Abnormal Development - Environmental
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
Introduction
Materal effects should really be called environmental (in contrast to genetic) removing the association of mother with the deleterious agent. Accepting this caveat, there are several maternal effects from lifestyle, environment and nutrition that can be prevented or decreased by change which is not an option for genetic effects.
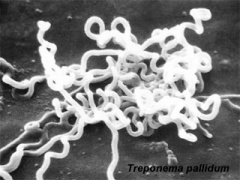
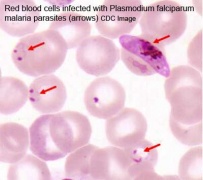
Infections, collectively grouped under the acronym TORCH for Toxoplasmosis, Other organisms (parvovirus, HIV, Epstein-Barr, herpes 6 and 8, varicella, syphilis, enterovirus) , Rubella, Cytomegalovirus and Hepatitis. See related pages on maternal hyperthermia, viral and bacterial infections.
Maternal diet the best characterised is the role of low folic acid and Neural Tube Defects (NTDs) see also abnormal neural development and Neural Tube Defects and the sample environmental effects listed below.
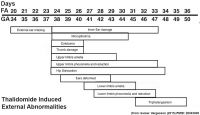
Maternal drugs effects either prescription drugs (therapeutic chemicals/agents, thalidomide limb development), non-prescription drugs (alcohol, smoking, herbal drugs), and illegal drugs (Cannabis/Marijuana, Methamphetamine/Amphetamine, Cocaine, Heroin, Lysergic Acid Diethylamide)
Environment (smoking, chemical, heavy metals) and maternal endocrine function (maternal diabetes, thyroid development) and maternal stress.
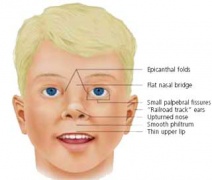
Different environmental effects can act individually or in combination on the same developing system. For example, neural development can be impacted upon by alcohol (fetal alcohol syndrome), viral infection (rubella) and/or inadequate dietry folate intake (neural tube defects). These effects may also not be seen as a direct effect on a system or systems but result in a reduced birth weight and the potential postnatal developmental effects.
Finally, when studying this topic remember the concept of "critical periods" of development that will affect the overall impact of the above listed factors. This can be extended to the potential differences between prenatal and postnatal effects, for example with infections and outcomes.
This current page provides only a general overview of the topic, use the links below to get detailed information about specific environmental effects.
| Bacterial Links: bacterial infection | syphilis | gonorrhea | tuberculosis | listeria | salmonella | TORCH | Environmental | Category:Bacteria |
| Abnormality Links: abnormal development | abnormal genetic | abnormal environmental | Unknown | teratogens | ectopic pregnancy | cardiovascular abnormalities | coelom abnormalities | endocrine abnormalities | gastrointestinal abnormalities | genital abnormalities | head abnormalities | integumentary abnormalities | musculoskeletal abnormalities | limb abnormalities | neural abnormalities | neural crest abnormalities | placenta abnormalities | renal abnormalities | respiratory abnormalities | hearing abnormalities | vision abnormalities | twinning | Developmental Origins of Health and Disease | ICD-11 | ||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Environmental Abnormal Development | teratogen |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
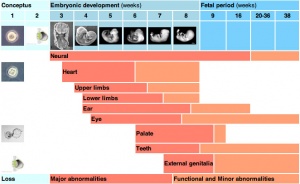
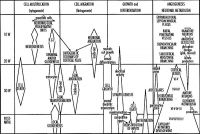
Critical Periods
The table below identifies approximate windows of time, a "critical period", that following exposure to teratogens can lead to developmental abnormalities (anomalies, congenital). In general, the effects for many system are more severe (major anomalies) in the embryonic period during organogenesis in the first trimester. Later teratogen exposure are less severe (minor anomalies) for many systems in the fetal period during continued growth and differentiation in the second and third trimester. Note that different systems have different critical periods within the developmental timeline and extend for different lengths of time.
| Critical Periods of Human Development | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conceptus | Embryonic development (weeks) | Fetal period (weeks) | ||||||||||||||||||
|
||||||||||||||||||||
| Neural | ||||||||||||||||||||
| Heart | ||||||||||||||||||||
| Upper limbs | ||||||||||||||||||||
| Lower limbs | ||||||||||||||||||||
| Ear | ||||||||||||||||||||
| Eye | ||||||||||||||||||||
| Palate | ||||||||||||||||||||
| Teeth | ||||||||||||||||||||
| External genitalia | ||||||||||||||||||||
| Loss | Major abnormalities | Functional and Minor abnormalities | ||||||||||||||||||
| ||||||||||||||||||||
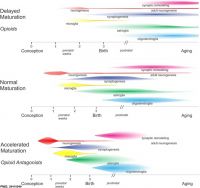
| Genital | Opioids | Neural | Thalidomide | |
|---|---|---|---|---|

|
|
|
| |
| genital abnormalities | neural abnormalities | neural abnormalities | thalidomide | |
| ||||
Scheuerle AE & Aylsworth AS. (2016). Birth defects and neonatal morbidity caused by teratogen exposure after the embryonic period. Birth Defects Res. Part A Clin. Mol. Teratol. , 106, 935-939. PMID: 27511745 DOI.
Fetal Period
A 2016 study classified teratogenic effects during the fetal period.[12]
- "Defects with documented first trimester pathogenesis (e.g., anencephaly, heterotaxy) were eliminated from consideration, as were chromosomal and single gene disorders (e.g., trisomy 21, achondroplasia). The remaining defects include the following: (1) those that are known to or could reasonably originate or manifest after the embryonic period (e.g., porencephaly, cataracts); (2) those for which pathogenesis is unclear or variable enough that exposure at any gestational age might be considered relevant (e.g., club foot, microcephaly); and (3) those that include some component of abnormal growth (e.g., hemihyperplasia). "Unspecified" defects (e.g., "abnormality of the leg") were included by default because there is insufficient information to assume first trimester embryogenesis. The final result is a list of major and minor anomalies in 11 organ system categories that may be caused by teratogen exposure during the fetal period."
DevTox
The potential of a pesticide or biocide to cause adverse effects in the developing embryo or fetus is an important consideration in any health risk assessment for humans and wildlife.
Report of the 8th Berlin Workshop on Developmental Toxicity held in May 2014.[13]
- "The main aim of the workshop was the continuing harmonization of terminology and innovations for methodologies used in the assessment of embryo- and feto-toxic findings. The following main topics were discussed: harmonized categorization of external, skeletal, visceral and materno-fetal findings into malformations, variations and grey zone anomalies, aspects of developmental anomalies in humans and laboratory animals, and innovations for new methodologies in developmental toxicology. The application of Version 2 terminology in the DevTox database was considered as a useful improvement in the categorization of developmental anomalies."
- Links: DevTox
Travel
There is an increasing number of women travelling during pregnancy that may carry some additional environmental risks. The following information is summarised from a recent BMJ article.[14]
- second trimester of pregnancy is considered the safest in which to travel
- air travel may carry risk of miscarriage, preterm birth, and thromboembolism
- obstetric and neonatal care facilities at destinations is varied
- obtain adequate insurance and check with their airline for restrictions on travel
- communicable diseases acquired abroad may increase risks of perinatal morbidity
References
- ↑ Beames TG & Lipinski RJ. (2020). Gene-environment interactions: aligning birth defects research with complex etiology. Development , 147, . PMID: 32680836 DOI.
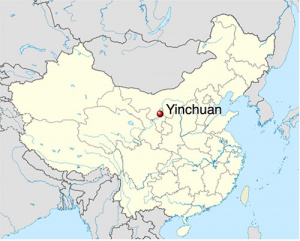
- ↑ Liu C, Li Q, Yan L, Wang H, Yu J, Tang J, Yao H, Li S, Zhang Y & Guo Y. (2019). The association between maternal exposure to ambient particulate matter of 2.5 μm or less during pregnancy and fetal congenital anomalies in Yinchuan, China: A population-based cohort study. Environ Int , 122, 316-321. PMID: 30455103 DOI.
- ↑ Jacob S, Dötsch A, Knoll S, Köhler HR, Rogall E, Stoll D, Tisler S, Huhn C, Schwartz T, Zwiener C & Triebskorn R. (2018). Does the antidiabetic drug metformin affect embryo development and the health of brown trout (Salmo trutta f. fario)?. Environ Sci Eur , 30, 48. PMID: 30595998 DOI.
- ↑ Hong Li Y & Marren A. (2018). Recurrent pregnancy loss: A summary of international evidence-based guidelines and practice. Aust J Gen Pract , 47, 432-436. PMID: 30114870
- ↑ Jenkins MM, Reefhuis J, Herring AH & Honein MA. (2017). Impact of sample collection participation on the validity of estimated measures of association in the National Birth Defects Prevention Study when assessing gene-environment interactions. Genet. Epidemiol. , 41, 834-843. PMID: 29071735 DOI.
- ↑ Wander PL, Hochner H, Sitlani CM, Enquobahrie DA, Lumley T, Lawrence GM, Burger A, Savitsky B, Manor O, Meiner V, Hesselson S, Kwok PY, Siscovick DS & Friedlander Y. (2014). Maternal genetic variation accounts in part for the associations of maternal size during pregnancy with offspring cardiometabolic risk in adulthood. PLoS ONE , 9, e91835. PMID: 24670385 DOI.
- ↑ Palmer KT, Bonzini M & Bonde JP. (2013). Pregnancy: occupational aspects of management: concise guidance. Clin Med (Lond) , 13, 75-9. PMID: 23472500
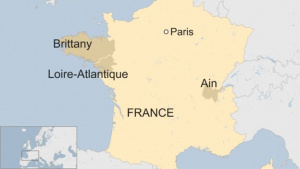
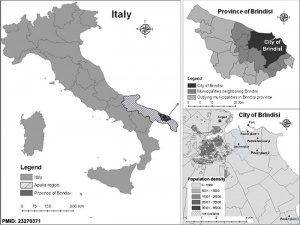
- ↑ Gianicolo EA, Bruni A, Rosati E, Sabina S, Guarino R, Padolecchia G, Leo C, Vigotti MA, Andreassi MG & Latini G. (2012). Congenital anomalies among live births in a polluted area. A ten-year retrospective study. BMC Pregnancy Childbirth , 12, 165. PMID: 23270371 DOI.
- ↑ Alexander PG & Tuan RS. (2010). Role of environmental factors in axial skeletal dysmorphogenesis. Birth Defects Res. C Embryo Today , 90, 118-32. PMID: 20544699 DOI.
- ↑ Zeller R. (2010). The temporal dynamics of vertebrate limb development, teratogenesis and evolution. Curr. Opin. Genet. Dev. , 20, 384-90. PMID: 20537528 DOI.
- ↑ West PR, Weir AM, Smith AM, Donley EL & Cezar GG. (2010). Predicting human developmental toxicity of pharmaceuticals using human embryonic stem cells and metabolomics. Toxicol. Appl. Pharmacol. , 247, 18-27. PMID: 20493898 DOI.
- ↑ Scheuerle AE & Aylsworth AS. (2016). Birth defects and neonatal morbidity caused by teratogen exposure after the embryonic period. Birth Defects Res. Part A Clin. Mol. Teratol. , 106, 935-939. PMID: 27511745 DOI.
- ↑ Solecki R, Rauch M, Gall A, Buschmann J, Clark R, Fuchs A, Kan H, Heinrich V, Kellner R, Knudsen TB, Li W, Makris SL, Ooshima Y, Paumgartten F, Piersma AH, Schönfelder G, Oelgeschläger M, Schaefer C, Shiota K, Ulbrich B, Ding X & Chahoud I. (2015). Continuing harmonization of terminology and innovations for methodologies in developmental toxicology: Report of the 8th Berlin Workshop on Developmental Toxicity, 14-16 May 2014. Reprod. Toxicol. , 57, 140-6. PMID: 26073002 DOI.
- ↑ Hezelgrave, NL, Whitty, CJM, Shennan, AH, and Chappell, LC. Advising on travel during pregnancy BMJ 2011; 342:d2506 doi: 10.1136/bmj.d2506 (Published 28 April 2011) BMJ
Journals
- Environmental Health Perspectives (EHP) is a monthly journal of peer-reviewed research and news on the impact of the environment on human health. EHP | Pubmed EHP
Reviews
Leeper C & Lutzkanin A. (2018). Infections During Pregnancy. Prim. Care , 45, 567-586. PMID: 30115342 DOI.
Arora N, Sadovsky Y, Dermody TS & Coyne CB. (2017). Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe , 21, 561-567. PMID: 28494237 DOI.
Sadler TW. (2017). Establishing the Embryonic Axes: Prime Time for Teratogenic Insults. J Cardiovasc Dev Dis , 4, . PMID: 29367544 DOI.
Foster WG, Evans JA, Little J, Arbour L, Moore A, Sauve R, Andrés León J & Luo W. (2017). Human exposure to environmental contaminants and congenital anomalies: a critical review. Crit. Rev. Toxicol. , 47, 59-84. PMID: 27685638 DOI.
Brent RL. (2004). Environmental causes of human congenital malformations: the pediatrician's role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics , 113, 957-68. PMID: 15060188
Articles
Search Pubmed
June 2010 "teratogens" All (25401) Review (3026) Free Full Text (3991) "TORCH Infections" All (183) Review (37) Free Full Text (18)
Search Pubmed: teratogens | TORCH Infections | maternal abnormalities
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Environmental Health Perspectives (EHP) is a monthly journal of peer-reviewed research and news on the impact of the environment on human health. EHP
- REPROTOX - contains summaries on the effects of medications, chemicals, infections, and physical agents on pregnancy, reproduction, and development.
- DevTox
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Abnormal Development - Environmental. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Environmental
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G