Introduction
| The heart rate (beats / minute) is a measurement that can be made from early in development (when the heart first starts) through embryonic and fetal stages into labor and birth. It is a common clinical diagnostic tool, but data can be variable between countries and institutions.
Early ultrasonographic measurement of embryonic heart rate (EHR) shows a steady increase from Stage 9 - 10 (75 beats/minute) to Stage 18 (130 beats/minute) and on to Stage 20, following which a gradual decrease in EHR occurs. This increase correlates with heart development and a low EHR is used as an indicator of developmental failure and likely abortion. Late stethoscope measurements of fetal heart rate can monitor fetal stress and identifies the characteristic "lub-dub" heart valve sounds.
|
<html5media height="280" width="320">File:Chicken_heartloop 01.mp4</html5media>
Click Here to play on mobile device
Chicken embryo heart beat. Normal Chick Heart Movie
|
Some Recent Findings
- Conversion of human cardiac progenitor cells into cardiac pacemaker-like cells[1] "We used a screening strategy to test for reprogramming factors for the conversion of human cardiac progenitor cells (CPCs) into Pacemaker-like cells. Human transcription factors SHOX2, TBX3, TBX5, TBX18, and the channel protein HCN2, were transiently induced as single factors and in trio combinations into CPCs, first transduced with the connexin 30.2 (CX30.2) mCherry reporter. Following screens for reporter CX30.2 mCherry gene activation and FACS enrichment, we observed the definitive expression of many pacemaker specific genes; including, CX30.2, KCNN4, HCN4, HCN3, HCN1, and SCN3b. These findings suggest that the SHOX2, HCN2, and TBX5 (SHT5) combination of transcription factors is a much better candidate in driving the CPCs into Pacemaker-like cells than other combinations and single transcription factors. Additionally, single-cell RNA sequencing of SHT5 mCherry+ cells revealed cellular enrichment of pacemaker specific genes including TBX3, KCNN4, CX30.2, and BMP2, as well as pacemaker specific potassium and calcium channels (KCND2, KCNK2, and CACNB1). In addition, similar to human and mouse sinoatrial node (SAN) studies, we also observed the down-regulation of NKX2.5. Patch-clamp recordings of the converted Pacemaker-like cells exhibited HCN currents demonstrated the functional characteristic of pacemaker cells. These studies will facilitate the development of an optimal Pacemaker-like cell-based therapy within failing hearts through the recovery of SAN dysfunction."
- Non-invasive fetal electrocardiography for the detection of fetal arrhythmias[2] "A total of 500 echocardiography and NI-FECG recordings were collected from pregnant women during a routine medical visit in this multicenter study. All the cases with fetal arrhythmias (n = 12) and a matching number of control (n = 14) were used. Two perinatal cardiologists analyzed the extracted NI-FECG while blinded to the echocardiography. The NI-FECG based diagnosis was compared to the reference fetal echocardiography diagnosis....It is possible to diagnose fetal arrhythmias using the NI-FECG technique. However, this study identifies that improvement in algorithms for reconstructing the P-wave is critical to systematically resolve the mechanisms underlying the arrhythmias. The elaboration of a NI-FECG Holter device will offer new opportunities for fetal diagnosis and remote monitoring of problematic pregnancies because of its low-cost, non-invasiveness, portability and minimal set-up requirements."
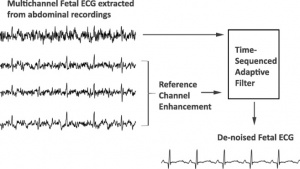 Fetal Electrocardiogram Enhancement [3]- Enhancement of low-quality fetal electrocardiogram based on time-sequenced adaptive filtering[3] "Extraction of a clean fetal electrocardiogram (ECG) from non-invasive abdominal recordings is one of the biggest challenges in fetal monitoring. An ECG allows for the interpretation of the electrical heart activity beyond the heart rate and heart rate variability. However, the low signal quality of the fetal ECG hinders the morphological analysis of its waveform in clinical practice. The time-sequenced adaptive filter has been proposed for performing optimal time-varying filtering of non-stationary signals having a recurring statistical character. In our study, the time-sequenced adaptive filter is applied to enhance the quality of multichannel fetal ECG after the maternal ECG is removed. To improve the performance of the filter in cases of low signal-to-noise ratio (SNR), we enhance the ECG reference signals by averaging consecutive ECG complexes. The performance of the proposed augmented time-sequenced adaptive filter is evaluated in both synthetic and real data from PhysioNet. This evaluation shows that the suggested algorithm clearly outperforms other ECG enhancement methods, in terms of uncovering the ECG waveform, even in cases with very low SNR. With the presented method, quality of the fetal ECG morphology can be enhanced to the extent that the ECG might be fit for use in clinical diagnostics. Graphical abstract The extracted fetal ECG signals from non-invasive abdominal recordings still contain a substantial amount of noise. The time-sequenced adaptive filter provides a relatively accurate estimate of the underlying fetal ECG signal when the quality of the reference channels is enhanced prior to filtering."
- Computerized analysis of cardiotocograms and ST signals is associated with significant reductions in hypoxic-ischemic encephalopathy and cesarean delivery: an observational study in 38 466 deliveries[4] "Intrapartum cardiotocography (CTG) is widely used in high-resource countries and remains at the centre of fetal monitoring and the decision to intervene, but there is ample evidence of poor reliability in visual interpretation, as well as limited accuracy in identifying fetal hypoxia. Combined monitoring of CTG and ST segment signals was developed to increase specificity, but analysis relies heavily on CTG interpretation and is therefore also affected by the previously referred problems. Computerized analysis was developed to overcome these limitations, aiding in the quantification of parameters that are difficult to evaluate visually, such as variability, integrating the complex guidelines of combined CTG and ST analysis, and using visual and sound alerts to prompt healthcare professionals to re-evaluate features associated with fetal hypoxia."
- Quantile Score: A New Reference System for Quantitative Fetal Echocardiography Based on a Large Multicenter Study[5] "Normative ranges of fetal echocardiographic measurements are important for quantitative diagnosis of fetal cardiovascular disease. The current normative ranges were derived from small samples and were based on the hypothesis of a normal distribution of these measurements during fetal cardiovascular growth. The aims of this study were to test the hypothesis of a normal distribution of fetal echocardiographic measurements in a large multicenter cohort and to propose a reference system without the normal distribution hypothesis to improve accuracy of fetal echocardiographic measurements. ...All fetal echocardiographic measurements showed non-normal distributions (P < .001). The normal range was underestimated by ordinary least squares regression compared with quantile regression by 30 ± 11%. The partial normalized areas under the receiver operating characteristic curve within the 20% false-positive rate were 0.62 and 0.50 for the q and Z scores, respectively."
- Diurnal rhythm of fetal heart rate in third trimester of pregnancy[6] (Article in Chinese) "To investigate the diurnal rhythms of fetal heart rate in third trimester of pregnancy. Methods: From June 2014 and October 2017, 97 cases of low-risk pregnancy women who received antenatal care and deliveried in Peking University Third Hospital were collected. Totally 130 cases of fetal heart rate and maternal holter monitoring data were analyzed. All cases were singleton pregnancy, cephalic position and had normal perinatal outcome. ...Fetal heart baseline (FHB)、fetal heart baseline variation (FHBV)、fetal heart rate acceleration area and maternal heart rate all presented diurnal rhythms. (1) FHB rose in daytime and decreased at night with the minimum value at 2:00-5:00, and didn't decline further at night with the advancing of gestational age (P=0.548). (2) FHBV was similar to FHB, which rose in daytime and decreased at night, but declined smaller at night with the advancing of gestational age, especially after 37 weeks (P<0.01). (3) Fetal heart rate acceleration area reduced in daytime and enlarged at night, and enlarged more with the advancing of gestational age. (4) The diurnal rhythm of maternal heart rate was consistent with fetal heart rate. FHB lagged behind maternal heart rate for 1-2 hours when declining to the nocturnal nadir but been basically in sync with maternal heart rate when recovered."
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- Antenatal cardiotocography for fetal assessment[7] "Cardiotocography (CTG) is a continuous recording of the fetal heart rate obtained via an ultrasound transducer placed on the mother's abdomen. CTG is widely used in pregnancy as a method of assessing fetal well-being, predominantly in pregnancies with increased risk of complications. ...There is no clear evidence that antenatal CTG improves perinatal outcome, but further studies focusing on the use of computerised CTG in specific populations of women with increased risk of complications are warranted."
- Normal Ranges of Embryonic Length, Embryonic Heart Rate, Gestational Sac Diameter and Yolk Sac Diameter at 6-10 Weeks[8] "We examined 4,698 singleton pregnancies with ultrasound measurements of CRL, HR, GSD and YSD at 6-10 weeks and CRL at 11-13 weeks resulting in the live birth after 36 weeks of phenotypically normal neonates with birth weight above the 5th centile. Gestational age was derived from CRL at the 11- to 13-week scan using the formula of Robinson and Fleming."
|
Textbooks
- Human Embryology (2nd ed.) Larson Ch7 p151-188 Heart
- The Developing Human: Clinically Oriented Embryology (6th ed.) Moore and Persaud Ch14: p304-349
- Before we Are Born (5th ed.) Moore and Persaud Ch12; p241-254
- Essentials of Human Embryology Larson Ch7 p97-122 Heart
- Human Embryology Fitzgerald and Fitzgerald Ch13-17: p77-111
Embryonic Heart Rate
In a 1996 study normal successful human gestations were defined by EHR criteria at different early embryonic (34-56 days from last menstrual period) developmental stages (at the earliest stages when embryo length is difficult to measure gestational sac diameters are included).[9]
- Stage 9 - 10 2 mm embryo (gestational sac diameter of 20 mm) EHR at least 75 beats / minute
- Stage 11 - 12 5 mm embryo (gestational sac diameter of 30 mm) EHR at least 100 beats / minute
- Stage 16 10 mm embryo EHR at least 120 beats / minute
- Stage 18 15 mm embryo EHR at least 130 beats / minute
Fetal Heart Rate
Week 15 (GA week 17) Fetal Heart Rate audio recording of human embryo heart sounds .
Fetal Heart Sounds
<html5media>File:Week17 fetal heart rate.mp3</html5media>
Ultrasound Measurement
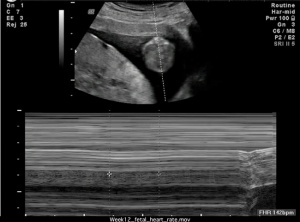
Measuring the fetal heart rate from ultrasound.
This movie is a realtime ultrasound recording of the week 12 fetus. The bottom window shows doppler analysis to measure the fetal heart rate.
<html5media height="560" width="680">File:Week12_fetal_heart_rate.mp4</html5media>
Click Here to play on mobile device
Electrocardiogram
Electrocardiogram (ECG) with a normal ECG on the left and an ECG showing T-wave inversion on the right.
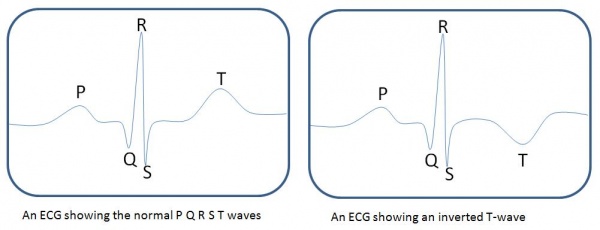
The T-waves represents the recovery/repolarisation of the ventricles. Inversion of T-waves relate to repolarisation abnormalities which may indicate a problem with the ventricles (in the recovery beat).
Heart Innervation
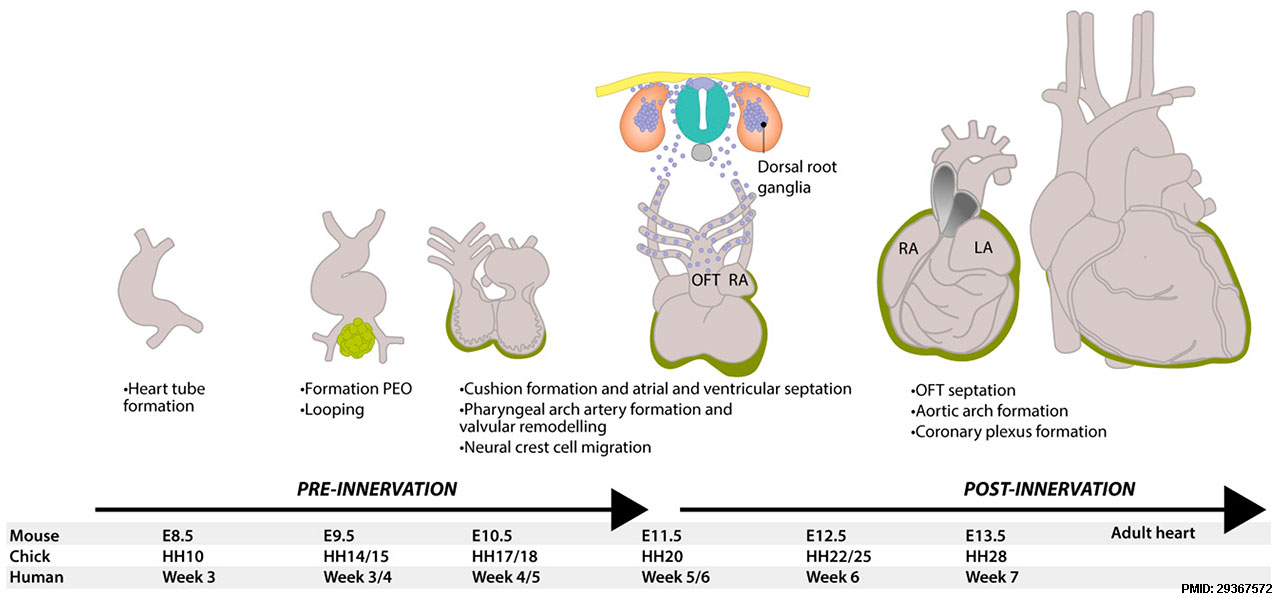
The 1976 study by Gardner and O'Rahilly[10], summarised below, described the developmental innervation of the heart at the end of the embryonic period (Week 8, Carnegie stage 23).
| Left side
|
Right Side
|
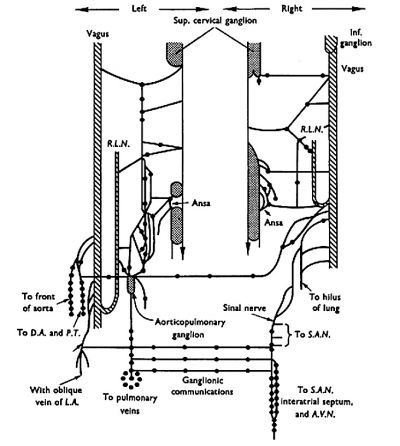
|
| from cervical sympathetic and from cervical and thoracic vagal (CN X) filaments.
|
from cervical sympathetic and from cervical and thoracic vagal (CN X) filaments.
|
Form several descending, ganglionated, vagosympathetic filaments that descended to the right of the arch of the aorta and entered the aorticopulmonary ganglion.
- Filaments leaving the ganglion supplied the pulmonary trunk, ascending aorta, interatrial septum, pulmonary veins, and, as the left sinal nerve, the fold of the left vena cava.
|
Interconnexions form vagoxympathetic nerves that:
- sent a branch in front of the trachea to the aorticopulmonary ganglion, supplying arterial and venous structures
- form the right sinal nerve, supplying the sinu-atrial node, and gave filaments to the interatrial septum which could be traced to the atrioventricular node and pulmonary veins.
|
- The thoracic vagal filaments descended to the left of the arch of the aorta and supplied chiefly the arterial end of the heart.
- No thoracic sympathetic cardiac filaments were found.
Sinu-Atrial Node
- began as a crescentic mass in front of the lower part of the superior vena cava.
- gradually extended on each side of the superior vena cava
- came to form its posterior wall at a more caudal level.
- atrial myocardium that formed the septum spurium, venous valves, and interatrial septum could be traced from the sinu-atrial to the atrioventricular node.
- Myocardium also encircled the atrial aspects of the atrioventricular orifices, and could be traced caudally to the atrioventricular node.
Atrioventricular Node
- was a mass in the anterior and lower part of the interatrial septum
- from which a defined bundle left to enter the interventricular septum.
- Right and left limbs were observed
- Right limb forms a rounded bundle that passed immediately in front of the root of the aorta.
References
- ↑ Raghunathan S, Islas JF, Mistretta B, Iyer D, Shi L, Gunaratne PH, Ko G, Schwartz RJ & McConnell BK. (2019). Conversion of human cardiac progenitor cells into cardiac pacemaker-like cells. J. Mol. Cell. Cardiol. , 138, 12-22. PMID: 31678351 DOI.
- ↑ Behar JA, Bonnemains L, Shulgin V, Oster J, Ostras O & Lakhno I. (2019). Non-invasive fetal electrocardiography for the detection of fetal arrhythmias. Prenat. Diagn. , , . PMID: 30602066 DOI.
- ↑ 3.0 3.1 Fotiadou E, van Laar JOEH, Oei SG & Vullings R. (2018). Enhancement of low-quality fetal electrocardiogram based on time-sequenced adaptive filtering. Med Biol Eng Comput , , . PMID: 29938302 DOI.
- ↑ Lopes-Pereira J, Costa A, Ayres-de-Campos D, Costa-Santos C, Amaral J & Bernardes J. (2018). Computerized analysis of cardiotocograms and ST signals is associated with significant reductions in hypoxic-ischemic encephalopathy and cesarean delivery: an observational study in 38 466 deliveries. Am. J. Obstet. Gynecol. , , . PMID: 30594567 DOI.
- ↑ Gu X, Zhu H, Zhang Y, Han J, Zhang H, Liu Y, Wang A, Liu B, Xue J, Sun B, Weng Z, Ge S & He Y. (2018). Quantile Score: A New Reference System for Quantitative Fetal Echocardiography Based on a Large Multicenter Study. J Am Soc Echocardiogr , , . PMID: 30591282 DOI.
- ↑ Li SF, Wang Y, Li GF, Zhao YY, Chen L & Zhang S. (2018). [Diurnal rhythm of fetal heart rate in third trimester of pregnancy]. Zhonghua Fu Chan Ke Za Zhi , 53, 849-854. PMID: 30585024
- ↑ Grivell RM, Alfirevic Z, Gyte GM & Devane D. (2012). Antenatal cardiotocography for fetal assessment. Cochrane Database Syst Rev , 12, CD007863. PMID: 23235650 DOI.
- ↑ Papaioannou GI, Syngelaki A, Poon LC, Ross JA & Nicolaides KH. (2010). Normal ranges of embryonic length, embryonic heart rate, gestational sac diameter and yolk sac diameter at 6-10 weeks. Fetal. Diagn. Ther. , 28, 207-19. PMID: 20847544 DOI.
- ↑ Coulam CB, Britten S & Soenksen DM. (1996). Early (34-56 days from last menstrual period) ultrasonographic measurements in normal pregnancies. Hum. Reprod. , 11, 1771-4. PMID: 8921130
- ↑ Gardner E & O'Rahilly R. (1976). The nerve supply and conducting system of the human heart at the end of the embryonic period proper. J. Anat. , 121, 571-87. PMID: 1018009
Reviews
Strasburger JF & Wakai RT. (2010). Fetal cardiac arrhythmia detection and in utero therapy. Nat Rev Cardiol , 7, 277-90. PMID: 20418904 DOI.
Bennet L & Gunn AJ. (2009). The fetal heart rate response to hypoxia: insights from animal models. Clin Perinatol , 36, 655-72. PMID: 19732619 DOI.
Butcher JT & Markwald RR. (2007). Valvulogenesis: the moving target. Philos. Trans. R. Soc. Lond., B, Biol. Sci. , 362, 1489-503. PMID: 17569640 DOI.
Articles
Hashima JN, Frias AE, Bernard L, Spindel ER, Hobbs TR & Rasanen J. (2010). Fetal ventricular diastolic filling characteristics in a primate model: the role of fetal heart rate and pulmonary vascular impedance. Reprod Sci , 17, 760-6. PMID: 20595708 DOI.
Park YS, Koh SK, Hoh JK & Park MI. (2010). Difference of fetal heart rate accelerations based on 10 and 15 beats per minute. J. Obstet. Gynaecol. Res. , 36, 291-5. PMID: 20492379 DOI.
Lunshof S, Boer K, Wolf H, van Hoffen G, Bayram N & Mirmiran M. (1998). Fetal and maternal diurnal rhythms during the third trimester of normal pregnancy: outcomes of computerized analysis of continuous twenty-four-hour fetal heart rate recordings. Am. J. Obstet. Gynecol. , 178, 247-54. PMID: 9500482
Mandarim-de-Lacerda CA, Le Floch-Prigent P & Hureau J. (1985). [Study of atrial conduction tissue in the 17 mm V-C human embryo. Morphological contribution to the pathogenesis of sinoauricular node dysfunction]. Arch Mal Coeur Vaiss , 78, 1504-9. PMID: 3938216
Gardner E & O'Rahilly R. (1976). The nerve supply and conducting system of the human heart at the end of the embryonic period proper. J. Anat. , 121, 571-87. PMID: 1018009
Orts Llorca F, Domenech Mateu JM & Puerta Fonolla J. (1979). Innervation of the sinu-atrial node and neighbouring regions in two human embryos. J. Anat. , 128, 365-75. PMID: 438095
Search Pubmed
Search Nov 2010 "Embryonic Heart Rate" All (7589) Review (405) Free Full Text (794)
Search Pubmed: Embryonic Heart Rate
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 2) Embryology Cardiovascular System - Heart Rate Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cardiovascular_System_-_Heart_Rate_Development
- What Links Here?
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G




