Paper - On the Development of the Human Heart
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Mall FP. On the development of the human heart. (1912) Amer. J Anat. 13: 249-298.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
On the Development of the Human Heart
From the Anatomical Laboratory of the Johns Hopkins University.
Thirty-Seven figures
In my recent study[1] on the musculature of the adult human
heart it was necessary to refer constantly to the development of this organ, and in general my description of the course of the muscle bundles was also based upon their development. This made it necessary to study numerous serial sections of embryo hearts, as well as Whole hearts which had been rem.oved from the embryo and dissected under the binocular microscope.
It is my purpose now to give as accurate a description as possible of several points which were obscure to me at the beginning of my study, so this report is to be viewed as supplementary to the excellent study by His as well as the recent one by Tandler. first of all an attempt was made to study the course of the muscle bundles and the formation of the vortex in the smallest hearts by means of direct observation upon whole hearts, stained and unstained, under the binocular microscope. This study was controlled by that of serial sections of other hearts, an abundance of material of both kinds being available. It soon became apparent that the muscle wall of the entire heart had to be included in this study, which soon showed that the critical point lay in the Wall of the atrial canal. that is the common canal between the atria and ventricles. The study led back to the study of the valves at this point, an understanding of which is really the key to the whole situation. This resulted in locating definitely the atrio-ventricular muscle bundle (bundle of His) in all stages of development.
The points to be discussed will be considered in the following order: A, Subdivisions of the early heart; B, Formation of the septum and atrio-ventricular valves; C, The atrio-ventricular bundle; D, Musculature of the left ventricle.
A. Subdivisions of the Primary Heart Tube
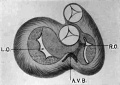
In an embryo about 2 mm. long (No. 391), which was modeled in wax by Dr. Dandy, the heart is shown as a relatively straight tube with its arterial end directed towards the head (fig. 1). Its muscle wall is of even thickness and communicates throughout its whole length along the dorsal midline with the rest of the mesoderm, that is it has a complete dorsal mesentery.[2] At its anterior end the heart tube shows a slight dilatation just before the arteries arise from it. Midway between the two ends of the muscle tube there is an indentation on the left side which marks the beginning of the bulbo-ventricular groove, that is, it separates the atria and left ventricle on the one hand from the right ventricle and the bulb on the other. Within the muscle tube there is suspended by means of numerous fine fibrils the collapsed endocardial lining. These fibrils will be considered later when the development of the valves is discussed.
The heart now separates rapidly from the rest of the mesoderm in subsequent stages and is soon suspended in the pericardial coelom, remaining attached to the body only at its venous and arterial ends. The indentation on the left side of the heart, mentioned above, becomes more pronounced, the heart rolls upon itself quite rapidly as succeeding stages of development show. In an embryo 2.5 mm. long (No. 3) the heart is separated from the dorsal midline in its middle, while in another of the same length (318) the separation is more pronounced. In one 3.5 mm. long (164) the separation is complete and the various subdivisions of the final heart tube can be outlined with precision (fig. 2).
Legends for All figures
|
|
|
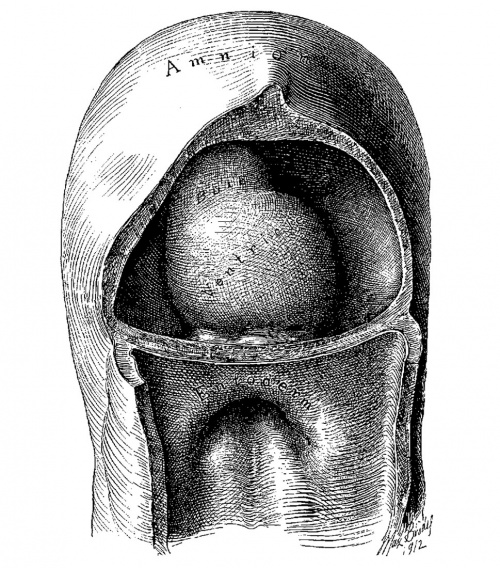
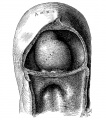
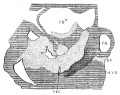
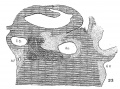
Fig. 1. Ventral view of the model of an embryo 2 mm long; (No. 391). X 1.00. The pericardium has been removed from in front of the heart.
I mentioned above in describing the heart of the embryo 2 mm. long that its endothelial lining is collapsed and suspended by a mass of fine fibrils Within the muscular tube. In slightly older stages the arrangement of the endothelial lining is changing, becoming dilated on the venous side of the heart. This change is beginning in No. 3, is more advanced in No. 318 and is complete in No. 164 (fig. 2). His[3] has pointed out that the endothelial lining hugs the muscle wall closely in the embryonic atrium, While it remains suspended for a time in the rest of the heart. This arrangement is so pronounced in the‘ early heart that it affords a Way by which we may determine with precision the exact portion of the heart tube from which the atrium arises. My specimens show conclusively that the atrium arises exclusively from the free heart tube and that the sinus venosus does not contribute to its formation. This being established it follows that as soon as the heart tube is fully separated from the body walls that the anlage of the entire adult heart is to be found between its arterial and venous attachments.
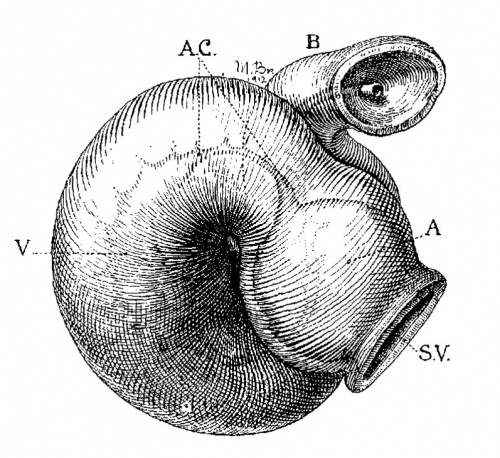
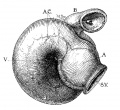
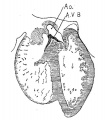
Fig. 2 From the reconstruction of a heart of an embryo 3.5 mm. long (No. 164). X 66. View from the left side.
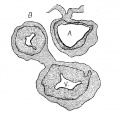
In the embryo 3.5 mm. long (No. 164) the completed heart tube is seen, which is S-shaped and twisted upon itself so that the arterial and venous ends are brought close together. At the venous end the muscle wall is slightly dilated which marks the atrium; this is lined closely with endothelium which encircles the cavity within. No delicate fibrils are here seen between the muscle wall and its endothelial lining. Then follows an upper bend to the heart after which there is a dilatation projecting towards the left side, the former marking the atrial: canal and the latter the left ventricle. The lower connecting piece unites the left ventricle with the bulb which later on gives rise to the right ventricle. In the atrial canal (Haller’s auricular canal) the endothclial tube is seen as a solid strand of cells suspended freely in the muscle wall by the delicate fibrils already mentioned. In the left ventricle the tube shows a distinct cavity, while throughout the rest of the heart tube the cavity is irregular but not pronounced. The form of the endothelial tube is shown in fig. 2 and again in a semi-diagrammatic figure of a transverse section through the atrium, ventricle and bulb in fig. 3. The delicate fibrils, which no doubt belong to the endothelial cells are present in large number throughout the whole heart tube, excepting in that which forms the atrium. In another embryo (No. 384, 2 mm. long), considerably smaller than the one just described and probably pathological, the degree of development of the heart is practically identical with the one 3.5 mm. long (No. 164).
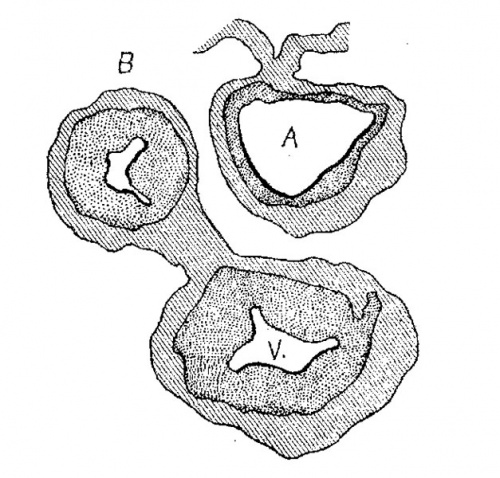
Fig. 3 Section of the heart of the embryo 3.5 mm. long. X 66.
In my collection there are two other embryos slightly more advanced in development than the one just described which bear upon the exact origin of the atria from the heart tube. They are Nos. 486 (4 mm. long) and 470 (4 mm. long). Neither of these specimens has been studied carefully as a whole, so the number of myotomes in each can not be given. Nor have the measurements been corrected by the drawings and the sections.
In No. 486 the single atrium as described above (No. 164) is more pronounced, is dilated and filled with blood, while the form of the endothelial tube is much the same. However, the atrium is sharply separated from the sinus venosus and there are a few fibrillae between the endothelial and muscular Walls. In the left ventricle the endothelial and the muscular layers are just beginning to interlock to form the first trabeculae.
Embryo No. 470 shows the heart more advanced than in No. 486. The atrium has become double, that is there are two atria. The endothelial tube in it is distended and its separation from the sinus venosus is still pronounced. The trabecular formation in the left ventricle is somewhat more pronounced than before.
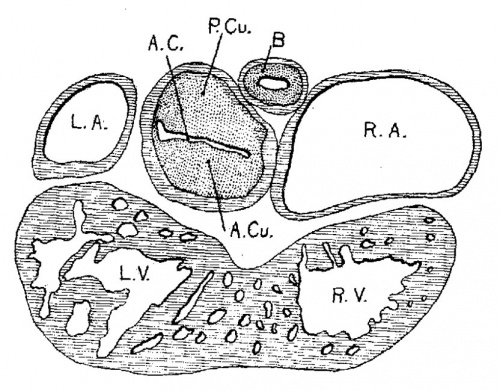
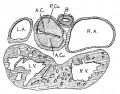
From now on the changes in the heart take place very rapidly, as the general form of the embryo also changes rapidly. The head is bent upon the body which is well curved upon itself with pronounced limb buds. In the next embryo, No. 289 (4.25 mm. long), the subdivisions of the heart are sharply defined and in the following stage, No. 463 (3.9 mm.), the preliminary subdivisions are complete (fig. 4). During this time the embryo curls upon itself and the limb buds are formed. In embryo 239 the two atria are very pronounced, the right communicating with the sinus venosus. The atrial canal is sharply defined, first as a constriction and secondly by a great increase of the fibrillar mass between the endothelial and muscular walls. The trabecular system is well formed in the left ventricle and has extended into the bulbus, that is into the right ventricle. In this specimen it is clear that the course of the circulation is from the right to the left atrium, then first to the left ventricle after which it enters the right. The right atrium still lies in the notch between the left ventricle and the bulb on the posterior side of the heart. Only a little later does it project to the right side of the bulb which becomes its permanent position. This is seen in No. 463 (fig. 4).
Although the two embryos just mentioned are practically of the same stage of development, the difference of the degree of development of their hearts is most pronounced. No. 463, which is perfectly preserved, has a heart with larger atria, a more constricted a_trial canal, a large left ventricle and a pronounced but contracted bulb. The muscular Wall of the whole heart is continuous without a single break in it. That surrounding the atrial canal is sharply defined forming a continuous ring connecting at all points with the atria above and the left ventricle below. This is menti.oned especially because a share of this connecting ring disappears while the remaining portion becomes the atrio-ventricular bundles.[4]
Fig. 4 Section of the heart of an embryo 3.9 mm. long (No. 463). X 66.
B. Formation of the Atrio-Ventricular Valves
In the earliest stages described (No. 391), while the muscle wall of the heart is still in the form of a straight tube and is connected throughout its length with the body wall, the endothelial tube separated from the muscular tube by a marked layer of delicate fibrils. In their papers upon the heart both His and Tandler speak of these fibrils but they give no very definite information regarding their nature. In his study of the chick His[5] describes the space between the endothelial tube and muscular wall of the heart, which later in development fills with connective tissue arising from the inner tube. In the atria, where this space is never pronounced, the secondary thickening is also insignificant. Later His[6] also observes this space in young human embryos. Many fibrils extend from the endothelial tube, which when they are pronounced, draw out the side of the tube in a characteristic way. This he pictures. He is uncertain whether these fibrils are natural or produced by the hardening reagents used. In the atrium the inner tube hugs the muscle wall closely. In the atrial canal the space is filled with two pronounced cushions of connective tissue, while in the ventricle the muscle forms trabeculae which are soon covered with endothelium. In the bulb this space is very marked and filled with a delicate connective tissue framework. He does not show conclusively the meaning of this tissue.
Tandler[7] describes and figures this substance well in a human embryo with fifteen somites. Although his figure shows beautifully the inwandering of nuclei from the endothelium, and although he speaks of a reduction and enlargement of these filbrils, he is unwilling to decide whether or not they are due to the method of preservation and of staining of the sections. He states expressly that the tissue resembles very much Wharton’s jelly. It seems to me, however, that the evidence of His and Tandler is sufficient to show that this tissue is not due to coagulation but a constant normal constituent of the developing heart. That it is distributed in a definite way in different portions of the heart and in different stages of development speaks almost conclusively for this opinion. Its origin and meaning is however a different question.
In the youngest embryos studied the reticular mass between the endothelial cells and the muscle wall appears either homogeneous, or as composed of most delicate fibrils, or of coarser fibers, according to the method of preservation. In general it appears like the most delicate reticulum of the mesenchyme and under all circumstances any stain which tinges the fibrils tinges also the endothelial cells. So intimate is this connection that it forces the conclusion that the fibrils together with the endothelial cells form a syncytium. In the younger hearts the endothelial nuclei lie altogether on the inner side of the fibrils as has been repeatedly observed, but as soon as the trabecular system begins to form in the left ventricle some of the endothelial nuclei invade the fibrillar layer. This is first seen in embryo No. 239 (43; mm. long). Here the trabecular system is quite completely formed in the left ventricle by an interlocking of processes from the muscular and endothelial layers. In the right ventricle the process is not so far advanced, while in the atrial canal and the bulb the reticular layer is invaded by endothelial nuclei but not by muscle cells. A similar arrangement is found in embryo No. 3, which as No. 239, is intensely stained. fig. 5 is from the posterior endocardial cushion of an embryo 4.3 mm. long, (No. 148), showing that the nuclei of the cushion are invading it from its endothelial side. All this is more pronounced in No. 463 (3.9 mm.) which is more advanced in development and is perfectly preserved. In this specimen it is quite easy to demonstrate that the nuclei of the endothelium and the reticular mass belong together, for they are distinctly intermingled and yet are separated from the muscular layer (fig. 6). Since the nuclei and fibrils belong together and since it has been demonstrated that the reticulum of the liver is developed from the endothelial cells, I shall speak of the reticulum between the endothelium and muscle layer of the heart as endothelial fibrils. The great importance of this distinction is at once apparent for it shows that connective tissue arises also from endothelial cells and that the intima of the entire vascular system including the the valves of the heart has a like origin.
I think that I have now shown that the endothelial fibrils are constant. in the heart and that we must hold the endothelial cells responsible for the production of the connective tissue of the endocardium as well as of the valves. Further study will probably show that endothelial connective tissue is by no means of rare occurrence. At any rate it has been definitely settled that the endothelial cells of the liver give rise to the connective tissue of the liver lobule.
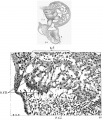
Fig. 5 Section of the posterior endocardialcushion of an embryo 4.3 mm. long (No. 148). X 360.
In my study on the development of the connective tissue I was astonished to find in macerated and digested frozen sections that the endothelial tube with its surrounding reticulum can be isolated.[8] In such specimens it is impossible to separate the nuclei from this continuous mass of reticulum; together they form a syncytium. This connection was demonstrated in pig embryos 20 mm. long. Although this Was entirely out of harmony with the results obtained for other connective tissues, which always arise from the niesenehyme, it had to be accepted and so it Was recorded.
Fig. 6 Section of the anterior endocardial cushion in the atrium of an embryo 3.9 mm. long (No. 463). X 360.
In a measure this was confirmed by Kon[9] who observed the development of the reticulum in the liver of a. foetus in the middle of pregnancy. Mollier[10] in his beautiful study on the development of the blood shows conclusively the connection between the endothelial cells of the liver and the surrounding reticulum. This he follows back to a. human embryo 10 mm. long and in subsequent stages the connection of the endothelial with the reticulum is complete, that is, it forms a syncytium. Although Mollier believes that the capillaries of the liver arise from the mesenchyme of the capsule, which is impossible, it answers our purpose to state that he shows that the connective tissue of the liver develops from the endothelial cells and not from other mesenchyme cells. Those of us who see the primary vascular tree of the liver arising from the endothelial Wall of the omphalomesenteric veins by a process of reduction of this large vessel to form sinusoids, recogn.ize Mollier’s “origin” of capillaries of the liver as only a secondary contact between the sprouting capillaries when they reach the mesenchyme of the capsule of the anlage of the liver. For the present purpose it is clear that Mollier demonstrates also that the connective tissue of the liver arises from endothelial cells. This relationship has been amply confirmed by F. T. Lewis in the liver of a human embryo 7.5 mm. long.[11] So for the liver the chain is complete; throughout development the connective tissue of the lobule is in direct continuity with the endothelial cells of the blood capillaries and therefore they give rise to them. Within the lobule there are only endothelial cells and epithelial cells and no one finds the reticulum arising from the latter.[12]
In embryo No. 239 the cndothelial fibrils are quite unequally distributed throughout the heart. In the atria, as mentioned above, they form but a very thin layer. In the atrial canal the fibrils are heaped up into two mounds to form the well known endocardial cushions, which have between them a transverse slit. The posterior cushion extends upward into the left atrium and then along its posterior surface into the right atrium and ends at the opening of the sinus venosus. The anterior cushion also extends into the left atrium along its anterior border and reaches to the septum primum which is just beginning to form. Below, in the left ventricle, the endocardial cushions blend with the endothelial reticulum covering the trabeculae. The interlaceinent of the endothelial and muscular layers to form the trabeculae extends into the right ventricle, but in the bulb the two layers are quite sharply defined and separated.
The nuclei of the endothelial syncytium form first of all the inner layer of the heart, but in the endocardial cushions of the atrial canal as well as in the bulb the nuclei gradually extend towards the muscular coat. In other words the nuclei of the inner coat are gradually invading their reticular layer.
In the heart of embryo 463 the differentiation of the endothelial syncytium is more pronounced than in the specimen just described. The heart is now well formed with two pronounced atria, a much constricted atrial canal and a marked constriction of the interventricular canal (fig. 4). The bulbo-ventricular and the interventrieular grooves are Well formed. The septum of the atria. and that of the ventricles are well marked. The endothelial syncytium is most pronounced in the endocardial cushions and in the bulbus. The cells are quite equally distributed throughout the syncytium but they are somewhat more numerous immediately under the endothelial covering than near the muscle layer of the heart (fig. .6). The posterior cushion does not reach as far into the left ventricle as the anterior and is also less extensive in the atria; it reaches nearly to the sinus venosus. The anterior endocardial cushion is a large sickle-shaped affair, encircles the heart in front as the border of the atrial septum (septum primurn) which is now forming. The anterior cushion ends on the medial side of the opening from the sinus venosus. The space in the atria between the cushions marks the primary foramen ovale (foramen ovale I).
In an embryo 4.3 mm. long (No. 148) practically the same conditions are seen as in the embryo just described. If anything it is a little more advanced in development. A section of the endocardial cushion is shown in fig. 5. From now on new conditions arise Which when concluded separate the heart into its right and left halves.
The anterior and posterior cushions are now well formed, the superior septum (primum) and the septum of the ventricles (septum inferior) are beginning but the septum aorto-pulmonale (aortic septum) is still absent. While these are forming, up to the next stage, when the muscle wall of the atrial canal begins to break down, the heart gradually enlarges without changing very markedly its external form. The steps which I am about to describe are well established in various mammals, but I shall repeat them hastily in order to confirm them all in the human heart. In doing this I shall include in the descriptions the following specimens:
- Embryo No. 80, C. R. length 5 mm.
- Embryo No. 136, C. R. length 4 mm.
- Embryo No. 116, C. R.. length 5 mm.
- Embryo No. 241, C. R. length 6 mm.
- Embryo No. 2, C. R. length 7 mm.
- Embryo No. 383, C. R.. length 7 mm.
- Embryo No. 380, C. R. length 7.5 mm.
- Embryo No. 113, C. R. length 8 mm.
- Embryo No. 397, C. R. length 8 mm.
- Embryo No. 422, C. R. length 9 mm.
- Embryo No. 163, C. R. length 9 mm.
In Embryo No. 80 the anterior and posterior cushions are considerably thicker than before but hold practically the same relation to the heart as in.Nos. 463 and 148. In the left ventricle the cushions are spread out and have attached themselves to the trabecular system. As there are two attachments which correspond in position to the anterior and posterior papillary muscles, it is proper to speak of them as such. The septum aorto-pulmonale is well formed and its two ridges reach well into the bulbus to the interventricular foramon. Much the same arrangement is found in Embryos No. 136, 116 and 380 which are of the same stage of development as No. 80.
Fig. 7 Transverse section of the atrial canal and bulbof the heart of an embryo 9 mm long; (No. 422). X 40.
Fig. 8 Transverse section of the heart of an embryo 7 mm long (No. 2). X 40.
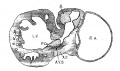
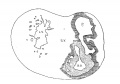
In Nos. 241, 422 and 2 the lumen of the bulb is oo—shaped, the endocardial cushions are much more pronounced than before; they are ready to fuse as is indicated in figs. 7 and 8. The septum primum and the interventricular septum are well marked. In 397 the septum primum is very thin above so that it is uncertain Whether or not it has broken through to form the foramen ovale II.
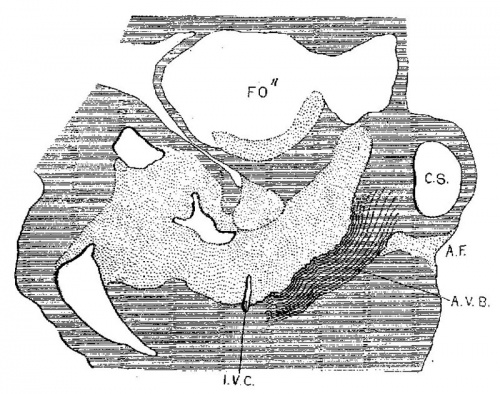
In 383, 113 and 163 the foramen ovale II has just formed, being smallest in the first and largest in the last. The cushions are Well developed in No. 383 ; the anterior reaches to the septum primurn and the posterior to the opening of the sinus into the right atrium. There is a large space between them connecting the two atria. High up in the atria the septum primum is broken though forming the foramen ovale II, as shown by Born. The arrangement of the endocardial cushions with the space between them and the foramen ovale II is well shown in No. 113 (fig. 9). Both cushions now course to the sinus venosus and are blended with the connective tissue above it. Behind the cushions there is a muscle strand from the sinus to the ventricle which marks the position of the atrio—ventrieular bundle. The thin septum primumreaehes to the two cushions, and high up it is perforated by an opening with sharply defined walls. Between the anterior and posterior cushions well within the atrial canal the two atria still communicate with each other; in this region the cushions are as yet not blended. In No. 163 the blending of the cushions is complete (fig. 10) and the permanent foramen ovale is fully established. Together the united endoeardial cushions form a cubical plug which blocks the center of the atrial canal leaving a channel on either side. It also projects into the left ventricle and is attached to its walls forming the two papillary muscles. The atrial canal is divided into two canals which now form the right and left ostia. The two cushions give rise to the medial cusps of the bicuspid and tricuspid valves, that is, the medial cusp of the tricuspid valve and the anterior cusp (B.N.A.) of the mitral valve; the right halves of each cushion make the former and the left halves the latter.
Fig. 9 Sagittal section. Embryo 5 mm. long (No. 113). X 40.
Fig. 10 Coronal section. Embryo 9 mm. long (No. 163). X 40.
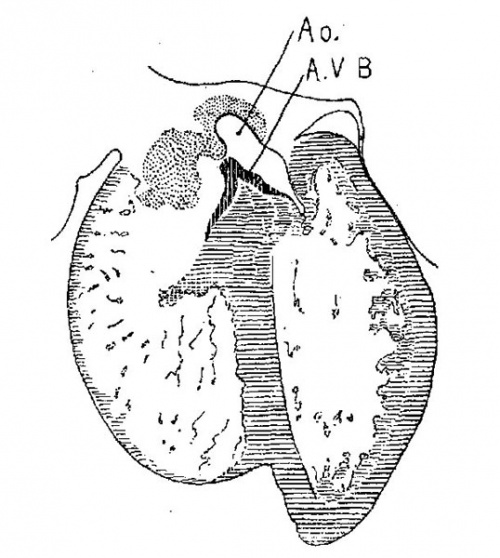
The septum aorto pulmonale is still incomplete in the specimens described, and.the interventricular foramen is wide open, but the septum of the ventricle is well formed, extends upward and blends with the posterior endocardial cushion behind. Behind the posterior endocardial cushion a marked band of muscle extends from the wall of the sinus venosus to the inter ventricular foramen where it ‘spreads over the inner walls of the two ventricles as shown in fig. 9. This is the sino- or atrio-ventricular bundle (bundle of His). These structures I shall describe in the heart of embryo No. 353 which is an unusually well preserved specimen, perfect in every respect and of the right stage of development for this purpose.
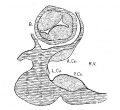
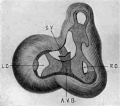
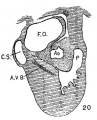
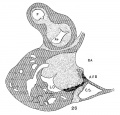
Embryo No. 353 is 11 mm. long with a well formed arm and hand plate. A profile outline of the embryo may be seen in the figure by Evans.[13] The sections are 10;; thick in a coronal direction and slightly oblique, that is, they strike the heart transversely. The heart which has been modeled in wax is 1.7 mm. wide and 2.2 mm. long. The apex is cleft as is so often the ease in this stage of development.[14] The septum aorto pulmonale is complete and the anterior and posterior cushions are fully united into a single mass of connective tissue. This mass extends to a point up in the atrial septum on its dorsal side, to the left valve of the opening into the sinus venosus. The complete union of the two cushions has obliterated the foramen ovale I and the foramen ovale II is Well above the common fibrous process of the united cushions. A yiew from below, that is after the apex of the heart is cut off, shows that the cushions mark the borders of the medial sides of the right and left ostia, these being notched to indicate the extent of the anterior and posterior cushions. This is well shown in fig. 11 which was drawn from the model. On the dorsal side, the posterior cushion extends well down the posterior border of the intervcntricular foramen, that is it makes part of the border of the septum of the ventricle. These arc- the primary connections of the two endocardial cushions. There are also secondary connections which in a measure involve the valves lateral to the ostia.
Fig. 11 View from below of a model of the heart of an embryo 11 mm.long; (No. 353). X 50. The ventricles have been cut off. The connective tissue septa are colored yellow.
On the lateral side of either ostium there is a rounded endocardial thickening which marks the beginning of the lateral valves. These are already observed in Embryo 422, 9 mm. long (fig. 7). To anticipate the description I may state that the right cushion marks the center of the anlage of the anterior and posterior cusps of the ‘tricuspid valve, and the left the anlage of the posterior cusp of the mitral valve as seen in fig. 11. The septum aorto pulmonale soon blends with the cushions through a dorso—latera1 wing which is divided into two branches to encircle in part the right Venous ostium, one of which blends with the right lateral endocardial thickening, and ‘the other, thepmedial, blends with the anterior process of the medial valves now represented by the right lower wing of the anterior endocardial cushion. It is thus seen that through the blending of the septum aorto pulmonale with the right side of the anterior endocardial cushion, the right venous ostium is nearly encircled by endothelial connective tissue. This connection may still be seen in the adult heart where the septum aorto-pulmonale (the tendon of the conus) is found to blend with the fibrous ring of the right ostium at the anterior border of the attachment of the medial cusp of the tricuspid valve.
The large space marked by the interventricular foramen at the root of the aorta remains constant in all subsequent stages of development and is termed by Quain[15] the vestibule of the aorta. This name, which is appropriate, I shall adopt and use in my description. The vestibule in fig. 11 is common to both ventricles, but as the septum of the ventricles and the septum aorto pulmonale approach each other more and more to form the permanent membranous septum, the vestibule becomes transferred to the left ventricle as may be seen in figs. 16 and 17. An open interventricular foramen in the adult always communicates between the aortic vestibule and the space below the medial cusp of the tricuspid valve, as is clear by observing Spalteholz’s figure.[16]
The topography in the wall of the left ventricle is much easier to define. The common endocardial mass borders the left veno us ostium and each of its two horns are continuous with pronounced muscular bands, the papillary muscles, which extend to the more solid muscular Wall of the heart. In their course from the valve to the outer wall of the heart muscle the papillary muscles communicate continuously with the trabecular system. Both the anterior and posterior papillary_muscles connect with the lateral valve which is being extended around the left ostium by an “undermining” process; already well described by His. So in this early stage of development the anterior papillary muscle unites the anterior tip of the medial and lateral valves (anterior and posterior B.N.A;) with the anterior wall of the left ventricle, and the posterior muscle unites the posterior tip with the posterior wall of the heart as seen in fig. 11. The vestibule of the aorta connects the aorta with the left ventricle; it is encircled by the border of the ventricular septum. In the course of time the border of the ventricular septum unites with the septum aorto pulmonale and thus finally separates the two ventricles of the heart. When viewed through the aorta the muscular interventricular septum usually makesin the adult the right border of the vestibule but often it projects into the vestibule as isnormally the case in the pig and the ox. In such specimens, as Well as in the pig and the ox, the right semilunar valve arises directlyfrom the interventricular muscular septum. This shows to what extent the valves must ‘sink into’ the bulbus in passing from the stage represented in No. 353 to the adult form.[17]
The anatomy of the heart of embryo No. 353 may serve as a basis to describe the final closure of the inter-ventricular opening and the formation of the membranous septum. In this specimen the septum aorto pulmonale has grown down to the interventricular foramen and blends with the right tip of the anterior endocardial cushion which is lodged in the foramen. The aortic septum also extends to the lateral side of the right venous ostium and the posterior cushion extends down the anterior border of the ventricular septum. So the interventricular foramen is bounded above by the union of the septum aorto pulmonale and the anterior cushion, in front by the septum aorto pulmonale, behind by the extended portion of the posterior cushion and below by the muscle of the ventricular septum.
Fig. 12 Coronal section. Embryo 13 mm. long (No. 175). X 40.
In embryos 109 (10.5) and 317 (12.5) the Ventricular septum is much more developed than in the stage just described. In these two specimens the fibrous tissue forming the septa and valves is much as in No. 353, but the muscle wall has grown up and nearly closes the interventricular foramen. However, there is still a free communication between the right ostium, with the right ventricle and the vestibule of the aorta. The interVentricular foramen is somewhat smaller in No. 175 (13 mm.) which is cut in a more fortunate plane to illustrate this point than the other two embryos (fig. 12). The opening is gradually becoming smaller in the following embryos in the order of their enumeration, No. 423 (15.2), 144 (14, fig. 13), 424 (17, fig. 14). In 390 (15.5) it is uncertain Whether it is closed or not, in 409 (16) it is just closed, and in 432 (18) it is closed but its connection with the right ventricle is still indicated. That the order of development does not correspond with the length of the embryo is due to the method of measuring; No. 144 was measured on the glass slide. But a comparison with the profile drawings of these specimens shows that the order of closure of the foramen corresponds with the degree of development of the external form. In No. 423 the interventricular foramen (0.1 mm. in diameter) is situated well anterior, at the point of junction between the septum aorto pulmonale and the right wing of the anterior endocardial cushion. It is under the medial cusp of the tricuspid valve in exactly the position taken by the atrio-ventricular bundle. In No. 424 the foramen is barely 0.02 mm. in diameter, and were not the vascular system injected with india ink the opening would probably be overlooked. It is present in but a single section. Here it is again located with the right limb of the atrio-ventricular bundle below the medial cusp of the tricuspid valve well anterior. On the left side it communicates with the vestibule of the aorta exactly in the position the atrio-ventricular bundle lies in the adult heart. That this is of significance will be considered when the atrio-ventricular bundle is discussed. In this stage the posterior cusp of the aortic valve still lies somewhat distant, but as the valves sink deeper and deeper into the vestibule of the aorta the position of the interventricular opening comes to lie behind the posterior cusp adjacent to the left limb of the atrio-ventricular bundle. In an adult heart with a-patent interventricular foramen I have found the atrio-ventricular system streaming through this opening, thus showing that there is an association between them.
Fig. 13 Sagittal section. Embryo 14 mm. long; (No. 144). X 40.
fig. 14 Coronal section. Embryo 17.2 mm. long (No. 424). X 24.
The atrio-ventricular valves are not as diflicult to trace in their development in the successive stages as has been that of the formation of the membranous septum. ' In the youngest embryos, that is those under 3.5 mm. long, the endocardial connective tissue which. was quite equally distributed in the earliest stages has gradually rearranged itself. first becoming less pronounced in the atria and then becoming well dove-tailed with the trabecular system in the ventricle and bulb. As soon as the atrial canal is well formed the endocardial connective tissue arranges itself there in the form of two cushions the anterior and posterior endocardial cushions. In the bulb two ridges are also formed which ultimately give rise to the septum aorto pulmonale, but as this latter structure has been considered by Greil and by Tandler, I have taken it up only in so far as it bears upon the formation of the membranous septum. In the embryo 3.9 mm. long (No. 463) the two endocardial cushions of the atrial canal are well formed (fig.4). The posterior is short and reaches from the sinus to the ventricle, while the anterior is much more extensive for it reaches from the sinus also around the upper and anterior part of the atrium through the atrial canal to the bottom of the ventricle. In general they repeat that which is shown in Greil’s fig. 3 taken from the heart of Lacerta.[18] The two cushions are confined almost wholly to the left ventricle. However,‘ the side of the lower tip of the anterior cushion passes through the interventricular foramen and continues as the anterior medial endocardial thickening of the bulb. A little later at 4.3 mm. (N0. 148) the same arrangement is still seen except that the left lateral tip of each of the two cushions is more intimately attached to the trabecular system of the ventricle. These attachments mark the beginning of the anterior and posterior papillary muscles. The cushions gradually become more and Inore pronounced until the embryo is 7 mm. long (N 0. 2) when their lower right tips begin to enter the interventricular canal. With the formation of the septum primum the anterior cushion gradually approaches the posterior with which it ultimately blends (fig. 9). Before this takes place the septum primum is perforated forming the foramen ovale II; somewhat later the foramen ovale I is completely obliterated.[19] By this time the two cushions have blended into a solid mass which obstructs the atrial canal, leaving on either side an ostium. The common cushion or valve mass is now wider than it is thick (fig. 15), hangs well into the left ventricle where its two corners or tips are well supported by the two papillary muscles (figs. 11 and 12). The right half rests upon the inferior septum behind (the right posterior tip, projects Well into the interventricular foramen, while the right anterior tip blends with the septum aorto pulmonale. It becomes clear, by comparing this stage with older ones, as well as with the adult (figs. 15 to 18), that this common central mass is destined to produce the medial valves of the two venous ostia, namely anterior cusp of_the mitral valve and the medial cusp of the tricuspid valve.
But in addition to the anterior and posterior endocardial cushions there are also two lateral cushions well demonstrated by His and by Greil. One of these, the left lateral, is first seen in an embryo 9 mm. (No. 422) long, (fig. 7), but both are not well pronounced until the embryo is 11 mm. long (No. 353, figs. 11 and 16). At this time a lateral process from the septum aorto pulmonale reaches to and blends with the right lateral cushion. The left lateral is still sharply defined and isolated. The line of connection between the right lateral cushion and the septum is always marked in the adult by a tendon which passes into the aortic septum or by a small papillary muscle which holds the same position.
This muscle is called medial papillary muscle by Henle.[20] Between the tendon or muscle and below the anterior tip of the medial cusp there is alway a clear field, the bottom of which is formed by the membranous septum.
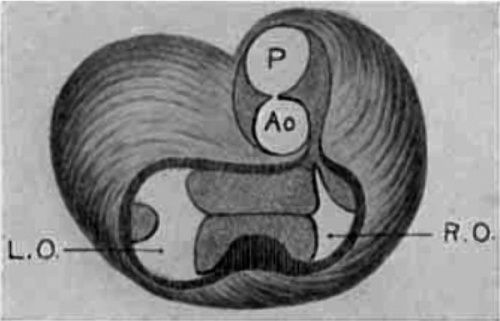
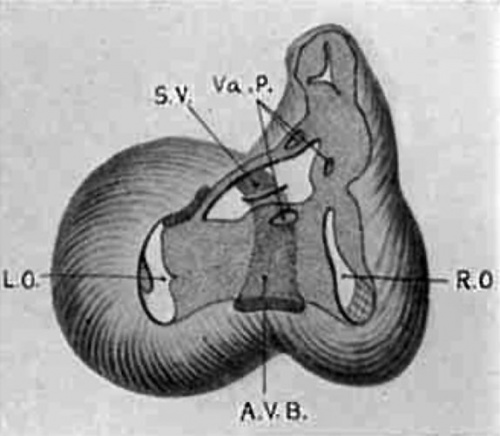
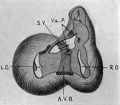
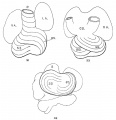
Semi-diagrammatic reconstructions of the tendinous masses at the base of the heart are shown in figs. 15 to 18. fig. 15 is from the heart of embryo No. 163 (9 mm.) with the left lateral cushion from N 0. 422 (9 mm.) added. The muscular wall of the atrial canal is indicated and the connective tissue is stippled. fig. 16 is from embryo No. 353 (11 mm.) which shows that the origin of the aorta has shifted well into the left ventricle. In fig. 17, No. 296 (17 mm.) the sinuses marking the three semilunar Valves are indicated upon the two wings of the septum aorto pulmonale, and upon the anterior endocardial cushion. fig. 18 shows the arrangement of the connective tissue at the base of the adult heart. The parts of the valves which are formed by the undermining process are indicated‘ by cross hatching. The course of the atrio-ventricular muscle bundle dividing within the interventricular foramen and crossing the ventricular septum,
Fig. 15 Semidiagrammatic reconstruction of the heart of an embryo 9 mm. long (No. 163),. The left lateral endocardial cushion has been added from another embryo of 9 mm. (No. 422). is also shown. These figures indicate clearly the fate of the primary dividing masses of connective tissue at the base of the heart in the embryo.
Fig. 16 Semidiagrammatic reconstruction of the heart of an embryo 11 mm. long (No. 353). The muscle encircling the atrial canal is reduced and the atriaventricular bundle is seen passing behind the endocardial cushions to spread over the ventricular septum below.
Fig. 17 The same as fig. 16 from the heart of an embryo 17 mm. long (No. 296). Most of the muscle wall of the atrial canal is absent. The atrio-ventricular bundle is shown and the semilunar valves of the aorta are indicated.
In young hearts the septum aorto pulmonale encircles the right ostium as shown in fig. 17 and from it the tricuspid valve arises. In the adult the septum aorto pulmonale blends With the anterior end of the medial and anterior cusp of the tricuspid Valve, While the same tip of the valve is tied to the conus by the medial tendon which frequently enlarges into a papillary muscle. In following its development it is found that this tendon marks the primary union of the septum aorto pulmonale to the valve and in its subsequent development is separated from the septum as the valve enlarges. The beginning of this process is shown in fig. 19 which is from a section of embryo 86 (30 mm.). It is here seen that the valve mass is enlarging and that its lower border is being separated from the muscle of the right ventricle as the tendinous cords are forming. In so doing the medial papillary muscle is forming and at the same time the trabeculae which encircle the base of the right ventricle as shown in fig. 11, become fused to produce the crista supraventricularis.[21]
Fig. 18 Sketch of the base of an adult heart showing the valves. The atrioventricular bundle is shown. The figure may be compared with figs. 15. 16 and 17.
Fig. 19 Section through the conus and right ostium of the heart of an embryo 30 mm. long (No. 86). X 20. The attachment of the tricuspid valve to the septum aorto pulmonale and the ventricular septum is shown.
This at first ends in the septum aortopulmonale but in the adult it passes around and over the medial tendon and passes down the anterior inner wall of the right ventricle. In the embryo the moderator band arises just below the medial cusp of the tricuspid valve, but in its further development is shifted towards the apex where it binds the large papillary muscle with the extension of the crista supraventricularis.[22] In the ox, pig and sheep, however, it retains its original position and contains the right limb of the atrio-ventricular bundle.
It is thus seen that the medial cusp of the tricuspid valve is attached in front by the medial tendon (or muscle), and behind by the large papillary muscle, and the inequality of these two structures accounts for the double appearance of the lateral valve. In reality no true tricuspid valve is present and correctly speaking there is no tricuspid Valve. Both are bicuspid with medial and lateral cusps. Both are tied down by two muscles, the two papillary muscles on the left side and the large papillary muscle and the median tendon on the right side.
The atrio-ventricular cushions expand not only by their own growth but to a greater extent by a process of undermining the ventricle wall all around the Venous ostia. To what extent this burrowing has taken place is marked by the attachment of the valves to the muscle walls of the heart. The tendons nearest the tips of the valves were the first to form, while those nearer the bases of the valves were formed subsequently. This has all been demonstrated by His. By this process of undermining the attachment of the base of the valve to the Wall of the ventricle, the atrial portion is telescoped into the ostia. The muscle of the atrium extends into the atrial part of the valves, which at first is continuous through the tendinous cords With the ventricular muscle. At first the atrio-ventricular muscle connection is through the main wall of the ventricle, but as this is resolved into the trabecular system with the growth of the valves and the formation of the papillary muscles the connection between atrium and ventricle is through the tendinous cords which are at first muscular. Later with the degeneration of the muscle in the cords the muscular connection between the atria and ventricles was believed to have been broken down completely. In lower vertebrates the muscular connection between the atria and ventricles through the trabecular system remained throughout life, and the significance of thishas been fully demonstrated by Gaskell[23] and His, Jr.[24] In man, however, all of the cords are converted into connective tissue, but the muscle of the atria extends well into the valves or it may reach to its free border (Kürchner).[25]It has also been stated that in some rare instances the tendinous cords of the mitral valve remain muscular in the adult (Oehl).[26] But the muscular connection through the valves is first interrupted at the free thin edges and later the muscular fibers in the tendinous cords disappear. The break in the muscular connection at the free edges of the valves will be considered in discussing the atrio-ventricular bundle.
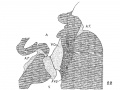
Returning to the description of embryo No. 353 (11 mm.), it is possible to determine with considerable precision the various adult connections of the valves. The most definite valve is the anterior cusp of the mitral which is formed by the union of the left lateral tips of the anterior and posterior endocardial cushions. Each tip is here bound to the trabecular system by well formed muscle strands, one of which passes to the anterior wall of the heart and the other to the posterior as well as to the septum. It is clear that these two strands, which appear much earlier than in this specimen and continue throughout development, are the anlages of the anterior and posterior papillarymuscles respectively. In addition a heavy strand of tissue encircles the lateral side of the left ostiuni and unites the apices of the two papillary muscles. In the middle of this, protruding into the left ostium, is seen the conspicuous left lateral cushion already noted in an embryo 9 mm. long (No. 422, fig. 7). This arrangement corresponds with what is found in the adult heart. Each papillary muscle not only attaches itself to the tips of the anterior cusp but also to the tips of the posterior cusp. V (It is confusing to use the B.N,A. terms anterior and posterior in naming the cusps when the embryological terms should be medial and lateral.) Between the two papillary muscles there is often a third or lateral papillary muscle, or numerous small muscles or the two muscles may be widened to fill this area. At any rate the structures found around the left ostium in embryo 353 represent fully what is present in the adult. We have in all cases two papillary muscles, each of which communicates freely with the tips of the two cusps of the initral valves. On the dorsal side and in front of the left ostium the muscle of the atrium and ventricle is continuous, as is shown in fig. 16.
As the heart grows larger more chordae tendinea are formed necessarily nearer to the base of the heart. V It follows that the primary condition found in No. 353 represents only the tips of the valves and that their subsequent enlargement is due partly to stretching of the anlage and partly to the undermining of the wall of the ventricle.
The united anterior and posterior endocardial cushions were projected at first into the left ventricle but subsequently its left tip becomes lodged in the interventricular forarnen (fig. 11). To this smaller portion the septum aorto pulmonale attaches itself anteriorly and the inferior muscular septum posteriorly. The left half hangs freely within the left ventricle throughout life, forming a loose flap or sail which is suspended between the left venous ostium and the vestibule of the aorta. While the semilunar Valves of the aorta are somewhat distant from this flap in embryo N o. 353, later on they are pushed down to it so that the aorta becomes finally attached to its base (fig. 17).
On the right side of the heart the septum aorto pulmonale soon blends with the lateral tip of the anterior cushion and by the time the septum is complete, as in No. 353, it divides, one portion of which is attached directly to the left anterior tip of the common cushion and the other encircles the right venous ostium and blends with the right lateral cushion. The posterior side of the ostium is formed by trabeculae which pass from the lateral cushion mainly to the muscular septum and others which course symmetrically into the trabecular system of the ventricle. It is by no means easy to determine three systems to correspond with the three cusps of this Valve. Neither is it easy to recognize the three cusps in the adult unless We associate the medial papillary muscle, which is constant, with the anterior cusp and the large papillary muscle With the posterior cusp. If this is done it is possible to name the valvesin the heart of embryo No. 356 as follows (figs. 15 to 18):
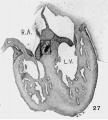
The median cusp is attached in this embryo to the septum aorto pulmonale in front and to the muscular septum behind. It has corresponding attachments in the adult. The anterior cusp is attached partly to the septum aorto pulmonale and to the large papillary muscle. The posterior cusp is attached to the large papillary muscle. If this division is correct the lateral cushion belongs to the anterior cusp While the posterior cusp is developed entirely from the lateral side of the ventricle. The medial papillary muscle is constant and always extends as a muscle, or as a tendon, from the region of the septum aorto pulmonale (tendon of the conus) to the anterior cusp which is marked by a similar strand of tissue from the septum to the lateral cushion in the embryo. That this is the case is shown in sections of older hearts, one of which is given in fig. 19. The posterior cusp, over the large papillary muscle, is of irregular formation and by a process of exclusion the muscle strands in the embryo which encircle the right ostium behind the right cushion, should give rise to it. At any rate the right and left ostia of numerous hearts of embryos less than 20 mm. long, are slit-like and similar, so that it is impossible to state that one is encircled by a tricuspid valve. Each is bordered by medial and lateral valves, and each medial and lateral valve is bound to the ventricle by anterior and posterior papillary muscles. In the course of time the valves and papillary muscles will be renamed to correspond with their development, which will also accord with the adult condition.[27]
C. The Atrio-Ventricular Bundle
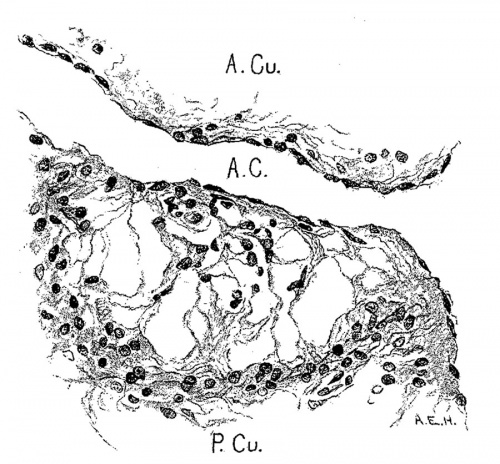
No complete description can be given to the formation of the tricuspid and mitral valves Without considering the fate of the muscular wall of the atrial canal. This at once defines definitely the atrio-ventricular bundle which is cmbryologically the remnant of the auricular canal after the greater portion of its muscular wall has been destroyed by the formation of the valves.
His, Sr., described the breaking down of the muscular wall of the atrial canal in small embryos and states that it is nearly broken down in an embryo 13.8 (T?) mm. long. Later His, Jr., showed the presence of a muscle bundle connecting atrium with ventricle in the new-born child and discusses the connections between atrium and ventricle in lower animals as well as in the embryo. He noted that the character of the heart beat changed in the chick at the time the atrial canal is forming, at the time the muscular connection between the atria and ventricles is reducing.[28] It is easy to read into this paper that His thought that the muscular connection between the atria and ventricles never does break down completely, although he never states it. Were there acomplete separation of the muscle wall of the atria and ventricles before the formation of the atrio-ventricular bundle is formed heart block should occur in the embryo. That the atrio-ventricular bundle is a remnant of the primary connection in the embryo is mentioned by Tandler as a possibility but he believes it improbable. He is rather inclined to think that it is a new formation, as has been asserted by Retzer.[29]
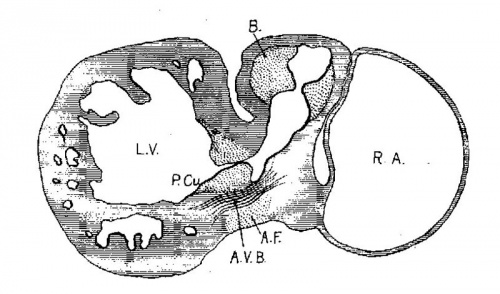
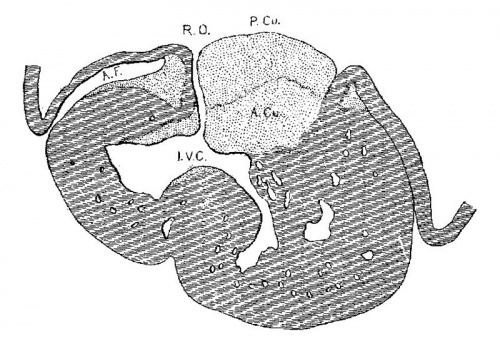
In following the /atrio-ventricular bundle in the foetus Fahr[30] found it in one of 160 mm. long, Tawara[31] in one 100 mm. long, Mönckeberg[32] at 75 mm., Tandler in human embryos 28.5 and 19 mm. long, and Retzer in the pig from 15 to 20 mm. long. His, Sr., states that the muscle wall between the atria and ventricles is nearly broken down in an embryo 13.8 (T?) mm. long. It is evident that if the bundle is a new formation it must appear in embryos about 15 mm. long. However, in order to anticipate my own report upon this subject it may be stated that the bundle is not a new formation but is the remnant of the wall of the atrial canal after its anterior and lateral sides have been broken in the formation of the tricuspid and mitral valves. The portion on the posterior wall which connects the sinus with the ventricle never breaks down; early in development it shows changes in structure which differentiate it from the rest of the heart muscle. ."It holds a constant position just behind the posterior endocardial cushion immediately over the septumof the ventricle, a position which is marked at first by the interventricular foramen and later by the membranous septum.
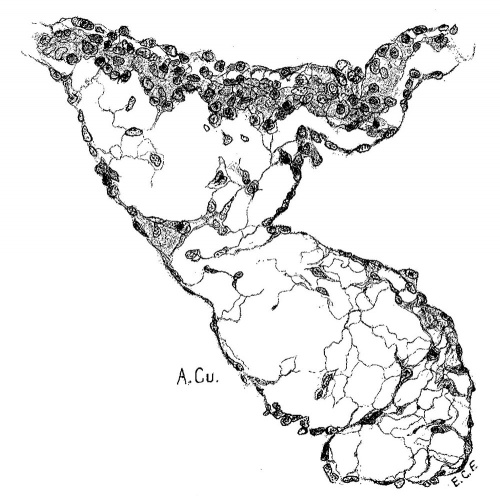
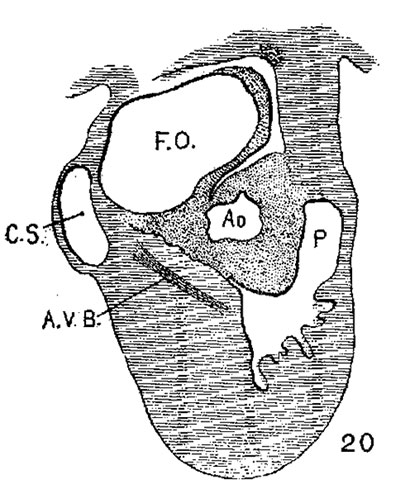
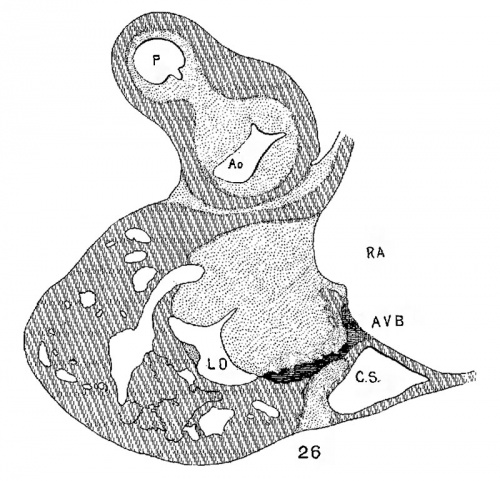
The atrial canal is first well formed in an embryo 3.9 mm. long (No. 463) ; it is now markedly constricted and is pretty well filled up by the two large endocardial cushions (fig. 4). Its outer wall is uniformly muscular throughout. There is practically no changes in subsequent stages until the lateral endocardial cushions make their appearance (fig. 15), both of which are present in an embryo 11 mm. long (No. 353, fig. 16). At this time there is a break in the region of the left cushion, and possiblyover the right cushion, as well as in the bottom of the bulbo-ventricular groove, that is between the aorta and the left ventricle. At 13 mm. (No. 406) the break is more marked over the left endocardial cushion, there are several breaks 011 the right lateral side, a large one just behind the aorta and one on the medial dorsal side of the left ostium, In other words the muscular connections between the atria and ventricles are as follows: A large one in front of and a large one behind the left ostium, a number of small ones lateral to the right ostium and a large one from the sinus to the ventricle dorsally just over its inferior septum. In another embryo of the same stage of development (N o. 317, 12.5 mm.) there are two marked muscular connections, one in front of each of the ostia. There is a very marked one from the sinus to the inferior septum below. This is composed of muscle fibers which arise as two horns to which the dorsal muscle fibers of the atria stream. This is clearly the atrio-ventricular muscle, as shown in fig. 17. From now on it is the chief muscular connection but for some time additional connections are often seen. For instance in embryos 14 mm. (No. 144) and 15.2 mm. (N o. 423) there is an additional connection just in front of the right ostium, in embryos 16 mm. (409), 18.5 mm. (431) and 20 mm. (368) there is an additional connection just in front of the left ostium. In a number of specimens the atrio—ventricular bundle is not confined to the region immediately over the inferior septum but is spread out over the dorsal side of the left ostium (432, 18 mm. ; 431, 18.5 mm. ; 368, 20 mm.), while in one specimen 21 mm. long (460) there is a single additional strip on the left lateral side. In general, however, the main bundle which is at first broad and associated more with the left ventricle than the right, gradually becomes constricted so that it is well formed and rounded by the time the embryo is 20 mm. long. It is beginning to be isolated at 11 mm. and well separated at 13 mm.
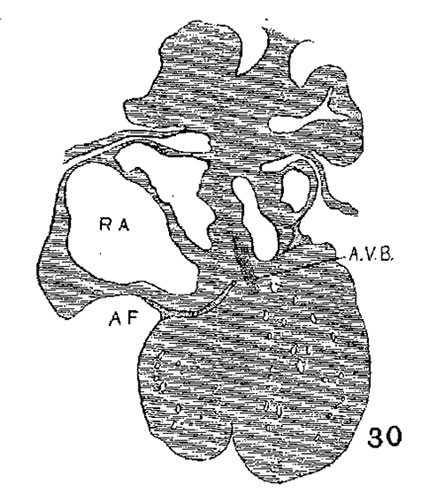
The position of the bundle is shown in sagittal sections in figs. 9 (No. 113, 8 mm. long), 13 (144, 14 mm.), 20 (390, 15.5 mm.), 21 (432, 18 mm.), 22 (431, 18.5 mm.) and 23 (368, 20mm.). It is clear in all of these hearts that the muscle connection is from the sinus between the posterior cushion and the annulus fibrosus into the septum of the ventricle. This is its position in the adult heart. In transverse sections the bundle is shown in figs. 8 (No. 2, 7 mm.), 24 (353, 11 mm.) and 26 (317, 16 mm.). Here again it is clear that the bundle lies just back of the endocardial cushion. Coronal sections ofthe heart giving the position of the bundle are shown in figs. 27 (46, 21 mm.), 29 (409, 16 mm.) and 30 (423, 15.2 mm.). In these figures it is clear that the bundle passes below the medial cusp of the tricuspid valve and between it and the interventricular septum. The extreme dorsal connection is shown in fig. 30. In earlier stages while the interventricular foramen is still present, it is easily ‘seen that the bundle forms its wall as indicated in figs. 12 (175, 13 mm.) and 14 (424, 17.2 mm.).
Fig. 20 Sagittal section. Embryo 15.5 mm. long (No. 390). x 18.
Fig. 21 Sagittal section. Embryo 18 mm. (No. 452). X 40.
These sections show definitely that the bundle is present in the youngest hearts after the atrial wall has begun to break down, and that its course is exactly the same as it is in the adult. After the embryo is over 20 mm. long the bundle is easily seen, as has been demonstrated by other observers. figs. 15 to 18 gives a summary of what I have stated.
The additional strips above connecting atria with ventricles in various stages in embryos less than 20 mm. long may be of significance in view of Romberg’s and Kent’s observations. Romberg[33] found muscular connections between the anterior and posterior cusps of the tricuspid valves. Kent[34] found such bundles in the left Wall of the heart as Well as in the right Wall in young rats and young rabbits. It is possible that some of these strips may be constant or they may be variations. At any rate their presence has not been established as has been the atrio-ventricular muscle of His.

|

|
| Fig. 22 Sagittal section. Embryo 18.5 mm long (No. 431). X 40. | Fig. 23 Sagittal section. Embryo 20 mm long (No. 368). X 40. |
So far I have shown that there is a band of tissue apparently muscular, which connects the sinus, or atria, with the ventricle. At first it is broad but it gradually becomes constricted. In an embryo 12.5 mm. long (317) the muscle fibers of the atria stream towards the connecting muscle as two horns, somewhat later it arises from a thickened mass in the neighborhood of the sinus and finally from the mass which has become converted into a nodule. About this time according to His, Jr., the nerves begin to invade this region. However, I have been able to trace the ganglion cells through the septum to the valves in but a single specimen 34 mm. long (No. 249) which shows that the nerve fibers really do enter the bundle on its atrial side. To what extent they penetrate the ventricular portion is still undecided.
It is not easy to determine with certainty the destruction of the lateral and anterior walls of the atrial canal, and my statements rest upon repeated studies of this region in all of my embryos. Not only does the endocardial thickening play a role in this process but the connective tissue of the epicardium also plays a part, as His, Sr., has shown. To what extent the outer connective tissue plays a part is well shown in the reconstruction of an embryo 11 mm. long (353). The model shows that it forms a collar encircling entirely the heart between the atria and ventricles and also extends into the anterior and posterior longitudinal sulci. On the two lateral sides the encircling connective tissue is drawn into the valves as they become larger and this process completes the separation of the atrial and ventricular musculature. Between the atria and the aorta it forms a large plug which soon comes into apposition and blends with the anterior endocardial cushion. Behind it encircles the atrio-ventricular bundle so that this bundle becomes lodged between the posterior cushion and the outer connective tissue. The relation here found corresponds with the position of the bundle in the adult, for it then lies behind the medial cusp of the tricuspid valve which arises from the endocardial cushion, and the right annulus fibrosus, which arises from the outer connective tissue.
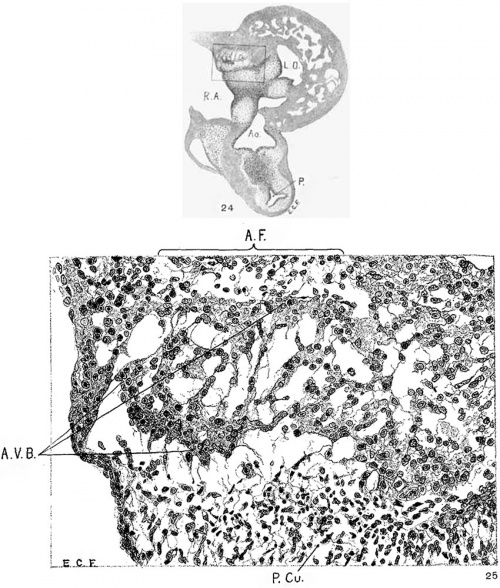
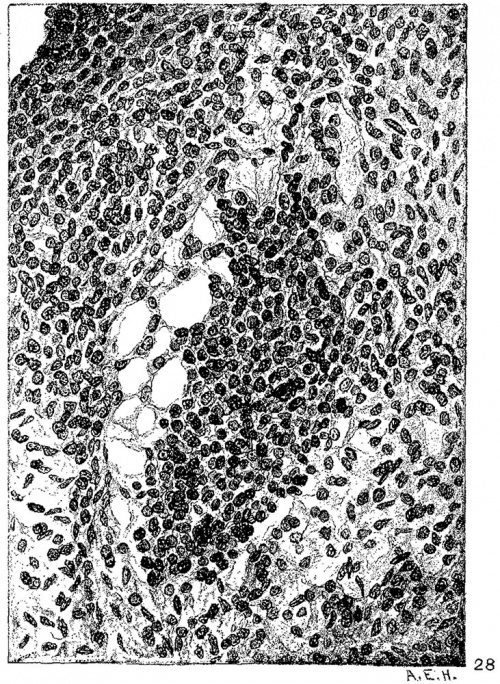
Fig. 24 Transverse section of the heart of an embryo 11 mm. long (No. 353). X 33. Fig. 25 Enlarged drawing of the square shown in fig. 24 including the atrio-ventricular muscle. X 360.
Fig. 26 Transverse section. Embryo 16 mm. (No. 317). X 40.
Fig. 27 Coronal section through the heart of an embryo 21 mm, long (No. 460). A square is around the atrio-ventricular muscle.
Fig. 28 Enlarged drawing of the atrio-ventricular muscle shown in fig. 27. X 360.
That the bundle differs in structure from the rest of the muscle of the heart of the foetus has been shown by Monckeberg, who describes and pictures it in a specimen of the fifth month. Fahr states that the bundle is less pronounced in the foetus than in the adult but that its position is the same. Tandler found it in an embryo 20 mm. long as “distinguishable even under low powers by its staining properties.” The nuclei are dark and the cell border stains faintly with eosin. At 20.5 mm. the cells of the bundle have become larger both in the atrium and the ventricle.
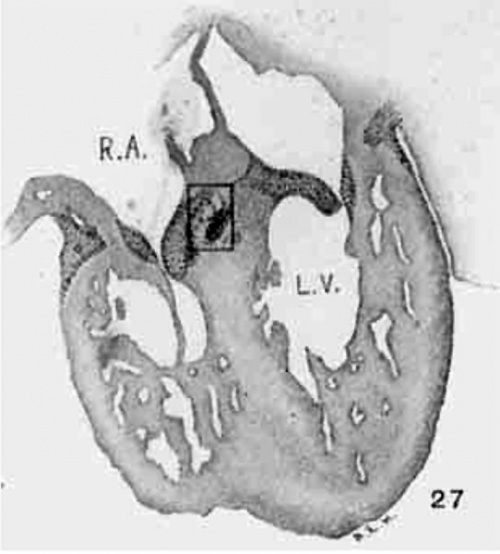
In suitable sagittal sections of the heart the atrio-ventricular bundle may be recognized in an embryo 8 mm. long (fig. 9) as a strand of tissue extending from the region of the sinus to the ventricle. This tissue is somewhat separated from the rest by delicate spaces and its cells stain somewhat more intensely than the rest. In embryo pigs 7 mm. long the bundle appears more like epithelium with a tendency towards vesicle formation in the immediate region of the sinus. In larger foetuses of the sheep the epithelial nature is most pronounced. In an embryo 11 mm. long (figs. 24 and 25) the numerous small spaces around the bundle encircle it from the region of the sinus just behind the posterior endocardial cushion, along its course behind the medial cusp of the tricuspid Valve to the upper border of the ventricular septum. This same arrangement is found in another embryo (12.5 mm. long, fig. 6) cut in the same plane and stained in the same Way. In this specimen the muscle fibrils are not present in the bundle but they are found in the muscle cells of the atria and the ventricles. In a sagittal section of an embryo 14 mm long (fig. 13) the bundle is recognizable throughout its extent by its surrounding spaces and not by its staining properties. In 175 (fig. 12) the bundle can be followed into the two ventricles. The same structure and arrangement is made out in embryos 15.2 mm. (423), 15.5 mm (390), 16 mm (409, fig. 29) and 17.2 mm (424, fig. 14). In older specimens the interventricular foramen is just closed and the bundle reaches to it from the region of the sinus. Two limbs are easily outlined, one of which reaches to the moderator band of the right ventricle, which is pronounced in the human embryo.
The atrio-ventricular muscle is well lodged between the valve and the annulus fibrosus in an embryo 18 mm. long (432, fig. 21); it again extends into the moderator band. The same is observed in another specimen of the same size and prepared in the same way (No. 43, 18.5 mm. long). At this stage the nerves have reached the region of the sinus as is seen in specimen (460, 21 mm. long). In this specimen the bundle can be followed by the spaces around it, figs. 27 and 28. Another specimen (368, 20 mm.), which is unusually Well preserved to show the fibrillae of the heart muscle, shows that the degree of development of the atrio-ventricular bundle is the same as that of the rest of the musculature of the heart.
Fig. 29 Coronal section to show the position of the atrio-ventricular muscle. Embryo 16 mm. long (No. 409). X 18.
Fig. 30 Coronal section, Well dorsalwards, to showthe extension of the atrio-ventricular muscle to the coronary sinus. Embryo 15.2 mm. long (No. 423). X 15.
The spaces around the bundle in embryos from 10 to 20 mm. long appear to continue in the adult; possibly they form the bursa of the bundle described by Curran[35] and the spaces which encircle the Purkinje fibers. Brilliant injections of these have been made by Lhamon.[36]
In an embryo 34 mm. long, No. 249, the atrio-ventricular bundle is chiefly muscular as it passes to the ventricle. Near the sinus it is invaded by nerve cells. From now on there is a change in the structure which is well shown in two foetuses 50 mm. long (Nos. 84 and 96). In both the cells are more ‘epithelial’ in appearance and in the former this differentiated band can be followed into both of its divisions, one of which reaches to the moderator band. The limb of the bundle which passes to the left ventricle is most sharply defined. In a foetus 80 mm. long (No. 172) this bundle is easily made out and appears much as it does in other foetuses, or as it is in the adult.
It is thus seen that the atrio-ventricular bundle is present in an embryo 8 mm. long and that as soon as the muscle of the rest of the atrial canal is broken down it becomes outlined by encircling spaces which are now formed. Later it is composed of muscle which resembles much the rest of the heart musculature. When the nerves invade the septum of the atria the atrio-ventricular muscle shows marked histological changeswhich remind one much of the Purkinje fibers. These I have followed into both ventricles to the moderator band on the right side in the human heart, and through it in the pig. In the foetal sheep up to 15 cm. long much the same conditions prevail and by using a great variety of stains I was able to follow the atrio—ventricular bundle beyond the moderator band; no Purkinje fibers were found. As we now know the continuity of the bundle with the Purkinje system it is not remarkable that in early stages the structure of the main bundle simulates that of this system. In the adult heart Purkinje fibers are found throughout the Ventricular portion of the atrio-ventricular bundle and this is all in harmony with what I have found. I can not leave the subject without expressing the suspicion that the differentiation of the Purkinje system is in some way due to the influence of nerves when they appear in the wall of the sinus.
D. Musculature of the Left Ventricle
It is practically impossible to unravel the musculature of ventricles from serial sections alone. However, it is possible to gain quite a clear picture of the muscle bundles by direct observation of the whole heart with the aid of the dissecting microscope. About two dozen hearts were removed from human embryos ranging from 10 to 40 mm. in length and these were first carefully studied with various enlarging lenses including the binocular. It was found that the hearts from embryos over 15 mm. long could be dissected under the binocular to great advantage, especially after they had been stained in alum cochineal. So all of the hearts were stained and preserved in alcohol. No dissection was made hastily and during the preparation numerous sketches made. In the course of time after many attempts had been made I finally satisfied myself regarding the various stages of the development of. the chief muscle bundles of the left ventricle.
The following specimens were dissected; the smallest embryo was an unusually good one from a tubal pregnancy.
Number and length of embryos whose hearts were removed entire, studied and dissected
| No. | Length |
|---|---|
| 426 | 10 12 |
| 360 | 14 |
| 434 | 15 |
| 90c | 17 17 |
| 90d | 18 |
| 283b | 19 |
| 293 | 19 |
| 263 | 23 25 |
| 118 | 25 |
| Davis | 27 |
| 33 | 27 |
| 90a | 30 |
| 227 | 40 |
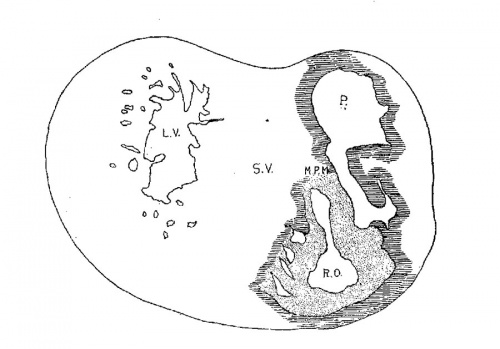
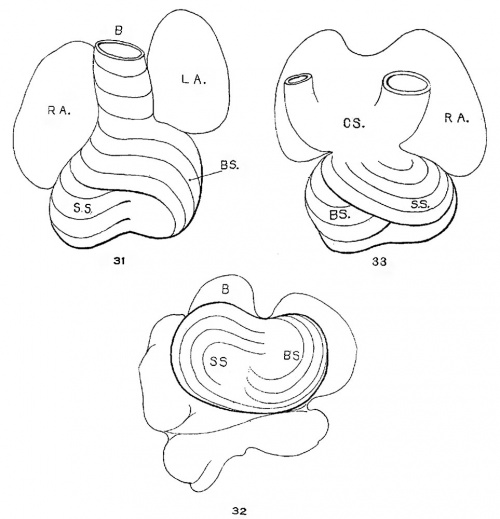
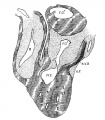
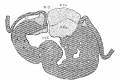
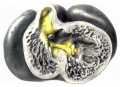
The heart was removed and examined many times in various fluids by transmitted and reflected light. The best drawings were obtained from the stained heart While it was in 60 per cent alcohol. These are shown as diagrams in figs. 31, 32 and 33. It is noticed at once that most of the striations on the surface of the heart are circular, which is probably the arrangement of all the fibers in younger specimens. However, at the apex certain changes have taken place which fully account for the arrangement of the muscle bundles in the adult heart. In front of the heart, at the bulboventricular furrow, the fibers leave the bulb and penetrate the medial side of the left ventricle, as indicated in fig. 31. Behind the fibers leave the surface of the left ventricle and enter the inferior septum Which is just beginning to form from below and behind (fig. 33). Thus it is seen that the vortex of the apex is laid down in the heart of an embryo 10 mm. long (fig. 32).
Fig. 31 Outline drawing of the front of the heart of an embryo 10 mm long (No. 426). The course of the fibers is indicated. Fig. 32 The same heart, viewed from below. fig. 33 The same heart, viewed from behind.
In a set of perfect serial sections of the heart of and embryo 11 mm. long (353) no such interlacing of the fibers can be seen, excepting well up near the base of the heart where the fibers from the right side- cross over the anterior longitudinal furrow, while in the posterior longitudinal furrow the fibers from the left side enter the septum. There is every indication from the study of older specimens that the ‘vortex’ formation is at first well up in the middle of the heart and that only later it is pushed down to the apex of the left ventricle.[37] I was unable to determine this point with certainty from my sections. However, it is clear that in an. embryo 3.9 mm. (463) long (fig. 1) the posterior bundle, that is the bulbo—spiral, enters the septum and forms its upper border just below the inter-ventricular foramen. Below this the sino—spiral should pass into the left ventricle in front, but sections do not show that the cells in this region of the heart are passing in any special direction. However, it is seen that the circular muscle of the left ventricle appears at the base of the heart and not at its apex. In this embryo the interventricular foramen is 0.15 mm. in diameter; in one 8 mm. long (113) it is 0.3 mm. in diameter and in one 11 mm. long (353) it is 0.4 mm. in diameter. This indicates that the ventricles grow longer by a down growth of their walls and not as an upgrowth of the inferior septum.[38] With this in view it is clear that the bulbo—spiral should be located near the base of the heart at the time of its earliest appearance.
In an embryo 12 mm. long the superficial fibers on the dorsal side of the heart, that is the bulbo-spiral, could be followed into the ventricular septum and the sino—spiral around the heart into the left ventricle. The structures showing the direction of the muscle fibers could only be seen after the whole heart had been stained with coéhineal and methylene blue. In a specimen 14: mm. long (360) the heart was dissected from behind under the binocular microscope and the sine-spiral bundle was followed. These fibers passed on both sides of the ventricular septum in front to the septum aorto pulmonale, that is they formed the longitudinal bundles of the right ventricle; the spreading out of the sinespiral in the left ventricle as well as the interpapillary muscle bundles were also seen. A large band of fibers crossing the right ventricle was incorporated with this system; no doubt it is the moderator band, which is Very pronounced in the embryo.
The two main bundles could be followed with precision in the heart of an embryo 17 mm. long, the bulbo-spiral passing into the upper part of the septum and the sino-spiral to the anterior wall of the left ventricle.
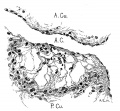
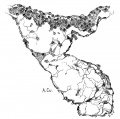
By the time the embryo reaches 25 mm. in length it is easy to demonstrate that the strands of muscle which were observed in younger specimens are destined to become the main muscle bundles of the heart in the adult. first of all there are fibers on the dorsal side of the heart which cross diagonally the posterior longitudinal sulcus to reach the apex of the right ventricle. From here they pass to the left ventricle and pierce it near the apex anteriorly to form the anterior horn of the vortex." From this horn fibers pass on the right side of the ventricular septum to the septum aorto pulmonale, that is they are the longitudinal fibers of the right ventricle. Under the sino-spiral band loops of fibers are seen which encircle the left ventricle and end in the posterior triangular field. All of these loops enter the septum from behind and form the bulbo-spiral fibers. This arrangement was clearly demonstrated in a heart from an embryo 17 mm. long, as well as in ‘specimens 25 and 27 mm. long (figs. 34 to 37). In one of these (No. 33) the sino-spiral turns upward at the apex of the right ventricle along the lower part of the anterior longitudinal sulcus and then enters the left ventricle. At this point the fibers blend with the trabeculae of the right ventricle and then give rise to the longitudinal fibers of the septum of the right ventricle. The bulbo-spiral bundles form loops reaching towards the apex of the left ventricle, that is, they are only an extension of the posterior triangular field. This, as has been shown in the previous study, remains in the adult as the circular fiber of the left venous ostium. At the extreme posterior border of the left ventricle there is a marked raphé to correspond with the base of the posterior papillary muscle much as in the adult pig’s heart. The sino-spiral bundles were also seen. A large band of fibers crossing the right ventricle was incorporated with this system; no doubt it is the moderator band, which is Very pronounced in the embryo.
The two main bundles could be followed with precision in the heart of an embryo 17 mm. long, the bulbo-spiral passing into the upper part of the septum and the sino-spiral to the anterior wall of the left ventricle.
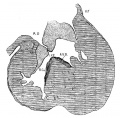
By the time the embryo reaches 25 mm. in length it is easy to demonstrate that the strands of muscle which were observed in younger specimens are destined to become the main muscle bundles of the heart in the adult. first of all there are fibers on the dorsal side of the heart which cross diagonally the posterior longitudinal sulcus to reach the apex of the right ventricle. From here they pass to the left ventricle and pierce it near the apex anteriorly to form the anterior horn of the vortex.[39] From this horn fibers pass on the right side of the ventricular septum to the septum aorto pulmonale, that is they are the longitudinal fibers of the right ventricle. Under the sino-spiral band loops of fibers are seen which encircle the left ventricle and end in the posterior triangular field. All of these loops enter the septum from behind and form the bulbo-spiral fibers. This arrangement was clearly demonstrated in a heart from an embryo 17 mm. long, as well as in ‘specimens 25 and 27 mm. long (figs. 34 to 37). In one of these (No. 33) the sino-spiral turns upward at the apex of the right ventricle along the lower part of the anterior longitudinal sulcus and then enters the left ventricle. At this point the fibers blend with the trabeculae of the right ventricle and then give rise to the longitudinal fibers of the septum of the right ventricle. The bulbo-spiral bundles form loops reaching towards the apex of the left ventricle, that is, they are only an extension of the posterior triangular field. This, as has been shown in the previous study, remains in the adult as the circular fiber of the left venous ostium. At the extreme posterior border of the left ventricle there is a marked raphé to correspond with the base of the posterior papillary muscle much as in the adult pig’s heart. The sino-spiral loops now form the anterior horn of the vortex and the lower end of the loops form the posterior horn.[40]
Fig. 34 Course of the fibers in the heart of an embryo 17 mm. long. The View is from behind. Fig. 35 The same as fig. 34 from the heart of an embryo 25 mm. long. The fibers which pass to the base on the right ventricle are present. Fig. 36 The same as fig‘. 35, from an embryo 27 mm. long. Fig. 37 The same as fig. 35, from another embryo 27 mm. long (No. 33).
To sum up: The simple heart tube is made up of circular fibers which in a general way remain circular throughout development. After the heart is well kinked upon itself the fiber bundles around the venous and arterial ends remain circular but there are marked deviations at the apex which correspond with the kinking at this point. Those fibers that arise from the bulbo-ventricular groove in front encircle the left ventricle and enter the ventricular septum behind quite high up so that they form its border just below the interventricular foramen. Those that arise behind encircle the right ventricle and enter the heart in front of through the anterior longitudinal sulcus. Now as the heart grows by an extension of the ventricles downward these two encircling bands also grow and ultimately become interlocked at the apex of the left ventricle, the fibers from the bulb forming the bulbo-spiral band and the posterior horn of the vortex; the fibers from the sinus side, the sino-spiral band form the anterior horn of the vortex. As the sino—spiral band becomes larger and larger it is shifted over the greater part of the bulbo-spiral band on the posterior side of the heart. On the anterior side of the heart with the growth of the anterior longitudinal sulcus, the sino-spiral band gradually extends sending bundles to both sides of the septum, that is it constantly straddles the septum from its lower and under side. By this extension it binds together the inner walls, especially the papillary muscles, of=the two ventricles with each other. These are the two strands shown in figs. 35 to 37.
The bulbo-spiral band first extends from the bulb over the left ventricle, and with its growth penetrates its wall, ultimately including its apex to end in the posterior horn of the vortex (figs. 31 to 33). In so doing it produces loops which constantly turn upon themselves to return to the base so that when viewed together the bulbo-spiral band appears as a series of telescoped loops of sheet fibers as is now well known. At the base of the left ventricle the fibers are more circular forming a thick fleshy mass on its dorsal side, the triangular field, which in the adult still marks the embryonic conditions; all loops of the bulbo-spiral band are but an extension'of this field as is shown by the development of the fibers as well as by the architecture of this muscle in the adult.
Figures
References
- ↑ Mall, F. P., On the muscular architecture of the ventricles of the human heart. Amer. Jour. Anat.. vol. 11, 1911.
- ↑ A complete description of this embryo is given by Dandy. Amer. Jour. Anat., vol. 10, 1910. Sections through the heart are shown in his figs. 1 and 2. Evans (Keibel—Mall, Manual of human embryology, vol. 2, fig. 409) also pictures sections through the heart of this embryo. Also by Mall, Ibid., vol. 1, figs. 3824386.
- ↑ His, W., Anatomic mensch. Embryonen. Theil 3, Leipzig, 1885.
- ↑ A few data regarding all of the embryos described in this paper are given in my catalogue of 500 specimens. Anat. Rec., vol. 5, 1911.
- ↑ His, Untersuch ucbcr die erste Anlage dos Wirbelthierleibes. Leipzig, 1868; S. 141.
- ↑ His, Anat. mensch. Ernbryonen. Th. 3, 1885, S. 141.
- ↑ Tandler, Entwicklungsgeschiehtc des Herzens. Keibel-Mall Handbuch d. Entwicklg. cl. Mensehen, Leipzig, 1911, Bd. 2, S. 524.
- ↑ Mall, Development of the connective tissues from the connective syncytium Amer. Jour. Anat., vol. 1, 1902, p. 354.
- ↑ Kon, Die Gittcz-fasergerust der Lcber, etc. A1-chiv. fur Entwickl.-Mcclianik, Bd. 25, 1903.
- ↑ Mollier, Die Blutbildung in dcr ernbryonalen Lel)e.1'desMenscl1en. Archiv fur mik. Anat., Bd. 74, 1909.
- ↑ Lewis, F. T., Entwicklung der Leber. Keibel—Mall, Handbuch der Entwir.:kl., Bd.2,1911. S.397,fig.291.
- ↑ Quite recently Mollier finds the endcthelial cells and reticulum of the spleen as one continuous reticulum from which he concludes that the endothelial cells arise directly from the mcsenchyme (Arch. f. mik. Anat., Bd. '76, 191.1). The opposite conclusion may be drawn equally Well. I have published figures which correspond with Mollier’s, giving at the same time a conclusive experiment to prove that the circulation of the spleen is entirely through the pulp spaces; these have the value of blood capillaries (Mall, Amer. Jour. Anat., vol. 2, p. 315). Pathologists have been of the opinion that in endarteritis the intima thickens by a proliferation of endothelial cells, and that connective tissue may arise from these cells. Marchand has questioned the truth of this statement, but recently the subject has been reinvestigated by Baumgarten (Arbeiten auf dem Gelmeite der Pathologischen Anatomic, Leipzig, 1904, Bd. 4) who showed that proliferation of endothelial cells may form a thickening of the intima without any rupture of the elastica interna, thus excluding entirely any participa1 ion of connective tissue cells in the process. See also Von Szily, Anatom. Hefte 35, 1908, Taf. 45-47.
- ↑ Evans, Keibel-Mall Handbuch, Bd. 2. fig. 469.
- ↑ Mall, Bifid apex in the human heart. Anat. Rec., vol. 6, 1912.
- ↑ Quain’s Anatomy, 10th Edition, vol. 2, fig. 317.
- ↑ Spalteholz, Hand atlas, vol. 2, fig. 420.
- ↑ The normal position of the membranous septum is described in an article on aneurysms arising from it, in the Anatomical Record, vol. 6, 1912.
- ↑ Greil, Beitrage zur vergleich. Anat. u. Entwicklungsg. d. Herzcns u. d. Truncus arteriosus d. Wirbelthiere. Morph. Jahrbuch, Bd. 31, 1903.
- ↑ See also Greil, l.c., figs. 5 and 12.
- ↑ Henle, Gefasslehre, II Aufl-age, 1876, fig. 33. This tendon is constant, in fact the most constant of all of the attachments of the valves in the right ventricle. It is pictured in all anatomies, but is not recognized in the B.N.A.
- ↑ The ‘crista’ is correctly figured in Toldt’s Atlas, fig. 946, while in Spalteholz, fig. 424, it is pictured as extending down to the base of the large papilary muscle. It is this extension which contains the right limb of the atrio-ventricular bundle and therefore must represent the moderator band. A study of the development of this portion of the heart shows that this is the case.
- ↑ Toldt’s ‘crista’ (l.c., fig. 946) ends in the medial papillary muscle (anterior), which according to its development is correct. Spalteholz (l.c., fig. 424) extends the crista past the anterior tendon down to the base of the large papillary muscle (posterior). This connecting band contains the right limb of the atrio—ventricular band and is pictured by Tawara on his plate 7. Retzer (J. H. H. Bull., vol. 20, 1909, and Anat. Rec., vol. 6, 1912) associates the moderator band with the erista, in fact he says that when absent it is represented by the crista. He recognizes fully the meaning of the anterior (medial) papillary muscle, but when it is recalled that the right limb of the atrio-ventricular band passes on the posterior side of this muscle and the crista on its anterior, the identity of these two structures is disproved. In fact in the embryo the crista reaches to the medialmuscle and the moderator band is considerably below it. In the adult heart this band is pushed to the base of the large (posterior) papillary muscle as pictured in Spalteholz and it contains the right limb of the atrio-ventricular bundle as shown by Tawara; this I have been able to confirm. His, who introduces this term ‘crista’ (Beitrage zur Anat. d. Mensch. Herzens, Leipzig, 1886, S. 9) is not clear in his description of its attachment to the septum. According to its development it should end in the septum aorto pulmonale, that is, at the point of origin of the medial papillary muscle.
- ↑ Gaskell, On the innervation of the heart with special reference to the heart of the tortoise. Jour. Phys., vol. 4, 1884.
- ↑ W. His, Jr., Die Thatikeit des embryonalen Herzens. Arbciten aus der med, Klinik zu Leipzig, F. C. W. Vogel, 1893.
- ↑ Kürchner, Wagner-’s Handworterbuch, vol. 2, 1844.
- ↑ Oehl, Henle’s Anatomic, Geffisslehre, Braunschweig, 1876.
- ↑ Direct dissection of the heart of an e1nbryo'30 mm. long (No.-90, a.) shows that the medial valves of the two ostia form a saddle hanging over the ventricular septum. Instead of single lateral cushions double lateral cushions are present on both sides, the posterior being larger than the anterior on the right side. A similar condition is sometimes seen in serial sections. In case the ventricles are cut through transversely the ‘valve system can be beautifully seen from below, and this method of investigation will probably settle this question definitely.
- ↑ His, Jr., l.c., p. 19.
- ↑ Retzer, Anat. Rec., vol. 2, 1908.
- ↑ Fahr, Virchow’s Archiv, Bd. 188, 1907.
- ↑ Tawara, Das Reizleitungssystem des Saugethierherzens, Jena, 1906.
- ↑ Mönckeberg, Untersuchungen fiber des Atrioyentriklarbiindcl in menschlichen Herzens. Jena, 1908.
- ↑ Krohl and Rornberg, Ueber die Bedeutung des Herzmuskels, etc., Arbeiten aus der mod. Klinik zu Leipzig, 1893, p. 72; and His, Jr., Die Théitigkeit dos embryonalen Herzens, Ibid., p. 25.
- ↑ Kent, Researches on the development of the mammalian heart. Journal of Physiology, vol. 14, 1893.
- ↑ Curran, A constant bursa in relation with the bundle of His. Anat. Rec., vol. 3,, 1909.
- ↑ Lhamon, The sheath of the sino-ventricular bundle. Amer. Jour. Anat., vol. 13. 1912.
- ↑ Mall, Bifid apex in the human heart. Anat. Rec, Vol.6, 1912.
- ↑ flack (Further advances in physiology, New York, 1909) says that the ventricular septum grows downward as the ventricles expand and unite. The atrio-ventricular bundle is thus associated from the first with the oldest part of the septum, that is, its upper border which encircles the interventricular foramen.
- ↑ Mall, Amer. Jour; Anat., vol. 11, 1911.
- ↑ His, Anat., mcnsch. Embryonen, Bd. 3, S. 177, makes a statement which is just the opposite of mine.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - On the Development of the Human Heart. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_On_the_Development_of_the_Human_Heart
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G