Paper - Teratogenecity in the setting of cardiac development and maldevelopment
| Embryology - 27 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Anderson RH. Teratogenecity in the setting of cardiac development and maldevelopment. (2016)
Teratogenecity in the Setting of Cardiac Development and Maldevelopment
Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom
Introduction
Investigation of a causal association between a drug therapy and congenital cardiac malformation must include knowledge of the timeline of normal cardiac development (Table 1). It should then investigate how timely perturbation of such normal development might have produced specific cardiac lesions. This, in turn, requires knowledge concerning the precise morphology of the cardiac lesions themselves. To take interatrial communications as an example, as I will explain subsequently in greater detail, the time line for production of the so-called “ostium primum” defect would be significantly different from that required to produce the sinus venosus defect. The timing to produce the commonest lesion involving deficient atrial septation, furthermore, namely the defect found within the oval fossa, usually known as the “secundum” defect, could involve insults overlapping the time frames required to produce the other variants. My purpose in producing this review, therefore, is to provide an overview of current knowledge regarding the timeline of human cardiac development, comparing this information with knowledge of development in the mouse. I will then seek to show how our knowledge of cardiac development might then be used as a guide to the likelihood of ingestion of a pharmaceutical drug at key periods producing the congenital malformations listed in Table 2.
Time Lines of Normal Cardiac Development
The stages of development in the human embryo are usually graded using the so-called Carnegie stages (Table 1).
| Table 1. Comparison of Human and Murine Development | |||||
|---|---|---|---|---|---|
| Human | Mouse | ||||
| Carnegie Stage |
Post-ovulatory Days |
Size (mm) Greatest Length |
Theiler Stage |
Post-conception Days |
Size (mm) Crown-rump |
| 11 | 24 | 2.5 - 4.5 | 14 | 9 - 9.5 | 2.1 |
| 12 | 26 | 3 - 5 | 15 | 9.5 - 10.25 | 2.5 |
| 13 | 28 | 4 - 6 | 16 | 10.25 - 10.5 | 3.6 |
| 14 | 32 | 5 - 7 | 17 | 10.5 | 4.1 |
| 15 | 33 | 7 - 9 | 18 | 11 | 4.6 |
| 16 | 37 | 8 - 11 | 19 | 11.5 | 6 - 7 |
| 17 | 41 | 11 - 14 | 20 | 12 | 7 |
| 18 | 44 | 13 - 17 | 21 | 12.5 - 13 | 8.2 |
| 19 | 47.5 | 16 - 18 | 21 | 12.5 - 13 | 8.2 |
| 20 | 50.5 | 18 - 22 | 22 | 13.5 - 14 | 9.1 |
| 21 | 52 | 22 - 24 | 22 | 13.5 - 14 | 9.1 |
| 22 | 54 | 23 - 28 | 22 | 13.5 - 14 | 9.1 |
| 23 | 56.5 | 27 - 31 | 22 | 13.5 - 14 | 9.1 |
| 26 | 17.5 - 18 | 17.8 | |||
|
Notes: Human Embryonic Development | Carnegie Stages | Mouse Development | Theiler Stage | Post-conception Days | Carnegie Stage Comparison Reference: Anderson RH. Teratogenecity in the setting of cardiac development and maldevelopment. (2016) | |||||
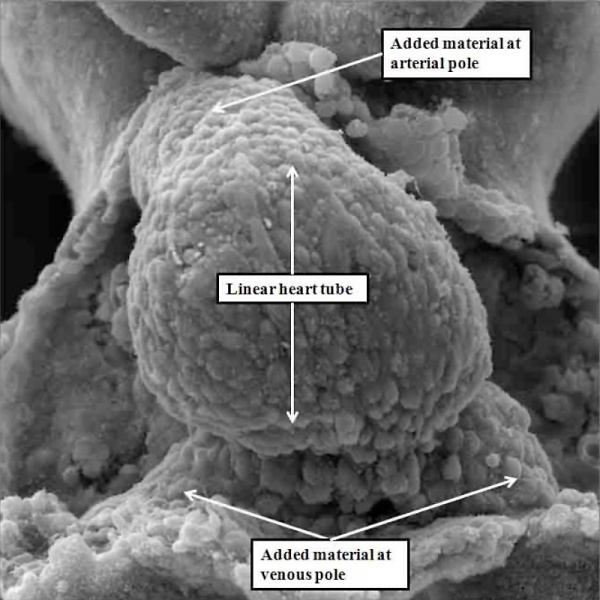
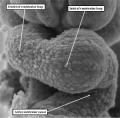
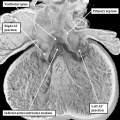
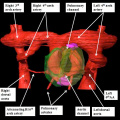
These span from 1 through 23, with stage 23 representing approximately 8 weeks after ovulation. Stage 23 is significant because it represents the situation at which the embryonic interventricular communication is closed. There are further significant changes that occur subsequent to this stage, and I will make reference to these at the appropriate point. By stage 23, nonetheless, the heart is septated, albeit with persistent patency of the oval foramen (foramen ovale) and the arterial duct (ductus arteriosus). In this respect, therefore, it is comparable in most respects with the postnatal organ. In the mouse, the comparable stages of key development of the heart are usually described in terms of embryonic (E) days. Closure of the embryonic interventricular communication in the mouse occurs during E13.5, with term in the mouse occurring at {{ME18.5]}}. In the mouse, the developing heart first becomes recognizable as a linear tube within its pericardial cavity during the eighth day of development (E8 – Figure 1)
Fig. 1. The image is a scanning electron micrograph showing the linear heart tube of the mouse within the pericardial cavity.
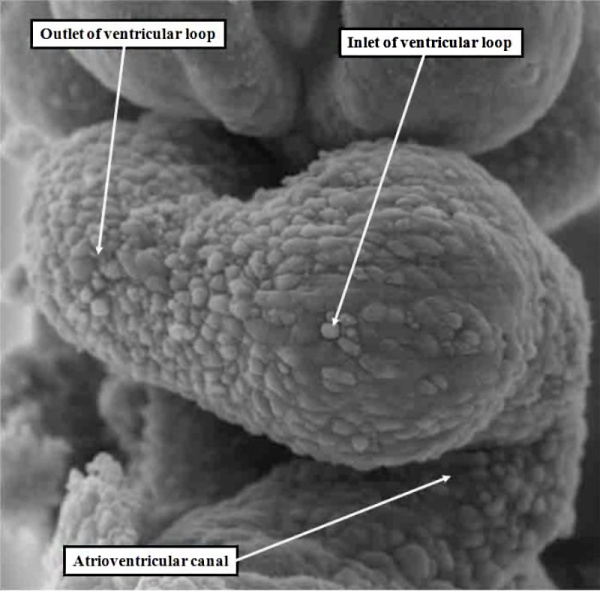
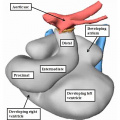
We know that the linear part of the tube is derived from the so-called “first heart field”. This is achieved by migration of cells, which become cardiomyocytes, from the heart-forming areas within the embryo. The embryo itself is initially little more than a disc when these first migrations take place. With ongoing development, further migrations take place from the heart-forming areas, so that new material is added at the top of the tube, known as the arterial pole, and the bottom, which is the venous pole. These new additions can be seen in Figure 1. As the new material is added to the tube, so it expands, and undergoes the process known as “looping” (Figure 2).
Fig. 2. The addition of material at the arterial and venous poles of the heart tune in the mouse during E8 have produced lengthening of the tube, which is now recognizable as having a ventricular loop. The inlet of the loop is continuous with the developing atrial component of the heart via the atrioventricular canal, while the outlet supports the outflow tract, which leads into the developing arterial channels that extend extrapericardially through the pharyngeal mesenchyme.
The new material is added from the so-called second heart field. The initial part of the loop eventually forms little more than the definitive left ventricle, while the new material produces the large part of the atrial chambers, along with the right ventricle and the outflow tract. When first seen, the tube has an outer myocardial layer and an endocardial lining, with cardiac jelly interposed between these two parts. The jelly eventually becomes concentrated at the junction between the ventricular loop and the developing atrial chambers, this region being known as the atrioventricular canal, and in the outflow tract. These concentrations of cardiac jelly, with ongoing development, will eventually provide the basis for formation of the cardiac valves. At the level of the atrioventricular canal, the structures derived from the jelly are known as the atrioventricular cushions, and are located superiorly and inferiorly. In the outflow tract, they are described as the septal and parietal outflow cushions, which spiral as they extend through the length of the outflow tract. These latter structures will not only provide the scaffold for formation of the leaflets of the arterial valves, but will also contribute to separating the initially common channel into its pulmonary and aortic components.
Formation of the Cardiac Chambers
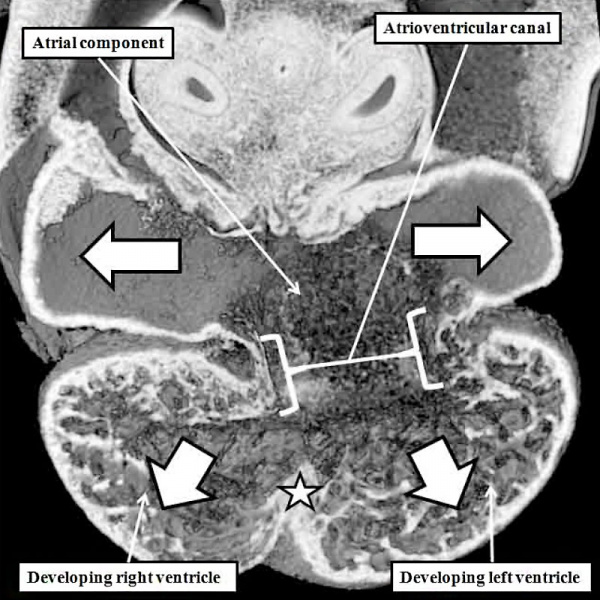
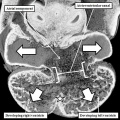
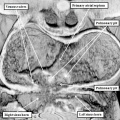
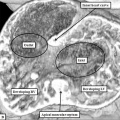
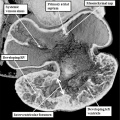
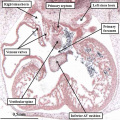
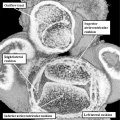
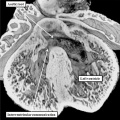
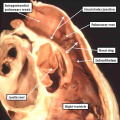
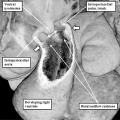
The atrial chambers are developed at the venous pole of the heart, while the initial linear heart tube itself lengthens to form the ventricular loop, from which will develop the ventricular chambers. Subsequent to formation of the ventricular loop, the outflow tract extends from its outlet part to the pericardial boundaries, where its lumen becomes continuous extrapericardially with the arterial channels. These arise from an extrapericardial manifold in the pharyngeal region of the developing embryo known as the aortic sac. Subsequent to the process of looping, all the components of the heart tube still have a continuous lumen. The formation of the initially common atrial chamber at the venous pole, and the commonality of the lumen at this stage with the ventricular loop via the atrioventricular canal, is well seen when the looped tube is sectioned along its length (Figure 3).
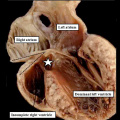
Fig. 3. The images shows a cut across the developing heart tube early on E10.5 in the developing mouse. This is equivalent to Carnegie stage 12 or 13 in man, when the human embryo is almost 4 weeks old. The atrial appendages are ballooning in parallel fashion (horizontal white arrows with black borders) from the atrial component of the initial heart tube, shown in the upper part of the frame. The atrioventricular (AV) canal at this early stage is supported by the inlet part of the ventricular loop, which is itself beginning to expand apically by the process of ballooning. Ballooning from the ventricular loop, however, occurs in serial fashion, so that the muscular ventricular septum (white star) is beginning to appear between the expanding ventricular apical components (downward pointing white arrows with black borders).
The process of expansion from the initial solitary lumen of the heart tube to form the atrial and ventricular chambers is known as ballooning. Ballooning in the atrial part of the tube produces the atrial appendages, while ballooning from the ventricular loop gives rise to the apical components of the ventricles. The atrial changes occur in parallel, with the right-sided and left-sided appendages ballooning from either side of the initial atrial component of the tube. At the ventricular level, in contrast, the ballooning takes place in series, with the apical component of the left ventricle ballooning from the inlet of the loop, and the apical component of the right ventricle ballooning from the outlet component (Figure 3). The parallel nature of the ballooning within the atrial component of the heart tube, compared to the serial nature of the ballooning from the ventricular loop, explains why so-called isomerism, in which structures are mirror-images of themselves in the same individual, is found only in the atrial chambers of the human heart.
Remodelling of the Venous Tributaries
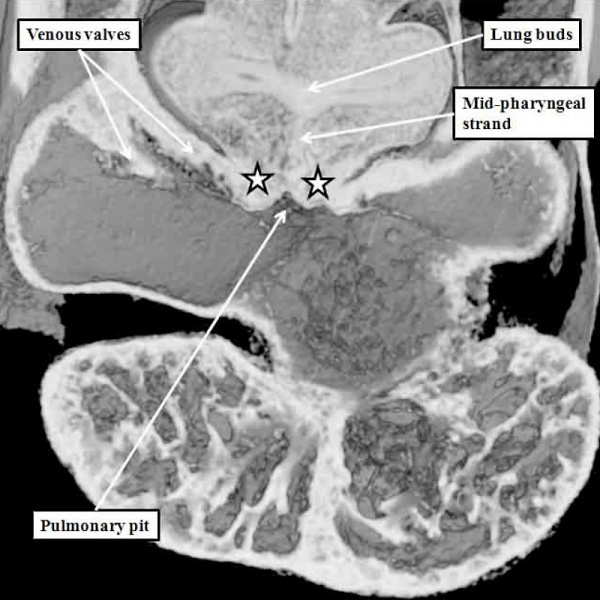
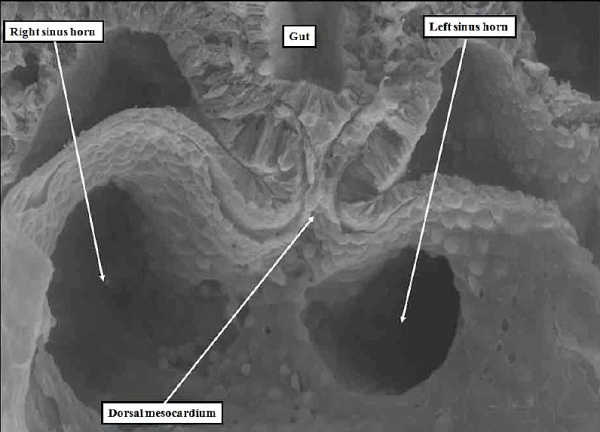
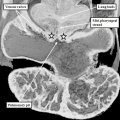
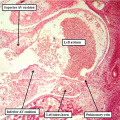
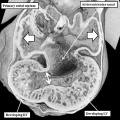
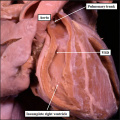
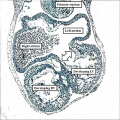
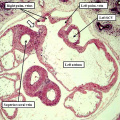
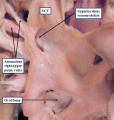
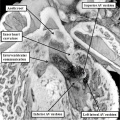
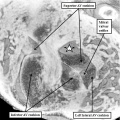
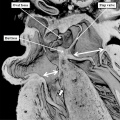
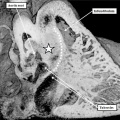
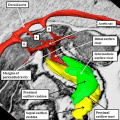
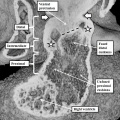
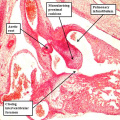
Further development of the atrial chambers, which occurs concomitant with the process of ballooning, requires remodeling of the venous channels which bring the blood back to the heart. There are three such sets of venous tributaries.They bring blood from the embryo, the yolk sac, and the placenta, respectively. The three sets unify to form the so-called sinus horns, which then drain to the initial atrial part of the heart tube. At the initial stages, when the blood enters the atrial component of the heart on each side through the so-called sinus horns, there is minimal formation of the lungs, so there are no pulmonary veins. It is the systemic venous return, therefore, which enters both sides of the atrial part of the tube. This part of the tube, moreover, remains connected to the body of the embryo through a stalk, known as the dorsal mesocardium. The dorsal mesocardium is bordered by two prominences known as the pulmonary ridges. The connection with the pharyngeal mesenchyme through the heart stalk will eventually, subsequent to the development of vascular channels within the lung buds, function as the portal of entry for the pulmonary vein (Figure 4).
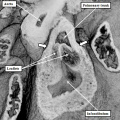
Fig. 4. The section is from another mouse embryo early at E10.5. It shows the continuity of the atrial component of the heart tube with the pharyngeal mesenchyme through the connection known as the dorsal mesocardium, or heart stalk. This connects the heart with the body of the embryo, producing raised flanges known as the pulmonary ridges. These flank the pulmonary pit (white stars with black borders). Note the presence of a midline strand within the pharyngeal mesenchyme. This will eventually canalize to form the pulmonary vein.
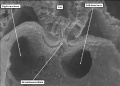
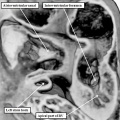
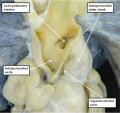
Prior to canalization of the pulmonary vein, however, there is a shift of the blood entering the heart through the systemic venous tributaries. As already emphasized, the channels bringing the blood from the embryo back to the heart initially enter the atrial component of the heart tube in symmetrical fashion (Figure 5).
Fig. 5. The image is a scanning electron micrograph through the dorsal mesocardium prepared from a mouse embryo at E8.5, earlier than the specimen shown in Figure 4. The lungs have yet to develop at this early stage. The venous tributaries open in relatively symmetrical fashion to the atrial component of the heart tube through the channels known as the horn of the systemic venous sinus.
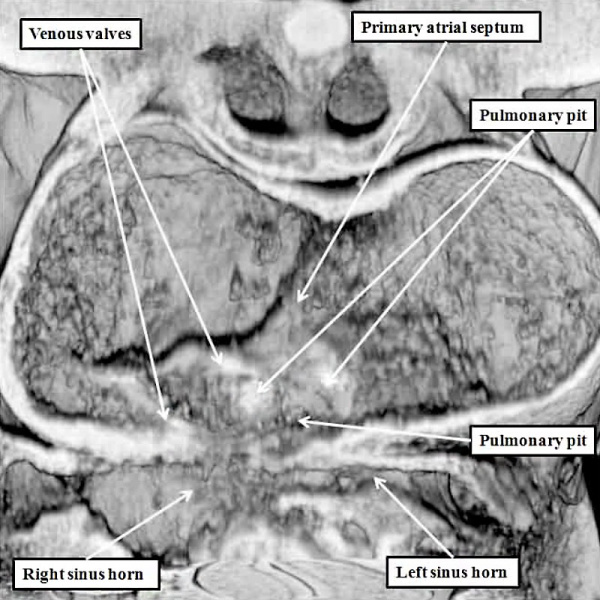
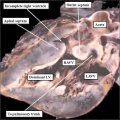
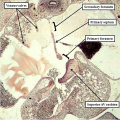
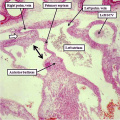
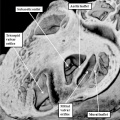
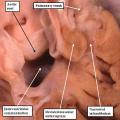
At the earlier stage, as shown in Figure 5, there are no obvious boundaries between the atrial chamber and the systemic venous channels, with the lungs not yet having been formed. With ongoing development, the venous channels move rightwards so that, eventually, they open only to the right side of the initially common atrial chamber. As the channels move rightwards, so flaps of myocardium are formed at their junction with the atrial chamber, producing the structures known as the venous valves. It is at this stage, furthermore, during the early part of E10.5 in the mouse, that the first sign is seen of atrial septation (Figure 6).
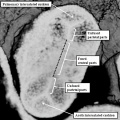
Fig. 6. The image is prepared from the same dataset as Figure 4. It shows a cut through the junction between the venous tributaries and the atrial component of the heart tube, with the boundary between the two now marked by the venous valves. As can be seen, the junction is to the right side of the common atrial chamber. Note the pulmonary pit between the pulmonary ridges, which mark the site of the dorsal mesicardium. Note also the appearance of the atrial septum in the roof of the common atrial chamber.
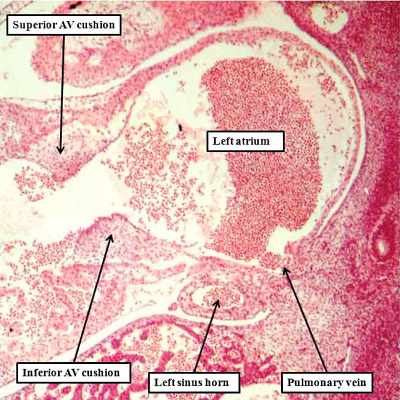
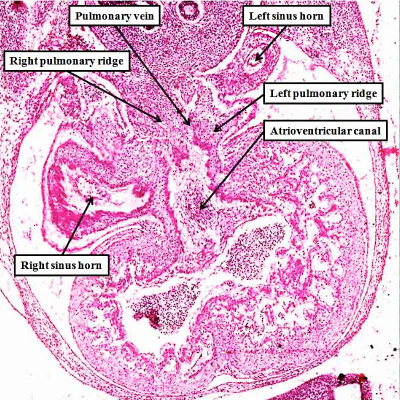
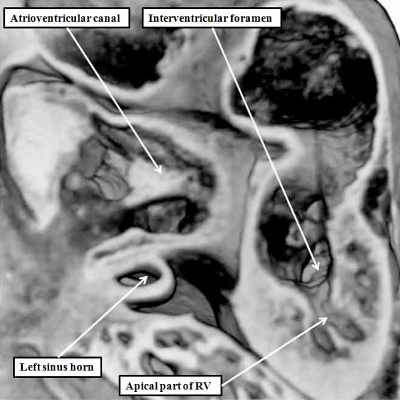
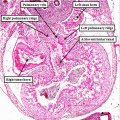
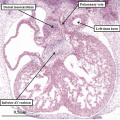
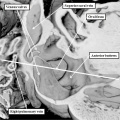
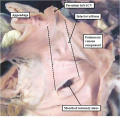
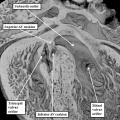
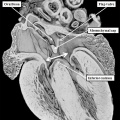
It is subsequent to the rightward shift of the systemic venous tributaries, as shown in Figure 6, that the lung buds grow and develop their own vasculature. The intraparenchymal pulmonary veins initially form a plexus that has connections with the adjacent systemic venous channels developing within the body of the embryo itself. With normal development, however, the pulmonary veins unite in the midline, with formation of a common pulmonary vein. This canalizes from the mid-pharyngeal strand, which was recognizable even before the establishment of the intraparenchymal plexuses (Figure 4). The common pulmonary vein then opens to the heart through the pulmonary pit, itself adjacent to the left sinus horn in the left atrioventricular junction (Figure 7).
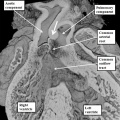
Fig. 7. The images are from different human embryos at Carnegie stage 14, at the end of the fourth week of development. The left hand panel, which is a sagittal section, shows the entrance of the pulmonary vein adjacent to the left sinus horn and the atrioventricular junction. The right hand panel, sectioned in frontal fashion, shows how the vein enters the atrial component of the heart tube between the pulmonary ridges, confirming its adjacency to the atrioventricular canal.
Should the intrapulmonary plexuses fail to unite so as to drain to the heart through the common pulmonary vein, however, they are able to retain and expand their connections to the systemic venous plexuses developing within the embryo itself. It is retention of these initial connections, with failure of junction with the pulmonary vein, which explains the lesion known as anomalous pulmonary venous connection. Depending on how much of the developing lungs drain anomalously, the lesion can be partial or complete. The pulmonary venous blood is able to return to the heart through any of the systemic venous channels. This means that, when the connection is totally anomalous, the blood can return to the heart through a superior caval venous channel, through the left sinus horn directly to the heart, or through the inferior caval vein, usually via the hepatic portal venous system. The situation in which there is only partial anomalous return can then, as we will see, influence the overall development of the atrial chambers, with one variant of partially anomalous pulmonary venous connection underscoring the appearance of the interatrial communication known as the sinus venosus defect.
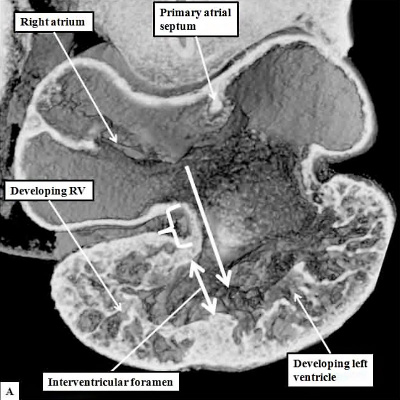
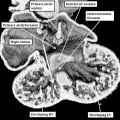
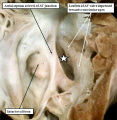
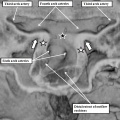
If we return to consider the stage at which the initial atrial septum, known as the primary septum, or “septum primum”, was first seen growing as a protuberance from the atrial roof (Figure 6), the atrial chamber itself is in connection only with the inlet component of the ventricular loop, this being the part that is ballooning to give rise to the developing left ventricle. This stage of development in the mouse, at E10.5, when the atrioventricular canal remains exclusively supported by the developing left ventricle, and the outflow tract is supported exclusively by the right ventricle, is equivalent to Carnegie stages 12 through 14 in the human embryo. The human embryo itself, at this early stage, is no more than four to five weeks old. At this early stage, furthermore, all the blood entering the atrial chambers is able to gain access only to the developing left ventricle (Figure 8, left hand panel), while the developing outflow tract is supported exclusively by the developing right ventricle. This can be likened to the situations, seen in malformed hearts, of double inlet left ventricle, and double outlet right ventricle (Figure 8, right hand panel).
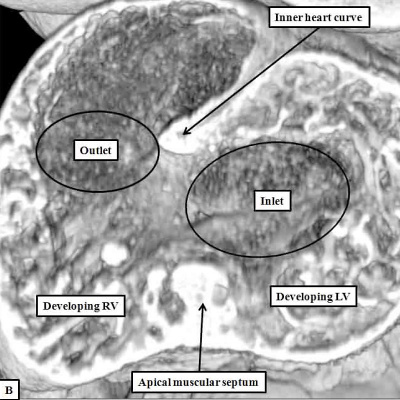
Fig. 8. The cut shown in Panel A, prepared from a mouse at E10.5, is taken through the atrioventricular canal, which opens exclusively to the apical part of the left ventricle, which is ballooning from the inlet part of the ventricular loop (long white arrow). The venous tributaries now open to the right side of the initially common atrium. The primary atrial septum can now be seen growing downwards from the atrial roof. The bracket shows the right side of the atrioventricular junction. There is no direct access from the right atrium to the developing right ventricle. Blood entering the right atrium must pass through the atrioventricular canal, and the interventricular foramen (double headed white arrow) so as to enter the cavity of the developing right ventricle. Panel B is taken from another mouse embryo at E10.5. As had been shown in panel A, the atrioventricular canal (inlet) opens exclusively to the developing left ventricle (LV). The cut in Panel B also shows that the outflow tract (outlet) is supported exclusively by the developing right ventricle (RV). Note that the muscular ventricular septum is developing between the ballooning apical components of the two ventricles. So as to reach the outflow tract, all the atrial blood must pass through the embryonic interventricular foramen, which is bounded cranially by the inner heart curvature, and caudally by the muscular ventricular septum.
The arrangement as found in the mouse heart, at E10.5, when the atrioventricular canal is supported exclusively by the developing left ventricle, with the outflow tract arising exclusively above the cavity of the developing right ventricle, is replicated in the developing human heart at Carnegie stage 14 (Figure 9).
Fig. 9. The images are prepared from a human heart at Carnegie stage 14 using high resolution episcopic microscopy. The left hand panel shows a section through the atrioventricular (AV) canal, which is supported exclusively above the cavity of the developing left ventricle. Note again the parallel expansion of the atrial appendages from the atrial component of the tube (white arrows with black borders), while the apical components of the ventricles are developing in series. As is shown in the right hand panel, from the same heart but sectioned so as to show the apical part of the developing right ventricle (RV), the outflow tract is supported by the developing right ventricular chamber.
Relationship to the Functionally Univentricular Heart
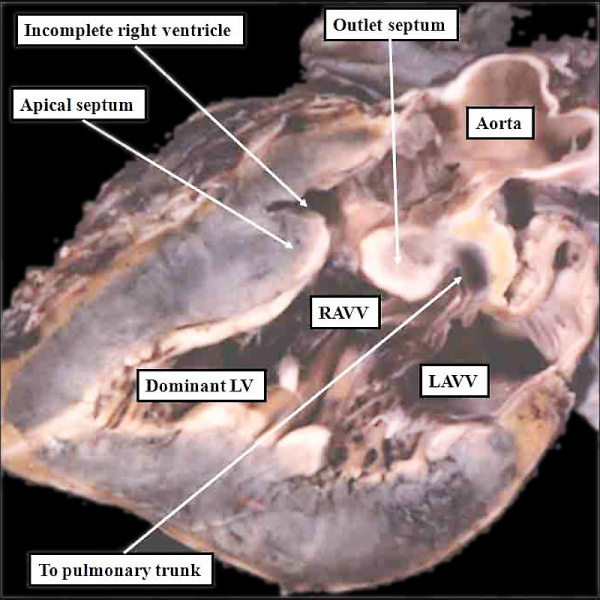
As I will show, the next key stage in cardiac development is rightward expansion of the atrioventricular canal, such that the cavity of the right atrium is able directly to drain to the developing right ventricle. As can be seen in Figures 8 and 9, however, the parietal wall of the right atrium at the rightward margin of the atrioventricular canal is already in continuity with the roof of the right ventricle, even though the circumference of the atrioventricular canal itself is supported above the cavity of the developing left ventricle (right hand panel, Figure 8). If the atrioventricular canal failed to expand, yet the remainder of the heart continued its development, the end result would be that the atrial chambers would retain their connection with the left ventricle. This is the derangement that produces the abnormal situation known as double inlet left ventricle (Figure 10). In this arrangement, the left ventricle remains as the dominant chamber in the ventricular mass, with the right ventricle being incomplete, since it self-evidently lacks its inlet component. In most instances, the right and left atriums, connecting to the dominant left ventricle, possess their own atrioventricular valves, but in a minority of cases, the atrioventricular junctions can be guarded by a common atrioventricular valve, replicating more closely the situation seen in the developing heart (right hand panel, Figure 7). The outflow tract, however, is usually divided in the malformed heart, showing that development of the outflow tract has continued, albeit not always in normal fashion, since in most instances it is the aorta that remains supported by the incomplete right ventricle, with the pulmonary trunk arising from the dominant left ventricle, as in the heart shown in Figure 10.
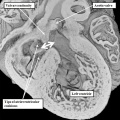
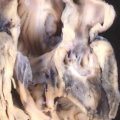
Fig. 10. The image shows a congenitally malformed heart in which both the right and left atrioventricular valves (RAVV. LAVV) are connected with the dominant left ventricle. The right ventricle is incomplete, and is supplied through a ventricular septal defect, comparable to the embryonic interventricular communication shown in Figure 8, right hand panel. Note that, in this heart, the aorta arises from the incomplete right ventricle, and the pulmonary trunk from the dominant left ventricle. This is the arrangement usually described as “transposition”, but better accounted for in terms of discordant ventriculo-arterial connections.
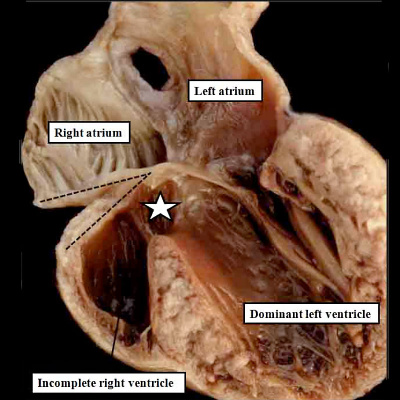
The heart with double inlet left ventricle has, for many years, been described as having a “single ventricle”. Because the atrial chambers connect with only the dominant left ventricle, and the left ventricle then pumps the entirety of the blood volume, the haemodynamic arrangement can appropriately be considered functionally univentricular. In anatomic terms, however, there is a small and incomplete right ventricle accompanying the dominant left ventricle, so the arrangement cannot logically be described in terms of a “single ventricle”. This is just one of the many terminological problems that confront those seeking to understand the anatomy of the congenitally malformed heart. When we consider the developmental implications, nonetheless, the lesion must have required an insult at around the time of 4.5 weeks during human development, or embryonic day 10.5 in the mouse. This is because, as I will describe in the next section, by 5 weeks of development in the human heart, the atrioventricular canal has expanded, such that the cavity of the right atrium is placed in direct communication with the cavity of the right ventricle. In the mouse, this process occurs during the early part of E11.5. A malformation in humans with close similarities to double inlet left ventricle, also involving the atrial chambers connecting only with the dominant left ventricle, is seen in so-called tricuspid atresia. The essence of this lesion is again failure of rightward expansion of the atrioventricular canal, but with growth of the atrial septum so that the right atrium is cut off from the atrioventricular canal. In the postnatal heart, this is seen as absence of the right atrioventricular connection (Figure 11).
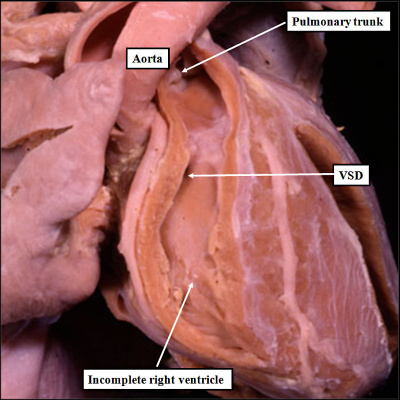
Fig. 11. The images show two different hearts with the lesion known as classical tricuspid atresia. As is seen in the left hand panel, there has been failure of expansion of the atrioventricular junctions, so that the floor of the right is separated from the roof of the right ventricle by the right atrioventricular groove (dashed black lines). Only the dominant left ventricle has an inlet, with the blood entering the incomplete right ventricle through the ventricular septal defect (star). The right hand panel, showing a different heart, is dissected to reveal the structure of the incomplete right ventricle. In this heart, as in most example of tricuspid atresia, it gives rise to the pulmonary trunk. The ventricular septal defect (VSD) is restrictive in this heart.
The heart with tricuspid atresia, like double inlet left ventricle, is also functionally univentricular. Other variants of hearts with double inlet and absent atrioventricular connection can have the right ventricle as the dominant ventricular chamber, implying excessive expansion of the atrioventricular canal, and hence later timing of the process disturbing normal morphological development. The lesions illustrated with the atrioventricular junctions, representing the remnants of the embryonic atrioventricular canal, connecting only with the morphologically left ventricle, nonetheless, serve to show the importance of appreciating the timing of the various events occurring during cardiac development. In the settings with univentricular connection to the dominant left ventricle, the insult must have occurred within the initial five weeks of human development. On very rare occasions, it is also possible to find double inlet to a truly solitary ventricle. This implies failure of ballooning of the apical ventricular components, with the insult presumably occurring even earlier than the one producing double inlet left ventricle.
Expansion of the Atrioventricular Canal
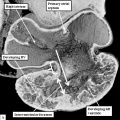
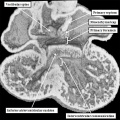
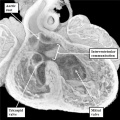
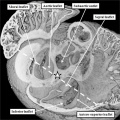
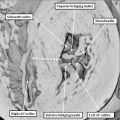
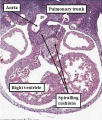
As indicated above, the next crucial event in cardiac development is rightward expansion of the atrioventricular canal. It was rightward shift of the systemic venous tributaries that brought all the blood from the developing embryo back to the right half of the common atrial chamber. The rightward expansion of the atrioventricular canal, occurring during the latter half of the fifth week of development in the human, and early on E11.5 in the mouse, now brings the cavity of the right atrium into direct continuity with the right ventricle, and thus produces the right ventricular inlet component (Figure 12).
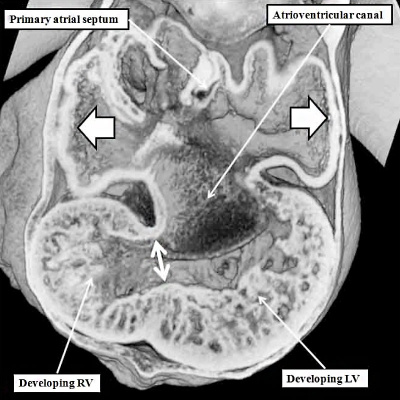
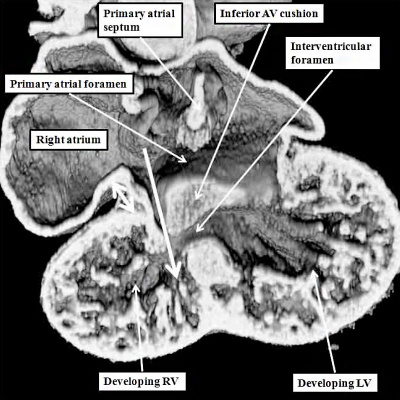
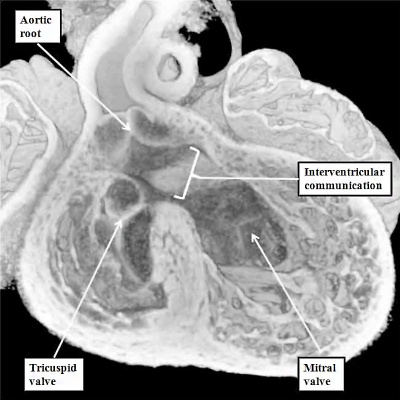
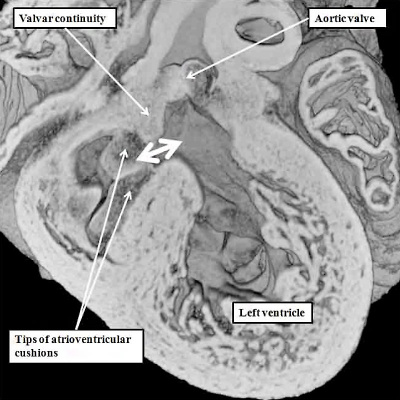
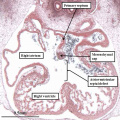
Fig. 12. The left hand image is from an episcopic dataset prepared from a developing mouse embryo early on E11.5. The section is taken in so-called “four chamber” plane. The atrioventricular canal has expanded rightward so that the right atrium is now in direct continuity (white arrow) with the developing right ventricle (RV). There has also been growth of the primary atrial septum, with the primary foramen now seen between its leading edge, which is capped by mesenchyme, and the cranial margin of the inferior atrioventricular (AV) cushions. Note that there is also a space between the caudal edge of the cushion and the crest of the muscular ventricular septum. This is the reorientated embryonic interventricular foramen. The right hand panel is a histological section, again taken in the so-called “four chamber plane”, from a human embryo at Carnegie stage 16, representing the end of the fifth week of development. As can be seen, it is directly comparable with the situation as shown in the mouse heart. The section in the human heart is taken between the atrioventricular cushions, which have yet to fuse at this stage of development. This shows that there is a large defect, well described as an atrioventricular septal defect, through which all four cardiac chambers are in free communication.
Atrial Septation
The images shown in Figure 12 also demonstrate the initial stages of atrial septation. It is the completion of atrial septation, along with ventricular septation, which converts the initial heart tube, with its common lumen throughout its length, into the four-chambered arrangement as seen in postnatal life. Throughout embryonic life, however, there is the requirement for an interatrial communication. This is because the richly oxygenated placental blood, which enters only the right atrium subsequent to the rightward shift of the systemic venous tributaries, must gain access to the left side of the heart so that blood is able to reach the aorta, once this channel has achieved its definitive position above the morphologically left ventricle. The process of atrial septation, therefore, requires initial separation of the right and left sides of the common atrial chamber, but with the capacity for ongoing passage of blood from the right to the left side.
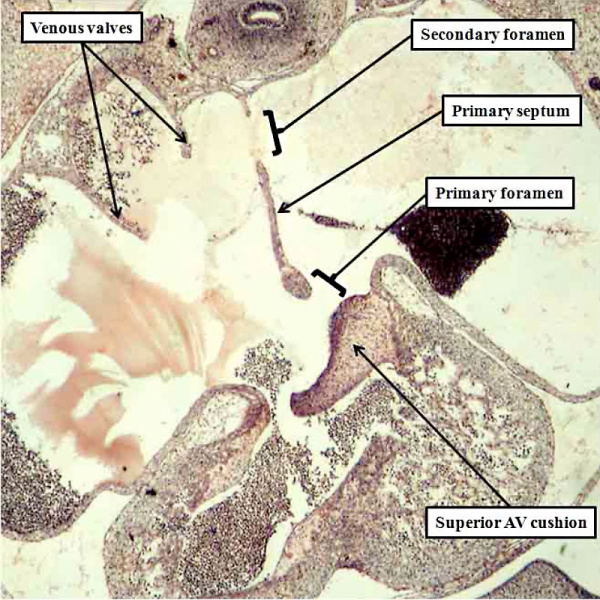
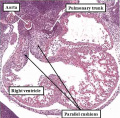
The initial stage of atrial septation is seen at Carnegie stage 13 in the human heart, comparable to the early part of E10.5 in the mouse, when a spur of myocardium grows down from the atrial roof (Figures 6, 13). As the atrial septum is growing initially from the atrial roof, the atrioventricular cushions have already formed superiorly and inferiorly within the atrioventricular canal. As already shown in the left hand panel of Figure 12, by the time the atrioventricular canal has expanded to permit the right atrium to communicate directly with the right ventricle, the primary atrial septum has extended well into the atrial chambers. This process is occurring during day 11.5 in the mouse. A comparable situation is seen at the beginning of the fifth week of development in man (Figure 14).
Fig. 13. The left hand image shows a cut through an episcopic dataset prepared from a mouse embryo at E10.5, while the right hand panel is a histological section from a human embryo at Carnegie stage 13 or 14, when the embryo is just over 4 weeks old. Both sections show the initial growth of the primary atrial septum (septum primum) from the atrial roof.
Fig. 14. The histological section, in four chamber plane, is from a human embryo at Carnegie stage 16, representing the turn from the fifth to the sixth week of development. The primary septum, carrying a mesenchymal cap, has extended well towards the atrial margin of the atrioventricular cushions, reducing the size of the primary foramen in the process. It has broken away from the atrial roof to form the secondary atrial foramen, which will permit the right atrial blood to continue to reach the left atrium subsequent to closure of the primary foramen. Note the remnant of the origin of the primary septum at the atrial roof.
Much is written in current textbooks of human anatomy and development concerning the formation of a “secondary atrial septum”, or “septum secundum”. This structure is alleged to grow down from the atrial roof, paralleling the growth of the primary septum, but situated on its right side. There is no such growth of a secondary atrial septum from the atrial roof. By the time the primary septum has approached the atrial margins of the atrioventricular cushions, however, the veins have developed within the lungs, with canalization of the pulmonary vein within the dorsal mesocardium permitting the pulmonary venous blood directly to enter the heart (Figure 7). At first, the pulmonary vein, canalizing as it does within the pharyngeal mesenchyme, is a relatively midline structure. And, when it gains its initial entrance to the atrial cavity, this is adjacent to the developing atrioventricular junction, which contains the left sinus horn (Figure 7). Entering the atrial cavity as it does adjacent to the atrioventricular junction, and initially through a solitary orifice which drains the blood from both developing lungs, the pulmonary venous orifice is also adjacent to the base of the primary atrial septum. We have already seen that the dorsal mesocardium, which anchors the atrial part of the heart tube to the body of the embryo through the heart stalk, produces the bulges known as the pulmonary ridges, which flank the opening of the pulmonary vein (Figure 4). With growth of the embryo during the beginning of the fifth week of development, there is unequal growth of the rightward of the two pulmonary ridges, with the new tissues growing into the heart from the pharyngeal mesenchyme. This produces the structure known as the vestibular spine, and also called the dorsal mesenchymal protrusion. Growth of this structure ensures that the orifice of the pulmonary vein is committed to the left atrium (Figure 15). It is then the fusion of the vestibular spine, along with the mesenchymal cap carried on the leading edge of the primary septum, with the atrial aspect of the atrioventricular cushions, which themselves fuse during this process, which obliterates the primary foramen. Once the primary foramen is closed, the secondary foramen is the only route through which the richly oxygenated blood entering the right atrium can cross to the left side. At the time at which the primary foramen is obliterated, the atrial roof remains flat, with no formation of the alleged “secondary septum”.
Fig. 15. The images are serial sections from a human embryo at Carnegie stage 16 in four-chamber projection, with the right hand panel anterior to the section shown in the left hand panel. They show how the rightward border of the dorsal mesocardium has expanded, with tissue growing through the mesocardium to reinforce the base of the primary septum. It is the growth of the right ridge into the heart that produces the vestibular spine.
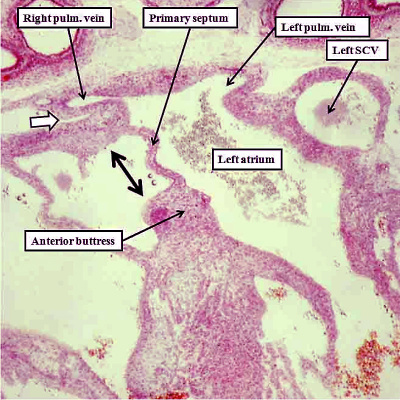
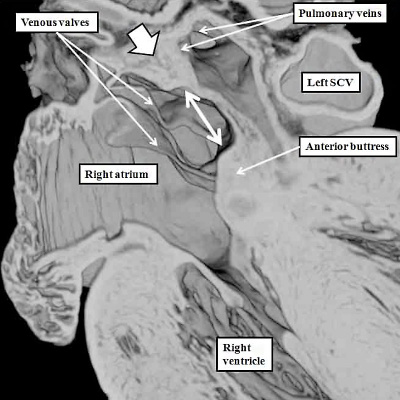
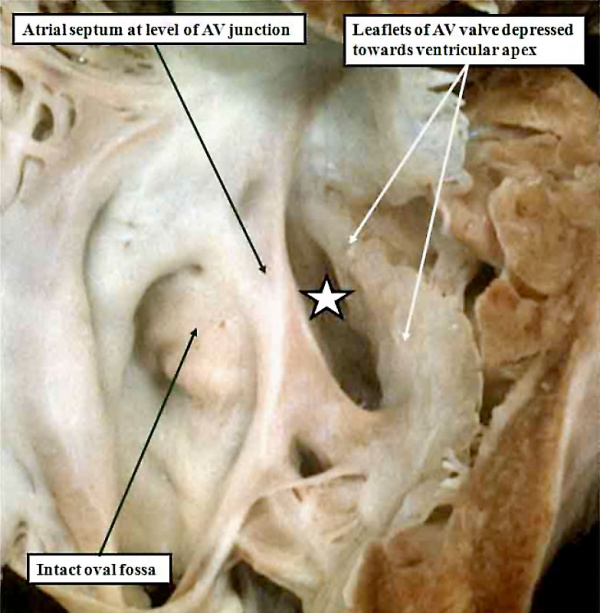
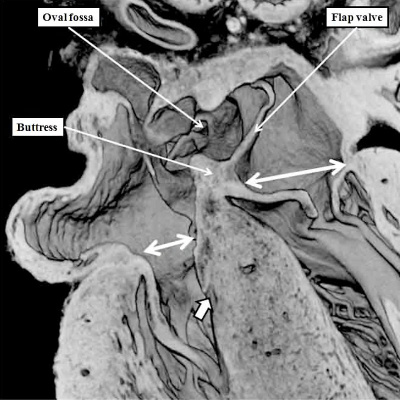
The persisting interatrial communication at the upper margin of the primary septum is now the primordium of the oval foramen, or foramen ovale. This will be required to close in postnatal life so as to remove the potential for interatrial shunting of blood. The arrangement permitting postnatal closure is achieved by the formation of a rim superiorly. The primary septum, which will form the floor of the oval fossa, will close against this rim once the left atrial pressure is higher than right atrial pressure in postnatal life. The superior rim is an infolding. It is formed concomitant with migration of the the pulmonary venous towards the roof of the left atrium, a process which occurs with ongoing atrial development. The process first becomes visible at the end of the seventh week of development (Figure 16). Incorporation of the pulmonary veins into the roof of the left atrium then continues beyond the completion of cardiac septation at Carnegie stage 23, when the embryo is 8 weeks old. It is at this stage that the roof of the atrium becomes fully infolded so as to produce the cranial rim of the oval fossa. By this time, an antero-inferior buttress, derived by muscularisation of the vestibular spine and the mesenchymal cap, has anchored the base of the primary septum to the insulating plane of the atrioventricular junction. This buttress forms the caudal rim and inferior rim of the oval fossa. These processes together establish the arrangement seen in postnatal life, whereby the flap valve forming the floor of the fossa, derived from the primary embryonic atrial septum, is able to close against its right atrial rims.
Fig. 16. The images are from human embryos, the left hand panel at CS20, and the right hand panel as CS21. The embryos are at the end of the seventh week of development. The pulmonary veins are migrating to the atrial roof, and as they do, a fold is produced between their atrial entrance and the right atrial structures (white arrows with black borders). By this time, the mesenchymal cap and vestibular spine have muscularised to form the inferior buttress of the atrial septum. The fold and buttress together form the right atrial rims of the oval foramen (double headed black arrow in right hand panel. The primary septum itself now forms the floor of the oval fossa. Abbreviations: SCV- superior caval vein; pulm. – pulmonary.
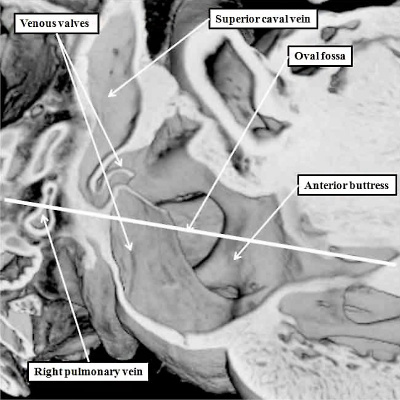
This situation is seen in the mouse heart just prior to term. A major difference, however, is that the pulmonary vein enters the mouse left atrium posteriorly, retaining its adjacency to the atrioventricular junction. The infolded rim of the fossa in the murine heart, therefore, is seen posteriorly (Figure 17), rather than cranially as in man (Figure 16).
Fig. 17. The images are taken from an episcopic dataset prepared from a mouse embryo at E18.5, just prior to birth. The left hand panel shows the oval fossa as seen from the right side, with the venous valves obscuring its posterior margin. The right hand panel, which is a cross section along the white line in the left hand panel, reveals the infolding (white arrow with black borders) which forms the posterior rim of the fossa (double headed white arrow). The anterior rim is the buttress formed by muscularisation of the vestibular spine and mesenchymal cap. SCV-superior caval vein.
Comparison with Postnatal Atrial Anatomy and Interatrial Communications
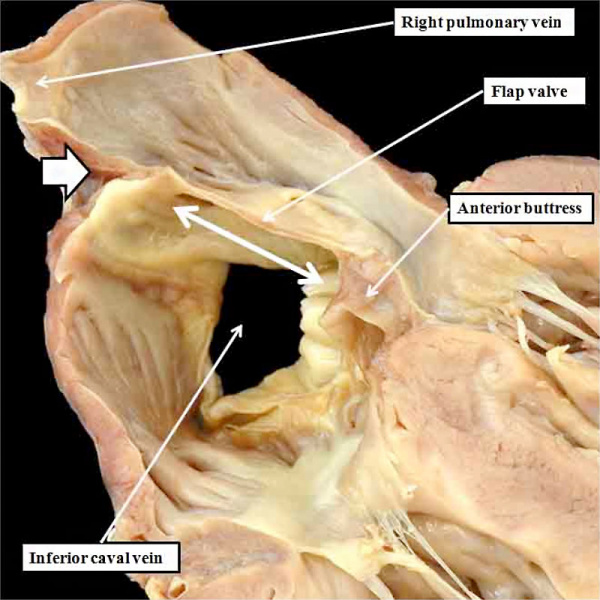
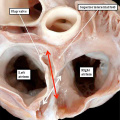
The developmental sequences as described above provide a rational platform to understand not only the structure of the oval fossa and its surrounds as seen in the postnatal heart, but also the anatomy of the congenital lesions that permit shunting to occur between the atrial chambers. Not all of these communications are found within the confines of the oval fossa. Because of this, although they are most often described as being “atrial septal defects”, it is more accurate to consider them as representing interatrial communications. The developmental sequence also permits inferences to be made regarding the likely times at which insults might have occurred so as to produce the various lesions. We can begin by considering the anatomy of the definitive atrial septum. Sectioning the postnatal atrial chambers in the human heart confirms that the so-called “septum secundum” is no more than an infolding between the attachments of the right pulmonary veins to the left atrium and the caval veins to the right atrium (Figure 18).
Fig. 18. The image shows a cross-section across the oval fossa (double headed white arrow) of a postnatal human heart. Comparison with the right hand panel of Figure 15 confirms that the cranial and posterior rim of the fossa is an infolding, while the anterior rim is the anterior buttress formed by muscularisation of the vestibular spine and mesenchymal cap. Prepared by kind permission of Diane Spicer, who made the initial photograph of the specimen.
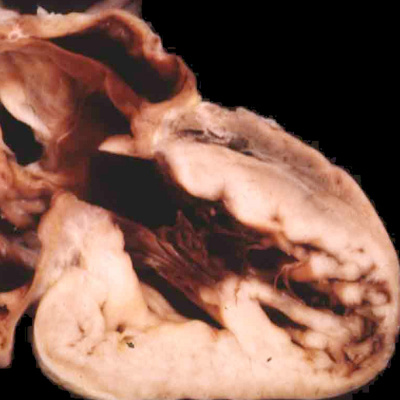
The commonest type of interatrial communication, the so-called “secundum defect”, is better described as a communication existing within the rims of the oval fossa. It exists because the floor of the fossa, derived as we have seen from the primary atrial septum, is either of insufficient dimensions to cover the right atrial rims of the fossa, or else is perforated. If perforate, then multiple holes can be found within the floor of the fossa. The defect itself, therefore, is an “ostium secundum” defect, rather than a “septum secundum” defect. It exists because of problems in formation, or alternatively subsequent dissolution, of the floor of the fossa, which is derived from the primary atrial septum (Figure 19).
Fig.19. The cross-section of a human heart (compare with Figure 18) shows defects in the floor of the oval fossa (double headed white arrow) due to deficiency and perforation of the flap valve derived from the primary atrial septum. The white arrow with black borders shows the intact infolded cranial rim of the fossa. The image is reproduced by kind permission of Diane Spicer, who made the original photograph of the specimen.
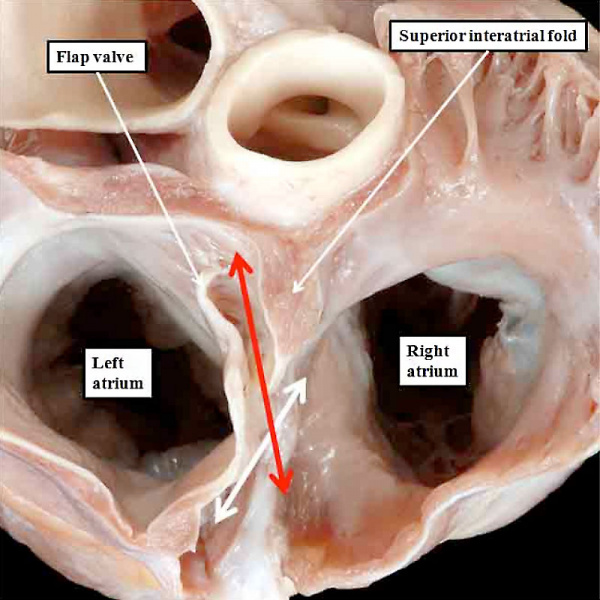
Defects within the oval fossa, therefore, might represent abnormal development at any time from the initial appearance of the primary septum, at the end of the fourth week of development, to term. The primary septum, during its growth, may be attenuated at its cranial border. This would mean that it is unable to overlap the rims of the fossa, which are produced by cranial infolding, and by muscularisation of the vestibular spine and mesenchymal cap. Alternatively, the substance of the septum may be dissolved at any time during fetal development so as to produce the perforations as seen in Figure 19. Defects within the floor of the oval fossa, nonetheless, should be distinguished from persistent patency of the oval foramen. The latter arrangement, whereby the flap valve of the fossa overlaps the rims, but is not anatomically fused to their left atrial side, is found in up to one-third of otherwise normal individuals (Figure 20). It is, therefore, a variation of normal anatomy, rather than a congenital cardiac malformation.
Fig. 20. The human heart has been sectioned across the short axis of the atrial chambers, and photographed from above, to show a persistently patent oval foramen (double headed red arrow). The double headed white arrow shows the normal rims of the oval fossa, which are overlapped by the flap valve, but in absence of fusion with the left atrial aspect of the rims. The heart is reproduced by kind permission of Diane Spicer, who took the original photograph of the specimen.
Defects can also be found within the inferior buttress of the oval fossa, due to failure of fusion of the muscularising components, themselves derived from the vestibular spine and the mesenchymal cap. Such vestibular defects are rarely recognized. Their existence implies improper fusion of the two components of the buttress, with fusion normally taking place during the fifth week of development in man. Should this particular form of atrial septal defect be recognised, therefore, it is possible to suggest a precise timing for its genesis.
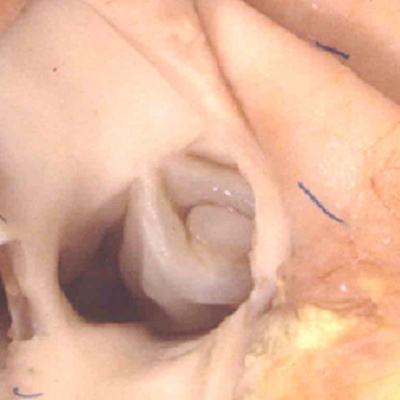
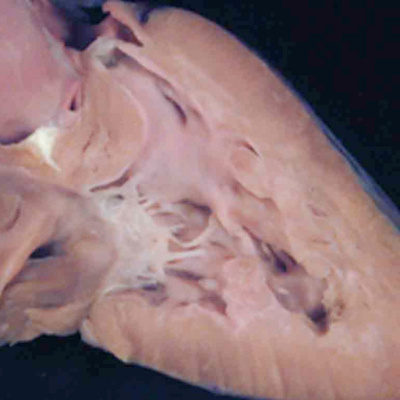
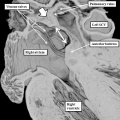
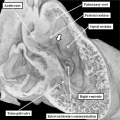
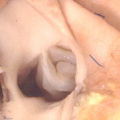
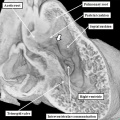
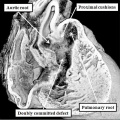
There are then additional defects that permit interatrial shunting, but which are outside the confines of the oval fossa. It follows that these lesions cannot be formed until after the fossa itself has achieved its normal rims, in other words the insult precipitating their formation must have occurred after the seventh week of development. The commonest of these interatrial communications, found outside the oval fossa, is the so-called sinus venosus defect. It can only exist when there is anomalous connection of one of more of the right pulmonary veins to a caval vein, with the pulmonary vein or veins retaining their initial left atrial connection (Figure 21).
Fig. 21. The right atrium is opened and photographed from the right side to show a sinus venosus defect, which permits interatrial shunting but is clearly outside the confines of the oval fossa, which itself shows minimal perforations. The defect exists because of the anomalous attachment of the right pulmonary veins, which retain their initial left atrial connection. Such a defect cannot be formed until after the pulmonary veins have migrated to the atrial roof, in other words after the eighth week of human development.
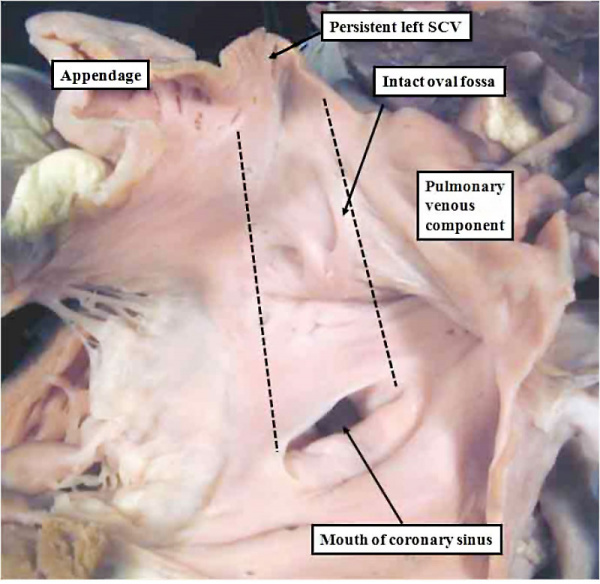
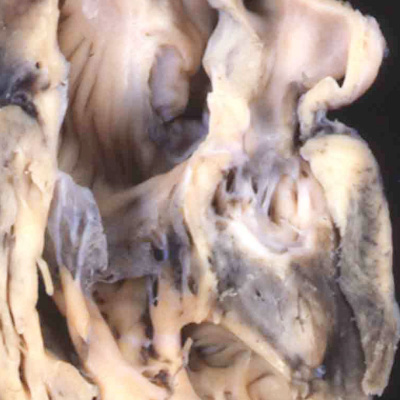
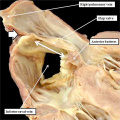
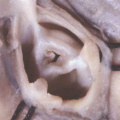
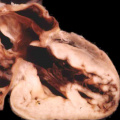
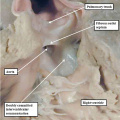
Another defect found outside the confines of the oval fossa, which also permits interatrial shunting, is also the rarest. It is the so-called coronary sinus defect. The shunting takes place through the mouth of the coronary sinus, which itself represents the termination of the embryonic left sinus horn. As we have already seen, the left sinus horn is incorporated into the left atrioventricular groove early in development, since rightward shift of the systemic venous tributaries is one of the first processes underscoring normal atrial septation. As the venous channel is taken into the left atrioventricular groove, it already possesses its own walls, which are discrete from those of the developing left atrium. The mouth of the coronary sinus is able to function as an interatrial communication only when a window develops between the atrial and venous cavities. In its extreme form, which produces the coronary sinus defect, the left superior caval vein remains as a patent channel, and connects to the leftward and cranial margin of the left atrium (Figure 22).
Fig. 22. The image shows a coronary sinus defect as viewed from the left atrial aspect. There is a persistent left superior caval vein (SCV) opening to the leftward and cranial margin of the left atrium. The caval vein would normally have been incorporated into the left atrioventricular groove, but the walls which would normally extend to its opening to the right atrium (black dotted lines) have disappeared. Note the integrity of the oval fossa.
We are unable, at the present time, to offer a rational explanation as to why the walls that would normally interpose between the sinus and the left atrium have disappeared. But, since the oval fossa itself is intact, and since the mouth of the coronary sinus is formed normally within the right atrium, we must presume that the insult occurred subsequent to normal formation, and closure, of the oval fossa itself, in other words after eight weeks of development in man. There is no way, however, of being confident of this assumption.
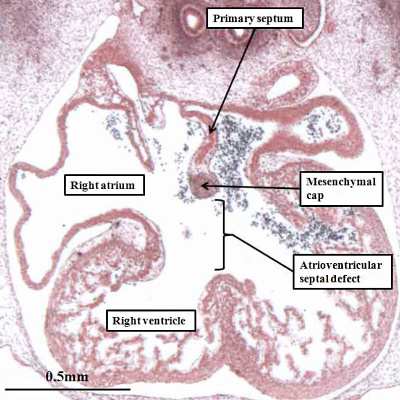
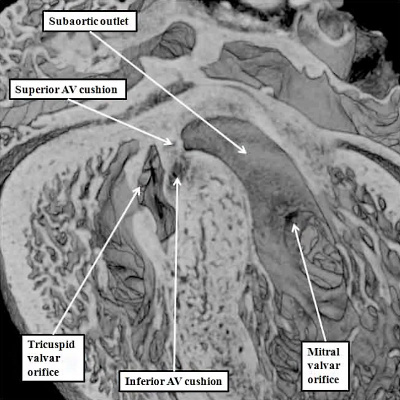
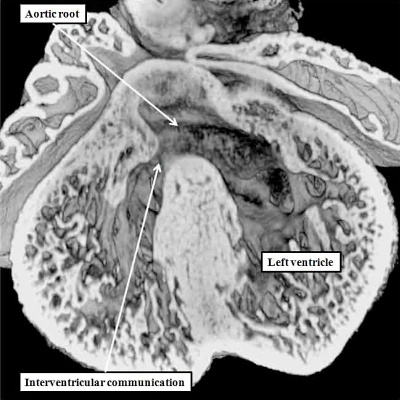
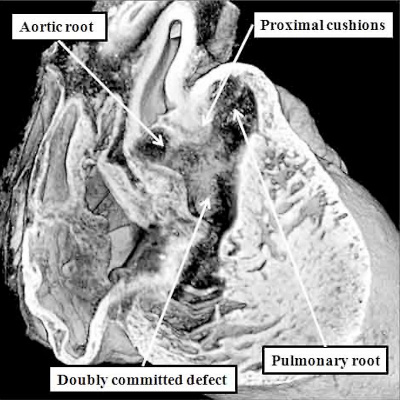
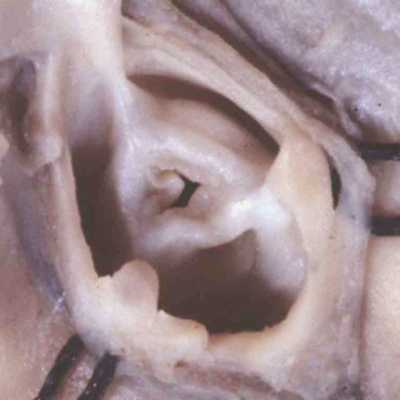
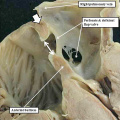
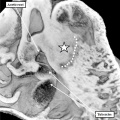
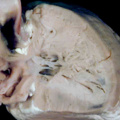
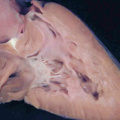
There is then one additional defect that permits interatrial shunting, and which is located outside the confines of the oval fossa. This is the so-called “ostium primum defect” (Figure 23).
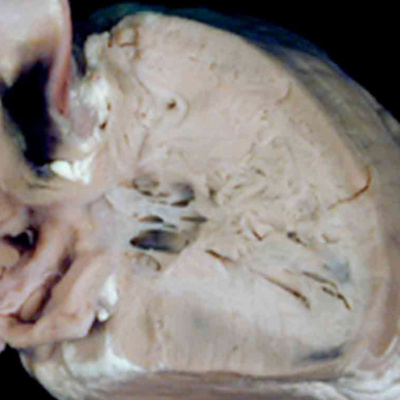
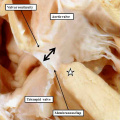
Fig. 23. The right atrium is opened, and the septal surface photographed from the right side to reveal the features of the so-called “ostium primum” defect (white star with black borders). The atrial septum itself extends apically to the level of the atrioventricular junction, and the oval fossa itself is well-formed and intact. The communication is an atrioventricular, rather than an atrial, septal defect. It is because the leaflets of the common atrioventricular valve are fused to each other, and also to the crest of the ventricular septum, that shunting through the defect is confined at atrial level.
This defect is an atrioventricular, rather than an atrial, septal defect. Its phenotypic feature is the commonality of the atrioventricular junction. Since the initially common atrioventricular junction of the developing heart is divided into its mitral and tricuspid components during the end of the fifth, and at the beginning of the sixth, week of human development, we can pinpoint the event producing the various types of atrioventricular septal defect to having occurred during or prior to the sixth week of development. To understand the variations in atrioventricular septal defects, also known as atrioventricular canal malformations, we must now return to consider the mechanisms of separation of the initially common atrioventricular junction.
Mechanisms of Atrioventricular Septation
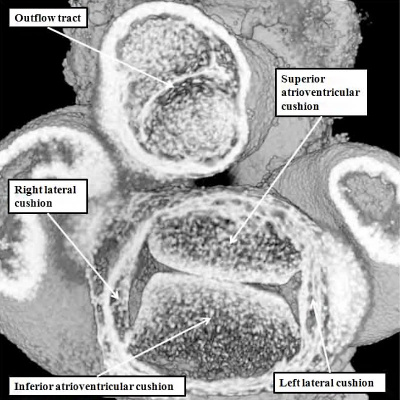
As we have already seen, obliteration of the primary atrial foramen requires growth of the vestibular spine by migration of tissues into the heart through the right pulmonary ridge. This structure then combines with the mesenchymal cap to close the primary interatrial foramen, at the same time binding the atrial septum to the atrioventricular cushions. During this process, the atrioventricular cushions themselves must themselves fuse to divide the initially common atrioventricular canal seen during E11.5 (Figure 24 – left hand panel) into the mitral and tricuspid valvar orifices (Figure 24 – right hand panel).
Fig. 24. The images, from mice at E11.5 (left hand panel) and E12.5 (right hand panel) show how the vestibular spine and the mesenchymal cap fuse with the atrial surface of the atrioventricular cushions to divide the initially common atrioventricular canal (see also Figure 25) into the definitive right and left atrioventricular (AV) junctions.
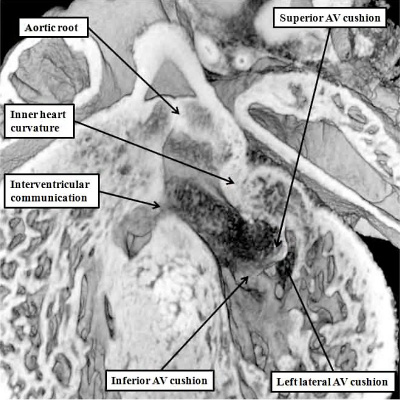
When assessing the developing heart in the so-called “four chamber projection” (Figure 24 – left hand panel), it is not immediately evident that, at E11.5, the major atrioventricular cushions have yet to fuse. The commonality of the canal at this stage is best appreciated when the heart is viewed in its short axis (Figure 25 – left hand panel). Images taken in the short axis then confirm that, at E11.5, the superior and inferior atrioventricular cushions have still to fuse along their facing surfaces. This process of fusion, along with the muscularisation of the vestibular spine and the mesenchymal cap, takes place during E12.5. Even subsequent to these stages, however, the aorta remains supported above the developing right ventricle. Then, although the inferior atrioventricular cushion has already fused dorsally with the crest of the muscular ventricular septum, thus obliterating the caudal component of the interventricular communication seen at E11.5, there remains of necessity a communication ventrally and cranially, so that the left ventricular blood is able to continue to flow into the aorta, and reach the developing brain (Figure 25 – right hand panel).
Fig. 25. The images are from mouse embryos at E11.5 (left hand panel) and E12.5 (right hand panel). Unlike those shown in Figure 23, these are sectioned to show the short axes of the atrioventricular junctions. At E11.5, as seen in the left hand panel, the superior and inferior atrioventricular cushions lie edge-to-edge, but have yet to fuse to each other. By the later stage (right hand panel – shown in a more oblique cut), the cushions have fused, but the aorta remains supported by the developing right ventricle. At this stage, therefore, the cranial and ventral part of the interventricular communication remains patent. This permits the blood entering the left ventricle to continue to reach the aortic root.
The completion of atrioventricular septation, therefore, cannot occur until the aortic root has been transferred from its initial location above the cavity of the right ventricle to its definitive position within the roof of the left ventricle. The transfer of the aortic root takes place during E13.5. Part and parcel of the transfer is the incorporation of the aortic root between the forming orifice of the mitral valve and the muscular ventricular septum. During this process, furthermore, there is expansion of the mural component of the developing mitral valve. The leaflet guarding this part of the mitral valvar orifice is derived from another cushion, itself formed leftward and laterally within the atrioventricular canal during E11.5 (Figure 25 – left hand panel). The process of fusion of the left ventricular components of the superior and inferior atrioventricular cushions has itself created the primordium of the aortic, or anterior, leaflet of the mitral valve (Figure 26).
Fig. 26. The images are from the same mouse heart at E13.5. The left hand panel, in the long axis, shows how the aortic root has become reorientated so as to be supported above the cavity of the left ventricle, although the interventricular communication remains patent. The superior and inferior atrioventricular (AV) cushions have now fused, and will eventually become the aortic, or anterior, leaflet of the mitral valve, which eventually achieves fibrous continuity with the developing leaflets of the aortic valve. At the stage shown in the left hand panel, however, the muscular inner heart curvature still interposes between the leaflets of the developing aortic and mitral valves. This is also well seen in the right hand panel, taken in short axis. The white dotted line shows the line of fusion between the left ventricular components of the superior and inferior atrioventricular cushions, with the white star showing the inner heart curvature. Note that, at this stage, the fused major cushions guard the larger part of the developing mitral valvar orifice, with the left lateral cushion being relatively small.
Subsequent to the fusion of the left ventricular components of the superior and inferior atrioventricular cushions to form the anterior component of the developing mitral valve, the valvar primordium derived from these components guards the greater part of the developing valvar orifice (Figure 25). As the aortic root is in the process of its transfer to the left ventricle, furthermore, the muscular inner heart curvature is interposed between the developing aortic and mitral valvar orifices within the roof of the left ventricle (Figure 25). This remains the situation after the persisting part of the interventricular communication is closed, completing the process of ventricular septation, and walling the aorta into the left ventricle (Figure 27).
Fig. 27. The images show how, at E14.5, when the aortic root is fully committed to the left ventricle, the persisting interventricular communication is closed by fusion of the rightward margins of the atrioventricular (AV) cushions (left hand panel). The right hand panel, which is from the same embryo but sectioned in short axis, shows how the left lateral atrioventricular cushion has expanded to form the mural leaflet of the mitral valve, which is now more extensive than the aortic leaflet, which is derived from the fused left ventricular components of the superior and inferior atrioventricular cushions (Compare with Figure 26). Note also that the aortic root is now fully wedged between the mitral valvar orifice and the muscular ventricular septum.
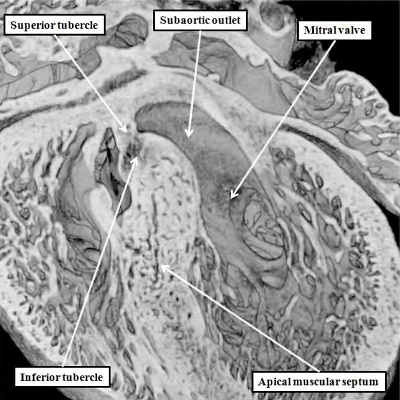
With closure of the interventricular communication, the aortic root itself is wedged more deeply between the mitral orifice and the muscular ventricular septum. Concomitant with the process of wedging, there is expansion of the leaflet of the mitral valve derived from the left lateral cushion formed within the atrioventricular canal (Figure 27 – right hand panel). At the stage of final closure of the interventricular communication, however, there has been no delamination of the septal leaflet of the developing tricuspid valve. This part of the tricuspid valve is formed from the larger parts of the rightward components of the superior and inferior atrioventricular cushions (Figure 26 – right hand panel), with smaller parts of the ventricular components of the cushions fusing together to close the embryonic interventricular communication (Figure 27 – left hand panel). As the atrioventricular junctions become separated, they also expand, with the mural leaflet of the mitral valve, derived from the left lateral cushion, eventually guarding two-thirds of the mitral valvar circumference. The right junction also expands, with the right lateral cushion forming the antero-superior and inferior leaflets of the tricuspid valve. This expansion of the right atrioventricular junction takes place inferiorly and caudally. The initial site of fusion of the cushions, reinforced on the atrial aspect by the site of fusion of the vestibular spine, then occupies a central location within the cardiac base (Figure 28).
Fig. 28. The images are from a mouse embryo at E15.5. Septation is now complete. The short axis cut, as seen in the left hand panel, shows how the right atrioventricular junction has expanded caudally and inferiorly, permitting formation of the septal, inferior, and anterro-superior leaflets of the tricuspid valve. The mitral valve now has aortic and mural leaflets, with the subaortic outlet interposed between the aortic leaflet and the septum. The star shows how the initial site of fusion of the cushions, the vestibular spine, and the mesenchymal cap now occupies a central location within the cardiac base. The four chamber section, taken along the plane showed by the dotted line, is illustrated in the right hand panel. The white double headed arrows show the separate nature of the right and left atrioventricular junctions. The septal leaflet of the tricuspid valve, however, has still to delaminate from the surface of the ventricular septum (white arrow with black borders).
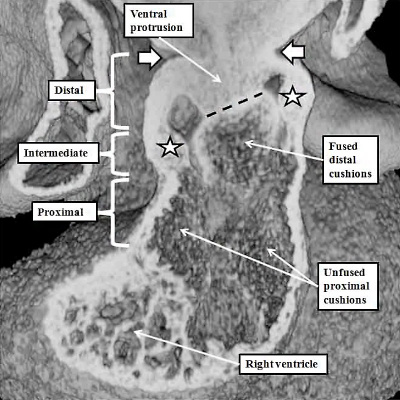
It is the failure to separate the atrioventricular junctions that is the essence of the so-called “atrioventricular canal defects”. These are best considered as atrioventricular septal defects in the setting of a common atrioventricular junction. The situation is exemplified by the arrangement now identified in mouse embryos prior to term with the so-called “ostium primum” defect. Usually described as an “atrial” septal defect, the sections from the abnormal mice show that the essence of the malformation is retention of an atrioventricular septal defect, but with the leaflets derived from the major atrioventricular cushions fused to the crest of the muscular ventricular septum, so that shunting across the atrioventricular septal defect can take place only at atrial level (Figure 29).
Fig. 29. The images are from a mouse embryo at E18.5 with an “ostium primum” atrioventricular septal defect. The left hand panel shows a four chamber cut, revealing the persisting primary atrial foramen, with a common atrioventricular junction (double headed white arrow), but with shunting possible only at atrial level because the inferior atrioventricular cushion is firmly fused to the crest of the muscular ventricular septum. The mesenchymal cap has muscularised to form the leading margin of the oval fossa, but there has been no growth of the vestibular spine. The short axis cut from the same heart, shown in the right hand panel as seen from the ventricular aspect, reveals that the superior and inferior atrioventricular cushions have fused not only with the ventricular septum, but also with each other (white dotted line). The left atrioventricular valve now has three leaflets, retaining the initial figuration of the undivided atrioventricular canal (see Figure 25 – left hand panel). The aortic root is committed to the left ventricle, but is no longer wedged between the left atrioventricular valvar orifice and the muscular ventricular septum (compare with Figure 27 – right hand panel).
The arrangement as seen in the mouse embryo at E18.5 with the ostium primum defect then permits an understanding of the various types of “atrioventricular canal defect”. These lesions used to be thought of as representing failure of fusion of the major atrioventricular cushions during development. We now know that is not the case, since the cushions themselves have fused in the abnormal mouse so as to produce separate right and left valvar orifices within the common atrioventricular junction (Figure 29 – right hand panel). The lesion underscoring the formation of the defect is failure of growth of the vestibular spine, permitting us to pinpoint the timing of production of the defect to E11.5 in the mouse heart, and to Carnegie stage 17, or six weeks of development, in the human heart. It follows, therefore, that infants born with any variant of atrioventricular septal defect and common atrioventricular junction must have had their development disturbed prior to the beginning of the seventh week of normal development.
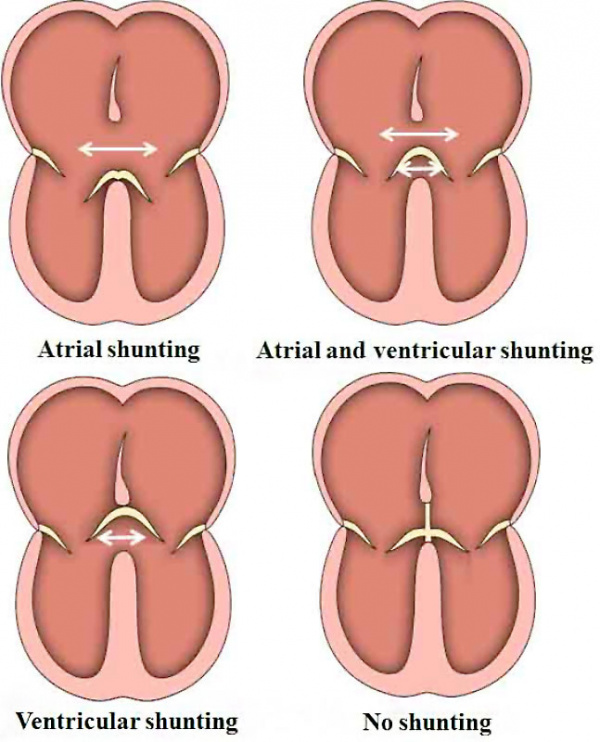
The various forms of atrioventricular septal defect with common atrioventricular junction are now recognized as representing the relationships between the bridging leaflets of the common atrioventricular valve, derived from the superior and inferior atrioventricular cushions, and the atrial and ventricular septal structures. When the bridging leaflets are attached to the crest of the muscular ventricular septum, as seen in the mouse heart with the “ostium primum” defect, then shunting across the atrioventricular septal defect can take place only at the level of the atrial chambers. In the commonest type of defect, however, the bridging leaflets are fused neither to the atrial nor ventricular septal structures, but are said to “float”. This means that shunting between the cardiac chambers can take place at both atrial and ventricular levels, with the extent of shunting reflecting the prevailing hemodynamic conditions, and the proximity of the floating leaflets to the septal structures. In the rarest type of defect, the bridging leaflets, rather than being attached to the crest of the ventricular septum, are tethered to the leading edge of the ventricular septum. This means that shunting can take place only at ventricular level. This parallels the situation seen typically in ventricular septal defects. Since the heart has a common atrioventricular junction, however, with a left atrioventricular valve having three rather than two leaflets, the lesion is properly described as the ventricular component of an atrioventricular septal defect (Figure 30).
Fig. 30. The cartoons show the variability in the structure of hearts with an atrioventricular septal defect and common atrioventricular junction depending on the relationship between the bridging leaflets of the common atrioventricular valve and the atrial and ventricular septal structures.
In all of the variants of atrioventricular septal defect with common atrioventricular junction, therefore, the problem during development must have occurred prior to separation of the common atrioventricular junction into its right and left components. This is not the case for patients having ventricular septal defects, although as we will see, there can be several phenotypic variants producing persistent interventricular shunting, including the lesion with a common atrioventricular junction. The more typical variants, however, have separate atrioventricular junctions. They too, nonetheless, can result from perturbations at different stages of normal development.
Normal as opposed to Abnormal Ventricular Septation
We have already discussed the changes taking place as the right ventricle attains its own inlet component subsequent to the rightward expansion of the atrioventricular canal. During this process, the dorsal part of the initial embryonic interventricular communication becomes reorientated concomitant with formation of the inlet component. Initially, the communication is roofed dorsally by the inner heart curvature (Figures 8 & 9, left hand panels). Subsequent to expansion, the interventricular communication is roofed by the atrioventricular cushions (Figure 24 – left hand panel).By this stage, the dorsal part of the communication has been closed as the inferior atrioventricular cushion fuses with the crest of the apical muscular ventricular septum (Figure 23 – right hand panel). At this stage, nonetheless, which is E12.5 in the mouse, and the beginning of the seventh week of development in man, the aorta retains its initial connection with the developing right ventricle (Figure 31).
Fig. 31. The image shows a mouse heart at E12.5. The aortic root, already separated from the pulmonary root by the fusing cushions spiraling through the outflow tract, remains supported by the right ventricle, which has acquired its own inlet through the tricuspid valve. The left ventricular blood must exit through the persisting interventricular communication so as to reach the aorta. Note the site of fusion between the outflow cushions (white arrow with black borders).
As can also be seen in Figure 31, the right ventricle has already obtained its own inlet through the tricuspid valve, although the valvar leaflets have yet to be formed. The key feature for the completion of ventricular septation is the transfer of the aortic root from the right to the left ventricle. This has already begun, in the mouse, by the end of E12.5 (Figure 32).
Fig. 32. The images come from the same mouse heart, showing the changes occurring later in E12.5. The left hand panel, a four chamber cut, shows that the aortic root is now overriding the crest of the apical muscular ventricular septum. As shown in the right hand panel, showing an “en face” view of the developing ventricular septum, as the root becomes transferred into the left ventricle, so the fused proximal parts of the outflow cushions (white star with black borders) are brought into line with the crest of the apical septum (white dashed line). This serves to reduce the size of the persisting interventicular communication, which eventually will be closed by fusion of the tubercles formed from the rightward ends of the ventricular surfaces of the atrioventricular cushions.
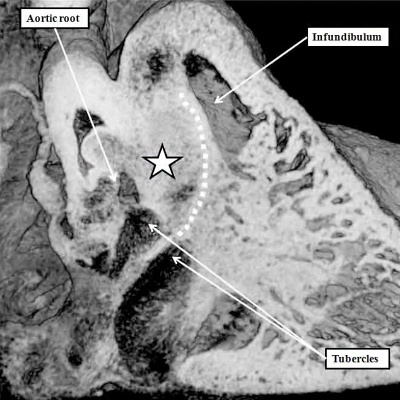
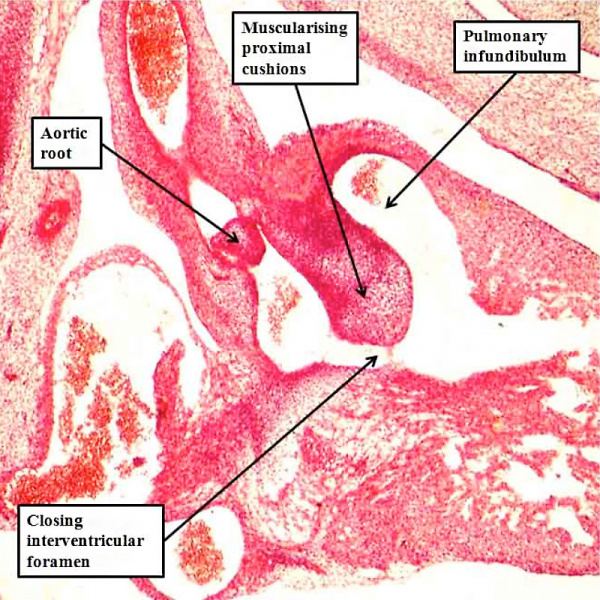
The shift of the aortic root to the left ventricle is accompanied by alignment of the leading edge of the fused outflow cushions with the crest of the apical muscular ventricular septum. The muscular septum itself was initially formed concomitant with the ballooning of the apical ventricular components from the inlet and outlet parts of the ventricular loop (Figure 3). For quite some time, we thought that, as the outflow cushions were brought into alignment with the apical septum, so their surface was muscularised to form a second component of the muscular septum, interposed between the aortic and pulmonary roots, and therefore forming an outlet septum. We now know that the surface component of the cushions does, indeed, muscularise. Rather than forming an outlet septum, however, it becomes transformed into the free-standing muscular sleeve that lifts the pulmonary root away from the base of the heart, in other words the right ventricular infundibulum (Figure 33).
Fig. 33. The left hand panel is from a mouse at E14.5. The tubercles of the atrioventricular cushions have fused with the crest of the apical muscular septum, walling the aorta into the left ventricle, and closing the embryonic interventricular communication. The surface of the proximal outflow cushions have now muscularised (white dotted line). This area will form the free-standing infundibulum subsequent to attenuation of the core of the cushions (white star with black border). The right hand panel, in four chamber section, is from the same episcopic dataset. It shows how the tubercles will become the membranous part of the septum, with the coalescing trabeculations which form the apical part of the septum also being well seen.
The initial apical septum formed between the apical ventricular components forms the entirety of the muscular ventricular septum. As can be seen from sections taken through its long axis even subsequent to the transfer of the aorta to the left ventricle, it is still possible to recognize the initial trabeculations that coalesced to form the septum during the process of ballooning (Figure 32 – right hand panel). The definitive ventricular septum, therefore, possesses a large muscular component, derived from the initial apical septum, and a much smaller fibrous part, which is formed from the tubercles of the atrioventricular cushions. This fibrous part, also known as the membranous septum, will eventually be divided into interventricular and atrioventricular components when the septal leaflet of the tricuspid valve, formed by the fused rightward margins of the atrioventricular cushions, has delaminated from the surface of the ventricular septum. This is a late event, and is not completed in the mouse heart until after the fetus is born. In the human heart, the delamination is not completed until the twelfth week of development, long after closure of the embryonic interventricular communication. The latter event is completed by the end of the eighth week of development.
Knowledge of the timing of these various steps observed during ventricular septation are pertinent to the analysis of ventricular septal defect. As we have seen, the muscular part of the ventricular septum is formed by coalescence of the initial trabeculations that form the larger part of the ventricular walls. Should these trabeculations fail to coalesce, then holes can persist in any part of the muscular septum. Such muscular defects, implying abnormal formation of the muscular septum, could represent abnormal development from any point after six weeks, when the right ventricle achieves its own inlet. Muscular defects are probably the commonest form of ventricular septal defect, but many close spontaneously during postnatal life, and many persisting defects are sufficiently small not to require surgical or interventional closure. The commonest variety requiring therapeutic closure is the defect representing persistent patency of the embryonic interventricular communication.
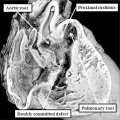
Fig. 34. The heart shown in the left hand panel is from a mouse embryo with knock-out of the Furin enzyme (OMIM). The embryo is at E15.5, by which time the interventricular communication should have closed. The persisting defect is comparable to the defect seen in the human heart, and known as the perimembranous defect (right hand panel). The problem producing the defect in man must have occurred between the sixth and eighth weeks of development.
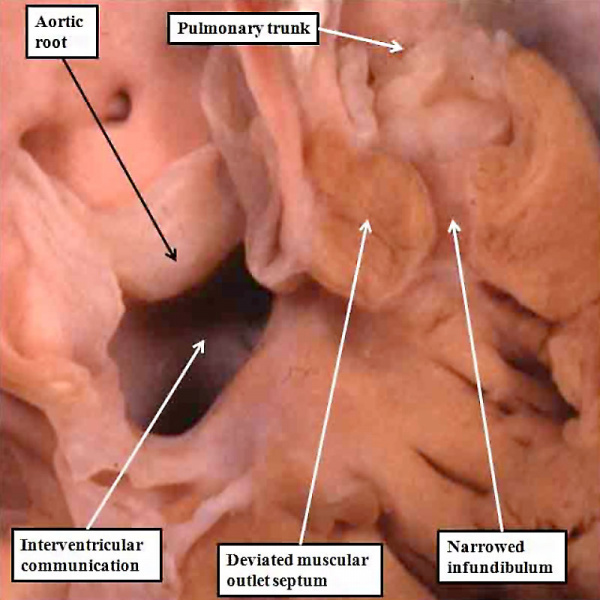
Since the embryonic communication is normally closed by the end of the eighth week of normal development, and since the right ventricular inlet is not formed until the sixth week, we are able to provide an accurate estimate of the insult responsible for this type of defect. We can also provide an accurate feature for its diagnosis, since it must be bounded posteriorly by the continuity between the tissues derived from the atrioventricular cushions, which normally close the embryonic ventricular communication, and the leaflets of the aortic valve. This is the feature than enables the defects to be recognized as being perimembranous (Figure 34 – right hand panel). They can open to the inlet or outlet of the right ventricle, but will always be bounded by fibrous continuity between the leaflets of the tricuspid and aortic valves. Some of these defects, however, are associated with so-called overriding of the aortic valve. This implies that the heart must have been disturbed earlier in its development when compared with those in which the aortic root is fully committed to the left ventricle. The lesions with overriding of the aorta are known as Eisenmenger defects. They are closely related to tetralogy of Fallot, which also is typically found with a perimembranous ventricular septal defect. In tetralogy of Fallot, however, the proximal outflow cushions are no longer aligned with the crest of the muscular septum, as is also the case with the Eisenmenger defect, but in association with narrowing of the subpulmonary muscular infundibulum. This means that tetralogy of Fallot can also be correlated with abnormal development during the seventh week.
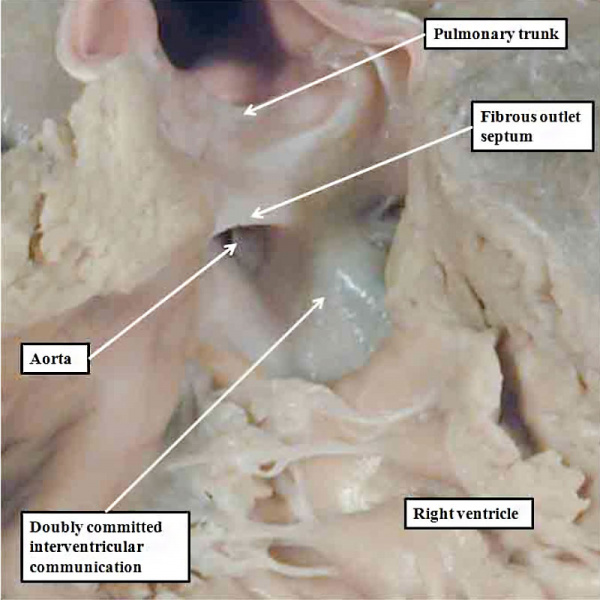
The final type of ventricular septal defect opens to the outlet of the right ventricle, and is due to failure of muscularisation of the proximal outflow cushions. Its phenotypic feature is fibrous continuity between the leaflets of the aortic and pulmonary valves in the roof of the defect, with the location in the ventricular outflow tract meaning that it is doubly committed, in other words related to both the aortic and pulmonary valves. We have also encountered this type of defect in our colony of mice in which the Furin enzyme (OMIM) has been knocked out. The findings in the abnormal mice show that there has been failure of muscularisation of the outflow cushions, which have nonetheless fused together so as to separate the aortic and pulmonary roots. In the absence of muscularisation, there is no formation of the free-standing sleeve of subpulmonary infundibulum. These features mean that the abnormality responsible for producing the defect must have occurred even earlier than for the defects seen in the Eisenmenger or Fallot lesions. These defects can reliably be considered as representing an abnormality occurring during the sixth week of human development (Figure 35).
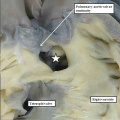
Fig. 35. The image in the left hand panel shows a defect in another mouse with knock-out of the Furin enzyme (OMIM). Both arterial trunks arise from the right ventricle, with the interventricular communication in doubly committed location. There has been failure of muscularisation of the proximal outflow cushions, so that there is no formation of the subpulmonary infundibulum. The right hand panel shows a comparable doubly committed defect (white star) from a patient. Because of the failure of muscularisation of the fused proximal outflow cushions, the defect can be correlated with an abnormality occurring during the seventh week of development.
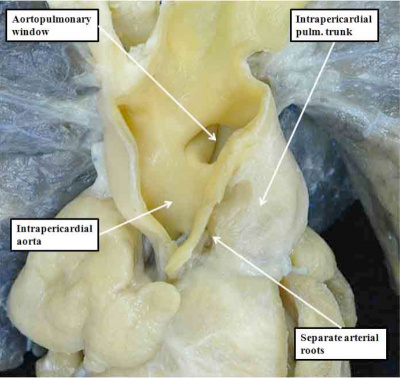
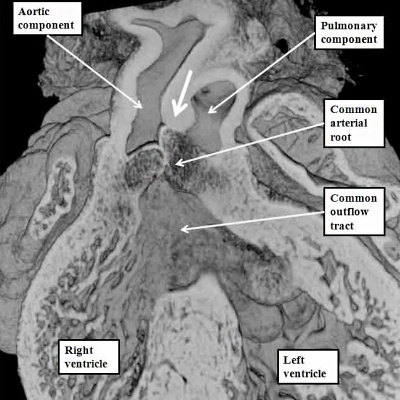
The essence of the doubly committed and juxta-arterial ventricular septal defect is that the proximal outflow cushions have themselves fused, but have then failed to muscularise. As we have seen, the proximal outflow cushions form the subpulmonary infundibulum of the right ventricle. The distal parts of the outflow cushions, however, fuse to separate the arterial roots. They also fuse with a protrusion from the dorsal wall of the aortic sac, thus separating the intrapericardial components of the arterial trunks. Failure of these processes results in common arterial trunk. Fully to appreciate this morphogenesis requires more detailed consideration of the mechanics of development of the outflow tracts.
Development of the Ventricular Outflow Tracts
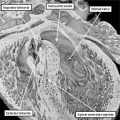
Understanding the development of the ventricular outflow tract, and relating the process of normal development to the congenital malformations that involve the outflow tract, is currently bedeviled by use of the term “conotruncal”. It is conventional wisdom that many, but not all, of the lesions involving the outflow tracts should be grouped under this banner. The use of the term itself reflect the suggestion made by Kramer, in a seminal work published in 1942, that the developing outflow tract could be considered in terms of two components, which he named the “truncus” and the “conus”. Currently, however, there is no consensus as to the boundaries of these two suggested components. The need for such definition becomes the more significant when we examine the postnatal outflow tracts, which possess three, rather than two, components. The normal arrangement is best seen in the right ventricular outflow tract, which is made up of the muscular subpulmonary infundibulum, the pulmonary root, and the intrapericardial component of the pulmonary trunk (Figure 36).
| Online Editor - Kramer 1942 |
|---|
| Kramer TC. The partitioning of the truncus and conus and the formation of the membranous portion of the interventricular septum in the human heart. (1942)
Amer. J Anat. 71(3): 343-370. |
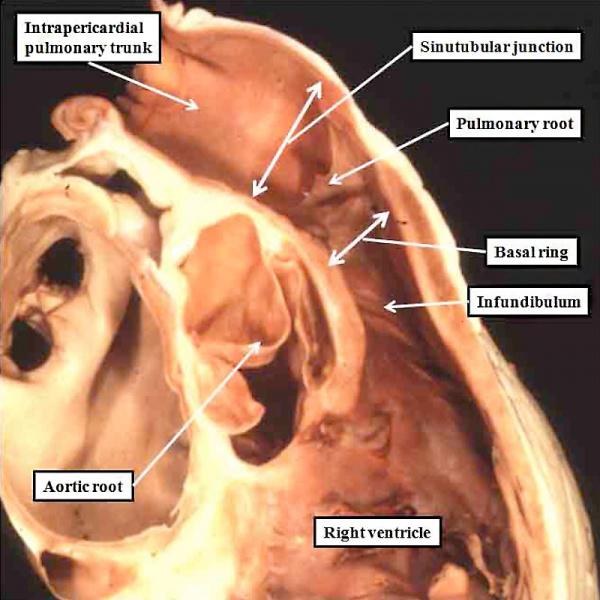
Fig. 36. The image shows the outflow tract of the right ventricle in the postnatal heart, sectioned along its length. It has three discrete anatomic components. The intermediate component, made up of the pulmonary root, is limited distally by the sinutubular junction, and proximally by the basal ring created by joining together the nadirs of attachment of the pulmonary valvar leaflets. The outflow tract then has a subvalvar proximal component, the right ventricular infundibulum, and a distal component, the intrapericardial pulmonary trunk.
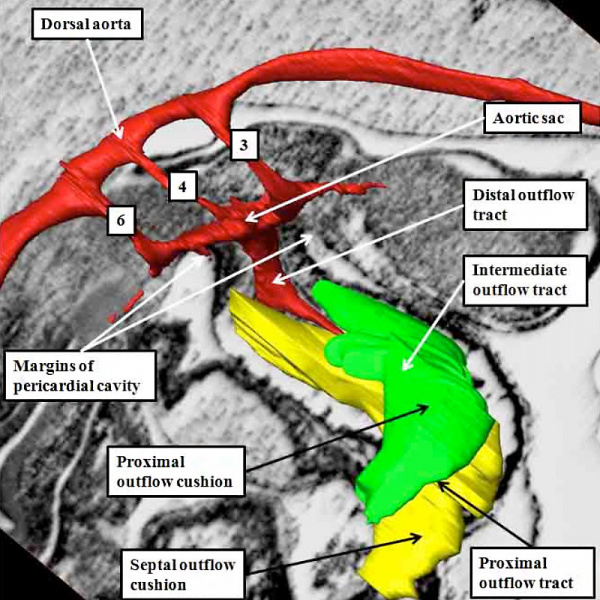
At the earliest stage of development, subsequent to looping of the heart tube (Figure 2), the developing outflow tract extends from the outlet of the primordium of the right ventricle to the margins of the pericardial cavity. The extent of the pericardial cavity is of particular value as an anatomic marker, since the cavity of outflow tract becomes continuous with that of the so-called aortic sac at this boundary. When reconstructed at this early stage of development, then the outflow tract, which has exclusively myocardial walls at this stage, also possesses three recognizable components. They are located in distal, intermediate, and proximal positions (Figure 37).
Fig. 37. The images shows a reconstruction of the developing heart in a human embryo at Carnegie stage 13, equivalent to four weeks of age subsequent to ovulation. The heart tube has already looped, with the outflow tract extending from the outlet of the developing right ventricle to the margins of the pericardial cavity, where its lumen becomes continuous with that of the aortic sac, shown in red. The reconstruction was made from an embryo in which myocardium was identified and coloured silver. The entirety of the outflow tract, at this stage, has myocardial walls, and is well described as possessing proximal, intermediate, and distal components.
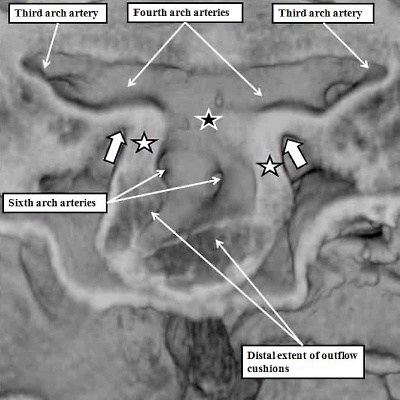
The division of the outflow tract into three components according to its shape is more obvious in the human heart than in the mouse. Comparable reconstructions made in the mouse, nonetheless, at a slightly later stage, namely early on E11.5 (Figure 38), again reveal the three components of the developing outflow tract. The reconstruction also shows well how the solitary lumen of the distal outflow tract continues as the lumens of the arteries coursing through the segments of mesenchyme known as the pharyngeal arches. These arteries arise in symmetrical fashion from the aortic sac, passing round the trachea-esophageal pedicle to unify dorsally as the dorsal aorta (Figure 38).
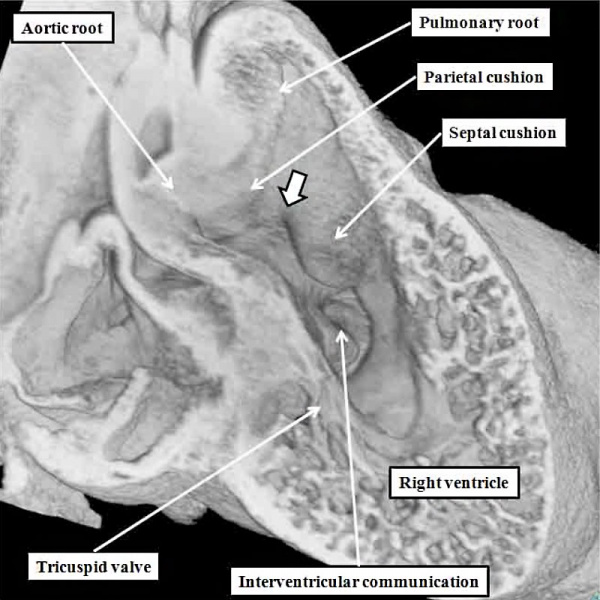
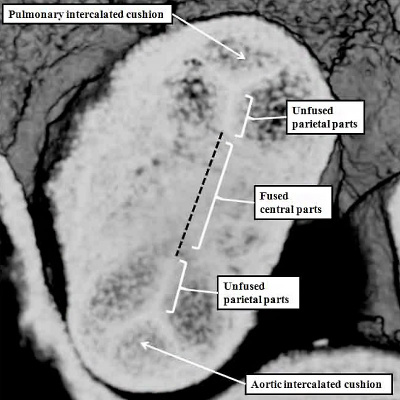
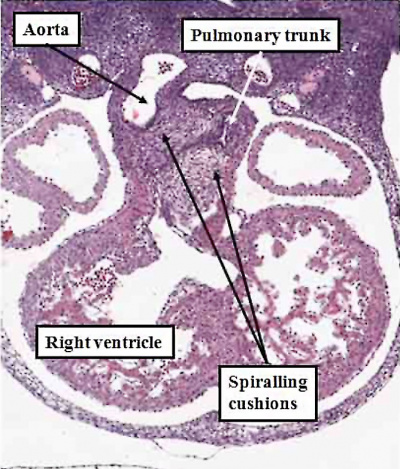
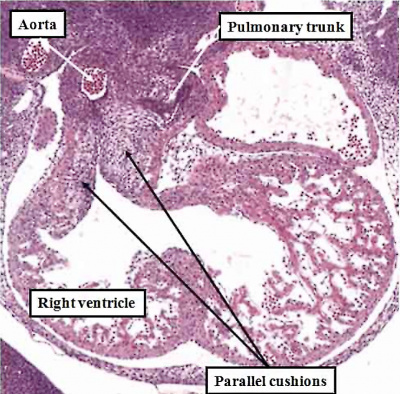
Fig. 38. The image shows a reconstructed episcopic dataset from a mouse embryo early during E11.5. The reconstruction is viewed from the right side. The lumen of the outflow tract, and its continuation as the pharyngeal arch arteries, are shown in red. The right third, fourth, and sixth arch arteries are seen extending through the pharyngeal mesenchyme. They unite dorsally to form the dorsal aorta. The margins of the pericardial cavity mark the boundary between the distal component of the outflow tract and the aortic sac. By this stage, the myocardial walls have regressed minimally from the pericardial border, with the most distal walls of the outflow tract now formed by non-myocardial tissues. Note the cushions, derived from the cardiac jelly, which spiral through the intermediate and proximal part of the outflow tract. They end distally at the level of the regressing myocardial border. The so-called septal cushion is shown in yellow, with the parietal cushion colored green. The reconstruction was made by Dr. Simon Bamforth, and is reproduced with his kind permission.
At the stage of development shown in the mouse heart in Figure 37, which is early during the twelfth day of development (E11.5), new tissue has migrated from the heart-forming areas to enter the distal component of the outflow tract. The new tissue is non-myocardial. As it enters the heart, so the distal boundary of the initial myocardial walls regresses towards the right ventricle. As the myocardial border moves proximally, the spiraling cushions also regress. Eventually, the cushions occupy only the intermediate and proximal components, where they fuse to separate these parts into the arterial roots and the ventricular outflow tracts. The distal part of the outflow tract, in contrast, is separated into aortic and pulmonary components by growth of a protrusion from the dorsal wall of the aortic sac. The processes separating the outflow tract, and eventually placing the extrapericardial pulmonary channels in continuity with the right ventricle, and the systemic channels with the left ventricle, are complicated.
As we have seen, the outflow tract, when first formed, arises exclusively from the part of the ventricular loop that will become the right ventricle. The default option for the developing heart, therefore, is double outlet right ventricle (Figure 8B). We have also seen, nonetheless, that during the final part of ventricular development, the dorsal part of the proximal outflow tract is transferred to the left ventricle. This part becomes the subvalvar component of the aortic channel (Figure 32). To understand how this subvalvar component becomes connected to the systemic channels, we need to turn our attention to changes taking place earlier in development within the aortic sac. During the overall stages of development, there are five pairs of arteries that take origin from the sac, and extent symmetrically through the pharyngeal mesenchyme to join the dorsal aorta. Not all are present at the same time. Indeed, the first two pairs are relatively insignificant, and become transformed into small arteries within the head of the developing embryo. The significant sets are seen at the beginning of E11.5, as was shown in Figure 38. Of the three pairs of arteries recognisable at this stage, it is the third and fourth pairs which will become the systemic arterial channels. The right and left pulmonary arteries, by now also developing within the pharyngeal mesenchyme, take their origin from the undersurfaces of the sixth arch arteries. If we now examine the aortic sac at this stage, it can be seen that the systemic channels, namely the third and fourth arch arteries, arise from the cranial component, which the sixth arch arteries, feeding the pulmonary arteries, arise caudally from the base of the aortic sac (Figure 39, left hand panel). It is these caudal pulmonary channels that need to be connected, with ongoing development, with the right ventricle, leaving the left ventricle left supplying the systemic channels. One of the processes needed to achieve these connections is the regression of the right-sided dorsal channels extending between the aortic sac and the dorsal aorta (Figure 39 – right hand panel).
Fig. 39. The images show, to the left hand, a frontal section through the aortic sac of an embryonic mouse early on E11.5, and to the right hand, a reconstruction of the arch arteries seen frontally later on E11.5. The left hand panel shows how the distal walls of the distal outflow tract are now non-myocardial (white stars with black borders), the myocardial border being confluent with the distal extent of the outflow cushions. The black star with white borders shows the dorsal wall of the sac between the origins of the systemic third and fourth arch arteries and the pulmonary arteries, which arise from the sixth arch arteries. The right hand panel shows a later stage, when the distal outflow tract has divided into the aortic and pulmonary channels, and shows the beginning of regression of the right sixth arch artery. The pericardium surrounding the distal extent of the outflow tract is shown in green, with the newly formed intercalated cushions reconstructed in violet.
The process of separation of the channels within the distal outflow tract then requires growth of the protrusion from the dorsal wall of the sac. It grows obliquely into the distal outflow tract so as to separate the cranial origins of the third and fourth arch arteries from the caudal origin of the sixth arch arteries (Figure 40).
Fig. 40. The images show, in the left hand panel, how at E11.5 the growth of the protrusion from the dorsal wall of the aortic sac (black star in right hand panel of Figure 39) has grown ventrally into the cavity of the distal outflow tract, separating it into the intrapericardial aortic and pulmonary channels. The white arrows show the regressing myocardial border, which remains confluent with the ends of the distal outflow cushions. There is an embryonic aortopulmonary foramen at this stage of development. Later in day E11.5, as shown in the right hand panel, the protrusion fuses with the ends of the outflow cushions, which themselves have also fused by this stage. The processes of fusion divide the distal outflow tract into the intrapericardial aorta and pulmonary trunk, at the same time closing the embryonic aortopulmonary foramen. By this stage, additional cushions, known as intercalated cushions, have been formed within the intermediate part of the outflow tract (white stars). These cushions were also seen as the violet structures reconstructed in the right panel of Figure 39. Together with the major cushions, they will provide the primordiums for formation of the leaflets of the arterial valves. The cushions within the proximal outflow tract, in contrast, remain unfused at this stage.
The fusion of the protrusion with the distal ends of the cushions, in combination with regression of the dorsal components of the initially bilaterally symmetrical arch arteries, has now connected the systemic extrapericardial channels with the right-sided and dorsal half of the distal outflow tract, leaving the pulmonary channels in continuity with the left-sided and ventral halft. It is then the spiraling configuration of the cushions within the intermediate and proximal parts of the outflow tract that places these distal channels in communication with the left and right ventricles, respectively. The fusion of the ventral protrusion from the dorsal wall of the aortic sac with the distal end of the outflow cushions also closes the embryonic aortopulmonary foramen (Figure 40). Should this process fail, but the cushions spiraling through the outflow tract succeed in fusing with each other, then there will be persistence of the foramen, producing the lesion known as an aortopulmonary window (Figure 41 – left hand panel).
Fig. 41. The images show the difference between aortopulmonary window (left hand panel) and common arterial trunk (right hand panel). The right hand panel shows an aortopulmonary window in a postnatal human heart . The intermediate and proximal cushions have fused to produce separate arterial roots, and separate ventricular outflow tracts, but the protrusion has failed to close the embryonic aortopulmonary foramen. The right hand panel is from a mouse genetically modified to perturb the Furin enzyme (OMIM). The outflow cushions have failed to fuse throughout the length of the outflow tract. The distal outflow, nonetheless, has been separated into balanced intrapericardial aortic and pulmonary trunks by growth of the protrusion from the dorsal wall of the aortic sac (white arrow).
If, in contrast, the outflow cushions themselves have failed to fuse, then the end result is retention of a common arterial trunk. Both of these lesions can be related to abnormal separation of the components of the outflow tract. Common arterial trunk (Figure 40 – right hand panel) reflects failure of separation of the intermediate and proximal part of the tract. Separation of the distal aortic and pulmonary channels, which depends on the growth of the protrusion, is itself variable in the setting of the common trunk, serving to explain the variations known to exist with this lesion. The aortopulmonary window, in contrast, does require fusion of the intermediate and proximal cushions. The window, therefore, is likely to depend on an abnormality occurring at a slightly later stage than required to produce the common trunk. Both lesions imply abnormal development during the fifth and sixth weeks of human development, since the pulmonary and systemic components of the distal and intermediate parts of the outflow tract are normally separated by the beginning of the seventh week subsequent to ovulation.
The growth of the new non-myocardial tissue into the distal outflow tract has effectively served to “push” the myocardial component, surrounding the outflow cushions, towards the right ventricle. This effective regression of the myocardial border is accompanied by the appearance of the intercalated cushions within the intermediate part of the outflow tract. The interdigitation of these newly formed intercalated cushions with the parietal ends of the major cushions then produces the scaffold for formation of the arterial valves. They appear during E11.5 (Figure 39 – right hand panel). By the end of E11.5, as we have seen, the central components of the major cushions have fused with each other, and also with the protrusion from the dorsal wall of the aortic sac (Figure 40 – right hand panel). By this stage, the cushions occupy the intermediate and proximal parts of the outflow tract. Within the intermediate part, it is only the central components of the major cushions that have fused. The peripheral parts remain unfused at their ends. It is the interdigitation of the intercalated cushions with these unfused margins that produces the scaffold for formation of the arterial valvar leaflets (Figure 42 – left hand panel). The leaflets are formed within the intermediate part of the outflow tract by excavation of the distal ends of the cushions (Figure 42 – right hand panel).
Fig. 42. The images show the stages involved in formation of the arterial roots within the intermediate component of the outflow tract. The left hand panel is a cross-section through the intermediate component at the end of E11.5. The central parts of the distal cushions have fused, but the parietal parts remain unfused. Together with the formation of the intercalated cushions, this sets the scene for formation of the leaflets of the arterial valves. This occurs during E12.5 and E13.5 by excavation of the distal ends of the cushions, as shown in the right hand panel for the pulmonary valve. At this relatively early stage, there has been minimal ingrowth of non-myocardial tissue to form the valvar sinuses, so that the distal myocardial border still remains adjacent to the tips of the developing valvar leaflets (white arrows with black borders). The sinuses continue to form subsequent to E13.5, which is the stage, in the mouse, of closure of the embryonic interventricular communication, equivalent to CS23, or eight weeks of development, in the human.
Ongoing migration of the non-myocardial tissues into the intermediate component then serves, over the next days of development, to produce the arterial walls of the valvar sinuses. It is the intermediate component of the outflow tract, therefore, that is the origin of the arterial valves and their supporting valvar sinuses. By the end of E12.5 in the mouse, the aortic and pulmonary roots will have separated one from the other, which is equivalent to six weeks after ovulation in the human embryo. If development proceeds normally, then as shown the ends of the major cushions will not have fused. Instead, they and the intercalated cushions will have cavitated so as to produce the leaflets of both arterial valves. Should the fusion of the peripheral margins of the cushions during these stages be excessive, or should one of the intercalated cushions fail to fuse, then the aortic or pulmonary valves will develop with two, rather than three leaflets. Such bicuspid valves are common congenital cardiac malformations, even though they may not produce symptoms until late in life. Excessive fusion of all three cushions during development then provides a similar explanation for so-called critical valvar stenosis, which can involve either the pulmonary or the aortic valves (Figure 43).
Fig. 43. The images show, to the left hand, a critically stenotic pulmonary valve, and to the right hand, a critically stenotic aortic valve. These lesions are well explained on the basis of excessive fusion of the distal outflow cushions and the intercalated cushion during the fifth or sixth week of development, or at any stage onwards to term. It is likely that the changes to produce the stenotic valves occur later in development, but the initial insult could occur during the fifth or sixth week.
Since these valves, along with their supporting arterial roots, are formed within the central components of the developing outflow tracts, there is no valid reason to exclude them from the category of so-called “conotruncal” malformations. At the moment, however, they are not grouped in this fashion. Abnormal development during the fifth and sixth weeks can produce all the lesions that involve the arterial valves. Ongoing fusion of the valvar leaflets subsequent to closure of the embryonic interventricular communication, nonetheless, could provide an equally valid explanation. Lesions of the aortic or pulmonary valves, therefore, could represent abnormal development anytime after the sixth week of development in man. The lesions involving the arterial valves also include atresia. This again can involve either the aortic or pulmonary valve, and typically is found when the ventricular septum is intact. In the setting of the aortic valve, this produces the lesion known as “hypoplastic left heart syndrome”. This exists in two primary variations, one with stenosis of the mitral valve (Figure 44 – left hand panel), and the other with mitral atresia (Figure 44 – right hand panel).
Fig. 44. The images show the variations in ventricular morphology to be found in the hypoplastic left heart syndrome. The left had panel shows the small globular left ventricle, lined with fibroelastosis, as found in mitral stenosis with aortic atresia. The right hand panel shows the slit-like left ventricle, without fibroelastosis, found when there is mitral atresia and aortic atresia.
The differences in the two phenotypes point to the timing in development at which the abnormality was likely induced. In both variations, the ventricular septum is intact, implying that the lesion was produced subsequent to closure of the embryonic interventricular communication at the end of the eighth week of human development. The finding of mitral atresia, however, suggests that the initial insult occurred during maturation of the atrioventricular canal, during the fifth week of development. It is equally possible that an insult occurred at this early stage when aortic atresia is accompanied by mitral stenosis. Evidence now available from fetal echocardiographic studies, nonetheless, has proven that the heart can be normal at 12 weeks of development, and only subsequently progress to become part of the hypoplastic left heart syndrome. This situation is also the case when it is the pulmonary valve that is atretic in the setting of an intact ventricular septum. Such examples with hypoplasia of the right ventricle are comparable to the hypoplastic left heart syndrome. The evidence now available from fetal echocardiographic studies shows convincingly that these lesions are produced subsequent to the closure of the embryonic interventricular communication. Thus, the size of the right ventricle almost certainly reflects the timing of the abnormality, with the larger ventricles suggesting that atresia did not occur until perhaps the final trimester of pregnancy (Figure 45).
Fig. 45. The images show the right ventricle from two cases of pulmonary atresia with intact ventricular septum. In the left hand panel, the right ventricular cavity is no more that the inlet, with gross hypertrophy of the ventricular walls. In the right hand panel, the ventricular cavity shows well-formed inlet, apical, and outlet components, with less mural hypertrophy. The findings suggest development much later in gestation for the right hand lesion.
It cannot be denied with certainty, of course, that the initial insult had occurred prior to closure of the embryonic interventricular foramen. The anatomic evidence, nonetheless, combined with the fetal echocardiographic findings, indicates that the balance of probabilities favours an insult occurring after eight weeks of development, this being the timing of closure of the embryonic interventricular foramen.
The various forms of arterial valvar stenosis and atresia found with intact ventricular septum all depend on abnormal formation of the intermediate component of the outflow tract. Several more malformations can be explained on the basis of abnormal development of the proximal components of the outflow tract. We have already shown that the key feature in this regard is the transfer of the dorsal part of the outflow tract to the left ventricle. This process is a pre-requisite to permit closure of the embryonic interventricular foramen (Figure 32). As was also discussed during the explanation of this process, the closure of the foramen itself cannot take place until the proximal outflow cushions have fused. Subsequent to their fusion, the muscularised surface of the cushions is brought into alignment with the crest of the muscular interventricular septum, with closure of the foramen taking place during the eighth week of human development (Figure 32 – right hand panel).
The proximal components are the last parts of the spiraling cushions to close during normal development of the outflow tract. Proximal closure in the human begins during the seventh week of development, by which time the distal cushions have already closed to separate the aortic and pulmonary roots (Figure 46).
Fig. 46. The left hand panel shows a normal mouse embryo at E12.5. The intermediate cushions have already closed to separate the aortic and pulmonary roots. The proximal cushions, however, have yet to complete their fusion (white arrow with black borders). At this stage, furthermore, both arterial trunks retain their initial location above the cavity of the developing right ventricle. An abnormality at this stage will produce double outlet from the right ventricle. This can occur when the proximal cushions themselves remain hypoplastic, and the interventricular communication remains doubly committed, as shown in the right hand panel, which is from a mouse at E14.5, but genetically programmed by perturbation of the Furin enzyme (OMIM).
Should an abnormality occur at this stage, and the proximal cushion fuse, but fail to muscularise, then the arterial trunks will retain their location above the right ventricle, but the interventricular communication will be doubly committed, with a fibrous rather than a muscular outlet septum (Figure 47).
Fig. 47. The image shows a human heart with double outlet from the right ventricle, but with a doubly committed interventricular communication. The proximal outflow cushions have fused, but have failed to muscularise. Because of this, the outlet septum, derived from the fused cushions, is fibrous and hypoplastic. The arrangement is directly comparable to the lesion shown in the right hand panel of Figure 46.
If development proceeds normally, the proximal cushions not only fuse, but also muscularise, with their surface then forming the free-standing subpulmonary infundibulum. It is the attenuation of the fused central component of the proximal cushions that eventually produces the extracavitary tissue plane that, in the postnatal heart, separates the pulmonary infundibular sleeve from the aortic root (Figure 36). The proximal cushions, during their development, nonetheless, begin the process of muscularisation before they are brought into line with the crest of the muscular interventricular septum. This process takes place during the eighth week of development in the human heart (Figure 48).
Fig. 48. The section is taken from a human embryo at Carnegie stage 20, in the eighth week of development, just prior to closure of the embryonic interventricular foramen. The proximal cushions have fused, and are beginning to muscularise. They are brought into alignment with the muscular ventricular septum as the aortic root is transferred to the left ventricle.
Should an abnormality occur around the stage of development shown in Figure 48, whilst the aortic root is being transferred to the left ventricle, but with the proximal cushions malpositioned within the outflow tract so that they obstruct the outlet to the pulmonary trunk, the resulting lesion is described as tetralogy of Fallot. The features of this lesion are an interventricular communication, overriding of the aortic root relative to the crest of the muscular ventricular septum, muscular obstruction of the subpulmonary outflow tract due to the malpositioned muscular outlet septum, and right ventricular hypertrophy. It is well accepted that the ventricular hypertrophy is an haemodynamic consequence of the embryonic malformation. The other three lesions are well explained on the basis of the abnormal location of the muscular outlet septum, derived by muscularisation of the proximal outflow cushions, coupled with an abnormal location of the parietal trabeculations formed with the right ventricle (Figure 49).
Fig. 49. The image shows the salient features of tetralogy of Fallot. The aortic root is overriding the crest of the muscular ventricular septum, in presence of a large interventricular communication. The muscular outlet septum is deviated antero-cranially, and is located exclusively within the right ventricle. Together with the hypertrophied trabeculations on the parietal wall of the right ventricle, it produces muscular subpulmonary obstruction. The walls of the right ventricle are hypertrophied, producing the fourth feature of the tetralogy.
There are several variable features found in hearts having the phenotypic feature of tetralogy, one of which is origin of both arterial trunks predominantly or exclusively from the right ventricle. This, along with the feature of the lesion shown previously with the doubly committed interventricular communication, show that double outlet right ventricle itself is no more than retention of the initial embryological situation, in which both arterial trunks began their development above the cavity of the developing right ventricle. There is, nonetheless, another variant of double outlet right ventricle that provides an important link to transposition, or discordant ventriculo-arterial connections. This is another of the lesions described under the banner of “conotruncal defects”, but better considered simply as a malformation of the outflow tracts. The essence of transposition, which can also be found in various phenotypic forms, is the presence of the aorta arising from the right ventricle, and the pulmonary trunk from the left ventricle. It is readily explained developmentally on the basis of straight, rather than spiraling, fusion of the outflow cushions (Figure 50).
Fig. 50. The images, prepared from mouse embryos at E12.5, show the difference between a normal mouse (left hand panel), and a mouse that will develop with transposition (right hand panel). The mouse embryo shown in the right hand panel had been genetically modified by knocking out the Pitx2 gene (OMIM). The outflow cushions are parallel, rather than being formed in spiraling fashion, as in the normal mouse.
Transposition in its various forms, therefore, can be explained on the basis of an abnormality occurring at or before E12.5 in the mouse heart, equivalent to the beginning of the seventh week of development in man. Subsequent changes can produce the variants, such as transposition with intact ventricular septum, or transposition with pulmonary stenosis. As can be seen from the right hand panel of Figure 49, in which the outflow cushions have fused in straight fashion, it is the pulmonary root, rather than the aortic root, which is closest to the embryonic interventricular communication. When development continues in this setting, it is then the pulmonary trunk that is transferred to the left ventricle, thus producing the transposition of the arterial trunks. Should, however, the pulmonary trunk retain its location above the morphologically right ventricle, then the end result will be yet another variant of double outlet right ventricle, specifically with a subpulmonary interventricular communication. This is the variant also known as the Taussig-Bing malformation.
With all these abnormalities, it is the location of the outlet septum, or its fibrous remnant, derived from the proximal cushions, and located within the proximal part of the outflow tract, which determines the presence of subaortic or subpulmonary obstruction. Excessive deviation of the outlet septum can produce pulmonary or aortic atresia, but then in association with a ventricular septal defect. These variants of arterial valvar atresia, therefore, require an abnormality to occur prior to normal closure of the embryonic interventricular communication, namely before the end of the eighth week of development of the human heart. Deviation of the outlet septum will, of necessity, obstruct the flow into either the pulmonary or the aortic outflow tracts, and hence reduce the flow into either the developing pulmonary or systemic circulations. In these situations, either the pulmonary arteries or the aortic arch will be hypoplastic. Hypoplasia of the aortic arch is described as coarctation, or in its extreme form is seen as interruption of the aortic arch. When found with an interventricular communication, these lesions reflect the timing of the ventricular lesion. Coarctation, and very rarely interruption, nonetheless, can also be found when the ventricular septum is intact. This then implies that the developmental abnormality occurred after closure of the embryonic interventricular communication, in other words subsequent to eight weeks after ovulation.
Conclusions
The relatively brief account of normal cardiac development outlined above shows that a rational explanation can now be offered for the greater majority of congenital cardiac malformations. Reasoned accounts can also be provided for the likely timing during development of the insult presumed to be responsible for the malformation (Table 2).
| Lesion | Start of Susceptibility to Malformation |
End of Susceptibility to Malformation | |||
|---|---|---|---|---|---|
| Interatrial communications | |||||
| Oval fossa defect | 6 weeks (E13.5) | Term | |||
| Sinus venosus defect | 8 weeks | 12 weeks | |||
| Coronary sinus defect | 8 weeks | Term | |||
| Vestibular defect | 7 weeks | 8 weeks | |||
| Ventricular Septal Defect | |||||
| Muscular | 8 weeks | Difficult to predict | |||
| Perimembranous | 6 weeks | 8 weeks | |||
| Doubly committed | 7 weeks | 8 weeks | |||
| Atrioventricular Septal Defect | |||||
| Ostium primum | 5 weeks | 6 weeks | |||
| “Complete” | 5 weeks | 6 weeks | |||
| Aortic coarctation | |||||
| With VSD | 5 weeks | 8 weeks | |||
| With intact ventricular septum | 8 weeks | Term | |||
| Double Outlet Right Ventricle | 6 weeks | 8 weeks | |||
| Transposition of Great Arteries | 6 weeks | 8 weeks | |||
| Ebstein’s malformation | 6 weeks | 8 weeks | |||
| Hypoplastic left heart syndrome | |||||
| With mitral atresia | 5 weeks | 8 weeks | |||
| With mitral stenosis | 8 weeks | Term | |||
| Pulmonary atresia | |||||
| With VSD | 6 weeks | 8 weeks | |||
| With intact ventricular septum | 8 weeks | Term | |||
| Other | |||||
| Functionally single ventricle | 5 weeks | 6 weeks | |||
| Tetralogy of Fallot | 7 weeks | 8 weeks | |||
| Totally anomalous pulmonary venous return | 8 weeks | 12 weeks | |||
| Tricuspid atresia | 5 weeks | 6 weeks | |||
| Common arterial trunk | 5 weeks | 7 weeks | |||
| Bicuspid aortic valve | 6 weeks | Term | |||
| Notes | |||||
| For approximate clinical Gestational Age GA add 2 weeks; number in brackets is mouse equivalent. Data Reference[1] | Links: heart | abnormal development | timeline | Category:Timeline | ||||
| Table 2. Summary of Timing (Post Fertilization) of Cardiac Susceptibility to a Drug-Induced Malformation | ||
|---|---|---|
| Lesion | Start of Susceptibility to Malformation |
End of Susceptibility to Malformation |
| Interatrial communications | ||
| Oval fossa defect | 6 weeks (E13.5) | Term |
| Sinus venosus defect | 8 weeks | 12 weeks |
| Coronary sinus defect | 8 weeks | Term |
| Vestibular defect | 7 weeks | 8 weeks |
| Ventricular Septal Defect | ||
| Muscular | 8 weeks | Difficult to predict |
| Perimembranous | 6 weeks | 8 weeks |
| Doubly committed | 7 weeks | 8 weeks |
| Atrioventricular Septal Defect | ||
| Ostium primum | 5 weeks | 6 weeks |
| “Complete” | 5 weeks | 6 weeks |
| Aortic coarctation | ||
| With VSD | 5 weeks | 8 weeks |
| With intact ventricular septum | 8 weeks | Term |
| Double Outlet Right Ventricle | 6 weeks | 8 weeks |
| Transposition of Great Arteries | 6 weeks | 8 weeks |
| Ebstein’s malformation | 6 weeks | 8 weeks |
| Hypoplastic left heart syndrome | ||
| With mitral atresia | 5 weeks | 8 weeks |
| With mitral stenosis | 8 weeks | Term |
| Pulmonary atresia | ||
| With VSD | 6 weeks | 8 weeks |
| With intact ventricular septum | 8 weeks | Term |
| Other | ||
| Functionally single ventricle | 5 weeks | 6 weeks |
| Tetralogy of Fallot | 7 weeks | 8 weeks |
| Totally anomalous pulmonary venous return | 8 weeks | 12 weeks |
| Tricuspid atresia | 5 weeks | 6 weeks |
| Common arterial trunk | 5 weeks | 7 weeks |
| Bicuspid aortic valve | 6 weeks | Term |
|
Notes: For approximate clinical Gestational Age GA add 2 weeks; number in brackets is mouse equivalent. Reference: Anderson RH. Teratogenecity in the setting of cardiac development and maldevelopment. (2016) | ||
Special thanks to Diane Spicer who photographed many of the human specimens in this review.
Table 1. Comparison of Human and Murine Development
| Human | Mouse | ||||
|---|---|---|---|---|---|
| Carnegie Stage |
Post-ovulatory Days |
Size (mm) Greatest Length |
Theiler Stage |
Post-conception Days |
Size (mm) Crown-rump |
| 11 | 24 | 2.5 - 4.5 | 14 | 9 - 9.5 | 2.1 |
| 12 | 26 | 3 - 5 | 15 | 9.5 - 10.25 | 2.5 |
| 13 | 28 | 4 - 6 | 16 | 10.25 - 10.5 | 3.6 |
| 14 | 32 | 5 - 7 | 17 | 10.5 | 4.1 |
| 15 | 33 | 7 - 9 | 18 | 11 | 4.6 |
| 16 | 37 | 8 - 11 | 19 | 11.5 | 6 - 7 |
| 17 | 41 | 11 - 14 | 20 | 12 | 7 |
| 18 | 44 | 13 - 17 | 21 | 12.5 - 13 | 8.2 |
| 19 | 47.5 | 16 - 18 | 21 | 12.5 - 13 | 8.2 |
| 20 | 50.5 | 18 - 22 | 22 | 13.5 - 14 | 9.1 |
| 21 | 52 | 22 - 24 | 22 | 13.5 - 14 | 9.1 |
| 22 | 54 | 23 - 28 | 22 | 13.5 - 14 | 9.1 |
| 23 | 56.5 | 27 - 31 | 22 | 13.5 - 14 | 9.1 |
| 26 | 17.5 - 18 | 17.8 | |||
|
Notes: Carnegie Stages | Theiler Stage | Post-conception Days | Carnegie Stage Comparison Reference: Anderson RH. Teratogenecity in the setting of cardiac development and maldevelopment. (2016) | |||||
| Table 1. Comparison of Human and Murine Development | |||||
|---|---|---|---|---|---|
| Human | Mouse | ||||
| Carnegie Stage |
Post-ovulatory Days |
Size (mm) Greatest Length |
Theiler Stage |
Post-conception Days |
Size (mm) Crown-rump |
| 11 | 24 | 2.5 - 4.5 | 14 | 9 - 9.5 | 2.1 |
| 12 | 26 | 3 - 5 | 15 | 9.5 - 10.25 | 2.5 |
| 13 | 28 | 4 - 6 | 16 | 10.25 - 10.5 | 3.6 |
| 14 | 32 | 5 - 7 | 17 | 10.5 | 4.1 |
| 15 | 33 | 7 - 9 | 18 | 11 | 4.6 |
| 16 | 37 | 8 - 11 | 19 | 11.5 | 6 - 7 |
| 17 | 41 | 11 - 14 | 20 | 12 | 7 |
| 18 | 44 | 13 - 17 | 21 | 12.5 - 13 | 8.2 |
| 19 | 47.5 | 16 - 18 | 21 | 12.5 - 13 | 8.2 |
| 20 | 50.5 | 18 - 22 | 22 | 13.5 - 14 | 9.1 |
| 21 | 52 | 22 - 24 | 22 | 13.5 - 14 | 9.1 |
| 22 | 54 | 23 - 28 | 22 | 13.5 - 14 | 9.1 |
| 23 | 56.5 | 27 - 31 | 22 | 13.5 - 14 | 9.1 |
| 26 | 17.5 - 18 | 17.8 | |||
|
Notes: Human Embryonic Development | Carnegie Stages | Mouse Development | Theiler Stage | Post-conception Days | Carnegie Stage Comparison Reference: Anderson RH. Teratogenecity in the setting of cardiac development and maldevelopment. (2016) | |||||
| Week: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Carnegie stage: | 1 2 3 4 | 5 6 | 7 8 9 | 10 11 12 13 | 14 15 | 16 17 | 18 19 | 20 21 22 23 |
- Mouse Stages: E1 | E2.5 | E3.0 | E3.5 | E4.5 | E5.0 | E5.5 | E6.0 | E7.0 | E7.5 | E8.0 | E8.5 | E9.0 | E9.5 | E10 | E10.5 | E11 | E11.5 | E12 | E12.5 | E13 | E13.5 | E14 | E14.5 | E15 | E15.5 | E16 | E16.5 | E17 | E17.5 | E18 | E18.5 | E19 | E20 | Timeline | About timed pregnancy
| Carnegie | Stage | |||||||||||||||||||||||
| Human | Days | 1 | 2-3 | 4-5 | 5-6 | 7-12 | 13-15 | 15-17 | 17-19 | 20 | 22 | 24 | 28 | 30 | 33 | 36 | 40 | 42 | 44 | 48 | 52 | 54 | 55 | 58 |
| Mouse | Days | 1 | 2 | 3 | E4.5 | E5.0 | E6.0 | E7.0 | E8.0 | E9.0 | E9.5 | E10 | E10.5 | E11 | E11.5 | E12 | E12.5 | E13 | E13.5 | E14 | E14.5 | E15 | E15.5 | E16 |
| Rat | Days | 1 | 3.5 | 4-5 | 5 | 6 | 7.5 | 8.5 | 9 | 10.5 | 11 | 11.5 | 12 | 12.5 | 13 | 13.5 | 14 | 14.5 | 15 | 15.5 | 16 | 16.5 | 17 | 17.5 |
| Note these Carnegie stages are only approximate day timings for average of embryos. Links: Carnegie Stage Comparison | ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| Timeline Links: human timeline | mouse timeline | mouse detailed timeline | chicken timeline | rat timeline | Medaka | Category:Timeline |
Table 2. Summary of Timing (Post Fertilization) of Susceptibility to a Drug-Induced Malformation
| Lesion | Start of Susceptibility to Malformation |
End of Susceptibility to Malformation | |||
|---|---|---|---|---|---|
| Interatrial communications | |||||
| Oval fossa defect | 6 weeks (E13.5) | Term | |||
| Sinus venosus defect | 8 weeks | 12 weeks | |||
| Coronary sinus defect | 8 weeks | Term | |||
| Vestibular defect | 7 weeks | 8 weeks | |||
| Ventricular Septal Defect | |||||
| Muscular | 8 weeks | Difficult to predict | |||
| Perimembranous | 6 weeks | 8 weeks | |||
| Doubly committed | 7 weeks | 8 weeks | |||
| Atrioventricular Septal Defect | |||||
| Ostium primum | 5 weeks | 6 weeks | |||
| “Complete” | 5 weeks | 6 weeks | |||
| Aortic coarctation | |||||
| With VSD | 5 weeks | 8 weeks | |||
| With intact ventricular septum | 8 weeks | Term | |||
| Double Outlet Right Ventricle | 6 weeks | 8 weeks | |||
| Transposition of Great Arteries | 6 weeks | 8 weeks | |||
| Ebstein’s malformation | 6 weeks | 8 weeks | |||
| Hypoplastic left heart syndrome | |||||
| With mitral atresia | 5 weeks | 8 weeks | |||
| With mitral stenosis | 8 weeks | Term | |||
| Pulmonary atresia | |||||
| With VSD | 6 weeks | 8 weeks | |||
| With intact ventricular septum | 8 weeks | Term | |||
| Other | |||||
| Functionally single ventricle | 5 weeks | 6 weeks | |||
| Tetralogy of Fallot | 7 weeks | 8 weeks | |||
| Totally anomalous pulmonary venous return | 8 weeks | 12 weeks | |||
| Tricuspid atresia | 5 weeks | 6 weeks | |||
| Common arterial trunk | 5 weeks | 7 weeks | |||
| Bicuspid aortic valve | 6 weeks | Term | |||
| Notes | |||||
| For approximate clinical Gestational Age GA add 2 weeks; number in brackets is mouse equivalent. Data Reference[1] | Links: heart | abnormal development | timeline | Category:Timeline | ||||
| Table 2. Summary of Timing (Post Fertilization) of Cardiac Susceptibility to a Drug-Induced Malformation | ||
|---|---|---|
| Lesion | Start of Susceptibility to Malformation |
End of Susceptibility to Malformation |
| Interatrial communications | ||
| Oval fossa defect | 6 weeks (E13.5) | Term |
| Sinus venosus defect | 8 weeks | 12 weeks |
| Coronary sinus defect | 8 weeks | Term |
| Vestibular defect | 7 weeks | 8 weeks |
| Ventricular Septal Defect | ||
| Muscular | 8 weeks | Difficult to predict |
| Perimembranous | 6 weeks | 8 weeks |
| Doubly committed | 7 weeks | 8 weeks |
| Atrioventricular Septal Defect | ||
| Ostium primum | 5 weeks | 6 weeks |
| “Complete” | 5 weeks | 6 weeks |
| Aortic coarctation | ||
| With VSD | 5 weeks | 8 weeks |
| With intact ventricular septum | 8 weeks | Term |
| Double Outlet Right Ventricle | 6 weeks | 8 weeks |
| Transposition of Great Arteries | 6 weeks | 8 weeks |
| Ebstein’s malformation | 6 weeks | 8 weeks |
| Hypoplastic left heart syndrome | ||
| With mitral atresia | 5 weeks | 8 weeks |
| With mitral stenosis | 8 weeks | Term |
| Pulmonary atresia | ||
| With VSD | 6 weeks | 8 weeks |
| With intact ventricular septum | 8 weeks | Term |
| Other | ||
| Functionally single ventricle | 5 weeks | 6 weeks |
| Tetralogy of Fallot | 7 weeks | 8 weeks |
| Totally anomalous pulmonary venous return | 8 weeks | 12 weeks |
| Tricuspid atresia | 5 weeks | 6 weeks |
| Common arterial trunk | 5 weeks | 7 weeks |
| Bicuspid aortic valve | 6 weeks | Term |
|
Notes: For approximate clinical Gestational Age GA add 2 weeks; number in brackets is mouse equivalent. Reference: Anderson RH. Teratogenecity in the setting of cardiac development and maldevelopment. (2016) | ||
Cardiac Abnormalities Identified in Text
|
|
| ICD-11 Structural developmental anomalies of the circulatory system (draft) |
|---|
| ICD-11 Beta Draft - NOT FINAL, updated on a daily basis, It is not approved by WHO, NOT TO BE USED for CODING except for agreed FIELD TRIALS.
20 Developmental Anomalies - Structural Developmental Anomalies Beta coding and tree structure for "structural developmental anomalies" within this section are shown in the table below. |
| Structural developmental anomalies of the circulatory system |
|
| CD-11 Beta Draft - NOT FINAL, updated on a daily basis, It is not approved by WHO, NOT TO BE USED for CODING except for agreed FIELD TRIALS.
|
| Heart Abnormal: Tutorial Abnormalities | atrial septal defects | double outlet right ventricle | hypoplastic left heart | patent ductus arteriosus | transposition of the great vessels | Tetralogy of Fallot | ventricular septal defects | coarctation of the aorta | Category ASD | Category PDA | Category ToF | Category VSD | ICD10 - Cardiovascular | ICD11 |
Figures
- Anderson (2016) Heart Figures
- Figure Links: 1 Heart tube mouse E8 | 2 Ventricular loop mouse E8 | 3 Heart mouse E10.5 | 4 Atrial component mouse E10.5 | 5 Sinus horns mouse E8.5 | 6 Venous valve mouse E10.5 | 7a Left atrium CS14 | 7b Atrioventricular canal CS14 | 8a Atrioventricular canal mouse E10.5 | 8b Outflow tract mouse E10.5 | 9a Atrioventricular canal CS14 | 9b Right ventricle CS14 | 10 Ventricular septal defect | fig 11a | fig 11b | fig 12a | fig 12b | fig 13a | fig 13b | fig 14 | fig 15a | fig 15b | fig 16a | fig 16b | fig 17a | fig 17b | fig 18 | fig 19 | fig 20 | fig 21 | fig 22 | fig 23 | fig 24a | fig 24b | fig 25a | fig 25b |fig 26a | fig 26b | fig 27a | fig 27b | fig 28a | fig 28b | fig 29a | fig 29b | fig 30 | fig 31 | fig 32a | fig 32b | fig 33a | fig 33b | fig 34a | fig 34b | fig 35a | fig 35b | fig 36 | fig 37 | fig 38 | fig 39a | fig 39b | fig 40a | fig 40b | fig 41a | fig 41b | fig 42a | 42b | 43a Stenotic pulmonary valve | 43b Stenotic aortic valve | fig 44a | fig 44b | fig 45a | fig 45b | fig 46a | fig 46b | fig 47 | fig 48 | fig 49 | fig 50a | fig 50b | Figure Gallery
Heart Terms
- angioblastic cords - Groups or ‘columns’ of embryonic precursor cells which will form the walls of both arteries and veins.
- apex - Anatomical term referring to the most inferior, left, downwards pointing part of the heart.
- aorta - The largest artery in the human body originating in the left ventricle. The aorta ascends, arches over the heart and then descends through the abdomen.
- aortic arch arteries - (pharyngeal arch arteries) Each early developing pharyngeal arch contains a lateral pair of arteries arising from the aortic sac, above the heart, and running into the dorsal aorta. Later in development these arch arteries are extensively remodelled to form specific components of the vascular system.
- aortic valve - Three-leaflet valve located at the junction between the left ventricle and aortic entrance.
- aortic vestibule - Smooth-walled portion of the left ventricle directly below the aortic valve.
- aorticopulmonary septum - Division between the aorta and the pulmonary trunk formed from the bulbar ridges.
- atresia - Abnormal closure or absence of a body vessel or orifice.
- atrioventricular canal - Junction between the primitive atrium and primitive ventricle in the embryo. This canal splits to later form two atrioventricular canals which consequently form the valves of the adult heart.
- bulbar ridges - Endocardial cushion tissue located in the bulbus cordis extending into the truncus arteriosus thus forming ridges. These fuse together to form the aorticopulmonary septum.
- bulbus cordis - A region of the early developing heart tube forming the common outflow tract, will differentiate to form three regions of the heart.
- cardiac jelly - Term used in early heart development to describe the initial gelatinous or sponge-like connective tissue separating the myocardium and heart tube endothelium.
- cardiogenic region - The area in the embryo where the precursor cells for heart development lie.
- chordae tendineae - Cord-like tendons connecting the papillary muscles to the leaflets of the mitral and tricuspid valves.
- congenital heart disease - Abnormal structure or function of the heart due to a developmental defect arising prior to birth.
- connective tissue - Fibrous tissue that acts to support body structures or bind other forms of tissue.
- conus arteriosus - An embryological heart outflow structure, that forms in early cardiac development and will later divides into the pulmonary artery and aorta. Term is also used clinically to describe the malformation of the cardiac outflow pattern, where only one artery arises from the heart and forms the aorta and pulmonary artery.
- coronary sinus - a venous sinus emptying into the right atrium that collects blood from the myocardium of the heart. The coronary sinus is the largest cardiac vein.
- cyanosis - Blue colouration of the skin and mucous membrane due to poor oxygenation of the blood.
- dorsal aortae - Two largest arteries either side of the midline which later fuse to form the descending portion of the aorta.
- dorsal mesocardium - The mesentery attaching the heart to the dorsal wall of the pericardial coelom. This breaks down to form a space known as the transverse pericardial sinus.
- dyspnoea - Shortness of breath.
- endocardial cushions - Swellings of migrated cells on the inner lining of the heart located in the atrioventricular canal.
- endocardial heart tubes - Two tubes formed from the cardiogenic plate in the developing embryo. These form the primordium of the truncus arteriosus, the atrium and the ventricles; later invested with myocardium.
- endocardium - The epithelial membrane lining the inside surface of heart, which along with the endothelial layer forms a continuous lining of the entire cardiovascular system. The endocardium, like the majority of the heart is mesoderm in origin.
- endothelium - A simple squamous epithelium lining blood vessels.
- epicardium - The outer layer of heart tissue.
- fibrous trigone - (trigonum fibrosum) term describing the dense connective tissue between the aortic ring and the atrioventricular ring and has a left and right component. The right fibrous trigone (trigonum fibrosum dextrum) lies between the aortic ring and the right atrioventricular ring. The left fibrous trigone (trigonum fibrosum sinistrum) lies between the aortic ring and the left atrioventricular ring.
- foramen ovale - Shunt allowing blood to enter the left atrium from the right atrium. It is located in the septum secundum.
- foramen primum - Original space between the septum primum and the fused endocardial cushions as the septum primum grows towards the cushions.
- foramen secundum - Refers to the coalesced perforations in the septum primum after it has fused with the endocardial cushions.
- fossa ovalis - (oval fossa, annulus ovalis) Region in the postnatal atrial septum where the foramen ovale was located during embryonic development. Appears as an indentation in the right atrium septum.
- heart murmur - Extra heart sounds appearing upon auscultation due to turbulent blood flow.
- hypertrophy - Increase in size of an organ or tissue due to enlargement of component cells.
- inferior vena cava - (IVC) Large vein which carries deoxygenated blood from the lower half of the body to the right atrium.
- inflow tract - Entrance of blood into the heart tube; the sinus venosus portion of the tube.
- infundibulum - Smooth-walled portion of the right ventricle directly below the pulmonary valve.
- interventricular septum - Wall of muscular tissue growing from the base of the heart dividing the primitive ventricle into the left and right ventricles.
- interventricular foramen - Space between the interventricular septum and the fused endocardial cushions. The foramen closes when the septum fuses with the endocardial cushions and bulbar ridges.
- intraembryonic coelom - Initial embryonic space in lateral plate mesoderm that will be separated to form the three major body cavities: pericardial, pleural and peritoneal cavity. The lateral plate mesoderm is divided by this space into splanchnic and somatic mesoderm.
- left horn of sinus venosus - The left side of the sinus venosus (initially symmetrical with the right) collecting blood from half of the paired veins: common cardinal veins, umbilical veins and vitelline veins. Later the left horn diminishes and becomes the small coronary sinus.
- mitral valve - (Bicuspid valve) two leaflet valve located on the left side of the heart, that is between the left atrium and ventricle.
- myocardium - The middle layer of the heart wall composed of cardiac muscle.
- neural crest mesenchyme - Connective tissue arising from critical cells in the cranial region of the embryo. These paired dorsal lateral streaks of cells migrate throughout the embryo and can differentiate into many different cell types (= pluripotential). Those that remain on the dorsal neural tube form the sensory spinal ganglia (DRG), those that migrate ventrally form the sympatheitic ganglia. Neural crest cells also migrate into the somites and regions through the entire embryo.
- outflow tract - Exit of blood from the heart tube formed by the truncus arterioles.
- oval fossa - (fossa ovalis, annulus ovalis) Region in the postnatal atrial septum where the foramen ovale was located during development. Appears as an indentation in the right atrium septum.
- papillary muscles - Small muscles found on the inner myocardium of the left and right ventricles. They are attached to the mitral and tricuspid valves via the chordae tendineae and serve to limit the movements of the valves.
- patent foramen ovale - Abnormality in atrial septum due to failure of the atrial septum to close at the foramen ovale. (More? Atrial Septal Defects)
- pericardial coelom - (pericardial cavity) The anatomical body cavity in which the heart lies. The pericardial cavity forms in the lateral plate mesoderm above the buccopharyngeal membrane, as part of the early intraembryonic coelom. This cavity is initially continuous with the two early pleural cavities. Note the single intraembryonic coelom forms all three major body cavities: pericardial cavity, pleural cavity, peritoneal cavity.
- primordial atrium - Common cavity in the upper portion of the developing heart. Later divides to form the left and right atria.
- primordial ventricle - Common cavity in the lower portion of the developing heart. Later divides to form the left and right ventricles.
- pulmonary circulation - Carries blood between the heart and lungs.
- pulmonary trunk - A vessel that arises from the right ventricle of the heart, extends upward, and divides into the right and left pulmonary arteries that transport deoxygenated blood to the lungs.
- pulmonary valve - Three-leaflet valve located at the junction between the right ventricle and the pulmonary trunk.
- pulmonary veins - Four veins that allow oxygenated blood from the lungs to empty into the left atrium.
- right horn of sinus venosus - The right side of the sinus venosus (initially symmetrical with the left) collecting blood from half of the paired veins: common cardinal veins, umbilical veins and vitelline veins. Later the right horn dilates, receiving all the veins, and becomes the sinus venarum of the right atrium.
- second heart field - (SHF) splanchnic mesoderm that forms progenitors important for heart formation.
- semilunar valves - Flaps of endocardium and connective tissue reinforced by fibres which prevent the valves from turning inside out. They are shaped like a half moon, hence the name semilunar. The semilunar valves are located between the aorta and the left ventricle and between the pulmonary artery and the right ventricle.
- septum primum - Original structure growing from the roof of the heart towards the endocardial cushions dividing the primitive atrium.
- septum secundum - Second structure growing to the right of the septum primum dividing the primitive atrium.
- sinoatrial node - Specialised cardiomyocyte pacemaking region of the heart located at the entry of the right superior caval vein (SVC) into the right atrium. (More? Detailed Cardiac - Sinus Node)
- sinus venosus - (systemic venous sinus) An early developmental cardiovascular structure, thin walled cavity, forming the input to developing heart which has 3 venous inputs (vitelline vein, umbilical vein, common cardinal vein). Later in heart development this structure gets incorporated into the wall of the future right atrium. (More? Systemic Venous Sinus)
- sinuatrial orifice - The opening between the sinus venosus and right atrium which has two valve leaflets to prevent backflow of blood.
- sinus venarum - Smooth-walled portion of the adult right atrium; originally the left horn of the sinus venous.
- splanchnic mesoderm - Gastrointestinal tract (endoderm) associated mesoderm formed by the separation of the lateral plate mesoderm into two separate components by a cavity, the intraembryonic coelom. Splanchnic mesoderm is the embryonic origin of the second heart field, gastrointestinal tract connective tissue, smooth muscle, blood vessels and contribute to organ development (heart, pancreas, spleen, liver).
- stenosis - Abnormal narrowing of a blood vessel or orifice.
- superior vena cava - (SVC) Short vein which carries deoxygenated blood from the upper half of the body to the right atrium.
- systemic circulation - Carries oxygenated blood away from the heart to the other body organs and returns to the heart deoxygenated.
- tachypnoea - Abnormally rapid breathing rate.
- trabeculae - (trabeculations)Muscular beams located within the ventricles and parts of the atria of the heart.
- transverse pericardial sinus - Dorsal area within the pericardial coelom, initially occupied by the dorsal mesocardium.
- tricuspid valve - Three leaflet valve located in the right atrioventricular canal i.e. between the right atrium and ventricle.
- tricuspid atresia - Abnormality of the tricuspid valve between the right atrium and right ventricle.
- truncus arteriosus - An embryological heart outflow structure, that forms in early cardiac development and will later divides into the pulmonary artery and aorta. Term is also used clinically to describe the malformation of the cardiac outflow pattern, where only one artery arises from the heart and forms the aorta and pulmonary artery.
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
References
Cite this page: Hill, M.A. (2026, February 27) Embryology Paper - Teratogenecity in the setting of cardiac development and maldevelopment. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Teratogenecity_in_the_setting_of_cardiac_development_and_maldevelopment
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G