Cardiovascular System - Heart Valve Development
| Embryology - 26 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
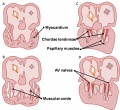
The heart valves form between the atria and ventricles (mitral valve, tricuspid valve) and between the atria and blood vessels (aortic valve, pulmonary valve). The cardiac cushions in the atrioventricular (AV) canal contain cells that are the primordia of the cardiac valves. The atrioventricular valves are attached to papillary muscles by chordae tendineae.
Scleraxis (SCX) is a transcription factor involved in tendon and ligament development and has been identified as also expressed in early heart valve development.[1]
Mitral valve also called the "bicuspid valve".
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Heart Valve Embryology <pubmed limit=5>Heart Valve Embryology</pubmed> |
Textbooks
- Human Embryology (2nd ed.) Larson Ch7 p151-188 Heart
- The Developing Human: Clinically Oriented Embryology (6th ed.) Moore and Persaud Ch14: p304-349
- Before we Are Born (5th ed.) Moore and Persaud Ch12; p241-254
- Essentials of Human Embryology Larson Ch7 p97-122 Heart
- Human Embryology Fitzgerald and Fitzgerald Ch13-17: p77-111
Tutorial Images
Fetal Heart Valve Sounds
| <html5media height="50" width="400">File:Week17 fetal heart rate.mp3</html5media>
|
The characteristic "lub-dup" sounds are associated with closing of heart valves.
|
Molecular
Scleraxis (Scx) - basic helix–loop–helix transcription factor expressed in the progenitors and cells of all tendon tissues (mouse).[7]
Periostin - regulates lineage commitment of valve precursor cells (chicken).[8]
Gata4 and Gata6
Tbx5
| Factor Links: AMH | hCG | BMP | sonic hedgehog | bHLH | HOX | FGF | FOX | Hippo | LIM | Nanog | NGF | Nodal | Notch | PAX | retinoic acid | SIX | Slit2/Robo1 | SOX | TBX | TGF-beta | VEGF | WNT | Category:Molecular |
Abnormalities
Noonan syndrome
An autosomal dominant single-gene cause of congenital heart disease. Patients also have proportionate short stature, facial abnormalities, and an increased risk of myeloproliferative disease. About half the patients have mutations in PTPN11, encoding the protein tyrosine phosphatase SHP2. A recent study in mice has identified PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation.[9]
Bicuspid Aortic Valve
GATA4 and NKX2.5 genes have been identified associated with this disorder.
A recent genetic screening study of GATA4 in 150 patients with bicuspid aortic valve (BAV)[10] identified a novel heterozygous GATA4 mutation, p.E147X, was identified in a family with BAV transmitted in an autosomal dominant pattern. The nonsense mutation was absent in 600 control chromosomes. The mutation disrupted the synergistic transcriptional activation between GATA4 and NKX2.5, another transcription factor responsible for BAV.
- GATA4 - (8p23.1) transcription factors that control gene expression and differentiation in a variety of cell types. Family of DNA-binding proteins recognize a consensus sequence known as the "GATA" motif, important cis-element in the promoters of many genes.
- NKX2-5 - (5q35.1) Homeobox-containing gene expressed only in the heart.
- Links: OMIM - GATA4 | OMIM - NKX2.5
Calcific Aortic Valve Disease
Calcification of the aortic valve shows calcific nodule formation on the aortic surface, leading to a less supple and more stiffened cusp, limiting movement and causing clinical stenosis.[4]
Pathogenic mechanism is unknown but may involve signaling pathways common to valvulogenesis and bone development:
- Transforming Growth Factor-β (TGF-β)
- bone morphogenetic protein (BMP)
- Wnt
- Notch
- Sox9
| Factor Links: AMH | hCG | BMP | sonic hedgehog | bHLH | HOX | FGF | FOX | Hippo | LIM | Nanog | NGF | Nodal | Notch | PAX | retinoic acid | SIX | Slit2/Robo1 | SOX | TBX | TGF-beta | VEGF | WNT | Category:Molecular |
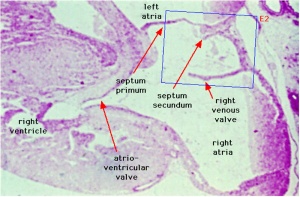
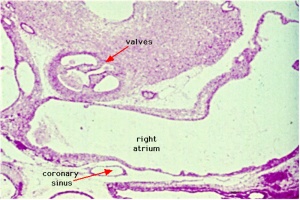
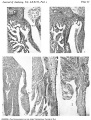
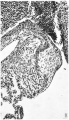
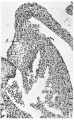
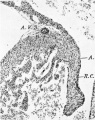
Images
Historic
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Odgers PNB. The development of the atrio-ventricular valves in man. (1939) J Anat. 73: 643-57. PMID 17104787
References
- ↑ Barnette DN, VandeKopple M, Wu Y, Willoughby DA & Lincoln J. (2014). RNA-seq analysis to identify novel roles of scleraxis during embryonic mouse heart valve remodeling. PLoS ONE , 9, e101425. PMID: 24983472 DOI.
- ↑ Chui J, Anderson RH, Lang RM & Tsang W. (2018). The Trileaflet Mitral Valve. Am. J. Cardiol. , 121, 513-519. PMID: 29304994 DOI.
- ↑ Richardson R, Eley L, Donald-Wilson C, Davis J, Curley N, Alqahtani A, Murphy L, Anderson RH, Henderson DJ & Chaudhry B. (2018). Development and maturation of the fibrous components of the arterial roots in the mouse heart. J. Anat. , 232, 554-567. PMID: 29034473 DOI.
- ↑ 4.0 4.1 Dutta P & Lincoln J. (2018). Calcific Aortic Valve Disease: a Developmental Biology Perspective. Curr Cardiol Rep , 20, 21. PMID: 29520694 DOI.
- ↑ Lee MP & Yutzey KE. (2011). Twist1 directly regulates genes that promote cell proliferation and migration in developing heart valves. PLoS ONE , 6, e29758. PMID: 22242143 DOI.
- ↑ Yalcin HC, Shekhar A, McQuinn TC & Butcher JT. (2011). Hemodynamic patterning of the avian atrioventricular valve. Dev. Dyn. , 240, 23-35. PMID: 21181939 DOI.
- ↑ Levay AK, Peacock JD, Lu Y, Koch M, Hinton RB, Kadler KE & Lincoln J. (2008). Scleraxis is required for cell lineage differentiation and extracellular matrix remodeling during murine heart valve formation in vivo. Circ. Res. , 103, 948-56. PMID: 18802027 DOI.
- ↑ Norris RA, Potts JD, Yost MJ, Junor L, Brooks T, Tan H, Hoffman S, Hart MM, Kern MJ, Damon B, Markwald RR & Goodwin RL. (2009). Periostin promotes a fibroblastic lineage pathway in atrioventricular valve progenitor cells. Dev. Dyn. , 238, 1052-63. PMID: 19334280 DOI.
- ↑ Araki T, Chan G, Newbigging S, Morikawa L, Bronson RT & Neel BG. (2009). Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation. Proc. Natl. Acad. Sci. U.S.A. , 106, 4736-41. PMID: 19251646 DOI.
- ↑ Li RG, Xu YJ, Wang J, Liu XY, Yuan F, Huang RT, Xue S, Li L, Liu H, Li YJ, Qu XK, Shi HY, Zhang M, Qiu XB & Yang YQ. (2018). GATA4 Loss-of-Function Mutation and the Congenitally Bicuspid Aortic Valve. Am. J. Cardiol. , 121, 469-474. PMID: 29325903 DOI.
Reviews
Hinton RB & Yutzey KE. (2011). Heart valve structure and function in development and disease. Annu. Rev. Physiol. , 73, 29-46. PMID: 20809794 DOI.
Markwald RR, Norris RA, Moreno-Rodriguez R & Levine RA. (2010). Developmental basis of adult cardiovascular diseases: valvular heart diseases. Ann. N. Y. Acad. Sci. , 1188, 177-83. PMID: 20201901 DOI.
Délot EC. (2003). Control of endocardial cushion and cardiac valve maturation by BMP signaling pathways. Mol. Genet. Metab. , 80, 27-35. PMID: 14567955
Barnett JV & Desgrosellier JS. (2003). Early events in valvulogenesis: a signaling perspective. Birth Defects Res. C Embryo Today , 69, 58-72. PMID: 12768658 DOI.
Articles
van den Berg G, Somi S, Buffing AA, Moorman AF & van den Hoff MJ. (2007). Patterns of expression of the Follistatin and Follistatin-like1 genes during chicken heart development: a potential role in valvulogenesis and late heart muscle cell formation. Anat Rec (Hoboken) , 290, 783-7. PMID: 17549728 DOI.
Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, Izumo S, Tabin CJ & Pu WT. (2006). Development of heart valves requires Gata4 expression in endothelial-derived cells. Development , 133, 3607-18. PMID: 16914500 DOI.
Odgers PN. (1939). The development of the atrio-ventricular valves in man. J. Anat. , 73, 643-57. PMID: 17104787
Search PubMed
Search Pubmed: heart valve development | heart valve morphogenesis | Valvulogenesis
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 26) Embryology Cardiovascular System - Heart Valve Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cardiovascular_System_-_Heart_Valve_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G