Abnormal Development - Rubella Virus
| Embryology - 27 Jun 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
| ICD-11 |
|---|
| KA62.8 Congenital rubella syndrome - A disease caused by an infection with the rubella virus in utero. This disease presents with symptoms depending on the timing of infection of the fetus and may present with birth defects (such as hearing loss), or intrauterine growth retardation. Transmission is by vertical transmission. Confirmation is by identification of rubella virus or detection of anti-rubella virus IgM antibodies in the neonate or infant.
|
Introduction
Rubella virus (Latin, rubella = little red), also known as "German Measles" (due to early citation in German medical literature), infection during pregnancy can cause congenital rubella syndrome (CRS) with serious malformations of the developing fetus. The type and degree of abnormality relates to the time of maternal infection.
Rubella peaked in 1964 and 1965, when 12.5 million cases were reported (USA). As a result, 20,000 babies were born with birth defects, 6,200 babies were stillborn, and an estimated 5,000 births were aborted, both naturally and assisted. At that time no treatment by vaccination existed and this only became available in 1969. The disease was dangerous because in children it was almost unnoticable and pregnant women often did not know that they had been exposed. Initial vaccination strategies varied between countries, in the United States infants were first to be vaccinated, while in the United Kingdom adolescent girls were first to be vaccinated.
Pregnancy effects of measles results in a higher risk of premature labor, spontaneous abortion, low-birth-weight, and possibly rare cases of birth defects with no definable pattern of malformation.[1]
Children infected with rubella before birth (a condition known as congenital rubella) are at risk for the following: growth retardation; malformations of the heart, eyes, or brain; deafness; and liver, spleen, and bone marrow problems.
The complete genomic sequence of Rubella is now known.[2] Rubella is a 9755 bp single stranded RNA positive-strand virus with no DNA stage (Togaviridae; Rubivirus) encoding nonstructural protein, capsid protein, glycoproteins E1 and E2. (More? Rubella Genome)
ICD-11 KA72.8 Congenital rubella syndrome.
Tinycc Rubella Virus page - http://tiny.cc/Rubella_Virus
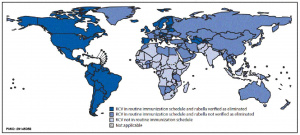
Some Recent Findings
|
| More recent papers | |
|---|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Abnormal Development Rubella Virus |
Congenital Rubella Syndrome |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Virus Structure
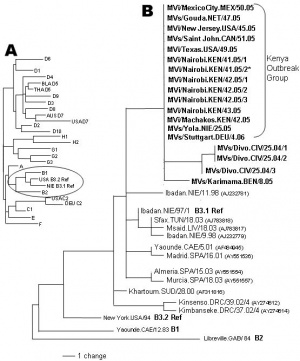
Lineage: Viruses; ssRNA viruses; ssRNA negative-strand viruses; Mononegavirales; Paramyxoviridae; Paramyxovirinae; Morbillivirus; Measles virus
- ssRNA; linear; Length: 15,894 nt Measles virus, complete genome
- virus replication involves a viral RNA-dependent RNA polymerase (vRdRp), using as a template a nucleocapsid (NC) made of a single strand of RNA in tight complex with the nucleoprotein (N).[11]
- negative-strand genome contains six transcription units encoding the N, phospho (P), matrix (M), fusion (F), hemagglutinin (H), and large (L) or polymerase protein.
- each N protein binds to 6 nucleotides.
- the N polymer entirely covers the 15,894-nucleotide genome.
- 23 known measles genotypes.
|
|
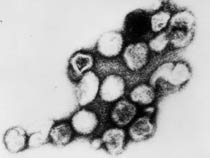
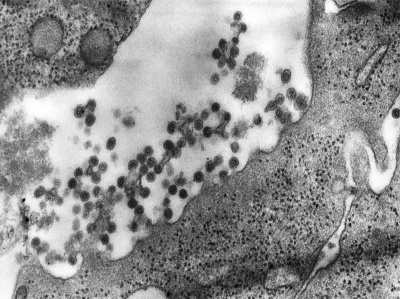
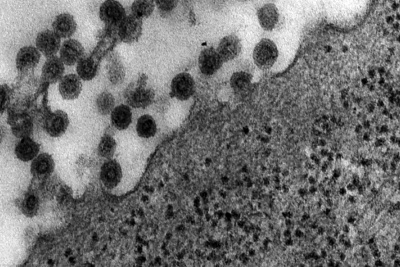
| This transmission electron micrograph (TEM) shows the presence of Rubella virus virions, as they were in the process of budding from the host cell surface to be freed into the host’s system. Inside the capsid lies the Rubella virus’ positive-sense single-stranded RNA ((+)ssRNA) genome. (Image: CDC) | The spherical virions' icosahedral capsid is enclosed in the host cell membrane, budding and producing an enveloped virus particle. (Image: CDC) |
Model of cell virus RNA accumulation
The following 5 -step model has been described for cell virus accumulation following hours post-infection (hpi)[11]
- 0 to ~5 hpi - incoming viral RNA-dependent RNA polymerase (vRdRp) initiated primary transcription from every gene with no detectable lag phase.
- ~5 to ~12 hpi - mRNA accumulates exponentially.
- ~12 to ~24 hpi - mRNAs, genomes, and antigenomes accumulate exponentially because of the increase of both newly available template and vRdRp.
- ~24 to ~30 hpi - genomes and antigenomes continue to accumulate exponentially at the same rate, whereas the accumulation of the transcripts slows down.
- 30+ hpi - genome and antigenome accumulation slows down, and the cell content in viral transcripts tends to decrease.
Vaccination
Japan - first introduced to Japan in 1966 and adopted in the national regular immunization program from 1978.
WHO Rubella Information
- Causative agent - Virus
- Reservoir - Humans
- Spread - Close respiratory contact and aerosolized droplets
- Transmission period - A few days before to seven days after rash; up to one year of age in congenitally infected
- Subclinical infection - Common
- Duration of natural immunity - Lifelong
- Risk factors for infection (for unvaccinated individuals) - Highly transmissible; crowding; low socioeconomic status
- Case-fatality rate - Less than 0.1 percent (dependent on care)
- Vaccine (number of doses); route - Rubella (one or two); subcutaneous
- Vaccine efficacy - 95 percent (at 12 months and up)
- Duration of immunity after primary series - Lifelong in most; presumed rare cases of waning immunity after one dose, not two
- Schedule - First dose at 12 to 15 months; when given, a second dose with measles vaccine
- Status as of the end of 2001 - 110 countries in 2003
- Comments - Lower efficacy when maternal antibody present
The World Health Organization recommends that the combination measles-rubella or measles-mumps-rubella vaccines be introduced only after careful evaluation of public health priorities within each country and following the establishment of an adequate program for measles control as demonstrated by high coverage rates as part of a well-functioning childhood immunization program.
Sources: WHO 2002, 2004.
Rubella History
1941
Norman Gregg (1892-1966) was a Sydney ophthalmologist who in 1941[12] identified the link between maternal rubella infection and developmental abnormalities (atypical congenital cataracts, congenital heart defects, infants small-for-gestational age) initially in his own practice. This had coincided with a rubella epidemic that occurred between 1940 to 1941.
An example of the types of congenital defects following first trimester infection was also described later by Swan (1944).[13]
- Links: Norman Gregg
1962
The rubella virus was initially isolated [14][15]
1969
United States first licensed live, attenuated rubella vaccines introduced.
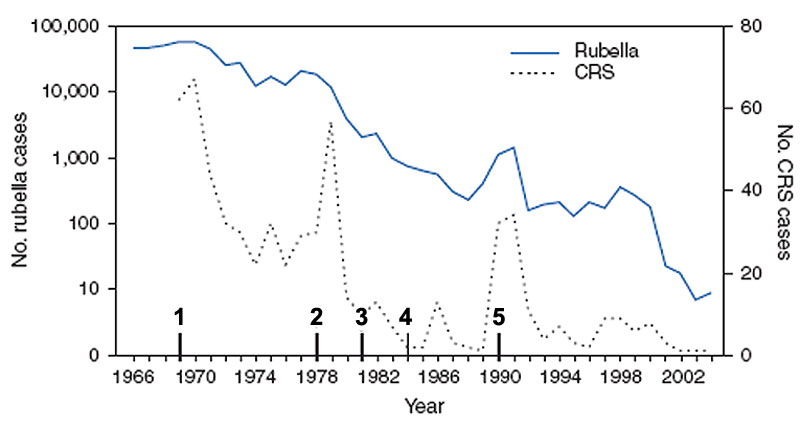
United States Elimination of rubella and congenital rubella syndrome, 1969-2004[16]
- 1969 - First official recommendations are published for the use of rubella vaccine. Vaccination is recommended for children aged 1 year to puberty.
- 1978 - Recommendations for vaccination are expanded to include adolescents and certain adults, particularly females.
- 1981 - Recommendations place increased emphasis on vaccination of susceptible persons in training and educational settings.
- 1984 - Recommendations are published for vaccination of workers in daycare centres, schools, colleges, companies, government offices, and industrial sites. Providers encouraged to conduct prenatal testing and postpartum vaccination of susceptible women. Recommendations for vaccination are expanded to include susceptible persons who travel abroad.
- 1990 - Recommendations include implementation of a new 2-dose schedule for measles-mumps-rubella vaccine.
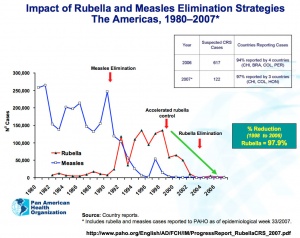
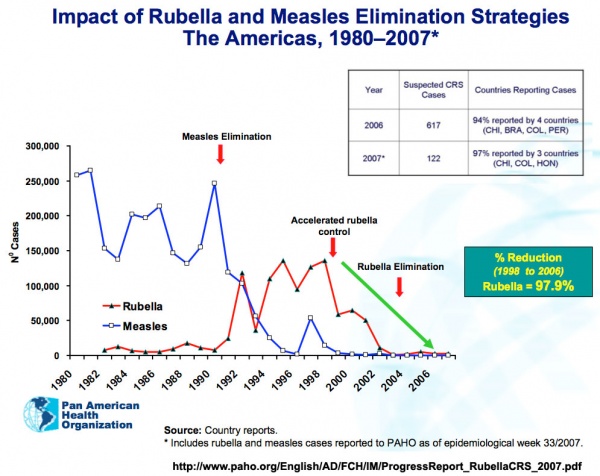
Rubella and measles elimination in the Americas[17]
2014
In 2014 a Philippines measles outbreak of over 50,000 cases occurred. Travellers to and from the Philippines during this period led to an increase in cases occurring in other countries. For example, the USA experienced the highest number of measles cases CDC had reported in 20 years, over 600, many of the people who got measles last year were linked to travelers who had gotten measles from the Philippines.
- Links: CDC 2014 travel advisory | NSW Health
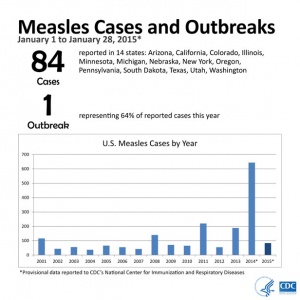
2015
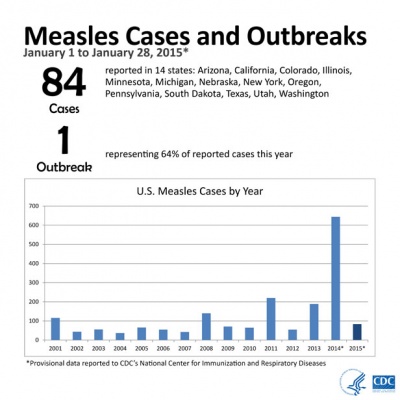
From January 1 to January 28, 2015, 84 people from 14 states were reported to have measles. Most of these cases are part of a large, ongoing outbreak linked to an amusement park in California. On January 23, 2015, CDC issued a Health Advisory to notify public health departments and healthcare facilities about this multi-state outbreak and to provide guidance for healthcare providers nationwide. For more information see CDC Press Briefing Transcript: Measles in the United States, 2015, January 29, 2015.
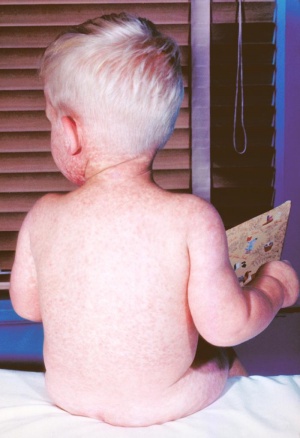
Congenital Rubella Syndrome
The following are some examples of developmental abnormalities associated with Congenital Rubella Syndrome (CRS).
| ICD-11 Beta - KA72.8 Congenital rubella syndrome | |
|---|---|
| Description
A disease caused by an infection with the rubella virus in utero. This disease presents with symptoms depending on the timing of infection of the fetus and may present with birth defects (such as hearing loss), or intrauterine growth retardation. Transmission is by vertical transmission. Confirmation is by identification of rubella virus or detection of anti-rubella virus IgM antibodies in the neonate or infant. | |
| Additional Information
Congenital rubella syndrome (CRS) is a group of anomalies that an infant may present as a result of maternal infection and subsequent foetal infection with rubella virus. In France, the prevalence at birth of CRS has decreased markedly to less than 1 case per 100,000 live births in 2002. The main defects caused by congenital rubella infection are sensorineural deafness (alteration of brainstem auditory evoked potentials) that may progress after birth, eye defects (such as cataract), cardiovascular defects, brain damage (occurring only after infection between the 3rd and 16th week of gestation) that may cause mild to severe intellectual deficit, microcephaly and spastic diplegia, and prematurity and low birth weight. Major structural malformations are rare. The prenatal diagnosis of foetal infection must be done in case of contact of the pregnant woman with an infected patient, with or without eruptive disease. If the woman was not vaccinated recently, a primary rubella infection is certain in case of:
In case of positive prenatal diagnosis of primary infection, the full congenital rubella syndrome is common when maternal infection occurs during the first two months of pregnancy. Up to 12 weeks gestation, about 80% of exposed foetuses are affected, and between 12 and 16 weeks, about half of exposed foetuses are affected. During this latter period of gestation, deafness is the most common abnormality encountered; the other defects and growth impairments appear to occur only after exposure during the first trimester. Congenital anomalies and growth impairment are rare with infection after 16 weeks gestation. | |
| ICD-10 : P35.0 | |
|
Vision
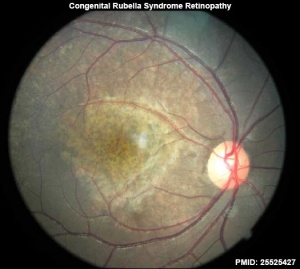
- retinopathy
- cataracts
- micropthalmia
- glaucoma
- retinitis
- Links: Vision Abnormalities | Vision Development
Hearing
- sensorineural deafness
- Links: Hearing Abnormalities | Hearing Development
Neural
- mental retardation
- meningoencephalitis
- (rare) progressive rubella panencephalitis
- microcephaly
Cardiovascular
- patent ductus arteriosis
- atrial septal defect
- ventricular septal defect
- peripheral pulmonic stenosis
Endocrine
- insulin dependent diabetes mellitus
- thyroiditis
Other Systems
- general growth retardation
- radiolucent bone disease
- heptosplenomegaly
- heamatologic abnormalities (thrombocytopenia, purpura)
- pneumonitis
References
- ↑ Chiba ME, Saito M, Suzuki N, Honda Y & Yaegashi N. (2003). Measles infection in pregnancy. J. Infect. , 47, 40-4. PMID: 12850161
- ↑ Dominguez G, Wang CY & Frey TK. (1990). Sequence of the genome RNA of rubella virus: evidence for genetic rearrangement during togavirus evolution. Virology , 177, 225-38. PMID: 2353453
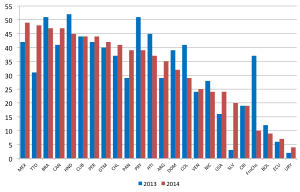
- ↑ 3.0 3.1 Grant GB, Reef SE, Patel M, Knapp JK & Dabbagh A. (2017). Progress in Rubella and Congenital Rubella Syndrome Control and Elimination - Worldwide, 2000-2016. MMWR Morb. Mortal. Wkly. Rep. , 66, 1256-1260. PMID: 29145358 DOI.
- ↑ Nóbrega YKM, de Carvalho BC, Nitz N, Vital TE, Leite FB, Sequeira IJ, Moreira EE, de Andrade JKB, Gandolfi L, Pratesi R & Hecht MM. (2017). Rubella Seropositivity in Pregnant Women After Vaccination Campaign in Brazil's Federal District. Viral Immunol. , 30, 675-677. PMID: 28972455 DOI.
- ↑ Lambert N, Strebel P, Orenstein W, Icenogle J & Poland GA. (2015). Rubella. Lancet , 385, 2297-307. PMID: 25576992 DOI.
- ↑ Centers for Disease Control and Prevention (CDC). (2013). Rubella and congenital rubella syndrome control and elimination - global progress, 2000-2012. MMWR Morb. Mortal. Wkly. Rep. , 62, 983-6. PMID: 24304830
- ↑ Barrabeig I, Torner N, Martínez A, Carmona G, Ciruela P, Batalla J, Costa J, Hernández S, Salleras L & Domínguez A. (2013). Results of the rubella elimination program in Catalonia (Spain), 2002-2011. Hum Vaccin Immunother , 9, 642-8. PMID: 23299566
- ↑ . (2010). Controlling rubella and preventing congenital rubella syndrome – global progress, 2009. Wkly. Epidemiol. Rec. , 85, 413-8. PMID: 20949700
- ↑ Oster ME, Riehle-Colarusso T & Correa A. (2010). An update on cardiovascular malformations in congenital rubella syndrome. Birth Defects Res. Part A Clin. Mol. Teratol. , 88, 1-8. PMID: 19697432 DOI.
- ↑ Rota J, Lowe L, Rota P, Bellini W, Redd S, Dayan G, van Binnendijk R, Hahné S, Tipples G, Macey J, Espinoza R, Posey D, Plummer A, Bateman J, Gudiño J, Cruz-Ramirez E, Lopez-Martinez I, Anaya-Lopez L, Holy Akwar T, Giffin S, Carrión V, de Filippis AM, Vicari A, Tan C, Wolf B, Wytovich K, Borus P, Mbugua F, Chege P, Kombich J, Akoua-Koffi C, Smit S, Bukenya H, Bwogi J, Baliraine FN, Kremer J, Muller C & Santibanez S. (2006). Identical genotype B3 sequences from measles patients in 4 countries, 2005. Emerging Infect. Dis. , 12, 1779-81. PMID: 17283637 DOI.
- ↑ 11.0 11.1 Plumet S, Duprex WP & Gerlier D. (2005). Dynamics of viral RNA synthesis during measles virus infection. J. Virol. , 79, 6900-8. PMID: 15890929 DOI.
- ↑ Gregg NM. Congenital cataract following German measles in the mother. (1941) Trans Ophthalmol Soc Aust. 3: 35–46. PubMed 1879476
- ↑ Swan C. A study of three infants dying from congenital defects following maternal rubella in the early stages of pregnancy. (1944) J. Pathol. Bact. 41(3): 289-295.
- ↑ PARKMAN PD, BUESCHER EL & ARTENSTEIN MS. (1962). Recovery of rubella virus from army recruits. Proc. Soc. Exp. Biol. Med. , 111, 225-30. PMID: 13941530
- ↑ WELLER TH, ALFORD CA & NEVA FA. (1964). RETROSPECTIVE DIAGNOSIS BY SEROLOGIC MEANS OF CONGENITALLY ACQUIRED RUBELLA INFECTIONS. N. Engl. J. Med. , 270, 1039-41. PMID: 14122801 DOI.
- ↑ Centers for Disease Control and Prevention (CDC). (2005). Elimination of rubella and congenital rubella syndrome--United States, 1969-2004. MMWR Morb. Mortal. Wkly. Rep. , 54, 279-82. PMID: 15788995
- ↑ Progress Report: Elimination of Rubella and CRS in the Americas, 2007 Powerpoint slide on Elimination of Rubella and Congenital Rubella Syndrome in the Americas: Progress Report. Pan American Health Organization World Health Organization.
- ↑ Jivraj I, Rudnisky CJ, Tambe E, Tipple G & Tennant MT. (2014). Identification of ocular and auditory manifestations of congenital rubella syndrome in mbingo. Int J Telemed Appl , 2014, 981312. PMID: 25525427 DOI.
Textbooks
- Medical Microbiology. 4th edition. Baron S, editor. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 55 Togaviruses: Rubella Virus | Chapter 54Alphaviruses (Togaviridae) and Flaviviruses (Flaviviridae) | Table 55-1 Abnormalities Associated with Congenital Rubella Syndrome | Figure 55-3 Incidence rates of rubella USA 1966-1993
- Molecular Biology of the Cell. 4th edition. Alberts B, Johnson A, Lewis J, et al. New York: Garland Science; 2002. Viruses Exploit Host Cell Machinery for All Aspects of Their Multiplication
- Disease Control Priorities in Developing Countries. 2nd edition. Jamison DT, Breman JG, Measham AR, et al., editors. Washington (DC): World Bank; 2006. Chapter 20Vaccine-preventable Diseases
- Antenatal Care: Routine care for the healthy pregnant woman. NICE Clinical Guidelines, No. 62. National Collaborating Centre for Women's and Children's Health (UK). London: RCOG Press; 2008 Mar. 10.8. Rubella
Reviews
George S, Viswanathan R & Sapkal GN. (2019). Molecular aspects of the teratogenesis of rubella virus. Biol. Res. , 52, 47. PMID: 31455418 DOI.
De Santis M, Cavaliere AF, Straface G & Caruso A. (2006). Rubella infection in pregnancy. Reprod. Toxicol. , 21, 390-8. PMID: 16580940 DOI.
Terada K. (2003). Rubella and congenital rubella syndrome in Japan: epidemiological problems. Jpn. J. Infect. Dis. , 56, 81-7. PMID: 12944671
Articles
Ye Z, Wang L, Yang T, Chen L, Wang T, Chen L, Zhao L, Zhang S, Zheng Z, Luo L & Qin J. (2019). Maternal Viral Infection and Risk of Fetal Congenital Heart Diseases: A Meta-Analysis of Observational Studies. J Am Heart Assoc , 8, e011264. PMID: 30995883 DOI.
Feng Y, Santibanez S, Appleton H, Lu Y & Jin L. (2011). Application of new assays for rapid confirmation and genotyping of isolates of rubella virus. J. Med. Virol. , 83, 170-7. PMID: 21108356 DOI.
Tipples G & Hiebert J. (2011). Detection of measles, mumps, and rubella viruses. Methods Mol. Biol. , 665, 183-93. PMID: 21116802 DOI.
Binnicker MJ, Jespersen DJ & Harring JA. (2010). Multiplex detection of IgM and IgG class antibodies to Toxoplasma gondii, rubella virus, and cytomegalovirus using a novel multiplex flow immunoassay. Clin. Vaccine Immunol. , 17, 1734-8. PMID: 20861325 DOI.
Fontana J, López-Iglesias C, Tzeng WP, Frey TK, Fernández JJ & Risco C. (2010). Three-dimensional structure of Rubella virus factories. Virology , 405, 579-91. PMID: 20655079 DOI.
Plotkin SA. (2006). The history of rubella and rubella vaccination leading to elimination. Clin. Infect. Dis. , 43 Suppl 3, S164-8. PMID: 16998777 DOI.
CORDES FC & BARBER A. (1946). Changes in lens of embryo after rubella; microscopic examination of eight week old embryo. Arch Ophthal , 36, 135-40. PMID: 20997667 DOI.
Search Pubmed
Search Pubmed: Rubella Virus | Congenital Rubella Syndrome | Congenital Rubella Infection
Historic
Pirrie GD. Rubella in pregnancy and congenital defects. (1947) Br Med J. 17;1(4506): 694. PMID 20343527
| Environmental Links: Introduction | low folic acid | iodine deficiency | Nutrition | Drugs | Australian Drug Categories | USA Drug Categories | thalidomide | herbal drugs | Illegal Drugs | smoking | Fetal Alcohol Syndrome | TORCH | viral infection | bacterial infection | fungal infection | zoonotic infection | toxoplasmosis | Malaria | maternal diabetes | maternal hypertension | maternal hyperthermia | Maternal Inflammation | Maternal Obesity | hypoxia | biological toxins | chemicals | heavy metals | air pollution | radiation | Prenatal Diagnosis | Neonatal Diagnosis | International Classification of Diseases | Fetal Origins Hypothesis |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- The Australian Immunisation Handbook 10th edition (updated June 2015) – Rubella http://www.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10-home~handbook10part4~handbook10-4-18
- World Health Organization (WHO)
- WHO position paper on rubella vaccines (2011). Weekly Epidemiological Record, No. 29, 2011, 86, 301–316 http://www.who.int/wer/2011/wer8629.pdf Webpage
- WHO-recommended surveillance standard of rubella and congenital rubella syndrome | PDF
- [http://www.euro.who.int/en/what-we-do/health-topics/communicable-diseases/me
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, June 27) Embryology Abnormal Development - Rubella Virus. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Rubella_Virus
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G