Abnormal Development - Drugs
| Embryology - 25 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
Introduction
This page introduces the possible effects of maternal use of some selected legal drugs (therapeutic chemicals/agents) on development.[1] In some cases these drugs are prescribed to treat pre-existing or pregnancy related maternal medical conditions. This is not a comprehensive drug list and includes some known teratogens as well as data from early studies that require further confirmation. In all cases, a discussion with a medical practioner should be had prior to any reproductive decision.
The placenta and fetal tissues may deal with drugs differently from adult target tissues. In particular, drugs are "cleared", metabolised and excreted, at a different rate in both the fetus and in newborn infants. In general there is a much lower rate of clearance.
Legal drugs are classified, usually by each country's appropriate regulatory body, on the safety of drugs during pregnancy. In Australia, the Therapeutic Goods Authority has classes (A, B1, B2, B3, C, D and X) to define their safety. The USA used to have (before 2015) a similar Food and Drug Administration (FDA) labelling classes (A, B, C, D, and X) to define their safety. Since 2015 these drug categories has been replaced with the "FDA Pregnancy and Lactation Labeling Rule" (PLLR).[2]
Note that pregnant and breastfeeding women are generally excluded from drug clinical trials[3] and most data relies on animal studies.
There are also a growing range of herbal drugs which may not have undergone this type of study and classification.
The importance of careful evaluation of drugs and differences between species[4] can be historically demonstrated with the teratogenic effects of thalidomide, a drug given to treat "morning sickness" in the first trimester of pregnancy, which affected musculoskeletal development. This current page also gives examples of some other current drugs which have been shown to impact on development.
Currently, there is a USA population specific abuse of the prescription drug Oxycodone, commercial name "OxyContin".[5] Oxycodone is a strong opioid analgesic, semisynthetic opioid synthesized from thebaine, that binds μ- and κ-opioid receptors.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Drug teratogenicity | Opioid teratogenicity |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Drug Use During Pregnancy
USA
A 2011 study of USA data from 1976-2008 has shown:[20]
- 6 million pregnancies every year
- 50% of pregnant women reported taking at least one medication
- Pregnant women take an average of 2.6 medications at any time during pregnancy
- First trimester use of prescription medications has increased by more than 60%
- Use of 4 or more medications in the first trimester has tripled (9.9% to 27.6%)
The USA has also recently (2015) replaced the drug classification with the "FDA Pregnancy and Lactation Labeling Rule" (PLLR).[2]
- Links: USA Drug Categories
Europe
A 2017 study of European data from 15 European countries from October 2011 to February 2012 has shown:[21]
- 69% of women used medications classified as safe
- 28% used medications classified as risky
- 3% used medications with no classification available
- Socio-demographic and medical factors were associated with use of risky medications during pregnancy
- Having a chronic disorder was the factor with the strongest association with the use of risky medications during pregnancy
Australia
A 2017 study of pregnancy-related calls to Australian national medicines call centre covering 8 years has shown:[22]
- 1166 calls with stage of pregnancy available concerned safety
- 34% of questions related to medication classified as 'safe' during pregnancy
- After antidepressants, most calls were made about over-the-counter (OTC) medicines
- paracetamol, dexchlorpheniramine, codeine
- Safe treatment for everyday conditions was of increasing concern as pregnancy progressed
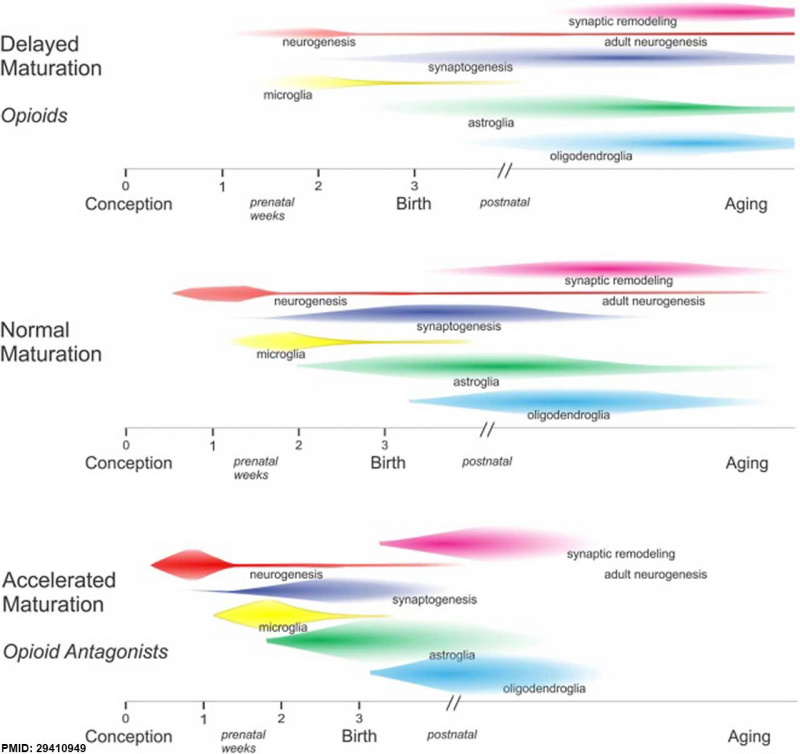
Neural Development
Opioids and neural development timeline effects.[11]
Advisory Committee on Medicines
- 1963 - Australian Drug Evaluation Committee (ADEC) was established in 1963 following the thalidomide experience.
- 2010 - the ADEC committee was replaced by the Advisory Committee on Prescription Medicines (ACPM)
- 2017 - ACPM committee was replaced by the Advisory Committee on Medicines (ACM).
- The ACM provides independent medical and scientific advice to the Minister for Health and the Therapeutic Goods Administration (TGA) on issues relating to the safety, quality and efficacy of medicines supplied in Australia including issues relating to pre-market and post-market functions for medicines.
| Australian Drug Categories |
|---|
| Legal drugs are classified, usually by each country's appropriate regulatory body, on the safety of drugs during pregnancy. In Australia, the Therapeutic Goods Authority has classes (A, B1, B2, B3, C, D and X) to define their safety. In the USA, drugs are classified by the Food and Drug Administration (FDA) into classes (A, B, C, D, and X) to define their safety. (More? Australian Drug Categories)
|
| Drug Testing |
|---|
| Typical testing of new drug compound today involves a lengthy series of animal and human studies.
Animal studies Usually tested in at least two mammalian species (rats and guinea pigs) using both single and repeated doses. For determining reproductive effects, tests on both male and female animals with dosing begins 4 weeks prior to mating are conducted to determine effects on fertility in both sexes, on embryogenesis, and on fetal malformation. Human Clinical trials Following animal studies to determine dose, efficacy and apparent safety, human studies can commence. Clinical trials are carried out under very strict conditions, set by international regulatory bodies in agreement with the principles espoused in the Declaration of Helsinki. There are four phases to the trials.
|
| After phase I to III the pharmaceutical company compiles all study data for independent assessment by government regulatory authorities in each country.
Regulatory Authorities: FDA in the USA, Therapeutic Goods Administration (TGA) in Australia, Medsafe in New Zealand, Medicines & Healthcare products Regulatory Agency (MHRA) in the UK, and Health Products and Food Branch (HPFB) in Canada. |
| Declaration of Helsinki |
| The Declaration of Helsinki was developed by The World Medical Association (WMA) as a statement of ethical principles for medical research involving human subjects, including research on identifiable human material and data. The Declaration is intended to be read as a whole and each of its constituent paragraphs should not be applied without consideration of all other relevant paragraphs. It is widely regarded as the cornerstone document on human research ethics. It is named after the location of its initial adoption in Helsinki, Finland, in June 1964. |
Australian Drug Categories
| Australian Drug Categories |
|---|
| Legal drugs are classified, usually by each country's appropriate regulatory body, on the safety of drugs during pregnancy. In Australia, the Therapeutic Goods Authority has classes (A, B1, B2, B3, C, D and X) to define their safety. In the USA, drugs are classified by the Food and Drug Administration (FDA) into classes (A, B, C, D, and X) to define their safety. (More? Australian Drug Categories)
|
Legal drugs are classified, usually by each country's appropriate regulatory body, on the safety of drugs during pregnancy. In Australia, the Therapeutic Goods Authority has classes (A, B1, B2, B3, C, D and X) to define their safety. In the USA, drugs are classified by the Food and Drug Administration (FDA) into classes (A, B, C, D, and X) to define their safety. (More? Australian Drug Categories)
Pregnancy Category A
Have been taken by a large number of pregnant women and women of childbearing age without an increase in the frequency of malformations or other direct or indirect harmful effects on the fetus having been observed.
Pregnancy Category B1
Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have not shown evidence of an increased occurrence of fetal damage.
Pregnancy Category B2
Have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals are inadequate or may be lacking, but available data show no evidence of an increased occurrence of fetal damage.
Pregnancy Category B3
Have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have shown evidence of an increased occurrence of fetal damage, the significance of which is considered uncertain in humans.
Pregnancy Category C
Have caused or may be suspected of causing, harmful effects on the human fetus or neonate without causing malformations. These effects may be reversible.
Pregnancy Category D
Have caused, are suspected to have caused or may be expected to cause, an increased incidence of human fetal malformations or irreversible damage. These drugs may also have adverse pharmacological effects.
Pregnancy Category X
Have such a high risk of causing permanent damage to the fetus that they should NOT be used in pregnancy or when there is a possibility of pregnancy.
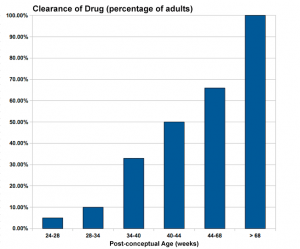
Infant Drug Clearance
The drug clearance data below are only approximate calculated rates for the fetus and infant from NZ Drug Safety in Lactation
| Post-conceptual Age (weeks) | Clearance of Drug (percentage of adults) |
| 24-28 | 5% |
| 28-34 | 10% |
| 34-40 | 33% |
| 40-44 | 50% |
| 44-68 | 66% |
| > 68 | 100% |
Drug Testing
| Drug Testing |
|---|
| Typical testing of new drug compound today involves a lengthy series of animal and human studies.
Animal studies Usually tested in at least two mammalian species (rats and guinea pigs) using both single and repeated doses. For determining reproductive effects, tests on both male and female animals with dosing begins 4 weeks prior to mating are conducted to determine effects on fertility in both sexes, on embryogenesis, and on fetal malformation. Human Clinical trials Following animal studies to determine dose, efficacy and apparent safety, human studies can commence. Clinical trials are carried out under very strict conditions, set by international regulatory bodies in agreement with the principles espoused in the Declaration of Helsinki. There are four phases to the trials.
|
| After phase I to III the pharmaceutical company compiles all study data for independent assessment by government regulatory authorities in each country.
Regulatory Authorities: FDA in the USA, Therapeutic Goods Administration (TGA) in Australia, Medsafe in New Zealand, Medicines & Healthcare products Regulatory Agency (MHRA) in the UK, and Health Products and Food Branch (HPFB) in Canada. |
| Declaration of Helsinki |
| The Declaration of Helsinki was developed by The World Medical Association (WMA) as a statement of ethical principles for medical research involving human subjects, including research on identifiable human material and data. The Declaration is intended to be read as a whole and each of its constituent paragraphs should not be applied without consideration of all other relevant paragraphs. It is widely regarded as the cornerstone document on human research ethics. It is named after the location of its initial adoption in Helsinki, Finland, in June 1964. |
Typical testing of new drug compound today involves a lengthy series of animal and human studies.
Animal studies
Usually tested in at least two mammalian species (rats and guinea pigs) using both single and repeated doses. For determining reproductive effects, tests on both male and female animals with dosing begins 4 weeks prior to mating are conducted to determine effects on fertility in both sexes, on embryogenesis, and on fetal malformation.
Human Clinical trials
Following animal studies to determine dose, efficacy and apparent safety, human studies can commence. Clinical trials are carried out under very strict conditions, set by international regulatory bodies in agreement with the principles espoused in the Declaration of Helsinki. There are four phases to the trials.
- Phase I trials - conducted in small groups of 10 to 20 healthy young male volunteers. Designed to examine how the drug is absorbed, distributed, metabolised and excreted by the body and to establish the safe dose for phase II trials.
- Phase II trials - conducted in 50 to 100 patients with the disease rather than healthy volunteers as in phase I. Designed to examine what effect the drug has on the body (heart rate, blood pressure and cognitive effects) depending on the disease the drug is being developed to treat.
- Phase III trials - conducted in 100’s of patients (larger numbers) with a particular disease or condition and are generally randomised comparative double-blinded studies. Using a comparator of either placebo, another active drug already used, or both. Several phase III trials are usually required by the regulatory authorities. Note that even these studies may not identify uncommon adverse effects, until used widely in the community.
- Phase IV trials - (post-registration) conducted in 1000’s of patients over several years, these trials are randomised controlled trials undertaken after the drug has been registered.
After phase I to III the pharmaceutical company compiles all study data for independent assessment by government regulatory authorities in each country.
- Regulatory Authorities: USA FDA | Australia Therapeutic Goods Administration (TGA) | New Zealand Medsafe | UK Medicines & Healthcare products Regulatory Agency (MHRA) | Canada Health Products and Food Branch (HPFB)
Declaration of Helsinki
The Declaration of Helsinki was developed by The World Medical Association (WMA) as a statement of ethical principles for medical research involving human subjects, including research on identifiable human material and data. The Declaration is intended to be read as a whole and each of its constituent paragraphs should not be applied without consideration of all other relevant paragraphs. It is widely regarded as the cornerstone document on human research ethics. It is named after the location of its initial adoption in Helsinki, Finland, in June 1964.
Teratology
Now consider how different environmental effects during pregnancy may influence developmental outcomes. The terms listed below are often used to describe these environmental effects
- Teratogen (Greek, teraton = monster) any agent that causes a structural abnormality (congenital abnormalities) following fetal exposure during pregnancy. The overall effect depends on dosage and time of exposure. (More? Critical Periods of Development)
- Absolute risk the rate of occurrence of an abnormal phenotype among individuals exposed to the agent. (e.g. fetal alcohol syndrome)
- Relative risk the ratio of the rate of the condition among the exposed and the nonexposed. (e.g. smokers risk of having a low birth weight baby compared to non-smokers) A high relative risk may indicate a low absolute risk if the condition is rare.
- Mutagen a chemical or agent that can cause permanent damage to the deoxyribonucleic acid (DNA) in a cell. DNA damage in the human egg or sperm may lead to reduced fertility, spontaneous abortion (miscarriage), birth defects and heritable diseases.
- Fetotoxicant is a chemical that adversely affects the developing fetus, resulting in low birth weight, symptoms of poisoning at birth or stillbirth (fetus dies before it is born).
- Synergism when the combined effect of exposure to more than one chemical at one time, or to a chemical in combination with other hazards (heat, radiation, infection) results in effects of such exposure to be greater than the sum of the individual effects of each hazard by itself.
- Toxicogenomics the interaction between the genome, chemicals in the environment, and disease. Cells exposed to a stress, drug or toxicant respond by altering the pattern of expression of genes within their chromosomes. Based on new genetic and microarray technologies.
Thalidomide
Thalidomide is a drug that was introduced on to the market on October 1, 1957 in West Germany. Thalidomide soon became a drug prescribed to pregnant women to combat symptoms associated with morning sickness. When taken during the first trimester of pregnancy, thalidomide prevented the proper growth of the fetus resulting in horrific birth defects in thousands of children around the world.
It was the linking of newborn abnormalities with the taking of thalidomide by an Australian clinician, William McBride, that identified it as a teratogenic agent causing a "thalidomide embryopathy".[23]
Not all species embryos are affected by the drug in the same way, with human and rabbit being most susceptible to the teratogenic effects. In addition, the effect on human development is also dependent upon the time and dose of the drug exposure, the "critical periods".
Antiepileptic Drugs
| ICD-11 LD2F.01 Foetal hydantoin syndrome - a fetopathy likely to occur when a pregnant woman takes the anticonvulsant drug phenytoin (diphenylhydantoin) for epileptic seizures. In utero exposure to this drug may result in a characteristic dysmorphic syndrome in the newborn, including low-set hair, short neck with pterygium colli, small nose, deep nasal bridge, epicanthus, hypertelorism, large mouth, malformed ears, hypoplastic distal phalanges of the fingers and toes and finger-like thumbs. These dysmorphic features are often associated with growth retardation and delayed psychomotor development. The mechanism underlying these anomalies has been shown to depend on maternal genetic characteristics, i.e. on maternal capacity to detoxify intermediate metabolites of phenytoin. |
This class of drugs are prescribed for a range of neurological conditions and are generally in a class of medications called anticonvulsants. There has been a recent review of information about the teratogenicity of antiepileptic medications.[24] Infant development affected by these drugs has also been called "fetal anticonvulsant syndrome", only a few drug examples are shown below.
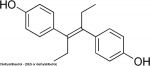
Valproic Acid
(Divalproex sodium, Valproate sodium, VPA)
Fetal Valproate Syndrome (FVS) results from prenatal exposure to valproic acid.
Valproic acid may also have direct effects on the placenta, altering the expression of transporters required for maternal thyroid hormone to cross to the fetus.[26] (More? Maternal Thyroid)
There has been recently identified in a Danish study is a risk of autism spectrum disorders and childhood autism.[14]
There is a risk of neural tube defects (NTDs) associated with this drug apparently due to a common polymorphism (677C→T) in humans for the methylene tetrahydrofolate reductase gene (MHTFR), that affects folate metabolism. Both genotypes (homozygote and heterozygote) show an increased risk with maternal heterozygotes greater.[27]
Increased risk of heart defects, craniofacial abnormalities, skeletal and limb defects. A recent study suggests that changes in cellular reactive oxygen species (ROS) levels may also lead to increased apoptosis during development.[28]
There have been several case studies of the limb development described with this drug.[29]
| Species | Neural tube defects | Skeletal defects | Route |
| Human | 30 | 20-30 | Oral |
| monkey | not observed | 150 | oral |
| rabbit | not observed | 150 | oral |
| rat | not observed | 150 | oral |
| hamster | 300 | not investigated | ip |
| mouse | 200 | 200, 250, 400 | ip, sc, oral |
| Table Data[4] Links: valproic acid | drugs | |||
Carbamazepine
Carbamazepine is an anticonvulsant and mood stabilizing drug used primarily in the treatment of epilepsy and bipolar disorder.
Phenytoin
| ICD-11 LD2F.01 Foetal hydantoin syndrome - a fetopathy likely to occur when a pregnant woman takes the anticonvulsant drug phenytoin (diphenylhydantoin) for epileptic seizures. In utero exposure to this drug may result in a characteristic dysmorphic syndrome in the newborn, including low-set hair, short neck with pterygium colli, small nose, deep nasal bridge, epicanthus, hypertelorism, large mouth, malformed ears, hypoplastic distal phalanges of the fingers and toes and finger-like thumbs. These dysmorphic features are often associated with growth retardation and delayed psychomotor development. The mechanism underlying these anomalies has been shown to depend on maternal genetic characteristics, i.e. on maternal capacity to detoxify intermediate metabolites of phenytoin. |
A study has identified mild abnormalities of the craniofacial skeletal, maxillary hypoplasia, among individuals exposed in utero to phenytoin monotherapy or phenytoin polytherapy.[30]
- Links: skull | MedlinePlus - Phenytoin | Phenytoin teratogenicity
Antithyroid Drugs
Graves' disease (GD) is the most common cause of hyperthyroidism during pregnancy (estimated 1 in 500 to 1,000 women) and has been treated with the antithyroid drugs propylthiouracil (PTU) and methimazole (MMI).
Propylthiouracil
Propylthiouracil (PTU) is an antithyroid drug used to treat maternal hyperthyroidism, commonly Graves' disease, during pregnancy. A recent study showed cranial neural tube defects in the mouse developmental model.[31]
Danish Study
The following information is from a recent Danish nationwide register-based cohort study of birth defects after early pregnancy use of antithyroid drugs.[32] (See also a recent Japanese study.[33])
Objective: Our objective was to determine to which degree the use of methimazole (MMI)/carbimazole (CMZ) and propylthiouracil (PTU) in early pregnancy is associated with an increased prevalence of birth defects.
Methods: This Danish nationwide register-based cohort study included 817 093 children live-born from 1996 to 2008.
Results: The prevalence of birth defects was high in children exposed to ATD in early pregnancy (PTU, 8.0%; MMI/CMZ, 9.1%; MMI/CMZ and PTU, 10.1%; no ATD, 5.4%; nonexposed, 5.7%; P < .001). Both maternal use of MMI/CMZ (adjusted OR = 1.66 [95% CI 1.35-2.04]) and PTU (1.41 [1.03-1.92]) and maternal shift between MMI/CMZ and PTU in early pregnancy (1.82 [1.08-3.07]) were associated with an increased OR of birth defects. MMI/CMZ and PTU were associated with urinary system malformation, and PTU with malformations in the face and neck region. Choanal atresia, esophageal atresia, omphalocele, omphalomesenteric duct anomalies, and aplasia cutis were common in MMI/CMZ-exposed children (combined, adjusted OR = 21.8 [13.4-35.4]).
Conclusions: Both MMI/CMZ and PTU were associated with birth defects, but the spectrum of malformations differed. More studies are needed to corroborate results in regard to early pregnancy shift from MMI/CMZ to PTU. New ATD with fewer side effects should be developed.
(above text edited from abstract)
- Links: neural abnormalities | thyroid
Anticoagulant Therapy
| ICD-11 LD2F.02 Embryofetopathy due to oral anticoagulant therapy - A condition caused by exposure of the embryo or fetus to anticoagulants during the antenatal period. This disease may present with optic nerve anomaly, optic atrophy, anomaly of the papilla, blindness, or choanal atresia. |
Search term: Anticoagulant Therapy Embryofetopathy
Pain Relief
There has been some recent interest in drugs used for maternal pain relief during pregnancy. Like all data from new research, these findings require more detailed and additional studies to confirm any finding related to pain relief during pregnancy. This section is included to show how today all drugs today can come under a more complex research spotlight, compared to earlier times.
Oxycodone
Currently, there is a USA population specific abuse of the prescription drug Oxycodone, commercial name "OxyContin".{{#pmid27513641|PMID27513641}} Oxycodone is a strong opioid analgesic, semisynthetic opioid synthesized from thebaine, that binds μ- and κ-opioid receptors. Animal studies appear to show no teratogenic effects at clinical doses (TGA 2010 report), though the drug can also pass into breast milk and there may also be untested polysubstance abuse by maternal abusers.
Acetaminophen
A single recent 2014 retrospective study of the Danish National Birth Cohort (1996 - 2002) 64 322 of live-born children using a phone interview has suggested a possible linkage between acetaminophen (paracetamol) and attention-deficit/hyperactivity disorder (ADHD).[34] More than half of all mothers reported acetaminophen use while pregnant.
A second earlier 2013 published study of the Norwegian Mother and Child Cohort (1999 - 2008) of 48 631 children on maternal use of paracetamol at gestational weeks GA 17 and 30 and at 6 months found: "Children exposed prenatally to short-term use of paracetamol (1-27 days) also had poorer gross motor outcomes, but the effects were smaller than with long-term use. Ibuprofen exposure was not associated with neurodevelopmental outcomes."[35]
- Links: MedLine Plus | MedLine Plus Overdose information | Search PubMed - acetaminophen+pregnancy | Search PubMed - ibuprofen+pregnancy
Antibiotics
There is some evidence that the use of some classes of antibiotics (macrolides) may have a weak association with neural teratogenic effects[8][36], see also the review.[37] Macrolides are the third most common prescribed antibiotic, derived from Saccharopolyspora erythraea, they inhibit the growth of bacteria (bacteriostatic) and are often used to treat common bacterial infections. Examples of macrolides include: erythromycin, roxithromycin, azithromycin and clarithromycin.
One suggested model[8] postulates short term fetal hypoxia results from the potential fetal cardiac arrhythmia effects of this drug, blocking the rapidly activating component of delayed rectifier K current ( I(Kr) ) associated with the Human-ether-a-go-go-related channel (hERG). Cardiac repolarization is controlled by both the rapidly ( I(Kr) ) and slowly ( I(Ks) ) activating delayed rectifier potassium channels, and decreases in I(Kr) or I(Ks) can also cause long QT syndrome (LQTS). Note that an isoform of the hERG channel (KCNH2-3.1) is also expressed at high levels in the fetal human brain.[38]
Antibiotics can also impact upon both the maternal and the neonatal microbiome.[9]
Oral Contraceptives
A recent 2016 Danish study[39] ofbirths from Danish registries between 1997 and 2011 identified that "Oral contraceptive exposure just before or during pregnancy does not appear to be associated with an increased risk of major birth defects."
- Links: menstrual cycle
Herbal Drugs
The following herbal drugs have been used for a number of different maternal conditions: Ginkgo Biloba, Kava (Piper methysticum), St. John's wort (Hypericum perforatum), Tian Ma (Gastrodia elata), Valerian (Valeriana officinalis). In some cases very little is known about the potential teratogenic effects of these drugs (More? Herbal Drugs).
HSTAT St. John's Wort | Appendix II: Side Effects, Adverse Effects, Precautions, and Warnings "The safety of using hypericum during pregnancy or lactation has not been proven so it should be avoided." "St. John's wort induces the CYP 450 3A4 metabolic pathway which is also used by many prescription drugs used to prevent conditions (transplant rejection or pregnancy oral contraceptives), health care providers should alert patients about these potential drug interactions."
- Links: herbal drugs
Anaesthesia
"Under usual circumstances, surgery is only conducted during pregnancy when it is absolutely necessary for the wellbeing of the mother, fetus, or both."[40] Maternal conditions requiring surgery, either related or not related to a pregnancy, may require anaesthesia and all general anaesthetic drugs cross the placenta. Teratogenic effects have not been identified with anaesthesia drugs, though there are suggestions of some impact on neurodevelopment.[41]
Opioids
Neonatal abstinence syndrome(NAS) describes neonatal affects of abrupt discontinuation at birth of opioids exposure and fetal dependence during development in the uterus. Prenatal opioid exposure can occur through prescription or illegal drug use. Prescription opioids include: codeine, dihydrocodeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocin, propoxyphene, buprenorphine, tapentadol, and tramadol.
A USA clinical study[42] has identified a trend increase in NAS live birth incidence (2000, 1.2/1,000; 2009 3.39/1000). Animal models have identified neural development abnormalities associated with prenatal opioid exposure.
Diethylstilbestrol
Diethylstilbestrol (DES or diethylstilbetrol) was a drug prescribed to women from 1938-1971 to prevent miscarriage in high-risk pregnancies. Acts as a potent estrogen (mimics natural hormone) and therefore a potential endocrine disruptor. Banned by the USA FDA in 1979 as a teratogen, previously used as livestock growth promoter.
- Female fetus - increased risk abnormal reproductive tract and cancer.
- Male fetus - abnormal genitalia.
DES induces vaginal abnormalities (vaginal adenosis) by inhibiting the BMP4/Activin A-regulated vaginal cell fate decision through a down-regulation of RUNX1.[43] Has also been shown to induces autophagy in thymocytes through epigenetic modulation.[44]
A recent French study of birth defects in children of men exposed in utero to diethylstilbestrol has shown a trans-generational effect in male offspring, with an increased incidence of cryptorchidism and hypoplasia of the penis.[45]
- Links: endocrine abnormalities
References
- ↑ Bologa-Campeanu M, Koren G, Rieder M & McGuigan M. (1988). Prenatal adverse effects of various drugs and chemicals. A review of substances of frequent concern to mothers in the community. Med Toxicol Adverse Drug Exp , 3, 307-23. PMID: 3054428
- ↑ 2.0 2.1 Pernia S & DeMaagd G. (2016). The New Pregnancy and Lactation Labeling Rule. P T , 41, 713-715. PMID: 27904304
- ↑ Illamola SM, Bucci-Rechtweg C, Costantine MM, Tsilou E, Sherwin CM & Zajicek A. (2018). Inclusion of pregnant and breastfeeding women in research - efforts and initiatives. Br J Clin Pharmacol , 84, 215-222. PMID: 28925019 DOI.
- ↑ 4.0 4.1 Nau H. (1986). Species differences in pharmacokinetics and drug teratogenesis. Environ. Health Perspect. , 70, 113-29. PMID: 3104022
- ↑ Jones CM, Muhuri PK & Lurie PG. (2017). Trends in the Nonmedical Use of OxyContin, United States, 2006 to 2013. Clin J Pain , 33, 452-461. PMID: 27513641 DOI.
- ↑ Azuine RE, Ji Y, Chang HY, Kim Y, Ji H, DiBari J, Hong X, Wang G, Singh GK, Pearson C, Zuckerman B, Surkan PJ & Wang X. (2019). Prenatal Risk Factors and Perinatal and Postnatal Outcomes Associated With Maternal Opioid Exposure in an Urban, Low-Income, Multiethnic US Population. JAMA Netw Open , 2, e196405. PMID: 31251378 DOI.
- ↑ Williams AL, Bates CA, Pace ND, Leonhard MJ, Chang ET & DeSesso JM. (2018). Impact of chloroform exposures on reproductive and developmental outcomes: A systematic review of the scientific literature. Birth Defects Res , 110, 1267-1313. PMID: 30350414 DOI.
- ↑ 8.0 8.1 8.2 Fan H, Li L, Wijlaars L & Gilbert RE. (2019). Associations between use of macrolide antibiotics during pregnancy and adverse child outcomes: A systematic review and meta-analysis. PLoS ONE , 14, e0212212. PMID: 30779772 DOI.
- ↑ 9.0 9.1 Miller JE, Wu C, Pedersen LH, de Klerk N, Olsen J & Burgner DP. (2018). Maternal antibiotic exposure during pregnancy and hospitalization with infection in offspring: a population-based cohort study. Int J Epidemiol , , . PMID: 29415232 DOI.
- ↑ Metz VE, Brown QL, Martins SS & Palamar JJ. (2018). Characteristics of drug use among pregnant women in the United States: Opioid and non-opioid illegal drug use. Drug Alcohol Depend , 183, 261-266. PMID: 29310077 DOI.
- ↑ 11.0 11.1 Hauser KF & Knapp PE. (2017). Opiate Drugs with Abuse Liability Hijack the Endogenous Opioid System to Disrupt Neuronal and Glial Maturation in the Central Nervous System. Front Pediatr , 5, 294. PMID: 29410949 DOI.
- ↑ AIHW 2016. Poisoning in children and young people 2012–13. Injury research and statistics series no. 97. Cat. no. INJCAT 173. Canberra: AIHW.
- ↑ Tanoshima M, Kobayashi T, Tanoshima R, Beyene J, Koren G & Ito S. (2015). Risks of congenital malformations in offspring exposed to valproic acid in utero: A systematic review and cumulative meta-analysis. Clin. Pharmacol. Ther. , 98, 417-41. PMID: 26044279 DOI.
- ↑ 14.0 14.1 Christensen J, Grønborg TK, Sørensen MJ, Schendel D, Parner ET, Pedersen LH & Vestergaard M. (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA , 309, 1696-703. PMID: 23613074 DOI.
- ↑ Levine M & O'Connor AD. (2012). Obstetric toxicology: teratogens. Emerg. Med. Clin. North Am. , 30, 977-90. PMID: 23137407 DOI.
- ↑ Adam MP, Polifka JE & Friedman JM. (2011). Evolving knowledge of the teratogenicity of medications in human pregnancy. Am J Med Genet C Semin Med Genet , 157C, 175-82. PMID: 21766440 DOI.
- ↑ van Gelder MM, Roeleveld N & Nordeng H. (2011). Exposure to non-steroidal anti-inflammatory drugs during pregnancy and the risk of selected birth defects: a prospective cohort study. PLoS ONE , 6, e22174. PMID: 21789231 DOI.
- ↑ Go HS, Seo JE, Kim KC, Han SM, Kim P, Kang YS, Han SH, Shin CY & Ko KH. (2011). Valproic acid inhibits neural progenitor cell death by activation of NF-κB signaling pathway and up-regulation of Bcl-XL. J. Biomed. Sci. , 18, 48. PMID: 21722408 DOI.
- ↑ Janneke Jentink, Helen Dolk, Maria A Loane, Joan K Morris, Diana Wellesley, Ester Garne, Lolkje de Jong-van den Berg, for the EUROCAT Antiepileptic Study Working Group Intrauterine exposure to carbamazepine and specific congenital malformations: systematic review and case-control study BMJ 2010; 341:c6581 BMJ
- ↑ Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C & Hernández-Díaz S. (2011). Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am. J. Obstet. Gynecol. , 205, 51.e1-8. PMID: 21514558 DOI.
- ↑ Trønnes JN, Lupattelli A & Nordeng H. (2017). Safety profile of medication used during pregnancy: results of a multinational European study. Pharmacoepidemiol Drug Saf , 26, 802-811. PMID: 28449197 DOI.
- ↑ Pijpers EL, Kreijkamp-Kaspers S, McGuire TM, Deckx L, Brodribb W & van Driel ML. (2017). Women's questions about medicines in pregnancy - An analysis of calls to an Australian national medicines call centre. Aust N Z J Obstet Gynaecol , 57, 334-341. PMID: 27624748 DOI.
- ↑ Bologa-Campeanu M, Koren G, Rieder M & McGuigan M. (1988). Prenatal adverse effects of various drugs and chemicals. A review of substances of frequent concern to mothers in the community. Med Toxicol Adverse Drug Exp , 3, 307-23. PMID: 3054428
- ↑ Kluger BM & Meador KJ. (2008). Teratogenicity of antiepileptic medications. Semin Neurol , 28, 328-35. PMID: 18777479 DOI.
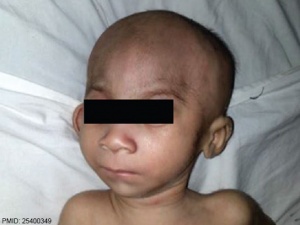
- ↑ Chandane PG & Shah I. (2014). Fetal valproate syndrome. Indian J Hum Genet , 20, 187-8. PMID: 25400349 DOI.
- ↑ Meir M, Bishara A, Mann A, Udi S, Portnoy E, Shmuel M & Eyal S. (2016). Effects of valproic acid on the placental barrier in the pregnant mouse: Optical imaging and transporter expression studies. Epilepsia , 57, e108-12. PMID: 27142887 DOI.
- ↑ Dean JC, Moore SJ, Osborne A, Howe J & Turnpenny PD. (1999). Fetal anticonvulsant syndrome and mutation in the maternal MTHFR gene. Clin. Genet. , 56, 216-20. PMID: 10563481
- ↑ Tung EW & Winn LM. (2011). Valproic acid increases formation of reactive oxygen species and induces apoptosis in postimplantation embryos: a role for oxidative stress in valproic acid-induced neural tube defects. Mol. Pharmacol. , 80, 979-87. PMID: 21868484 DOI.
- ↑ Pandya NA & Jani BR. (2000). Post-axial limb defects with maternal sodium valproate exposure. Clin. Dysmorphol. , 9, 143-4. PMID: 10826630
- ↑ Orup HI, Holmes LB, Keith DA & Coull BA. (2003). Craniofacial skeletal deviations following in utero exposure to the anticonvulsant phenytoin: monotherapy and polytherapy. Orthod Craniofac Res , 6, 2-19. PMID: 12627792
- ↑ Benavides VC, Mallela MK, Booth CJ, Wendler CC & Rivkees SA. (2012). Propylthiouracil is teratogenic in murine embryos. PLoS ONE , 7, e35213. PMID: 22529993 DOI.
- ↑ Andersen SL, Olsen J, Wu CS & Laurberg P. (2013). Birth defects after early pregnancy use of antithyroid drugs: a Danish nationwide study. J. Clin. Endocrinol. Metab. , 98, 4373-81. PMID: 24151287 DOI.
- ↑ Yoshihara A, Noh J, Yamaguchi T, Ohye H, Sato S, Sekiya K, Kosuga Y, Suzuki M, Matsumoto M, Kunii Y, Watanabe N, Mukasa K, Ito K & Ito K. (2012). Treatment of graves' disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malformation. J. Clin. Endocrinol. Metab. , 97, 2396-403. PMID: 22547422 DOI.
- ↑ Liew Z, Ritz B, Rebordosa C, Lee P, Olsen J. Acetaminophen Use During Pregnancy, Behavioral Problems, and Hyperkinetic Disorders. JAMA Pediatr. 2014;():. doi:10.1001/jamapediatrics.2013.4914.
- ↑ Brandlistuen RE, Ystrom E, Nulman I, Koren G & Nordeng H. (2013). Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol , 42, 1702-13. PMID: 24163279 DOI.
- ↑ Mallah N, Tohidinik HR, Etminan M, Figueiras A & Takkouche B. (2019). Prenatal Exposure to Macrolides and Risk of Congenital Malformations: A Meta-Analysis. Drug Saf , , . PMID: 31721138 DOI.
- ↑ Hantoushzadeh S, Anvari Aliabad R & Hossein Norooznezhad A. (2020). Antibiotics, Pregnancy, and Fetal Mental Illnesses: Where is the link?. Am. J. Obstet. Gynecol. , , . PMID: 32017921 DOI.
- ↑ Huffaker SJ, Chen J, Nicodemus KK, Sambataro F, Yang F, Mattay V, Lipska BK, Hyde TM, Song J, Rujescu D, Giegling I, Mayilyan K, Proust MJ, Soghoyan A, Caforio G, Callicott JH, Bertolino A, Meyer-Lindenberg A, Chang J, Ji Y, Egan MF, Goldberg TE, Kleinman JE, Lu B & Weinberger DR. (2009). A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat. Med. , 15, 509-18. PMID: 19412172 DOI.
- ↑ Charlton BM, Mølgaard-Nielsen D, Svanström H, Wohlfahrt J, Pasternak B & Melbye M. (2016). Maternal use of oral contraceptives and risk of birth defects in Denmark: prospective, nationwide cohort study. BMJ , 352, h6712. PMID: 26738512
- ↑ Reitman E & Flood P. (2011). Anaesthetic considerations for non-obstetric surgery during pregnancy. Br J Anaesth , 107 Suppl 1, i72-8. PMID: 22156272 DOI.
- ↑ Palanisamy A. (2012). Maternal anesthesia and fetal neurodevelopment. Int J Obstet Anesth , 21, 152-62. PMID: 22405978 DOI.
- ↑ Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM & Davis MM. (2012). Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA , 307, 1934-40. PMID: 22546608 DOI.
- ↑ Laronda MM, Unno K, Ishi K, Serna VA, Butler LM, Mills AA, Orvis GD, Behringer RR, Deng C, Sinha S & Kurita T. (2013). Diethylstilbestrol induces vaginal adenosis by disrupting SMAD/RUNX1-mediated cell fate decision in the Müllerian duct epithelium. Dev. Biol. , 381, 5-16. PMID: 23830984 DOI.
- ↑ Singh NP, Miranda K, Singh UP, Nagarkatti P & Nagarkatti M. (2018). Diethylstilbestrol (DES) induces autophagy in thymocytes by regulating Beclin-1 expression through epigenetic modulation. Toxicology , 410, 49-58. PMID: 30153466 DOI.
- ↑ Tournaire M, Devouche E, Epelboin S, Cabau A, Dunbavand A & Levadou A. (2018). Birth defects in children of men exposed in utero to diethylstilbestrol (DES). Therapie , 73, 399-407. PMID: 29609831 DOI.
Reviews
Koren G, Berkovitch M & Ornoy A. (2018). Dose-Dependent Teratology in Humans: Clinical Implications for Prevention. Paediatr Drugs , 20, 331-335. PMID: 29725877 DOI.
Saunders EJ & Saunders JA. (1990). Drug therapy in pregnancy: the lessons of diethylstilbestrol, thalidomide, and bendectin. Health Care Women Int , 11, 423-32. PMID: 2228814 DOI.
Pacifici GM. (2009). Clinical pharmacokinetics of aminoglycosides in the neonate: a review. Eur. J. Clin. Pharmacol. , 65, 419-27. PMID: 19104791 DOI.
Al-Saleh E, Al-Harmi J, Nandakumaran M & Al-Shammari M. (2008). Transport kinetics of cisplatin in the perfused human placental lobule in vitro. J. Matern. Fetal. Neonatal. Med. , 21, 726-31. PMID: 19012189 DOI.
Articles
McBride WG. (1992). Prescription drugs in the first trimester and congenital malformations. Aust N Z J Obstet Gynaecol , 32, 386. PMID: 1290446
Search Pubmed
June 2010 "infant drug clearance rates" All (168) Review (22) Free Full Text (45)
Search Pubmed: infant drug clearance rates | thalidomide teratogenicity |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
WHO - The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD)
- Australia Advisory Committee on Prescription Medicines (ACPM)
- United Nations International Drug Control Programme
- USA TOXNET - Databases on toxicology, hazardous chemicals, environmental health, and toxic releases. mobile
- Centre for Education and Information on Drugs and Alcohol (CEIDA) (Australia)
- Australian Congenital Anomalies Monitoring System (ACAMS)
- Australian Drug Foundation (ADF)
- Child Health and Safety (Australia)
- NIDA (USA)- Consequences of Prenatal Drug Exposure
- Australian Medicines Handbook (no electronic version yet)
- Australian Institute of Health and Welfare (AIHW)
- DrugBank Canadian database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information.
- The Teratogen Information System (TERIS) a computerized database designed to assist physicians or other health care professionals in assessing the risks of possible teratogenic exposures in pregnant women. (subscription only, information is not freely available).
Terms
| Drug Terms | ||
|---|---|---|
| ||
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 25) Embryology Abnormal Development - Drugs. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Drugs
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G