Renal System Development
| Embryology - 26 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The paired adult kidneys consist of a functional unit called the "nephron", that filters blood, excretes waste, reabsorbs water (and other compounds) and has endocrine functions. Each adult human kidney typically contains about 750,000 nephrons, though the total number can vary significantly from as few as 250,000 to as many as 2,000,000.[1][2]
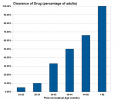
In the embryo, nephron development, nephrogenesis, occurs through several stages involving classical epithelial/mesenchyme type of interactions. Nephrogenesis continues into the late fetal period (GA week 34–35) and while the fetal kidney does produce urine, not until after birth does the glomerular filtration rate (GFR) increases rapidly due to a postnatal drop in kidney vascular resistance and an increase in renal blood flow.
The urinary system is developmentally and anatomically associated with genital development, often described as the "urogenital system". (More? genital)
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Renal Embryology | Renal Development | Nephrogenesis | Intermediate Mesoderm | Ureter Development | Urethera Development | Renal Pelvis Development | Urinary Bladder Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Objectives
- Understand the 3 main stages of kidney development.
- Understand development of the nephron and renal papilla.
- Brief understanding of the mechanisms of nephron development.
- Understand the development of the cloaca, ureter and bladder.
- Brief understanding of abnormalities of the urinary system.
Textbook References
- The Developing Human: Clinically Oriented Embryology (8th Edition) by Keith L. Moore and T.V.N Persaud - Moore & Persaud Chapter 13 p303-346
- Larsen’s Human Embryology by GC. Schoenwolf, SB. Bleyl, PR. Brauer and PH. Francis-West - Chapter 10 p261-306
Renal Movies
|
|
|
| ||||||||||||
|
|
|
Background
- Mesoderm then intermediate mesoderm
- Vascular Development
- Gastrointestional
- Cloacal development
- Endocrine - covered in future lecture/lab
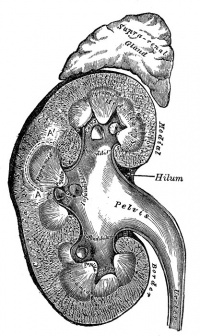
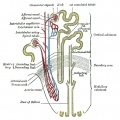
Kidney Anatomy
- Nephron - Functional unit of kidney
- Humans up to 1 million
- Filtration of waste from blood
- Endocrine
- Blood pressure regulation
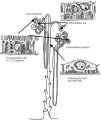
The key structure of the adult nephron is the glomerulus (renal corpuscle), which represents the initial vascular/renal interface.
Related Images: Nephron histology overview | glomerulus structure | vascular and renal poles
Ureter
- Bladder - Urine storage
- Endoderm allantois
Mesoderm
- Intermediate mesoderm - Lies between somites and lateral plate
Intermediate Mesoderm
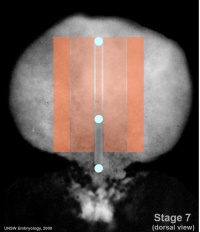
Week 3 - Stage 7 dorsal view |
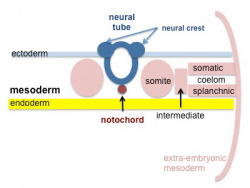
Cross-section showing mesoderm regions |
|
Mesonephric Duct
Later in development, both the mesonephric duct and the cloaca both continue to differentiate and undergo extensive remodelling (and renaming)
Ureteric Bud
- arise near the cloacal connection of the mesonephric duct
- branch from the mesonephric duct laterally into the intermediate mesoderm
- induce the surrounding mesoderm to differentiate - metanephric blastema
- this mesoderm will in turn signal back to differentiate the ureteric bud
Epithelial - mesenchymal interaction
Ureteric Bud forms - ureter, pelvis, calyces, collecting ducts
Metanephric Blastema
- forms glomeruli, capsule, nephron tubules
- this development continues through fetal period
Nephros Development
Three pairs appearing in sequence within intermediate mesoderm during development.
- pronephros
- mesonephros
- metanephros
Pronephros
- week 4 few cells in cervical region fish
- Human E18, Mouse E7.5pronephric duct forms first with associated nephrogenic mesenchyme
- grows rostro caudally cervical -> cloaca
- E22 nephrogenic mesenchyme differentiates to form pronephroi not functional in mammals degenerates rapidly
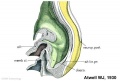
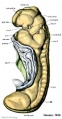
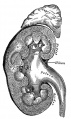
Mesonephros
The mesonephros, historically called the Wolffian body, is the embryonic stage of renal development that is entirely lost during development of the later fetal /adult metanepros. The only developmental structure not lost is the mesonephric duct.
Human E24, Mouse E9.5 caudal to pronephros
|

Week 5 - Stage 13 mesonephros |
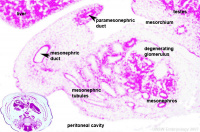
Week 8 - 22 mesonephros |
Wolffian body
Historic name for the developing combined renal (mesonephros) and genital (paramesonephrotic blastema) structures. This term is no longer used in describing development.
| Historic Embryology |
|
Caspar Friedrich Wolff (1734-1794) was a German embryologist and anatomist best known today for identifying the Wolffian duct (mesonephric duct; ductus deferens, epididymis), Wolffian body (mesonephros) and Wolffian cyst (mesonephric origin uterine broad ligament cyst) that bear his name. Thought also to be a founder of the germ layer theory. His doctorate dissertation Theoria generationis (1774) discarded the developmental theory of preformation. Later in his career, his teaching in Berlin was opposed by the professors of the Medical-Surgical College, who had guild privileges to teach medicine. |
Metanephros
- Human E35-37, Mouse E11 epithelia bud at end of mesonephric duct ureteric bud and associated metanephric mesenchyme
Ureteric Bud
- induced by metanephric mesenchyme to differentiate
- forms collecting tubules, renal pelvis, ureter
- metanephric mesenchyme induced by ureteric to differentiate forms nephron
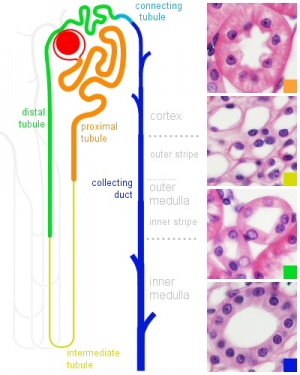
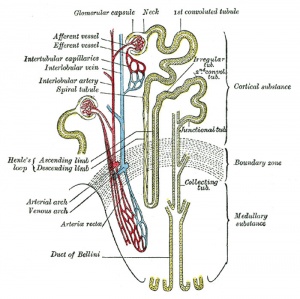
Nephron
| In humans, nephrogenesis only occurs before birth, though nephron maturation continues postnatally. Mean glomerular number shown to level at 36 weeks, increasing from about 15,000 at 15 weeks to 740,000 at 40 weeks. | 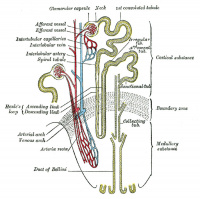
Adult nephron structure |
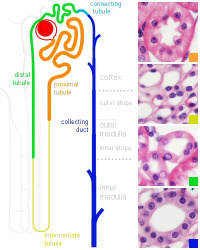
Nephron histology |
|
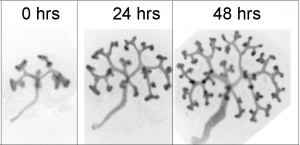
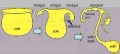
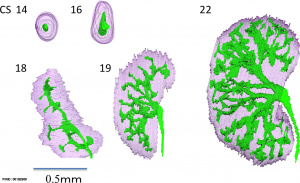
Nephron development has four identifiable developmental stages:
- Vesicle (V) stage (13-19 weeks, second trimester)
- S-shaped body (S) stage ( 20-24 weeks, second trimester)
- Capillary loop (C) stage (25-29 weeks, third trimester)
- Maturation (M) stage (infants aged 1-6 months, neonatal and postnatal)
|
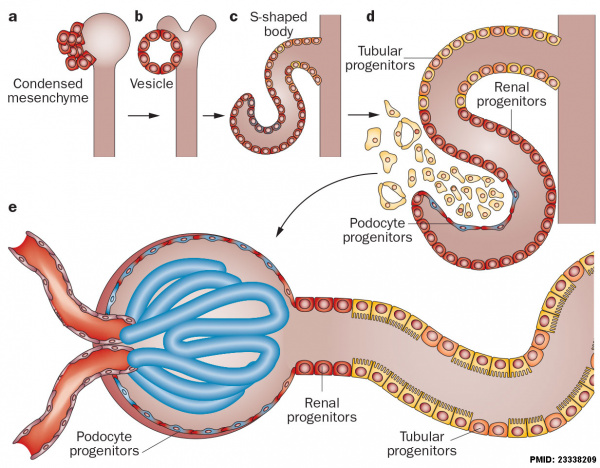
Nephron development[13]
|
- cyst invaginates twice to form a comma
- then a S-shaped body one invagination site later becomes the glomerular cleft
- At about this time blood vessel progenitors invade cleft to begin construction of vascular component of glomerulus
- Tubule maturation specialised transporting segments of nephron differentiate complex of convoluted tubules is created
|

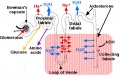
Renal Development Interactions[14] |
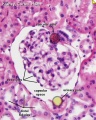
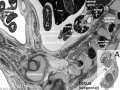
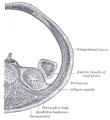
| Bowmans Capsule | |
|---|---|
Bowmans Capsule forms two layers with a space between these layers.
|
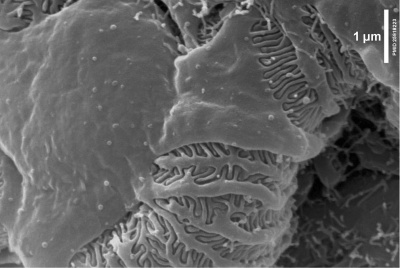
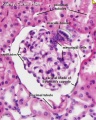
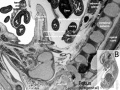
Within the glomerulus a high magnification view of a podocyte showing the interdigitated foot processes (pedicels) that are wrapped around the exterior of glomerular capillaries.[15] |
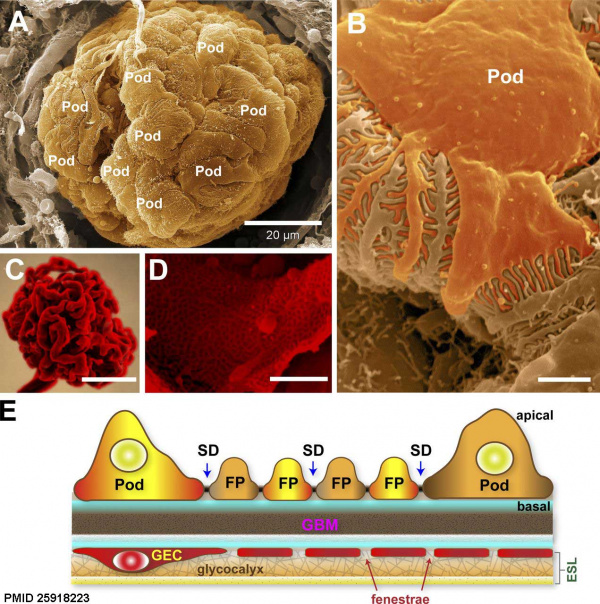
| |
Embryonic Kidney
Embryonic stage descriptions based on Carnegie Collection embryos.[16]
Week 4
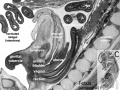
- Carnegie stage 12 - 29 somite embryo mesonephros tubules begin at the level of somite 8 and are distinct as far caudally as somite 20, whence they extend as a continuous nephrogenic cord to t he level of somite 24. Opposite each somite there are two or more tubules. Thus they are not metameric, any more than the mesonephric duct is metameric, or the umbilical vein. The number of nephric vesicles is being increased by progressive differentiation caudally from the nephrogenic cord.[17] The mesonephric duct at first ends blindly immediately short of the cloaca, but soon becomes attached to the cloaca (i.e., to the terminal part of the hindgut) and acquires a lumen.
- Carnegie stage 13 - Mesonephros glomeruli begin to develop, and nephric tubules become S-shaped.[18] A ureteric bud may possibly be present in some specimens[19] fig. 4), although further confirmation is needed. The mesonephric duct, which becomes separated from the surface ectoderm except in its caudal portion, is fused to the cloaca, into which it may open. A urorectal cleavage line is apparent.
Week 5
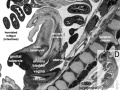
- Carnegie stage 14 - In embryos of this stage the mesonephros is well along in its organogenesis. The steps in this process are made easier to follow by the fact that the development occurs progressively in a rostrocaudal direction. Here again is an organ in which the epithelial elements constitute its primary tissue and seem largely to determine its form. The non-epithelial mesonephric elements, though necessary complements for epithelial-mesenchymal interaction, give the appearance of being subsidiary. It has already been seen that the coelomic surface cells possess various inherent potentialities. The surface of the coelom can be mapped in definite areas in accordance with the distribution of these various kinds of surface cells. Running along each side of the median plane is a narrow strip of coelom where, by the proliferation and delamination of its surface cells, there is produced a longitudinal series of epithelial tubules that constitute the units of the mesonephros. This follows the manner in which nephric elements were formed in previous stages, and it is now about to be repeated, with certain modifications, in the development of the metanephros, which is still in the primordial state of a budding ureter with its nephrogenic capsule (fig. 14-6). One can go a step further in regard to the inherent constitution of these coelomic epithelial tubules. Not only do they become tubules, but from the beginning they show regional differentiation. The proximal end promptly blends with and opens into the mesonephric duct, and this part of the tubule persists as a collecting duct. The distal free end at the same time begins its expansion into a highly specialized part of the tubule, namely the mesonephric corpuscle. The intervening central segment of the tubule becomes the convoluted secretory portion. Embryos in this stage are especially favorable for the study of the process of formation of the mesonephric corpuscle. The proliferation of the tubular epithelium at the free end results in its maximum expansion. This occurs in such a way as to produce an indented flattened vesicle, known as a glomerular capsule. As seen in section, it has an arched floor-plate several cells thick and a thin, single-layered roof membrane. The two are continuous with each other but are very different in their potentialities. The roof membrane becomes attenuated as an impermeable membrane. The floor plate continues active proliferation and many of its cells were believed by Streeter to delaminate and apparently become angioblasts, participating in the formation of the vascular glomerulus and its supporting tissues. It is now maintained, however, that the glomerular capillaries (in the metanephros) come from adjacent vessels and never develop in situ from epithelial cells (Potter, 1965). Further details of renal development have been provided by several authors (e.g., Potter, 1972). The residual cells facing the capsular lumen in the mesonephros at stage 14 are reduced in the more advanced phases to a single layer, covering and conforming everywhere to the tabulations of the underlying capillary tufts. Angiogenesis around the secretory part of the tubule is not far advanced. Angiogenic strands connect with the caudal cardinal vein, and throughout the mesonephros there are isolated clumps of angioblasts, particularly around the capsules. These show the typical difference in complexity of the three parts of the tubule: (1) collecting duct, (2) secretory segment, and (3) glomerular capsule.
- Carnegie stage 15 - The ureteric bud is longer, and its tip is expanded as the pelvis of the ureter (fig. 15-10). The primary urogenital sinus is distinguishable.
Week 6
- Carnegie stage 16 - The metanephros, which is now reniform, is still sacral in level. The ureter is elongating and, in more advanced embryos, the pelvis of the ureter divides into rostral and caudal poles. The urorectal septum, the formation of which is disputed, is well marked.
- Carnegie stage 17 - The mesonephros shows epithelial plaques in the visceral layer of the glomerular capsule and hence can produce urine (Silverman, 1969)[20]. The pelvis of the ureter usually shows three main divisions, and calices appear. The urogenital sinus presents a pelvic part (vesico-urethral canal) and a phallic part (definitive urogenital sinus).
Week 7
- Carnegie stage 18 - Collecting tubules develop from the calices at stages 17 and 18. They are surrounded by sharply outlined condensed primordia in the process of forming secretory tubules. Renal corpuscules are not yet present. By stage 18 the mesonephric duct and the ureter open almost independently into the vesico-urethral canal[18]: i.e., the common excretory duct is disappearing. The cloacal membrane is ready to rupture.
- Carnegie stage 19 - Lack of orientation in metanephrogenic tissue. Beginning formation of renal vesicles.[21]
Week 8
- Carnegie stage 20 - The external surface of the metanephros is said to be slightly lobulated. A reconstruction of the urinary system has been published by Shikinami[18] (fig. 5). S-shaped lumen in renal vesicles. Spoon-shaped capsules (Bowman’s).[21]
- Carnegie stage 21 - Metanephros is spoon-shaped glomerular capsules are developing, no large glomeruli.[21]
- Carnegie stage 22 - Metanephros few large glomeruli.[21]
- Carnegie stage 23 - Short secretory tubules. Numerous large glomeruli. Long secretory tubules. High epithelium in some tubules. Increased number and convolutions of tubules.[21] Comparison between the mesonephros and the metanephros in staged embryos is lacking. In metanephros, the kidneys have ascended from a sacral level at stages 13–15 to a lumbar level at stages 17–23. At stage 23 they are generally at the level of lumbar vertebrae 1–3..
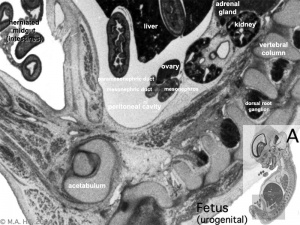
Fetal Kidney
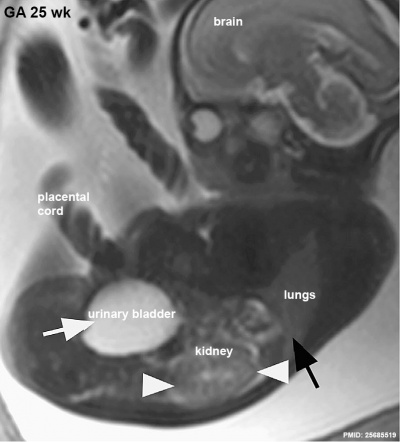
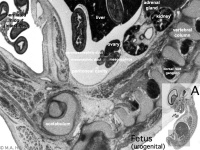
|
MRI appearance of normal fetal kidney.[22] Sagittal T2- SSFSE of a fetal abdomen at GA 25 week. Adequate volume of the amniotic fluid and the developing lungs indicate good renal function.
Note that the urinary bladder can occupy a considerable portion of the abdomen as a normal finding.
|
| Fetal nephron development[13] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
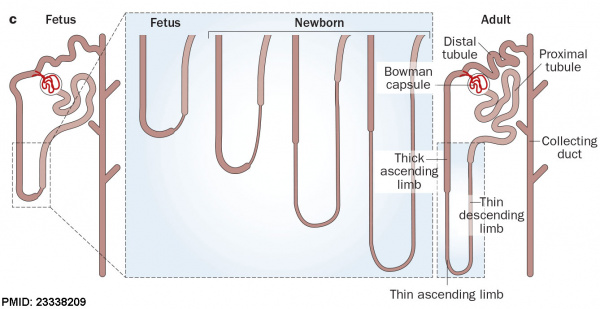
After nephron development has completed and concomitant with the development of the renal papilla in the newborn, the thin ascending limb of Henle’s loops is generated as an outgrowth from the S3 segment of the proximal tubule and from the distal tubule anlage of the nephron. Endocrine KidneyCovered also in Endocrine Development lecture
Cloaca
Common Urogenital Sinus
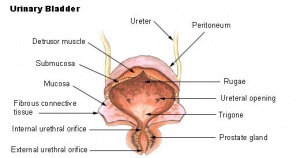
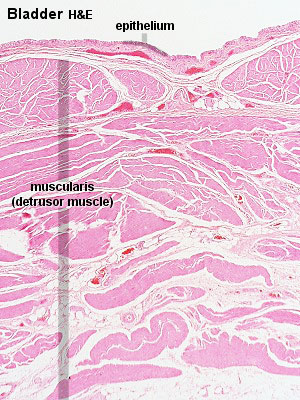
Urinary Bladder
Bladder StructureCan be described anatomically by its 4 layers from outside inward:
Detrusor Muscle
Ureter Development
Trigone Development
Urethra DevelopmentThe entire human male and female urethra is endodermal in origin based on the presence of FOXA1, KRT 7, uroplakin, and the absence of KRT10 staining.[23] A recent study of male penile urethra describes a theory of a "two-step process of urethral plate canalization and urethral fold fusion to form the human penile urethra. Canalization ("opening zipper") opens the solid urethral plate into a groove, and fusion ("closing zipper")."[24][25] Kidney Ascent
Renal Arteries
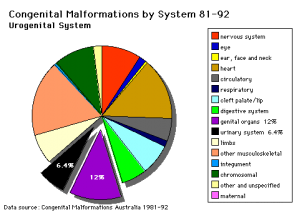
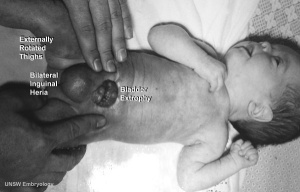
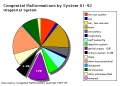
Note: Frequently a second renal artery (inferior renal) from abdominal aorta at a lower level, supplies lower portion of kidney AbnormalitiesThere are many different forms of renal development abnormalities associated with kidney, ureters, bladder and urethra. There are many genetic disorders associated with failure or abnormal renal development. Prenatal diagnosis of obstructive and renal agenesis/dysgenesis disorders are also important for early reproductive decisions by the parents. For example, with bilateral renal agenesis, failure of both kidneys to development, is not compatible with fetal/neonatal survival. Because of their close developmental association, often described as the urogenital system, there can be an associated genital abnormalities.
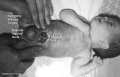
Horseshoe Kidney
Urorectal Septum Malformation
Bladder
Bladder Exstrophy
Ureter and Urethra
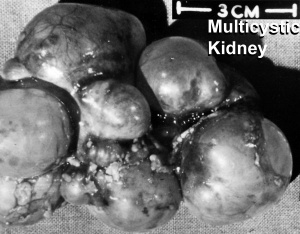
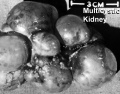
Polycystic Kidney Disease
Wilms' Tumor
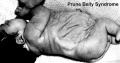
Prune Belly Syndrome
Renal CystsThe Bosniak classification system (Category I - IV) was designed to separate identified cystic renal masses by analysis of computed tomography (CT) features into surgical and nonsurgical categories.[26] Named after Morton Bosniak, Yale University School of Medicine, the developer of this classification system. Molecular
References
ReviewsLumbers ER, Kandasamy Y, Delforce SJ, Boyce AC, Gibson KJ & Pringle KG. (2020). Programming of Renal Development and Chronic Disease in Adult Life. Front Physiol , 11, 757. PMID: 32765290 DOI.
Textbooks
Online TextbooksSearch Bookshelf intermediate mesoderm | kidney development | renal development | ureteric bud | nephron development | bladder development
ReviewsScott RP & Quaggin SE. (2015). Review series: The cell biology of renal filtration. J. Cell Biol. , 209, 199-210. PMID: 25918223 DOI. Hohenstein P, Pritchard-Jones K & Charlton J. (2015). The yin and yang of kidney development and Wilms' tumors. Genes Dev. , 29, 467-82. PMID: 25737276 DOI. Herzlinger D & Hurtado R. (2014). Patterning the renal vascular bed. Semin. Cell Dev. Biol. , 36, 50-6. PMID: 25128732 DOI. Upadhyay KK & Silverstein DM. (2014). Renal development: a complex process dependent on inductive interaction. Curr Pediatr Rev , 10, 107-14. PMID: 25088264 Blake J & Rosenblum ND. (2014). Renal branching morphogenesis: morphogenetic and signaling mechanisms. Semin. Cell Dev. Biol. , 36, 2-12. PMID: 25080023 DOI. Jacob M. Yusuf F. and Jacob HJ. Development, Differentiation and Derivatives of the Wolffian and Müllerian Ducts. (2012) The Human Embryo, Dr. Shigehito Yamada (Ed.), ISBN: 978-953-51-0124-6, InTech, Available from: https://www.intechopen.com/books/the-human-embryo/development-differentiation-and-derivatives-of-the-wolffian-and-m-llerian-ducts Little M, Georgas K, Pennisi D & Wilkinson L. (2010). Kidney development: two tales of tubulogenesis. Curr. Top. Dev. Biol. , 90, 193-229. PMID: 20691850 DOI. Dressler GR. (2009). Advances in early kidney specification, development and patterning. Development , 136, 3863-74. PMID: 19906853 DOI. Michos O. (2009). Kidney development: from ureteric bud formation to branching morphogenesis. Curr. Opin. Genet. Dev. , 19, 484-90. PMID: 19828308 DOI. Reidy KJ & Rosenblum ND. (2009). Cell and molecular biology of kidney development. Semin. Nephrol. , 29, 321-37. PMID: 19615554 DOI. Quaggin SE & Kreidberg JA. (2008). Development of the renal glomerulus: good neighbors and good fences. Development , 135, 609-20. PMID: 18184729 DOI. Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT & Herzlinger D. (2007). Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development , 134, 1967-75. PMID: 17442697 DOI. Costantini F. (2006). Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation , 74, 402-21. PMID: 16916378 DOI. Forefronts Symposium on Nephrogenetics: from development to physiology March 8-11, 2007 Danvers, MA A meeting to synthesize an integrated view of the normal development and function of the kidney from the genetic standpoint.
ArticlesSutherland MR, Vojisavljevic D & Black MJ. (2020). A practical guide to the stereological assessment of glomerular number, size, and cellular composition. Anat Rec (Hoboken) , , . PMID: 31960613 DOI. Desgrange A, Heliot C, Skovorodkin I, Akram SU, Heikkilä J, Ronkainen VP, Miinalainen I, Vainio SJ & Cereghini S. (2017). HNF1B controls epithelial organization and cell polarity during ureteric bud branching and collecting duct morphogenesis. Development , 144, 4704-4719. PMID: 29158444 DOI. Hinata N, Suzuki R, Ishizawa A, Miyake H, Rodriguez-Vazquez JF, Murakami G & Fujisawa M. (2015). Fetal development of the mesonephric artery in humans with reference to replacement by the adrenal and renal arteries. Ann. Anat. , 202, 8-17. PMID: 26335195 DOI. Grinstein M, Yelin R, Herzlinger D & Schultheiss TM. (2013). Generation of the podocyte and tubular components of an amniote kidney: timing of specification and a role for Wnt signaling. Development , 140, 4565-73. PMID: 24154527 DOI. Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, Chatelut E, Grubb A, Veal GJ, Keir MJ & Holford NH. (2009). Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr. Nephrol. , 24, 67-76. PMID: 18846389 DOI.
Search PubMedSearch Pubmed: Renal System Development | Renal Development | intermediate mesoderm | kidney development | renal development | ureteric bud | nephron development | bladder development Additional Images
Historic
TermsOpen table below to see list of renal terms.
External LinksExternal Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
Cite this page: Hill, M.A. (2026, February 26) Embryology Renal System Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Renal_System_Development
|