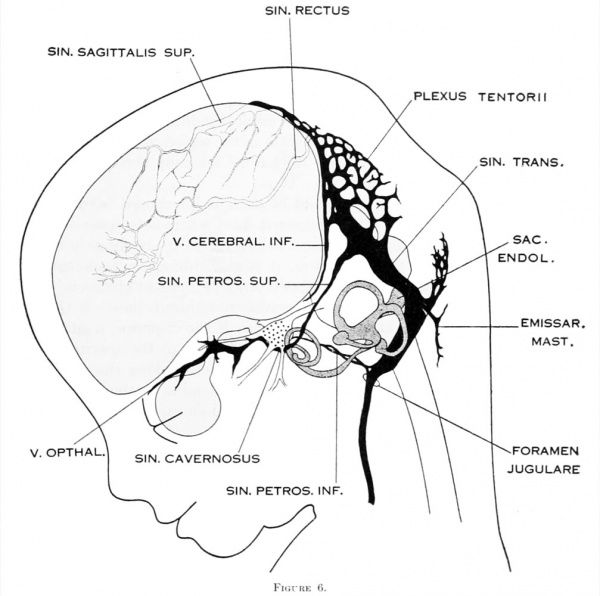
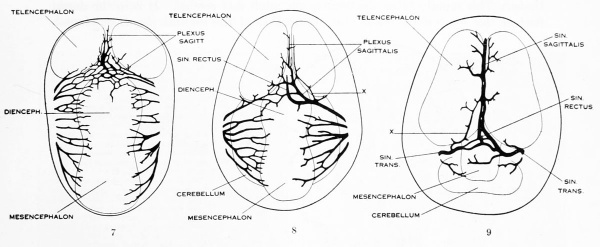
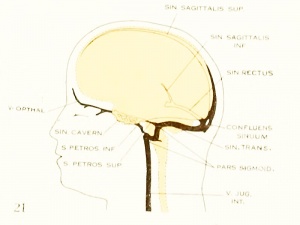
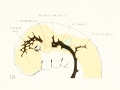
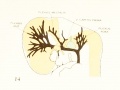
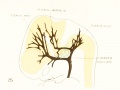

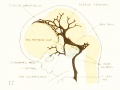
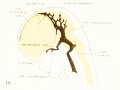
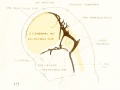
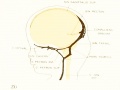
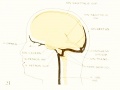
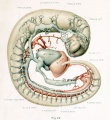
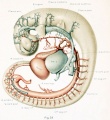
![Right profile view human embryo 11.5 mm long]]](/embryology/images/thumb/2/28/Streeter1921_fig24.jpg/110px-Streeter1921_fig24.jpg)
![Left profile view human embryo 11.5 mm long]]](/embryology/images/thumb/f/f4/Streeter1921_fig25.jpg/110px-Streeter1921_fig25.jpg)