Abnormal Development - Biological Toxins
Introduction
There are a variety of toxins produced by organisms that may impact upon development. Several types of bacteria also produce toxins (More? Bacterial Infection).
Some biological compounds are also used in research and the specific effects of each compound/chemical is detailed in a Safety and Data Sheet (SDS). These sheets are now generally required to be supplied along with the compound purchased from a supplier and give a standardised description of the chemical, its physical properties, handling and health effects/toxicity. The information relating to a compound safety is continuously changing and the most current source of Safety Data Sheet (SDS) should be used for accurate information. (More? Chemicals)
There are also several internet sites that have searchable databases of SDSs information and also that handling chemical saftey may vary from country to country. Note that this information used to be called a Material Safety and Data Sheet (MSDS).
In addition, there is much information about chemicals in relation to food safety and poisoning.
Some Recent Findings
|
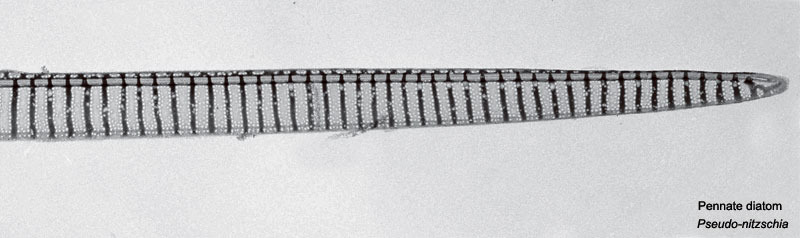
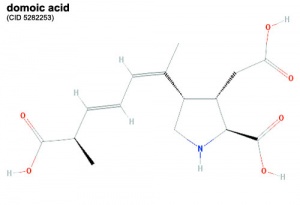
Domoic Acid
A single toxin example and study is show below, text has been modified from the rat development study.[2]
A marine toxin domoic acid (DA) is produced by the cosmopolitan diatom species Pseudonitzchia and known to form harmful algal blooms. The toxin has been shown to affect numerous organisms in the wild through trophic transfer including: sea birds, manatees, dolphins, sea lions, as well as humans. In human causes the illness amnesic shellfish poisoning.
- acts as excitotoxin that binds to kainate subtypes of ionotropic glutamate receptors as a high affinity partial agonist that prevents normal channel inactivation.
- behavioral effects of DA exposure, such as scratching, ataxia, tremors, and seizures.
- identified as potential cause of cause fatal loss in stranded pregnant sea lions.
- single injection into maternal rat led to detectable levels in amniotic fluid and embryonic brain tissue within 1 hour.
Shiga Toxin
Shiga toxin and Shiga-like toxins are protein toxins that binds to the cell surface and another activity that after entry into the cytosol inhibits protein synthesis enzymatically.[3]
- toxins can also cause apoptosis.
- toxins secreted by Shigella dysenteriae , some strains of Escherichia coli and other bacteria during infections.
- Produced by Escherichia coli (STEC) O104:H4 can produce haemolytic uraemic syndrome (HUS).
- haemolytic uraemia syndrome is most common in children.
- most common cause of acute kidney failure in children.
- Links: bacteria | MedlinePlus - HUS | PMID 21871215 | PMID 21749817
Alpha-toxin
The bacteria Staphylococcus aureus (S. aureus) produces alpha-toxin that induces cell death in a caspase-independent, necrotic-like manner. The process of necrosis (Greek, nekros = corpse) is a pathological cell death from extrinsic injury (tissue damage) and is irreversible. Necrosis is induced by tumor necrosis factor, double-stranded RNA, viral infection or bacterial infection toxins.
In the early stages, cell and organelles (mitochondria) swell (oncosis) (Greek, onkos = 'swelling') previously described as a separate form of cell death. This is due to disruption of plasma membrane leading to cell contents leak out leading to inflammation and necrosis.
In the late stages, there is a loss of cell membrane integrity, finally cell disintegration. This cell lysis can also trigger an inflammatory response, leading to further inflammation and damage, and triggering a cycle of death
Verotoxin
Produced by Escherichia coli O157 (VTEC O157).
- Links: PMID 21794221
Chemical Terms
Below are listed some terms which relate to a chemicals harmful effects.
Acute Toxicity
Adverse effects occurring following oral or dermal administration of a single dose of a substance, or multiple doses given within 24 hours, or an inhalation exposure of 4 hours. This is further classified by five toxicity categories based upon exposure route.
Carcinogen
A chemical known or believed to cause cancer in humans. The number of known carcinogens is comparatively small, but many more chemicals are suspected to be carcinogenic.
Effective Dose
(ED50) The amount of material required to produce a specified effect in 50% of an animal population. (See qualification in the definition of LD50).
Lethal Dose
(LD50) The dose of a chemical which kills 50% of a sample population. In full reporting, the dose, treatment and observation period should be given. Further, LD50 and ED50 values are strictly only comparable when the age, sex and nutritional state of the animals is specified. Nevertheless, LD50 values are widely reported as a measure of the potential toxicity of chemicals.
Material Safety Data Sheet
(MSDS) A defined set of information about a specific chemical's properties, risks, hazards and toxicity. This term in Australia is being replaced by Safety Data Sheet (SDS), under the United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS) program to standardise chemical data around the world.
Mutagen
An agent that changes the hereditary genetic material which is a part of every living cell. Such a mutation is probably an early step in the sequence of events that ultimately leads to the development of cancer.
Reproductive Toxicity
Adverse effects on sexual function and fertility in adult males and females, as well as developmental toxicity in the offspring.
Risk
The probability that a hazard will give rise to an adverse effect at a level in a specified period and is normally indicated in descriptive terms; high, modest, negligible. A hazard is the potential for physical harm to life, health or property.
Safety Data Sheet
(SDS) Under the United Nations "Globally Harmonized System of Classification and Labelling of Chemicals" (GHS) program to standardise chemical data around the world.
Threshold Limit Value
(TLV) The maximum permissible concentration of a material, generally expressed in parts per million in air for some defined period of time (often 8 hours). These values, which may differ from country to country, are often backed up by regulation and are therefore often legally enforceable.
United Nations - Globally Harmonized System of Classification and Labelling of Chemicals
(GHS) The new system, which was called "Globally Harmonized System of Classification and Labelling of Chemicals (GHS)", addresses classification of chemicals by types of hazard and proposes harmonized hazard communication elements, including labels and safety data sheets. It aims at ensuring that information on physical hazards and toxicity from chemicals be available in order to enhance the protection of human health and the environment during the handling, transport and use of these chemicals. The GHS also provides a basis for harmonization of rules and regulations on chemicals at national, regional and worldwide level, an important factor also for trade facilitation.
(Text from UN Website)
Center for the Evaluation of Risks to Human Reproduction
The National Toxicology Program (NTP) Center for the Evaluation of Risks to Human Reproduction (CERHR) was established in 1998 to serve as an environmental health resource to the public and regulatory and health agencies. CERHR publishes monographs that assess the evidence that environmental chemicals, physical substances, or mixtures (collectively referred to as "substances") cause adverse effects on reproduction and development and provide opinion on whether these substances are hazardous for humans.
Australia
Therapeutic Goods Administration
Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP) - produced by the National Health and Medical Research Council (NHMRC) is the basis for State and Territory Poisons Act legislation (which may differ between regions in detail). The legislation applies restrictions at the point of sale. There are nine Poisons Schedules listing substances or types of substances which require certain labelling and description, packaging (inner and outer), controls on advertising and supply, storage, and for some, the permitted level of impurities.
Revised medicines and chemicals scheduling arrangements (23 Nov 2010)
- the National Drugs and Poisons Schedule Committee (NDPSC) will be replaced by the Secretary of the Department of Health and Ageing (DoHA) - or her delegate - as the decision maker for the scheduling of medicines and chemicals.
- two new expert advisory committees, the Advisory Committee on Medicines Scheduling and the Advisory Committee on Chemicals Scheduling, will be established to provide advice and make recommendations to the Secretary (or delegate) on medicines and chemicals scheduling decisions.
- a single Secretariat, supporting both Advisory Committees, will ensure ongoing consistency and cohesiveness of processes and decisions.
- closer integration of the revised scheduling arrangements with existing Commonwealth evaluation and product registration schemes.
- Links: Poisons Standard 2010 | TGA - About The Poisons Standard | Therapeutic Goods Administration | Revised Scheduling Nov 2010
Safe Work Australia
An independent statutory agency responsible to improve occupational health and safety and workers' compensation arrangements across Australia.
- Links: [Work Australia | Hazardous Substances Information System | PDF - Approved Criteria for Classifying Hazardous Substances [NOHSC: 1008 (2004)
Food Standards Australia
This government body's responsibility is to develop and administer the Australia New Zealand Food Standards Code (the Code), which lists requirements for foods such as additives, food safety, labelling and GM foods. Enforcement and interpretation of the Code is the responsibility of State/Territory departments and food agencies within Australia and New Zealand.
National Measurement Institute
National Measurement Institute (NMI) is Australia's peak measurement body responsible for biological, chemical, legal, physical and trade measurement.
National Research Centre for Environmental Toxicology (Queensland)
(EnTox) This centre is a Joint Venture of the University of Queensland and the Queensland Department of Health.
- Links: Food Standards Australia | Food Standards Code | Standard 1.4.1 - Contaminants and Natural Toxicants PDF | Standard 1.4.2 Maximum Residue Limits (Australia Only) | National Measurement Institute | Persistent Organic Pollutants | EnTox
Poison Control Centres
Generally throughout the world at national, state and hospital levels are centres available to provide information on poisons. These centres advise on, or assists with, the prevention, diagnosis and management of poisoning. The structure and function of poisons centres varies around the world. At a minimum a poisons centre is an information service. Some poisons centres may also include a toxicology laboratory and/or a clinical treatment unit.
Australian Poison Control Centres
Australians should initially telephone 13 11 26 and they will be directed to their local Poisons Information Centre.
- Links: WHO - International Programme on Chemical Safety | WHO - World directory of poisons centres | IPCS - Chemicals in food | IPCS directory of poison centres - Western Pacific Region
Toxicogenomics
This term is a combination of toxicology associated with chemicals and the effects on our genome. It is thought that molecular-based approaches, such as transcriptomics, proteomics and metabolomics for studying the impact of chemicals on human and wildlife populations will have an important role in hazard and risk assessment. There have subsequently been several Organisation for Economic Co-operation and Development (OECD) and the International Programme on Chemical Safety (IPCS) co-operatively organised workshops to explore the potential regulatory applications of toxicogenomics.
References
- ↑ Oluwafemi F & Ibeh IN. (2011). Microbial contamination of seven major weaning foods in Nigeria. J Health Popul Nutr , 29, 415-9. PMID: 21957681
- ↑ Maucher JM & Ramsdell JS. (2007). Maternal-fetal transfer of domoic acid in rats at two gestational time points. Environ. Health Perspect. , 115, 1743-6. PMID: 18087593 DOI.
- ↑ Sandvig K. (2001). Shiga toxins. Toxicon , 39, 1629-35. PMID: 11595626
Reviews
Wigle DT, Arbuckle TE, Turner MC, Bérubé A, Yang Q, Liu S & Krewski D. (2008). Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev , 11, 373-517. PMID: 18470797 DOI.
Articles
Konishi K, Sasaki S, Kato S, Ban S, Washino N, Kajiwara J, Todaka T, Hirakawa H, Hori T, Yasutake D & Kishi R. (2009). Prenatal exposure to PCDDs/PCDFs and dioxin-like PCBs in relation to birth weight. Environ. Res. , 109, 906-13. PMID: 19683226 DOI.
Search Pubmed
Search Pubmed: Biological Toxin
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- USA - National Toxicology Program | Reports
- USA - Center for the Evaluation of Risks to Human Reproduction
- USA - Toxicogenomics Research Consortium
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 27) Embryology Abnormal Development - Biological Toxins. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Biological_Toxins
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G