Introduction
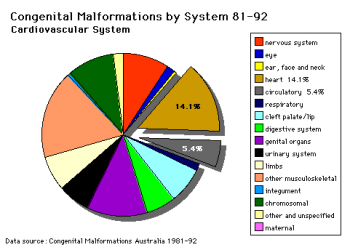
Data shown as a percentage of all major abnormalities based upon published statistics using the same groupings as Congenital Malformations Australia 1981-1992 P. Lancaster and E. Pedisich ISSN 1321-8352.
Heart defects and preterm birth are the most common causes of neonatal and infant death. The long-term development of the heart combined with extensive remodelling and post-natal changes in circulation lead to an abundance of abnormalities associated with this system. A good recent review of cardiac abnormalities is available online.[1]
A UK study literature showed that preterm infants have more than twice as many cardiovascular malformations (5.1 / 1000 term infants and 12.5 / 1000 preterm infants) as do infants born at term and that 16% of all infants with cardiovascular malformations are preterm. (0.4% of live births occur at greater than 28 weeks of gestation, 0.9% at 28 to 31 weeks, and 6% at 32 to 36 weeks. Overall, 7.3% of live-born infants are preterm)[2]
"Baltimore-Washington Infant Study data on live-born cases and controls (1981-1989) was reanalyzed for potential environmental and genetic risk-factor associations in complete atrioventricular septal defects AVSD (n = 213), with separate comparisons to the atrial (n = 75) and the ventricular (n = 32) forms of partial AVSD. ...Maternal diabetes constituted a potentially preventable risk factor for the most severe, complete form of AVSD."[3]
In addition, there are in several congenital abnormalities that exist in adults (bicuspid aortic valve, mitral valve prolapse, and partial anomalous pulmonary venous connection) which may not be clinically recognized.
Developmental abnormalities of the cardiovascular system are classified under ICD-11 includes a coding based upon the International Pediatric Cardiac Code.[4][5]
Some Recent Findings
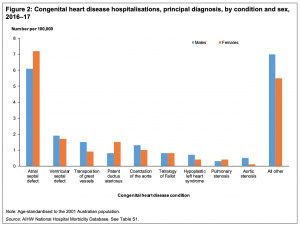
Australia Congenital heart disease 2016–17
- Congenital heart disease in Template:Australia (Report 07 Nov 2019)[6] "Globally, congenital heart disease affects around 9 in every 1,000 babies born. In Template:Australia, a large number of children, young people and adults live with congenital heart disease. This report presents latest statistics and trends on the incidence of congenital heart disease, and on hospitalisation and mortality. There were almost 5,000 congenital heart disease hospitalisations in 2016–17. There were 70 infant deaths from congenital heart disease in 2017, comprising 6.9% of all infant deaths. Around 9 in every 1,000 live births are affected by congenital heart disease. Advances in paediatric cardiac care has led to more adults living with congenital heart disease."
- First trimester combined screening biochemistry in detection of congenital heart defects[7] "To evaluate the performance of first trimester biochemical markers, pregnancy-associated plasma protein-A (PAPP-A), free beta human chorionic gonadotropin (fβ-hCG), and nuchal translucency (NT) in detection of severe congenital heart defects (CHDs). Methods: During the study period from 1 January 2008 to 31 December 2011, biochemical markers and NT were measured in 31,144 women as part of voluntary first trimester screening program for Down's syndrome in Northern Finland. Data for 71 severe CHD cases and 762 controls were obtained from the hospital records and from the National Medical Birth Register, which records the birth of all liveborn and stillborn infants, and from the National Register of Congenital Malformations that receives information about all the CHD cases diagnosed in Finland. Results: Both PAPP-A and fβ-hCG multiple of median (MoM) values were decreased in all severe CHDs: 0.71 and 0.69 in ventricular septal defects (VSDs), 0.58 and 0.88 in tetralogy of Fallot cases (TOFs), 0.82 and 0.89 in hypoplastic left heart syndromes (HLHSs), and 0.88 and 0.96 in multiple defects, respectively. NT was increased in all study groups except of VSD group. ROC AUC was 0.72 for VSD when combining prior risk with PAPP-A and fβ-hCG. Adding NT did not improve the detection rate. With normal NT but decreased (<0.5 MoM) PAPP-A and fβ-hCG odds ratios for VSD and HLHS were 19.5 and 25.6, respectively. Conclusions: Maternal serum biochemistry improves the detection of CHDs compared to NT measurement only. In cases with normal NT measurement but low concentrations of both PAPP-A and fβ-hCG, an alert for possible CHD, especially VSD, could be given with thorough examination of fetal heart in later ultrasound scans."
- Association of NKX2-5, GATA4, and TBX5 polymorphisms with congenital heart disease in Egyptian children[8] "Venous blood samples from 150 congenital heart disease children (including a ventricular septal defect, atrial septal defect, tetralogy of Fallot, and patent ductus arteriosus) and 90 apparently healthy of matched age and sex were studied by polymerase chain reaction followed by direct sequencing in order to study two single-nucleotide variants of NKX2-5 (rs2277923, rs28936670), two single-nucleotide variants of GATA4 (rs368418329, rs56166237) and one single-nucleotide variant TBX5 (rs6489957). The distribution of genotype and allele frequency in the congenital heart diseases (CHD) group and control group were analyzed. Significantly higher frequency of different allelle of five variants was observed in cases when compared to the control group, with significant risky effect for the development of septal defect. In addition to two polymorphisms of NKX2-5 (rs2277923, rs28936670) variant in the cardiac septal defect, two variants in GATA4 (rs368418329, rs56166237) and one variant in TBX5 (rs6489957) seem to have a role in the pathogenesis of congenital heart disease."
- Non-invasive fetal electrocardiography for the detection of fetal arrhythmias[9] "A total of 500 echocardiography and NI-FECG recordings were collected from pregnant women during a routine medical visit in this multicenter study. All the cases with fetal arrhythmias (n = 12) and a matching number of control (n = 14) were used. Two perinatal cardiologists analyzed the extracted NI-FECG while blinded to the echocardiography. The NI-FECG based diagnosis was compared to the reference fetal echocardiography diagnosis....It is possible to diagnose fetal arrhythmias using the NI-FECG technique. However, this study identifies that improvement in algorithms for reconstructing the P-wave is critical to systematically resolve the mechanisms underlying the arrhythmias. The elaboration of a NI-FECG Holter device will offer new opportunities for fetal diagnosis and remote monitoring of problematic pregnancies because of its low-cost, non-invasiveness, portability and minimal set-up requirements." heart rate
- Early fetal ultrasound screening for major congenital heart defects without Doppler[10] "Congenital heart defects are the most common major structural fetal abnormalities. Color flow mapping has played a dominant role in the detection of abnormalities during the first trimester, regardless of the International Society of Ultrasound in Obstetrics and Gynecology warning on the use of Doppler during early pregnancy. The aim of our study was to investigate the use of transvaginal two-dimensional sonography without Doppler for assessing the four-chamber view and the outflow tract view of fetuses at 11-13 weeks of gestation for cardiac screening of major congenital heart defects. ...Routine first-trimester ultrasonagraphy without Doppler, when performed by experienced sonographers, can effectively identify major congenital heart defects. Additional multicenter well designed studies should clarify the feasibility of this approach."
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Cardiovascular Abnormality
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- Utility of fetal cardiac MRI to assess fetuses with right aortic arch and right ductus arteriosus[11] "To evaluate the utility of fetal cardiac magnetic resonance imaging (MRI) to diagnose right aortic arch (RAA) with right ductus arteriosus. ...Fetal cardiac MRI diagnosed the six fetal cases with RAA with right ductus arteriosus correctly. Among the six fetuses, four were associated with other congenital heart defects. In three of six cases, the diagnoses established using prenatal echocardiography (echo) was correct when compared with postnatal diagnosis. Fetal cardiac MRI is a useful complementary tool to assess fetuses with RAA and right ductus arteriosus." Magnetic Resonance Imaging
- High throughput in vivo functional validation of candidate congenital heart disease genes in Drosophila[12] "We developed a Drosophila-based functional system to screen candidate disease genes identified from Congenital Heart Disease (CHD) patients. 134 genes were tested in the Drosophila heart using RNAi-based gene silencing. Quantitative analyses of multiple cardiac phenotypes demonstrated essential structural, functional, and developmental roles for more than 70 genes, including a subgroup encoding histone H3K4 modifying proteins. We also demonstrated the use of Drosophila to evaluate cardiac phenotypes resulting from specific, patient-derived alleles of candidate disease genes. We describe the first high throughput in vivo validation system to screen candidate disease genes identified from patients." fly
- Long-Term Survival of Individuals Born With Congenital Heart Disease: A Systematic Review and Meta-Analysis[13] "Estimates of long-term survival are required to adequately assess the variety of health and social services required by those with congenital heart disease (CHD) throughout their lives. ... Among persons with CHD, the mortality rate is greatest during the first year of life; however, this systematic review and meta-analysis showed that survival decreases gradually after infancy and into adulthood."
- Spontaneous Closure of Muscular Trabecular Ventricular Septal Defect: Comparison of Defect Positions[14] "We performed a historical cohort study for which 150 patients <3 months of age (median age, 9 days) diagnosed as having a muscular trabecular VSD were selected. ...We infer that midventricular muscular trabecular VSD tends to close spontaneously earlier and more frequently than either anterior or apical muscular trabecular VSD."
- Cyp26 Enzymes Facilitate Second Heart Field Progenitor Addition and Maintenance of Ventricular Integrity[15]"Although retinoic acid (RA) teratogenicity has been investigated for decades, the mechanisms underlying RA-induced outflow tract (OFT) malformations are not understood. Here, we show zebrafish embryos deficient for Cyp26a1 and Cyp26c1 enzymes, which promote RA degradation, have OFT defects resulting from two mechanisms: first, a failure of second heart field (SHF) progenitors to join the OFT, instead contributing to the pharyngeal arch arteries (PAAs), and second, a loss of first heart field (FHF) ventricular cardiomyocytes due to disrupted cell polarity and extrusion from the heart tube." Zebrafish Development | retinoic acid
|
International Classification of Diseases
The International Classification of Diseases (ICD) World Health Organization's classification used worldwide as the standard diagnostic tool for epidemiology, health management and clinical purposes. This includes the analysis of the general health situation of population groups. It is used to monitor the incidence and prevalence of diseases and other health problems.
| ICD-11
|
| ICD-11 Structural developmental anomalies of the circulatory system (draft)
|
| ICD-11 Beta Draft - NOT FINAL, updated on a daily basis, It is not approved by WHO, NOT TO BE USED for CODING except for agreed FIELD TRIALS.
20 Developmental Anomalies - Structural Developmental Anomalies
Beta coding and tree structure for "structural developmental anomalies" within this section are shown in the table below.
|
| Structural developmental anomalies of the circulatory system
|
- Structural developmental anomaly of heart and great vessels
- LB00 Congenital heart or great vessel related acquired abnormality
- LB01 Congenital anomaly of atrioventricular or ventriculo-arterial connections
- LB01.1 Transposition of the great arteries
- LB01.2 Double outlet right ventricle
- LB01.3 Double outlet left ventricle
- LB01.4 Common arterial trunk
- LB01.Y Other specified congenital anomaly of atrioventricular or ventriculo-arterial connections
- LB01.Z Congenital anomaly of atrioventricular or ventriculo-arterial connections, unspecified
- LB02 Congenital anomaly of the mediastinal veins Congenital anomaly of atria or atrial septum
- LB20 Congenital anomaly of atrioventricular valves or septum
- LB21 Congenital anomaly of ventricles and ventricular septum
- LB21.1 Congenital right ventricular outflow tract obstruction
- LB21.2 Double-chambered right ventricle
- LB21.3 Tetralogy of Fallot
- LB21.4 Congenital left ventricular outflow tract obstruction
- LB21.5 Congenital ventricular septal defects
- LB21.Y Other specified congenital anomaly of ventricles and ventricular septum
- LB21.Z Congenital anomaly of ventricles and ventricular septum, unspecified
- LB22 Functionally univentricular heart
- LB23 Congenital anomaly of ventriculo-arterial valves and adjacent regions
- LB24 Congenital anomaly of great arteries including arterial duct
- LB.1 Congenital aorto-pulmonary window
- LB.2 Congenital anomaly of pulmonary arterial tree
- LB.3 Congenital anomaly of aorta and its branches
- LB.4 Tracheo-oesophageal compressive syndrome
- LB.5 Patent arterial duct
- LB.Y Other specified congenital anomaly of great arteries including arterial duct
- LB.Z Congenital anomaly of great arteries including arterial duct, unspecified
- LB25 Anomalous position-orientation of heart
- LB26 Total mirror imagery
- LB27 Left isomerism
- LB28 Congenital anomaly of coronary arteries
- LB29 Structural developmental anomalies of the pericardium
- LB2Y Other specified structural developmental anomaly of heart and great vessels
- LB2Z Structural developmental anomaly of heart and great vessels, unspecified
- LB30 Structural developmental anomalies of the peripheral vascular system
- LB30.1 Capillary malformations
- LB30.2 Lymphatic malformations
- LB30.21 Macrocystic lymphatic malformation
- LB30.22 Microcystic lymphatic malformation
- LB30.23 Cystic hygroma in fetus
- BD23.1 Primary lymphoedema
- EK91 Yellow nail syndrome
- LC5F.26 Noonan syndrome
- LB30.2Y Other specified lymphatic malformations
- LB30.2Z Lymphatic malformations, unspecified
- LB30.3 Peripheral venous malformations
- LB30.4 Peripheral arteriovenous malformations
- LB30.5 Peripheral arterial malformations
- LB30.6 Pulmonary arteriovenous fistula
- LB30.Y Other specified structural developmental anomalies of the peripheral vascular system
- LB30.Z Structural developmental anomalies of the peripheral vascular system, unspecified
- LB3Y Other specified structural developmental anomalies of the circulatory system
- LB3Z Structural developmental anomalies of the circulatory system, unspecified
|
| CD-11 Beta Draft - NOT FINAL, updated on a daily basis, It is not approved by WHO, NOT TO BE USED for CODING except for agreed FIELD TRIALS.
See also International Classification of Diseases | Abnormalities
|
|
| ICD10 Congenital malformations of the circulatory system (Q20-Q28)
|
| Cardiovascular System - Abnormalities
|
| Q20 Congenital malformations of cardiac chambers and connections
Excl.: dextrocardia with situs inversus (Q89.3) mirror-image atrial arrangement with situs inversus (Q89.3)
- Q20.0 Common arterial trunk Persistent truncus arteriosus
- Q20.1 Double outlet right ventricle Taussig-Bing syndrome
- Q20.2 Double outlet left ventricle
- Q20.3 Discordant ventriculoarterial connection Dextrotransposition of aorta Transposition of great vessels (complete)
- Q20.4 Double inlet ventricle Common ventricle Cor triloculare biatriatum Single ventricle
- Q20.5 Discordant atrioventricular connection Corrected transposition Laevotransposition Ventricular inversion
- Q20.6 Isomerism of atrial appendages Isomerism of atrial appendages with asplenia or polysplenia
- Q20.8 Other congenital malformations of cardiac chambers and connections
- Q20.9 Congenital malformation of cardiac chambers and connections, unspecified
|
| Q21 Congenital malformations of cardiac septa
Excl.: acquired cardiac septal defect (I51.0)
- Q21.0 Ventricular septal defect
- Q21.1 Atrial septal defect Coronary sinus defect Patent or persistent: foramen ovale ostium secundum defect (type II) Sinus venosus defect
- Q21.2 Atrioventricular septal defect Common atrioventricular canal Endocardial cushion defect Ostium primum atrial septal defect (type I)
- Q21.3 Tetralogy of Fallot Ventricular septal defect with pulmonary stenosis or atresia, dextroposition of aorta and hypertrophy of right ventricle.
- Q21.4 Aortopulmonary septal defect Aortic septal defect Aortopulmonary window
- Q21.8 Other congenital malformations of cardiac septa Eisenmenger's defect Pentalogy of Fallot Excl.: Eisenmenger's complex (I27.8) syndrome (I27.8)
- Q21.9 Congenital malformation of cardiac septum, unspecified Septal (heart) defect NOS
|
Q22 Congenital malformations of pulmonary and tricuspid valves
- Q22.0 Pulmonary valve atresia
- Q22.1 Congenital pulmonary valve stenosis
- Q22.2 Congenital pulmonary valve insufficiency Congenital pulmonary valve regurgitation
- Q22.3 Other congenital malformations of pulmonary valve Congenital malformation of pulmonary valve NOS
- Q22.4 Congenital tricuspid stenosis Tricuspid atresia
- Q22.5 Ebstein's anomaly
- Q22.6 Hypoplastic right heart syndrome
- Q22.8 Other congenital malformations of tricuspid valve
- Q22.9 Congenital malformation of tricuspid valve, unspecified
|
Q23 Congenital malformations of aortic and mitral valves
- Q23.0 Congenital stenosis of aortic valve Congenital aortic: atresia stenosis Excl.: congenital subaortic stenosis (Q24.4) that in hypoplastic left heart syndrome (Q23.4)
- Q23.1 Congenital insufficiency of aortic valve Bicuspid aortic valve Congenital aortic insufficiency
- Q23.2 Congenital mitral stenosis Congenital mitral atresia
- Q23.3 Congenital mitral insufficiency
- Q23.4 Hypoplastic left heart syndrome Atresia, or marked hypoplasia of aortic orifice or valve, with hypoplasia of ascending aorta and defective develop-ment of left ventricle (with mitral valve stenosis or atresia).
- Q23.8 Other congenital malformations of aortic and mitral valves
- Q23.9 Congenital malformation of aortic and mitral valves, unspecified
|
| Q24 Other congenital malformations of heart
Excl.: endocardial fibroelastosis (I42.4)
- Q24.0 Dextrocardia Excl.: dextrocardia with situs inversus (Q89.3) isomerism of atrial appendages (with asplenia or polysplenia) (Q20.6) mirror-image atrial arrangement with situs inversus (Q89.3)
- Q24.1 Laevocardia Location of heart in left hemithorax with apex pointing to the left, but with situs inversus of other viscera and defects of the heart, or corrected transposition of great vessels.
- Q24.2 Cor triatriatum
- Q24.3 Pulmonary infundibular stenosis
- Q24.4 Congenital subaortic stenosis
- Q24.5 Malformation of coronary vessels Congenital coronary (artery) aneurysm
- Q24.6 Congenital heart block
- Q24.8 Other specified congenital malformations of heart Congenital: diverticulum of left ventricle malformation of: myocardium pericardium Malposition of heart Uhl's disease
- Q24.9 Congenital malformation of heart, unspecified Congenital: anomaly disease NOS of heart
|
Q25 Congenital malformations of great arteries
- Q25.0 Patent ductus arteriosus Patent ductus Botallo Persistent ductus arteriosus
- Q25.1 Coarctation of aorta Coarctation of aorta (preductal)(postductal)
- Q25.2 Atresia of aorta
- Q25.3 Stenosis of aorta Supravalvular aortic stenosis Excl.: congenital aortic stenosis (Q23.0)
- Q25.4 Other congenital malformations of aorta Absence Aplasia Congenital: aneurysm dilatation of aorta Aneurysm of sinus of Valsalva (ruptured) Double aortic arch [vascular ring of aorta] Hypoplasia of aorta Persistent: convolutions of aortic arch right aortic arch Excl.: hypoplasia of aorta in hypoplastic left heart syndrome (Q23.4)
- Q25.5 Atresia of pulmonary artery
- Q25.6 Stenosis of pulmonary artery Supravalvular pulmonary stenosis
- Q25.7 Other congenital malformations of pulmonary artery Aberrant pulmonary artery Agenesis Aneurysm, congenital Anomaly Hypoplasia of pulmonary artery Pulmonary arteriovenous aneurysm
- Q25.8 Other congenital malformations of great arteries
- Q25.9 Congenital malformation of great arteries, unspecified
|
Q26 Congenital malformations of great veins
- Q26.0 Congenital stenosis of vena cava Congenital stenosis of vena cava (inferior)(superior)
- Q26.1 Persistent left superior vena cava
- Q26.2 Total anomalous pulmonary venous connection
- Q26.3 Partial anomalous pulmonary venous connection
- Q26.4 Anomalous pulmonary venous connection, unspecified
- Q26.5 Anomalous portal venous connection
- Q26.6 Portal vein-hepatic artery fistula
- Q26.8 Other congenital malformations of great veins Absence of vena cava (inferior)(superior) Azygos continuation of inferior vena cava Persistent left posterior cardinal vein Scimitar syndrome
- Q26.9 Congenital malformation of great vein, unspecified Anomaly of vena cava (inferior)(superior) NOS
|
| Q27 Other congenital malformations of peripheral vascular system
Excl.: anomalies of: cerebral and precerebral vessels (Q28.0-Q28.3) coronary vessels (Q24.5) pulmonary artery (Q25.5-Q25.7) congenital retinal aneurysm (Q14.1) haemangioma and lymphangioma (D18.-)
- Q27.0 Congenital absence and hypoplasia of umbilical artery Single umbilical artery
- Q27.1 Congenital renal artery stenosis
- Q27.2 Other congenital malformations of renal artery Congenital malformation of renal artery NOS Multiple renal arteries
- Q27.3 Peripheral arteriovenous malformation Arteriovenous aneurysm Excl.: ac
- Quired arteriovenous aneurysm (I77.0)
- Q27.4 Congenital phlebectasia
- Q27.8 Other specified congenital malformations of peripheral vascular system Aberrant subclavian artery Absence Atresia of artery or vein NEC Congenital: aneurysm (peripheral) stricture, artery varix
- Q27.9 Congenital malformation of peripheral vascular system, unspecified Anomaly of artery or vein NOS
|
| Q28 Other congenital malformations of circulatory system
Excl.: congenital aneurysm: NOS (Q27.8) coronary (Q24.5) peripheral (Q27.8) pulmonary (Q25.7) retinal (Q14.1) ruptured: cerebral arteriovenous malformation (I60.8) malformation of precerebral vessels (I72.-)
- Q28.0 Arteriovenous malformation of precerebral vessels Congenital arteriovenous precerebral aneurysm (nonruptured)
- Q28.1 Other malformations of precerebral vessels Congenital: malformation of precerebral vessels NOS precerebral aneurysm (nonruptured)
- Q28.2 Arteriovenous malformation of cerebral vessels Arteriovenous malformation of brain NOS Congenital arteriovenous cerebral aneurysm (nonruptured)
- Q28.3 Other malformations of cerebral vessels Congenital: cerebral aneurysm (nonruptured) malformation of cerebral vessels NOS
- Q28.8 Other specified congenital malformations of circulatory system Congenital aneurysm, specified site NEC
- Q28.9 Congenital malformation of circulatory system, unspecified
|
| ICD-10
|
- Links: Neonatal Development | International Society for Nomenclature of Paediatric and Congenital Heart Disease (ISNPCHD)
Heart Abnormalities
Cardiac Critical Periods
Table 2. Summary of Timing (Post Fertilization) of Susceptibility to a Drug-Induced Malformation
| Lesion
|
Start of Susceptibility
to Malformation
|
End of Susceptibility
to Malformation
|
| Interatrial communications
|
| Oval fossa defect
|
6 weeks (E13.5)
|
Term
|
| Sinus venosus defect
|
8 weeks
|
12 weeks
|
| Coronary sinus defect
|
8 weeks
|
Term
|
| Vestibular defect
|
7 weeks
|
8 weeks
|
| Ventricular Septal Defect
|
| Muscular
|
8 weeks
|
Difficult to predict
|
| Perimembranous
|
6 weeks
|
8 weeks
|
| Doubly committed
|
7 weeks
|
8 weeks
|
| Atrioventricular Septal Defect
|
| Ostium primum
|
5 weeks
|
6 weeks
|
| “Complete”
|
5 weeks
|
6 weeks
|
| Aortic coarctation
|
| With VSD
|
5 weeks
|
8 weeks
|
| With intact ventricular septum
|
8 weeks
|
Term
|
| Double Outlet Right Ventricle
|
6 weeks
|
8 weeks
|
| Transposition of Great Arteries
|
6 weeks
|
8 weeks
|
| Ebstein’s malformation
|
6 weeks
|
8 weeks
|
| Hypoplastic left heart syndrome
|
| With mitral atresia
|
5 weeks
|
8 weeks
|
| With mitral stenosis
|
8 weeks
|
Term
|
| Pulmonary atresia
|
| With VSD
|
6 weeks
|
8 weeks
|
| With intact ventricular septum
|
8 weeks
|
Term
|
| Other
|
| Functionally single ventricle
|
5 weeks
|
6 weeks
|
| Tetralogy of Fallot
|
7 weeks
|
8 weeks
|
| Totally anomalous pulmonary venous return
|
8 weeks
|
12 weeks
|
| Tricuspid atresia
|
5 weeks
|
6 weeks
|
| Common arterial trunk
|
5 weeks
|
7 weeks
|
| Bicuspid aortic valve
|
6 weeks
|
Term
|
| Notes
|
| For approximate clinical Gestational Age GA add 2 weeks; number in brackets is mouse equivalent. Data Reference[1]
|
Links: heart | abnormal development | timeline | Category:Timeline
|
| Table 2. Summary of Timing (Post Fertilization) of Cardiac Susceptibility to a Drug-Induced Malformation
|
| Lesion
|
Start of Susceptibility
to Malformation
|
End of Susceptibility
to Malformation
|
| Interatrial communications
|
| Oval fossa defect
|
6 weeks (E13.5)
|
Term
|
| Sinus venosus defect
|
8 weeks
|
12 weeks
|
| Coronary sinus defect
|
8 weeks
|
Term
|
| Vestibular defect
|
7 weeks
|
8 weeks
|
| Ventricular Septal Defect
|
| Muscular
|
8 weeks
|
Difficult to predict
|
| Perimembranous
|
6 weeks
|
8 weeks
|
| Doubly committed
|
7 weeks
|
8 weeks
|
| Atrioventricular Septal Defect
|
| Ostium primum
|
5 weeks
|
6 weeks
|
| “Complete”
|
5 weeks
|
6 weeks
|
| Aortic coarctation
|
| With VSD
|
5 weeks
|
8 weeks
|
| With intact ventricular septum
|
8 weeks
|
Term
|
| Double Outlet Right Ventricle
|
6 weeks
|
8 weeks
|
| Transposition of Great Arteries
|
6 weeks
|
8 weeks
|
| Ebstein’s malformation
|
6 weeks
|
8 weeks
|
| Hypoplastic left heart syndrome
|
| With mitral atresia
|
5 weeks
|
8 weeks
|
| With mitral stenosis
|
8 weeks
|
Term
|
| Pulmonary atresia
|
| With VSD
|
6 weeks
|
8 weeks
|
| With intact ventricular septum
|
8 weeks
|
Term
|
| Other
|
| Functionally single ventricle
|
5 weeks
|
6 weeks
|
| Tetralogy of Fallot
|
7 weeks
|
8 weeks
|
| Totally anomalous pulmonary venous return
|
8 weeks
|
12 weeks
|
| Tricuspid atresia
|
5 weeks
|
6 weeks
|
| Common arterial trunk
|
5 weeks
|
7 weeks
|
| Bicuspid aortic valve
|
6 weeks
|
Term
|
|
Notes: For approximate clinical Gestational Age GA add 2 weeks; number in brackets is mouse equivalent.
Reference: Anderson RH. Teratogenecity in the setting of cardiac development and maldevelopment. (2016)
|
Sex Ratios
- Links: sex bias
Ventricular Septal Defect
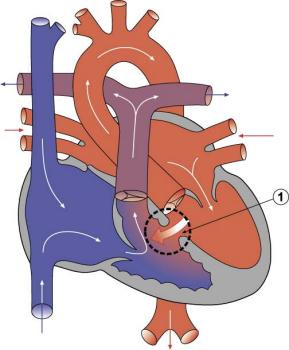
|
The Ventricular Septal Defect (VSD) usually occurs in the membranous (perimembranous) rather than muscular interventricular septum, and is more frequent in males that females.
Perimembranous defects are located close to the aortic and tricuspid valves and adjacent to atrioventricular conduction bundle.
- Links: Ventricular Septal Defects | Search PubMed
|
Atrial Septal Defects
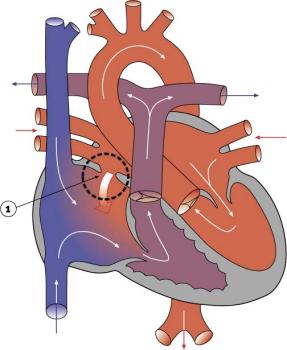
|
Atrial Septal Defects (ASD) are a group of common (1% of cardiac) congenital anomolies defects occuring in a number of different forms and more often in females.
- patent foramen ovale- allows a continuation of the atrial shunting of blood, in 25% of people a probe patent foramen ovale (allowing a probe to bepassed from one atria to the other) exists.
- ostium secundum defect
- endocardial cushion defect involving ostium primum
- sinus venosus defect - contributes about 10% of all ASDs and occurs mainly in a common and less common form. Common ("usual type") - in upper atrial septum which is contiguous with the superior vena cava. Less common - at junction of the right atrium and inferior vena cava.
- common atrium
Treatment: The surgical repair requires a cardiopulmonary bypass and is recommended in most cases of ostium secundum ASD, even though there is a significant risk involved. Ostium primum defects tend to present earlier and are often associated with endocardial cushion defects and defective mitral or tricuspid valves. In such cases, valve replacement may be necessary and the extended operation has a considerable chance of mortality.
- Increasingly closure by a transcatheter device closure has been applied.
- Repair of atrial septal defects on the perfused beating heart (atrial septal defect size 2 cm - 4.5 cm)[17]
- Links: Atrial Septal Defects | OMIM: Atrial Septal Defect | Search PubMed | Medline Plus - ASD Repair Video
|
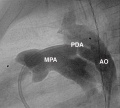
Patent Ductus Arteriosus
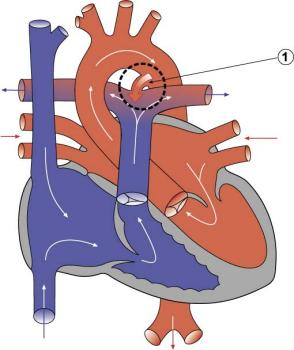
|
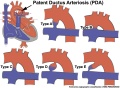
Patent ductus arteriosus (PDA), or Patent arterial duct (PAD), or common truncus, occurs commonly in preterm infants, and at approximately 1 in 2000 full term infants and more common in females (to male ratio is 2:1). Can also be associated with specific genetic defects, Trisomy 21 and Trisomy 18, and the Rubinstein-Taybi syndrome and CHARGE syndrome. The opening is asymptomatic when the duct is small and can close spontaneously (by day three in 60% of normal term neonates), the remainder are ligated simply and with little risk, with transcatheter closure of the duct generally indicated in older children. The operation is always recommended even in the absence of cardiac failure and can often be deferred until early childhood.
Patent ductus arteriosus classification
- Links: Patent Ductus Arteriosus | Search PubMed
|
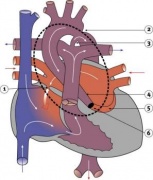
Tetralogy of Fallot
Hypoplastic Left Heart
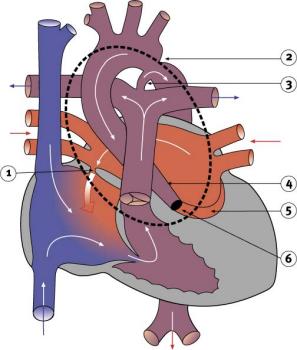
|
Characterized by hypoplasia (underdevelopment or absence) of the left ventricle obstructive valvular and vascular lesion of the left side of the heart.
- Links: Hypoplastic Left Heart | Search PubMed
|
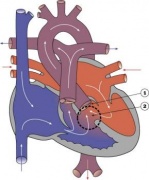
Double Outlet Right Ventricle
Tricuspid Atresia
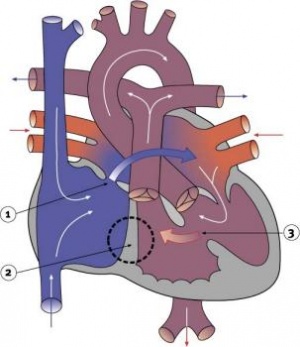
|
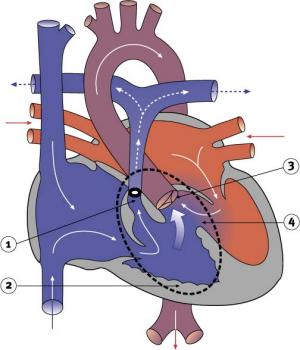
Blood is shunted through an atrial septal defect to the left atrium and through the ventricular septal defect to the pulmonary artery. The shaded arrows indicate mixing of the blood.
Fontan Procedure: a surgical procedure developed by Fontan and Baudet (1971) to restore a circulation in patients with tricuspid atresia.
- Links: Search PubMed | Fontan procedure
|
Dextrocardia
|
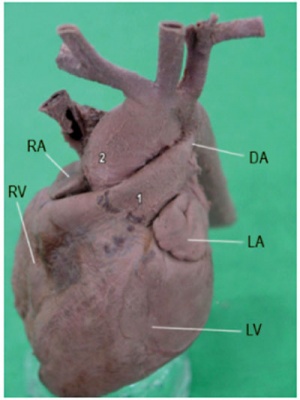
|
| Dextrocardia anatomical heart position[18]
|
Dextrocardia (postnatal 1 year old)[18]
|
Initial malrotation of the heart tube bending left instead of right. Results in heart and greater vessels reversed. Can also occur with situs invertus, where viscera are transposed LR.
Anatomical left-right normal asymmetry is called situs solitus. The alternative heterotaxy can be either randomization (situs ambiguus) or a complete reversal (situs inversus) of normal organ position.
- Links: Chicken Abnormal Heart Movie | Search PubMed
Abnormalities of Conducting System
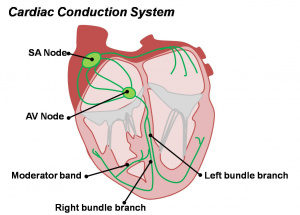
Cardiac Conduction System
Also variously called the cardiac conduction system (CCS), cardiac pacemaking and conduction system (CPCS), or atrioventricular conduction system (AVCS). Recently animal models (CCS-lacZ transgenic mouse) have helped identify key processes in the development of this specialized conduction system.
"Known arrhythmogenic areas including Bachmann's bundle, the pulmonary veins, and sinus venosus derived internodal structures, demonstrate lacZ expression." (Jongbloed et al, 2004)
Long QT Syndrome
Congenital long QT syndrome (LQTS) is a group of rare genetic disorders with prolonged ventricular repolarization and a risk of ventricular tachyarrhythmias. Cause is mutations in genes encoding either cardiac ion channels or channel interacting proteins.
- Tachyarrhythmia is defined as a heart rhythm with a ventricular rate of 100 beats/min or greater.
- Links: Search PubMed LQTS
Heart Vessel Abnormalities
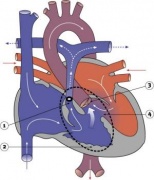
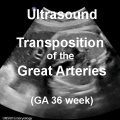
Transposition of the Great Vessels
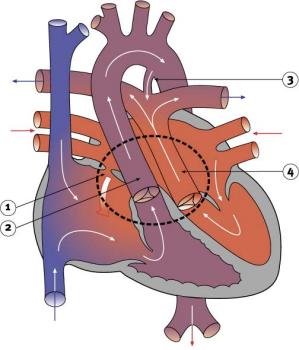
|
Characterized by aorta arising from right ventricle and pulmonary artery from the left ventricle and often associated with other cardiac abnormalities (e.g. ventricular septal defect).
- Australian national rate (1982-1992) 3.6/10,000 births.
- Of 988 infants 4.1% were stillborn and 23.2% liveborn died during neonatal period.
- slightly more common in twin births than singleton.
- Congenital Malformations Australia 1981-1992 P. Lancaster and E. Pedisich ISSN 1321-8352
- Neonates with transposed great arteries die without an arterial switch operation, first carried out in 1975.
|
|
- LInks: transposition of the great vessels | Search PubMed | PMID 15987628 | PMID 18851735
|
Coarctation of the Aorta
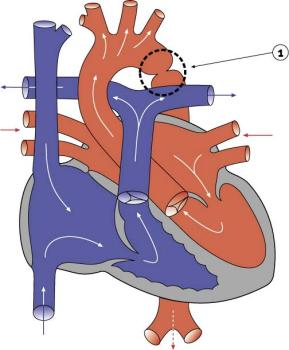
|
- 5-8% of Congenital Heart Disease
- Aortic constriction.
- Treatment aims at maintaining the ductus arteriosus via prostaglandins and by surgical intervention.
- Recent review[19] suggests it may:
- affect the aortic arch in a highly variable manner
- be associated with a host of other left sided heart lesions
- represent a wider vasculopathy within the pre-coarctation arterial tree
- LInks: coarctation of the aorta | Search PubMed | PMID 21947983 | 2011 Review PDF
|
Interrupted Aortic Arch
- LInks: Search PubMed
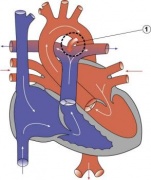
Pulmonary Atresia
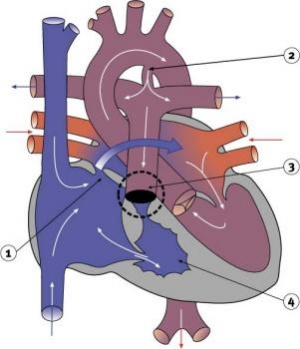
|
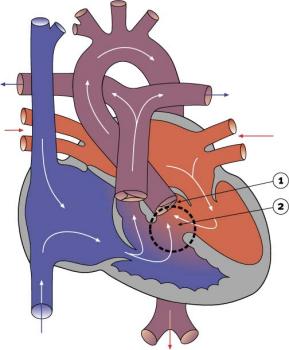
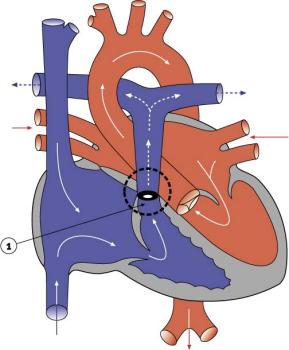
- Abnormal blood flow (as indicated by the shaded blue arrow) is from the right atrium and right ventricle through an atrial septal defect to the left side of the heart.
- Blood can reach the pulmonary arteries only through a patent ductus arteriosus.
- 10% of Congenital Heart Disease
- Unequal division of trunks causes cusps to fuse to form a dome with a narrow/non-existent lumen.
- Heart-lung transplantation may be the only therapy.
- LInks: Search PubMed
|
Total Anomalous Pulmonary Venous Connection
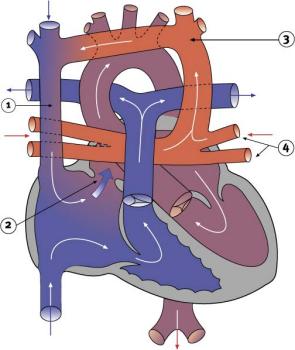
|
Total Anomalous Pulmonary Venous Connection (TAPVC) or Total Anomalous Pulmonary Venous Return (TAPVR) occurs when pulmonary veins connect to the right atrium (RA) and not the left atrium (LA). This abnormal connection returns oxygenated pulmonary blood from the lungs back to the right atrium or a vein flowing into the right atrium.
- < 4% of Congenital Heart Disease
- more common in females
- Total or partial lack of connection of the pulmonary veins with LA.
- They open into RA, one of the systemic veins or both.
- The overloaded pulmonary circuit leads to cyanosis, tachypnoea and dyspnoea.
- Treatment is via surgical redirection
- LInks: Search PubMed
|
Complete Atrioventricular Canal
Partial Anomalous Pulmonary Venous Drainage
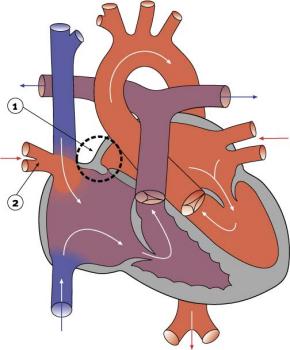
|
- < 4% of Congenital Heart Disease
- more common in females
- Total or partial lack of connection of the pulmonary veins with left atria (LA).
- They open into right atria (RA), one of the systemic veins or both.
- The overloaded pulmonary circuit leads to cyanosis, tachypnoea and dyspnoea.
- Treatment is via surgical redirection
- LInks: Search PubMed | PMID 22119121 | PMID 12652510
|
Aortic Stenosis
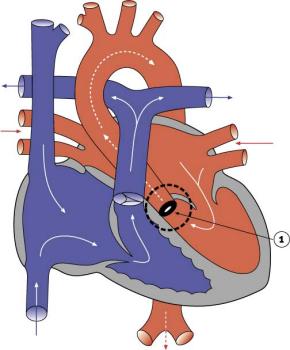
|
- 7% of Congenital Heart Disease
- Persistence of tissue that normally degenerates.
- Results in left ventricle hypertrophy, heart murmurs.
- LInks: Search PubMed
|
Pulmonary Stenosis
|
- 10% of Congenital Heart Disease
- Unequal division of trunks causes cusps to fuse to form a dome with a narrow/non-existent lumen.
- Heart-lung transplantation may be the only therapy.
- LInks: Search PubMed
|
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a postnatal cardiac abnormality with left ventricular hypertrophy following pre-hypertrophic crypts, abnormal mitral leaflets and trabeculae. This heart anomaly is due to mutations in the sarcomeric protein genes, most commonly myosin-binding protein C (MYBPC3).
Blood Disorders
Sickle Cell Anemia
| People who have this form of sickle cell disease inherit two sickle cell genes (“S”), one from each parent. This is commonly called “sickle cell anemia”, and is usually the most severe form of the disease. The name comes from the "sickle" shape of the RBC compared to the normal "donut" shape.
The scanning electron micrograph (SEM) on the right shows the ultrastructural morphology of a sickle cell RBC found in a blood specimen of an 18 year old female patient with sickle cell anemia, (HbSS).
|
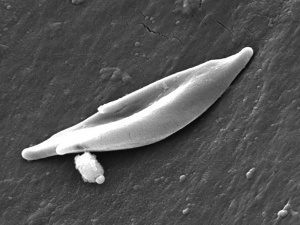
Sickle cell RBC (Image CDC)
|
Thalassemia
Thalassemia is a group of inherited (genetic) blood disorders most frequently in people of Italian, Greek, Middle Eastern, Southern Asian and African Ancestry. The most severe form of alpha thalassemia, affecting mainly people of Southeast Asian, Chinese and Filipino ancestry, results in fetal or newborn death.
The two main types of thalassemia are called "alpha" and "beta," depending on which part of an oxygen-carrying protein in the red blood cells is lacking. Both types of thalassemia are inherited in the same manner. A child who inherits one mutated gene is a carrier, which is sometimes called "thalassemia trait." Most carriers lead completely normal, healthy lives.
- Links: Blood Development | Medline Plus - sickle cell anemia | Medline Plus - thalassemia
Statistics
Australia
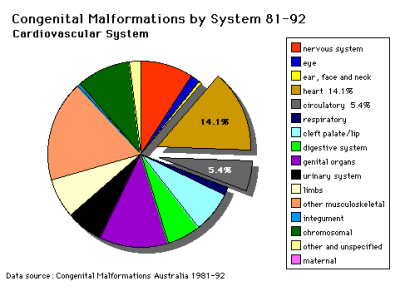
Data shown as a percentage of all major abnormalities based upon published statistics using the same groupings as Congenital Malformations Australia 1981-1992 P. Lancaster and E. Pedisich ISSN 1321-8352.
Belgium
Congenital heart disease in 111 225 births in Belgium 2008 study[20]:
- 921 children with congenital heart disease (birth prevalence of 8.3 per 1000).
- 33% ventricular septal defects most frequently occurring condition
- 18% ostium secundum atrial septal defects
- 10% pulmonary valve abnormalities
- 39% had either cardiosurgical operation or catheter intervention.
- 4% of the children died (higher in univentricular physiology, pulmonary atresia with VSD, left ventricle outflow obstruction and tetralogy of Fallot).
South America
Paediatric and congenital heart disease in South America: an overview.[21]
- Ventricular septal defects - Surgical mortality with repair in infancy, mainly in the muscular septum, probably slightly higher in South America than in North America and Europe.
- Secundum atrial septal defects - trans-catheter closure is the preferred method of treating most patients.
- Pulmonary valve stenosis and atresia with intact ventricular septum - balloon pulmonary valvuloplasty for PVS.
- Aortic stenosis - balloon valvuloplasty.
- Coarctation of the aorta - children balloon angioplasty, adolescents and adults a trend towards the use of bare and covered stents.
- Tetralogy of Fallot - patients with severe hypoxaemia have a modified Blalock–Taussig shunt.
USA
"Baltimore-Washington Infant Study data on live-born cases and controls (1981-1989) was reanalyzed for potential environmental and genetic risk-factor associations in complete atrioventricular septal defects AVSD (n = 213), with separate comparisons to the atrial (n = 75) and the ventricular (n = 32) forms of partial AVSD. ...Maternal diabetes constituted a potentially preventable risk factor for the most severe, complete form of AVSD."[3]
((USA 1997-2009 Abnormalities Sex Ratio table}}
References
- ↑ 1.0 1.1 Anderson RH. Teratogenecity in the setting of cardiac development and maldevelopment. (2016)
- ↑ Tanner K, Sabrine N & Wren C. (2005). Cardiovascular malformations among preterm infants. Pediatrics , 116, e833-8. PMID: 16322141 DOI.
- ↑ 3.0 3.1 Loffredo CA, Hirata J, Wilson PD, Ferencz C & Lurie IW. (2001). Atrioventricular septal defects: possible etiologic differences between complete and partial defects. Teratology , 63, 87-93. PMID: 11241431 <87::AID-TERA1014>3.0.CO;2-5 DOI.
- ↑ Franklin RC, Jacobs JP, Krogmann ON, Béland MJ, Aiello VD, Colan SD, Elliott MJ, William Gaynor J, Kurosawa H, Maruszewski B, Stellin G, Tchervenkov CI, Walters Iii HL, Weinberg P & Anderson RH. (2008). Nomenclature for congenital and paediatric cardiac disease: historical perspectives and The International Pediatric and Congenital Cardiac Code. Cardiol Young , 18 Suppl 2, 70-80. PMID: 19063777 DOI.
- ↑ Giroud JM, Jacobs JP, Spicer D, Backer C, Martin GR, Franklin RC, Béland MJ, Krogmann ON, Aiello VD, Colan SD, Everett AD, William Gaynor J, Kurosawa H, Maruszewski B, Stellin G, Tchervenkov CI, Walters HL, Weinberg P, Anderson RH & Elliott MJ. (2010). Report from the international society for nomenclature of paediatric and congenital heart disease: creation of a visual encyclopedia illustrating the terms and definitions of the international pediatric and congenital cardiac code. World J Pediatr Congenit Heart Surg , 1, 300-13. PMID: 23804886 DOI.
- ↑ Australian Institute of Health and Welfare 2019. Congenital heart disease in Australia. Cat. no. CDK 14. Canberra: AIHW.
- ↑ Alanen J, Korpimaki T, Kouru H, Sairanen M, Leskinen M, Gissler M, Ryynanen M & Nevalainen J. (2019). First trimester combined screening biochemistry in detection of congenital heart defects. J. Matern. Fetal. Neonatal. Med. , 32, 3272-3277. PMID: 29683008 DOI.
- ↑ Behiry EG, Al-Azzouny MA, Sabry D, Behairy OG & Salem NE. (2019). Association of NKX2-5, GATA4, and TBX5 polymorphisms with congenital heart disease in Egyptian children. Mol Genet Genomic Med , , e612. PMID: 30834692 DOI.
- ↑ Behar JA, Bonnemains L, Shulgin V, Oster J, Ostras O & Lakhno I. (2019). Non-invasive fetal electrocardiography for the detection of fetal arrhythmias. Prenat. Diagn. , , . PMID: 30602066 DOI.
- ↑ García Fernández S, Arenas Ramirez J, Otero Chouza MT, Rodriguez-Vijande Alonso B & Llaneza Coto ÁP. (2018). Early fetal ultrasound screening for major congenital heart defects without Doppler. Eur. J. Obstet. Gynecol. Reprod. Biol. , 233, 93-97. PMID: 30580230 DOI.
- ↑ Dong SZ & Zhu M. (2018). Utility of fetal cardiac magnetic resonance imaging to assess fetuses with right aortic arch and right ductus arteriosus. J. Matern. Fetal. Neonatal. Med. , 31, 1627-1631. PMID: 28438064 DOI.
- ↑ Zhu JY, Fu Y, Nettleton M, Richman A & Han Z. (2017). High throughput in vivo functional validation of candidate congenital heart disease genes inDrosophila. Elife , 6, . PMID: 28084990 DOI.
- ↑ Best KE & Rankin J. (2016). Long-Term Survival of Individuals Born With Congenital Heart Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc , 5, . PMID: 27312802 DOI.
- ↑ Miyake T, Shinohara T, Inoue T, Marutani S & Takemura T. (2011). Spontaneous closure of muscular trabecular ventricular septal defect: comparison of defect positions. Acta Paediatr. , 100, e158-62. PMID: 21517965 DOI.
- ↑ Rydeen AB & Waxman JS. (2016). Cyp26 Enzymes Facilitate Second Heart Field Progenitor Addition and Maintenance of Ventricular Integrity. PLoS Biol. , 14, e2000504. PMID: 27893754 DOI.
- ↑ Michalski AM, Richardson SD, Browne ML, Carmichael SL, Canfield MA, VanZutphen AR, Anderka MT, Marshall EG & Druschel CM. (2015). Sex ratios among infants with birth defects, National Birth Defects Prevention Study, 1997-2009. Am. J. Med. Genet. A , 167A, 1071-81. PMID: 25711982 DOI.
- ↑ Pendse N, Gupta S, Geelani MA, Minhas HS, Agarwal S, Tomar A & Banerjee A. (2009). Repair of atrial septal defects on the perfused beating heart. Tex Heart Inst J , 36, 425-7. PMID: 19876418
- ↑ 18.0 18.1 Faig-Leite FS & Faig-Leite H. (2008). Anatomy of a dextrocardia case with situs solitus. Arq. Bras. Cardiol. , 91, e64-6. PMID: 19142355
- ↑ Kenny D & Hijazi ZM. (2011). Coarctation of the aorta: from fetal life to adulthood. Cardiol J , 18, 487-95. PMID: 21947983
- ↑ Moons P, Sluysmans T, De Wolf D, Massin M, Suys B, Benatar A & Gewillig M. (2009). Congenital heart disease in 111 225 births in Belgium: birth prevalence, treatment and survival in the 21st century. Acta Paediatr. , 98, 472-7. PMID: 19046347 DOI.
- ↑ Pedra CA, Haddad J, Pedra SF, Peirone A, Pilla CB & Marin-Neto JA. (2009). Paediatric and congenital heart disease in South America: an overview. Heart , 95, 1385-92. PMID: 19174420 DOI.
Articles
Godfrey ME, Friedman KG, Drogosz M, Rudolph AM & Tworetzky W. (2017). Cardiac output and blood flow redistribution in fetuses with D-loop transposition of the great arteries and intact ventricular septum: insights into pathophysiology. Ultrasound Obstet Gynecol , 50, 612-617. PMID: 27873373 DOI.
Kumar SD, Dheen ST & Tay SS. (2007). Maternal diabetes induces congenital heart defects in mice by altering the expression of genes involved in cardiovascular development. Cardiovasc Diabetol , 6, 34. PMID: 17967198 DOI.
Search Pubmed
Search Pubmed: Cardiovascular System Abnormalities
Terms
| Cardiovascular Terms
|
Cardiovascular System Development See also Heart terms, Immune terms and Blood terms.
- angioblast - the stem cells in blood islands generating endothelial cells which will form the walls of both arteries and veins. (More? Blood Vessel)
- angiogenesis - the formation of new blood vessels from pre-existing vessels following from vasculogenesis in the embryo. (More? Blood Vessel)
- anlage (German, anlage = primordium) structure or cells which will form a future more developed or differentiated adult structure.
- blood islands - earliest sites of blood vessel and blood cell formation, seen mainly on yolk sac chorion.
- cardinal veins - paired main systemic veins of early embryo, anterior, common, posterior.
- cardiogenic region - region above prechordal plate in mesoderm where heart tube initially forms.
- ectoderm - the layer (of the 3 germ cell layers) which form the nervous system from the neural tube and neural crest and also generates the epithelia covering the embryo.
- endoderm - the layer (of the 3 germ cell layers) which form the epithelial lining of the gastrointestinal tract (GIT) and accessory organs of GIT in the embryo.
- endocardium - lines the heart. Epithelial tissue lining the inner surface of heart chambers and valves.
- endothelial cells - single layer of cells closest to lumen that line blood vessels.
- extraembryonic mesoderm - mesoderm lying outside the trilaminar embryonic disc covering the yolk sac, lining the chorionic sac and forming the connecting stalk. Contributes to placental villi development.
- haemocytoblasts - stem cells for embryonic blood cell formation.
- anastomose - to connect or join by a connection (anastomosis) between tubular structures.
- chorionic villi - the finger-like extensions which are the functional region of the placental barrier and maternal/fetal exchange. Develop from week 2 onward as: primary, secondary, tertiary villi.
- estrogens - support the maternal endometrium.
- growth factor - usually a protein or peptide that will bind a cell membrane receptor and then activates an intracellular signaling pathway. The function of the pathway will be to alter the cell directly or indirectly by changing gene expression. (eg VEGF, shh)
- intra-aortic hematopoietic cluster - (IAHC) blood stem cells associated with the endothelial layer of aorta and large arteries.
- maternal decidua - region of uterine endometrium where blastocyst implants. undergoes modification following implantation, decidual reaction.
- maternal sinusoids - placental spaces around chorionic villi that are filled with maternal blood. Closest maternal/fetal exchange site.
- Megakaryocytopoiesis - the process of bone marrow progenitor cells developMENT into mature megakaryocytes.
- mesoderm - the middle layer of the 3 germ cell layers of the embryo. Mesoderm outside the embryo and covering the amnion, yolk and chorion sacs is extraembryonic mesoderm.
- myocardium - muscular wall of the heart. Thickest layer formed by spirally arranged cardiac muscle cells.
- pericardium - covers the heart. Formed by 3 layers consisting of a fibrous pericardium and a double layered serous pericardium (parietal layer and visceral epicardium layer).
- pericytes - (Rouget cells) cells located at the abluminal surface of microvessels close to endothelial cells, mainly found associated with CNS vessels and involved in vessel formation, remodeling and stabilization.
- pharyngeal arches (=branchial arches, Gk. gill) series of cranial folds that form most structures of the head and neck. Six arches form but only 4 form any structures. Each arch has a pouch, membrane and groove.
- placenta - (Greek, plakuos = flat cake) refers to the discoid shape of the placenta, embryonic (villous chorion)/maternal organ (decidua basalis)
- placental veins - paired initially then only left at end of embryonic period, carry oxygenated blood to the embryo (sinus venosus).
- protein hormone - usually a protein distributed in the blood that binds to membrane receptors on target cells in different tissues. Do not easliy cross placental barrier.
- sinus venosus - cavity into which all major embryonic paired veins supply (vitelline, placental, cardinal).
- splanchnic mesoderm - portion of lateral plate mesoderm closest to the endoderm when coelom forms.
- steroid hormone - lipid soluble hormone that easily crosses membranes to bind receptors in cytoplasm or nucleus of target cells. Hormone+Receptor then binds DNA activating or suppressing gene transcription. Easliy cross placental barrier.
- syncitiotrophoblast extraembryonic cells of trophoblastic shell surrounding embryo, outside the cytotrophoblast layer, involved with implantation of the blastocyst by eroding extracellular matrix surrounding maternal endometrial cells at site of implantation, also contribute to villi. (dark staining, multinucleated).
- truncus arteriosus - an embryological heart outflow structure, that forms in early cardiac development and will later divides into the pulmonary artery and aorta. Term is also used clinically to describe the malformation where only one artery arises from the heart and forms the aorta and pulmonary artery.
- vascular endothelial growth factor - (VEGF) A secreted protein growth factor family, which stimulates the proliferation of vasular endotheial cells and therefore blood vessel growth. VEGF's have several roles in embryonic development. The VEGF family has 7 members (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF) that have a common VEGF homology domain. PIGF is the placental growth factor. They act through 3 VEGF tyrosine kinase membrane receptors (VEGFR-1 to 3) with seven immunoglobulin-like domains in the extracellular domain, a single transmembrane region, and an intracellular tyrosine kinase sequence.
- vasculogenesis - the formation of new blood vessels from mesoderm forming the endothelium. Compared to angiogenesis that is the process of blood vessel formation from pre-existing vessels.
- vitelline blood vessels - blood vessels associated with the yolk sac.
- waste products - products of cellular metabolism and cellular debris, e.g.- urea, uric acid, bilirubin.
|
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Cardiovascular System - Abnormalities. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cardiovascular_System_-_Abnormalities
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G