Trophoblast
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
(Greek, trophe = "nutrition" and -blast, a primordial cell) In early development the blastocyst outer trophectoderm (TE) layer will generate all the extra-embryonic trophoblast cell types: cytotrophoblast, syncytiotrophoblast, trophoblastic column and extra-villous trophoblast cells. These cells have an important contribution to extra-embryonic tissues (fetal placenta and membranes) and processes of early development (adplantation, implantation and endocrine support of pregnancy).
|
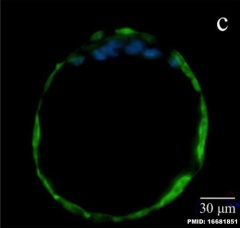
|
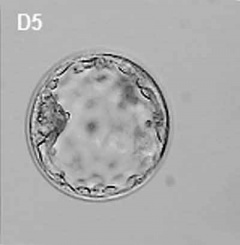
| Human Blastocyst (day 5), trophoblast cells form the peripheral flattened epithelial layer of cells directly under the zona pellucida.[1] | Mouse blastocyst labelled with trophoblast marker.[2] |
In humans, week 1 blastocyst formation the outer layer of cells (adjacent to the zona pellucida) form a flat squamous epithelial layer of cells, the trophectoderm layer. Week 2 following blastocyst hatching the trophoblast layer is involved with initial adhesion to the uterine wall and subsequent implantation within the wall. During this period the trophoblast layer proliferates and differentiates into two distinct layers (syncitiotrophoblast and cytotrophoblast).
Following implantation, trophoblast cells continue to contribute to the placenta. Prenatal diagnosis by invasive chorionic villus sampling and [[ non-invasive cervical cell sampling[3]uses mainly DNA from these cells.
| Historical - Who named the trophoblast cell? |
|---|
| From an 1905 paper on tubal implantation - "Hubrecht in 1889 published a monograph on the placentation of the hedgehog, showing that the developing ovum in this animal, after sequestration in a crypt, becomes imbedded in the sub-epithelial portion of the mucoa of the uterus by the action of the non-foetal ectodermal cells of the blastocyst. These cells Hubrecht named trophoblast because of their nutritive function, for by their agency the blastocyst “burrows” into the maternal tissues, both destroying and absorbing them." |
- Links: blastocyst | implantation | Week 2 | Week 3 | placenta | Prenatal Diagnosis | Trophoblast - Protein Expression | Ectopic Implantation Research
Historic Embryology: 1904 Hubrecht | 1925 Trophoblast at Implantation | 1932 Trophoblast Morphology | 1944 Trophoblast at previllous stage
| Blastocyst Links: blastocyst | morula | fertilization | Week 1 | trophoblast | implantation | Human Day 3-6 Movie | Mouse Model Movie | Category:Blastocyst |
| Molecular: Hippo | Notch |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Trophoblast | Cytotrophoblast | Syncytiotrophoblast | Extravillous Trophoblast | syncytins |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
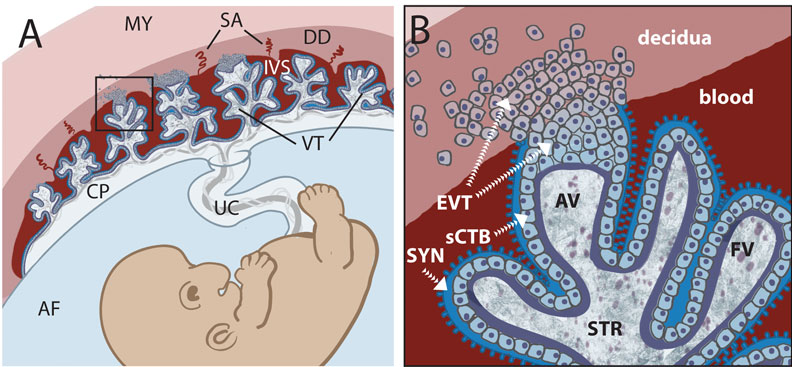
Trophoblast and Placental Villi
Early placental development cartoon showing trophoblast contribution to placental villi.[17]
Legend
- SYN - syncytiotrophoblasts
- sCTB - subsyncytial cytotrophoblasts (this layer grows increasingly discontinuous in later trimesters)
- EVT - extravillous cytotrophoblasts (anchor the villous tree in the decidua)
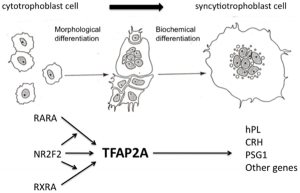
Cytotrophoblast
The cytotrophoblast cells are the initial unfused trophoblast cells that cover the implanting blastocyst surface. In the late pregnancy placenta this cellular layer becomes squamous and discontinuous, with syncytiotrophoblast cells forming the main cellular barrier.
Hyperglycosylated human Chorionic Gonadotropin (hCG) promotes the growth of cytotrophoblast cells and the endometrial invasion by these cells during implantation.[18]
Syncytiotrophoblast
The syncytiotrophoblast cells form by fusion of rapidly dividing cytotrophoblast cells. These form the main cellular interface/barrier between the maternal blood-filled space and the placental villi, particularly at term.
- secrete proteolytic enzymes, enzymes break down extracellular matrix around cells
- Allow passage of blastocyst into endometrial wall, totally surround the blastocyst
- generate spaces, lacunae, that fill with maternal blood
- secrete Human Chorionic Gonadotropin (hCG), hormone, maintains decidua and Corpus Luteum, basis of pregnancy diagnostic test, present in urine is diagnostic of pregnancy
- Later in development placenta will secrete hCG
Cell–cell Fusion Activity
Two pairs of envelope genes of retroviral origin, syncytins, have fusogenic properties. Human endogenous retroviruses (HERVs) make up about 8% of the human genome. Recent study has shown that placentae from intrauterine growth restriction have impaired cell fusion and differentiation that correlates with reduced levels of HERV envelope genes.[19]
Human - syncytin 1 and syncytin 2
- Syncytin 2 is an envelope gene from the human endogenous retrovirus FRD (HERV-FRD)[20]
- Syncytin 2 receptor is Major Facilitator Superfamily Domain Containing 2 (MFSD2) (at chromosomal position 1p34.2)
- belongs to family of presumptive carbohydrate transporters with 10-12 membrane-spanning domains
- Syncytin 1 (-FRD) receptor is ASCT-1/-2
Mouse - syncytin A and syncytin B
- syncytin A is essential for trophoblast cell differentiation and syncytiotrophoblast morphogenesis[21]
- Links: OMIM Syncytin-2
Human Chorionic Gonadotropin
hCG sources - produced by villous syncytiotrophoblast cells, hyperglycosylated hCG produced by cytotrophoblast cells, free beta-subunit made by multiple primary non-trophoblastic malignancies, and pituitary hCG made by the gonadotrope cells of the anterior pituitary.
| Pregnancy | |
|---|---|
| Time (GA) weeks | Levels (mIU/mL) |
| 0 - 1 weeks | 0 - 50 |
| 1 - 2 weeks | 40 - 300 |
| 3 - 4 weeks | 500 - 6,000 |
| 1 - 2 months | 5,000 - 200,000 |
| 2 - 3 months | 10,000-100,000 |
| second trimester | 3,000 - 50,000 |
| third trimester | 1,000 - 50,000 |
| Non-pregnant | < 5.0 |
| Post-menopausal | < 9.5 |
| Pregnancy test - greater than 20 mIU/mL (milli-international units per milliliter) is a positive result. Levels peak at 8 to 10 weeks of pregnancy, then decline and are lower for rest of pregnancy. | |
Extravillous Trophoblast
The extravillous trophoblast cells invade and maintain open the maternal spiral arteries emptying into the maternal lacunae[22] During the first trimester, trophoblast cells also invade both the venous and lymphatic vessels, a process shown to be compromised in recurrent spontaneous abortions.[23]
At term, depending on maternal decidual localization, there are several identifiable subtypes:[4]
- interstitial mononuclear (and multinuclear) - dispersed in decidual mesenchyme
- endovascular - in spiral arteries lumen (or replacing endothelial cells)
- intramural - in spiral arteries tunica media
- "epithelial" lines - maternal decidua basalis basal plate with maternal endothelial cells in a mosaic fashion
HLA-G
An acronym for histocompatibility antigen, class I, G (also called: Human Leukocyte Antigen G, (HLA-6.0; HLA60, T-CELL A LOCUS, TCA) and is expressed on placental cytotrophoblast cells and other adult tissues. This distinct tissue distribution differs from the other HLA antigens (HLA-A, HLA-B, HLA-C) leading to the description as a non-classical class I antigen. May have a role in protecting the fetus from the maternal immune response. For example, expression of the HLA-G class, instead of HLA-A and HLA-B, may be a mechanism for avoiding clearance by maternal natural killer cells.
Human gene is located at 6p22.1 and there exist several protein isoforms from alternative splicing of messenger RNAs, membrane-bound isoforms (HLA-G 1-4) and soluble soluble (HLA-G 5-6).[24] The molecule is a heterodimer consisting of both a heavy chain and a light chain (beta-2 microglobulin). The membrane-bound isoform heavy chain is anchored in the membrane and increased expression of the soluble form is related to higher implantation rates. Changes in HLA-G expression have been associated with increased miscarriage rates.[25] Killer cell immunoglobulin-like receptor (KIR) 2DL4 (KIR2DL4) has been shown to act as a receptor for the soluble HLA-G, leading to a stimulation of resting natural killer (NK) cells.[26]
- Links: HLA-G | OMIM142871
Trophoblast Infiltration
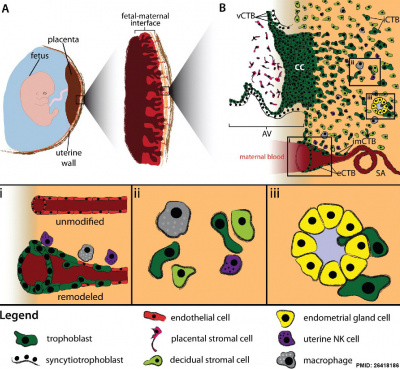
|
Human trophoblast invasion[27]
(A) The placenta connects the fetus to the uterine wall and establishes a vascular connection between mother and child. The placenta is structured as villous tree and is in direct contact with maternal blood and, thus referred to as hemochorial. The site where the placenta comes in direct contact with the maternal decidua is called the fetal-maternal interface. (B) During early pregnancy, vCTBs fuse to form multinucleated STs, which surround the placental villus. STs transport nutrients and gases from the maternal to the fetal circulation and represent the major endocrine unit of the placenta by secreting hormones such as chorionic gonadotropin, placental growth hormone or placental lactogen. AV form cell columns that attach to the maternal decidua and give rise to the EVT lineage. Invasive EVTs can be divided into iCTBs, which invade the decidual stroma and become terminally differentiated multinucleated GCs, or eCTBs.
|
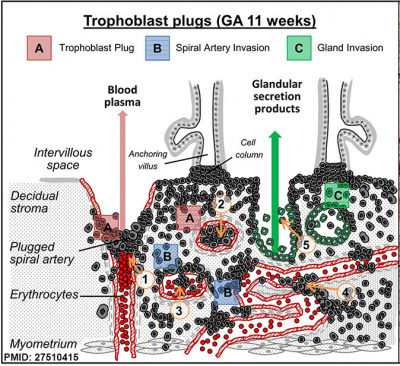
|
Trophoblast plugs within Spiral Arteries Week 9, GA week 11.[9]
|
Trophoblast Cell Lines
A useful component of current placentation and trophoblast research are a number of trophoblast cell lines that have been derived from a number of different sources including term placenta, choriocarcinomas and transformation of primary isolated cells. BeWo cell line is the earliest human choriocarcinoma, established in 1968.[28] Most appear to be examples of extravillous trophoblast cells, some examples of various human "trophoblast" cell lines are listed below.
- BeWo - human choriocarcinoma[28], hypotetraploid (modal number = 86; range = 71 to 178) (ATCC CCL-98)
- JEG-3 - choriocarcinoma HTR8/SVneo a transformed extravillous trophoblast line
- SGHPL-4 - [29][30] SV40 (pSV3neo) transformed primary cell line express cytokeratin-7, HLA class I antigen, HLA-G, BC-1, CD9, human chorionic gonadotrophin, and human placental lactogen.
- TEV-1 - (HPV16) E6/E7 infection first-trimester extravillous trophoblast cell line[31]
- ACH-3P - fusion of primary human first trimester trophoblasts (GA week 12) with a human choriocarcinoma cell line (AC1-1).[32]
- HChEpC1b - retroviral infection by E6/E7/hTERT[33]
History
The name "Trophoblast" was used for the first time by Ambrosius Arnold Willem Hubrecht (1853 – 1915) at the meeting of the Anatomical Congress at Wiirzburg in 1888, and its earliest definition is found in the report of that meeting in Nos. 17 and 18 of the Anatomischer Anzeiger, Bd. III. "We there read, concerning a very early stage of the hedgehog (p. 510) : Die aussere Wand der Keimblase ist verdickt (drei bis vierschichtig) und besitzt wabige Lacunen. Fur diese aussere (epiblastische) Schicht sei der. Name Trophoblast gewahlt."
- Links: The Trophoblast - A Rejoinder (1904) | Ambrosius Arnold Willem Hubrecht | Hubrecht Collection | Embryologists | Embryology History
Rauber's Layer
Rauber's layer is a thinned-out trophoblast membrane lying over the embryonic disk in developing carnivores and ungulates. A recent study of cattle has shown that prevention of the loss of this polar trophoblast layer leads to ectopic domains of BRACHYURY, a gastrulation marker.
The layer is named after August A. Rauber (1841-1917) a German embryologist and anatomist.
- Links: PNAS 2020
Abnormalities
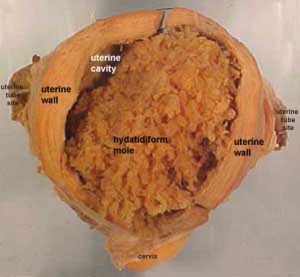
Hydatidiform Mole
A type of placental abnormality when only the conceptus trophoblast layers proliferates and not the embryoblast, no embryo develops, this is called a "hydatidiform mole", which is due to the continuing presence of the trophoblastic layer, this abnormal conceptus can also implant in the uterus. The trophoblast cells will secrete human chorionic gonadotropin (hCG), as in a normal pregnancy, and may appear maternally and by pregnancy test to be "normal". Prenatal diagnosis by ultrasound analysis demonstrates the absence of a embryo.
There are several forms of hydatidiform mole: partial mole, complete mole and persistent gestational trophoblastic tumor. Many of these tumours arise from a haploid sperm fertilizing an egg without a female pronucleus (the alternative form, an embryo without sperm contribution, is called parthenogenesis). The tumour has a "grape-like" placental appearance without enclosed embryo formation. Following a first molar pregnancy, there is approximately a 1% risk of a second molar pregnancy.
This topic is also covered in placenta abnormalities.
Trophoblast Infections
- Malaria (More? malaria)
- Chlamydia trachomatis[34]
- Herpesvirus 8 (HHV-8)[35]
- Group B streptococcus (GBS)[36]
References
- ↑ Zhang P, Zucchelli M, Bruce S, Hambiliki F, Stavreus-Evers A, Levkov L, Skottman H, Kerkelä E, Kere J & Hovatta O. (2009). Transcriptome profiling of human pre-implantation development. PLoS ONE , 4, e7844. PMID: 19924284 DOI.
- ↑ Tanaka N, Takeuchi T, Neri QV, Sills ES & Palermo GD. (2006). Laser-assisted blastocyst dissection and subsequent cultivation of embryonic stem cells in a serum/cell free culture system: applications and preliminary results in a murine model. J Transl Med , 4, 20. PMID: 16681851 DOI.
- ↑ Jain CV, Kadam L, van Dijk M, Kohan-Ghadr HR, Kilburn BA, Hartman C, Mazzorana V, Visser A, Hertz M, Bolnick AD, Fritz R, Armant DR & Drewlo S. (2016). Fetal genome profiling at 5 weeks of gestation after noninvasive isolation of trophoblast cells from the endocervical canal. Sci Transl Med , 8, 363re4. PMID: 27807286 DOI.
- ↑ 4.0 4.1 4.2 He N, van Iperen L, de Jong D, Szuhai K, Helmerhorst FM, van der Westerlaken LA & Chuva de Sousa Lopes SM. (2017). Human Extravillous Trophoblasts Penetrate Decidual Veins and Lymphatics before Remodeling Spiral Arteries during Early Pregnancy. PLoS ONE , 12, e0169849. PMID: 28081266 DOI.
- ↑ Saha B, Ganguly A, Home P, Bhattacharya B, Ray S, Ghosh A, Rumi MAK, Marsh C, French VA, Gunewardena S & Paul S. (2020). TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: An implication in early human pregnancy loss. Proc. Natl. Acad. Sci. U.S.A. , , . PMID: 32669432 DOI.
- ↑ West RC, Ming H, Logsdon DM, Sun J, Rajput SK, Kile RA, Schoolcraft WB, Roberts RM, Krisher RL, Jiang Z & Yuan Y. (2019). Dynamics of trophoblast differentiation in peri-implantation-stage human embryos. Proc. Natl. Acad. Sci. U.S.A. , 116, 22635-22644. PMID: 31636193 DOI.
- ↑ Jiang SW, Zhou W, Wang J, Little LM, Leaphart L, Jay J, Igbinigie E, Chen H & Li J. (2018). Gene expression patterns associated with human placental trophoblast differentiation. Clin. Chim. Acta , , . PMID: 29329728 DOI.
- ↑ Rothbauer M, Patel N, Gondola H, Siwetz M, Huppertz B & Ertl P. (2017). A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci Rep , 7, 5892. PMID: 28724925 DOI.
- ↑ 9.0 9.1 Weiss G, Sundl M, Glasner A, Huppertz B & Moser G. (2016). The trophoblast plug during early pregnancy: a deeper insight. Histochem. Cell Biol. , 146, 749-756. PMID: 27510415 DOI.
- ↑ Liang H, Zhang Q, Lu J, Yang G, Tian N, Wang X, Tan Y & Tan D. (2016). MSX2 Induces Trophoblast Invasion in Human Placenta. PLoS ONE , 11, e0153656. PMID: 27088357 DOI.
- ↑ Renaud, SJ. entail., OVO-like 1 regulates progenitor cell fate in human trophoblast development PNAS
- ↑ Ohinata Y & Tsukiyama T. (2014). Establishment of trophoblast stem cells under defined culture conditions in mice. PLoS ONE , 9, e107308. PMID: 25203285 DOI.
- ↑ Chuprin A, Gal H, Biron-Shental T, Biran A, Amiel A, Rozenblatt S & Krizhanovsky V. (2013). Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev. , 27, 2356-66. PMID: 24186980 DOI.
- ↑ Blois SM, Tirado-González I, Wu J, Barrientos G, Johnson B, Warren J, Freitag N, Klapp BF, Irmak S, Ergun S & Dveskler GS. (2012). Early expression of pregnancy-specific glycoprotein 22 (PSG22) by trophoblast cells modulates angiogenesis in mice. Biol. Reprod. , 86, 191. PMID: 22423048 DOI.
- ↑ Hubert MA, Sherritt SL, Bachurski CJ & Handwerger S. (2010). Involvement of transcription factor NR2F2 in human trophoblast differentiation. PLoS ONE , 5, e9417. PMID: 20195529 DOI.
- ↑ Lash GE, Naruse K, Innes BA, Robson SC, Searle RF & Bulmer JN. (2010). Secretion of angiogenic growth factors by villous cytotrophoblast and extravillous trophoblast in early human pregnancy. Placenta , 31, 545-8. PMID: 20338637 DOI.
- ↑ Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M & Bakardjiev AI. (2010). Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. , 6, e1000732. PMID: 20107601 DOI.
- ↑ Cole LA. (2010). Biological functions of hCG and hCG-related molecules. Reprod. Biol. Endocrinol. , 8, 102. PMID: 20735820 DOI.
- ↑ Ruebner M, Strissel PL, Langbein M, Fahlbusch F, Wachter DL, Faschingbauer F, Beckmann MW & Strick R. (2010). Impaired cell fusion and differentiation in placentae from patients with intrauterine growth restriction correlate with reduced levels of HERV envelope genes. J. Mol. Med. , 88, 1143-56. PMID: 20664994 DOI.
- ↑ Esnault C, Priet S, Ribet D, Vernochet C, Bruls T, Lavialle C, Weissenbach J & Heidmann T. (2008). A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl. Acad. Sci. U.S.A. , 105, 17532-7. PMID: 18988732 DOI.
- ↑ Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P & Heidmann T. (2009). Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. U.S.A. , 106, 12127-32. PMID: 19564597 DOI.
- ↑ Whitley GS & Cartwright JE. (2010). Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta , 31, 465-74. PMID: 20359743 DOI.
- ↑ Windsperger K, Dekan S, Pils S, Golletz C, Kunihs V, Fiala C, Kristiansen G, Knöfler M & Pollheimer J. (2017). Extravillous trophoblast invasion of venous as well as lymphatic vessels is altered in idiopathic, recurrent, spontaneous abortions. Hum. Reprod. , 32, 1208-1217. PMID: 28369440 DOI.
- ↑ Yao YQ, Barlow DH & Sargent IL. (2005). Differential expression of alternatively spliced transcripts of HLA-G in human preimplantation embryos and inner cell masses. J. Immunol. , 175, 8379-85. PMID: 16339579
- ↑ Papamitsou T, Toskas A, Papadopoulou K, Sioga A, Lakis S, Chatzistamatiou M, Economou Z & Adriopoulou L. (2014). Immunohistochemical study of immunological markers: HLAG, CD16, CD25, CD56 and CD68 in placenta tissues in recurrent pregnancy loss. Histol. Histopathol. , 29, 1047-55. PMID: 24557735 DOI.
- ↑ Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I & Long EO. (2006). Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. , 4, e9. PMID: 16366734 DOI.
- ↑ Velicky P, Knöfler M & Pollheimer J. (2016). Function and control of human invasive trophoblast subtypes: Intrinsic vs. maternal control. Cell Adh Migr , 10, 154-62. PMID: 26418186 DOI.
- ↑ 28.0 28.1 Pattillo RA, Gey GO, Delfs E & Mattingly RF. (1968). Human hormone production in vitro. Science , 159, 1467-9. PMID: 5753554
- ↑ Choy MY & Manyonda IT. (1998). The phagocytic activity of human first trimester extravillous trophoblast. Hum. Reprod. , 13, 2941-9. PMID: 9804259
- ↑ LaMarca HL, Ott CM, Höner Zu Bentrup K, Leblanc CL, Pierson DL, Nelson AB, Scandurro AB, Whitley GS, Nickerson CA & Morris CA. (2005). Three-dimensional growth of extravillous cytotrophoblasts promotes differentiation and invasion. Placenta , 26, 709-20. PMID: 16226120 DOI.
- ↑ Feng HC, Choy MY, Deng W, Wong HL, Lau WM, Cheung AN, Ngan HY & Tsao SW. (2005). Establishment and characterization of a human first-trimester extravillous trophoblast cell line (TEV-1). J. Soc. Gynecol. Investig. , 12, e21-32. PMID: 15866109 DOI.
- ↑ Hiden U, Wadsack C, Prutsch N, Gauster M, Weiss U, Frank HG, Schmitz U, Fast-Hirsch C, Hengstschläger M, Pötgens A, Rüben A, Knöfler M, Haslinger P, Huppertz B, Bilban M, Kaufmann P & Desoye G. (2007). The first trimester human trophoblast cell line ACH-3P: a novel tool to study autocrine/paracrine regulatory loops of human trophoblast subpopulations--TNF-alpha stimulates MMP15 expression. BMC Dev. Biol. , 7, 137. PMID: 18093301 DOI.
- ↑ Omi H, Okamoto A, Nikaido T, Urashima M, Kawaguchi R, Umehara N, Sugiura K, Saito M, Kiyono T & Tanaka T. (2009). Establishment of an immortalized human extravillous trophoblast cell line by retroviral infection of E6/E7/hTERT and its transcriptional profile during hypoxia and reoxygenation. Int. J. Mol. Med. , 23, 229-36. PMID: 19148547
- ↑ de la Torre E, Mulla MJ, Yu AG, Lee SJ, Kavathas PB & Abrahams VM. (2009). Chlamydia trachomatis infection modulates trophoblast cytokine/chemokine production. J. Immunol. , 182, 3735-45. PMID: 19265152 DOI.
- ↑ Di Stefano M, Calabrò ML, Di Gangi IM, Cantatore S, Barbierato M, Bergamo E, Kfutwah AJ, Neri M, Chieco-Bianchi L, Greco P, Gesualdo L, Ayouba A, Menu E & Fiore JR. (2008). In vitro and in vivo human herpesvirus 8 infection of placenta. PLoS ONE , 3, e4073. PMID: 19115001 DOI.
- ↑ Kaplan A, Chung K, Kocak H, Bertolotto C, Uh A, Hobel CJ, Simmons CF, Doran K, Liu GY & Equils O. (2008). Group B streptococcus induces trophoblast death. Microb. Pathog. , 45, 231-5. PMID: 18599257 DOI.
Reviews
de Souza EA & Pisani LP. (2020). The relationship among vitamin D, TLR4 pathway and preeclampsia. Mol. Biol. Rep. , , . PMID: 32654051 DOI.
Leiva NL, Nolly MB, Ávila Maniero M, Losinno AD & Damiani MT. (2020). Rab Proteins: Insights into Intracellular Trafficking in Endometrium. Reprod Sci , , . PMID: 32638281 DOI.
Aplin JD. (2010). Developmental cell biology of human villous trophoblast: current research problems. Int. J. Dev. Biol. , 54, 323-9. PMID: 19876840 DOI.
Articles
Ashary N, Laheri S & Modi D. (2020). Homeobox genes in endometrium: from development to decidualization. Int. J. Dev. Biol. , 64, 237-247. PMID: 32659011 DOI.
Ralston A, Cox BJ, Nishioka N, Sasaki H, Chea E, Rugg-Gunn P, Guo G, Robson P, Draper JS & Rossant J. (2010). Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development , 137, 395-403. PMID: 20081188 DOI.
Hayashi Y, Furue MK, Tanaka S, Hirose M, Wakisaka N, Danno H, Ohnuma K, Oeda S, Aihara Y, Shiota K, Ogura A, Ishiura S & Asashima M. (2010). BMP4 induction of trophoblast from mouse embryonic stem cells in defined culture conditions on laminin. In Vitro Cell. Dev. Biol. Anim. , 46, 416-30. PMID: 20033790 DOI.
Stoye JP. (2009). Proviral protein provides placental function. Proc. Natl. Acad. Sci. U.S.A. , 106, 11827-8. PMID: 19617545 DOI.
Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P & Heidmann T. (2009). Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. U.S.A. , 106, 12127-32. PMID: 19564597 DOI.
Viswanathan SR, Mermel CH, Lu J, Lu CW, Golub TR & Daley GQ. (2009). microRNA expression during trophectoderm specification. PLoS ONE , 4, e6143. PMID: 19582159 DOI.
Search PubMed
Search October 2010
- Syncytiotrophoblast - All (10230) Review (1104) Free Full Text (2508)
- Cytotrophoblast - All (9828) Review (1062) Free Full Text (2401)
Search Pubmed: Trophoblast | syncytiotrophoblast | cytotrophoblast |
Embryo Week: Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9
- Carnegie Stages: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | About Stages | Timeline
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Trophoblast. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Trophoblast
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G