Abnormal Development - Varicella Zoster Virus
| Embryology - 1 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
Introduction
Varicella Zoster Virus (VSV) or chickenpox maternal infection can be transmitted to the fetus. It is a herpesvirus belonging to the subfamily of Alphaherpesviridae that is transmitted by directly aerosol droplets or direct contact, or indirectly by touching freshly soiled contaminated items.
Fetal varicella syndrome (FVS), was first described in 1947[1] and is caused by transplacental infection by the varicella zoster virus following maternal infection.
Commonly called chickenpox or shingles in adults. The chickenpox vaccine is made from a weakened varicella virus producing an immune response that protects you against chickenpox infection. The United States has had a licensed chickenpox vaccine since 1995.
Fetal and Neonatal Risks
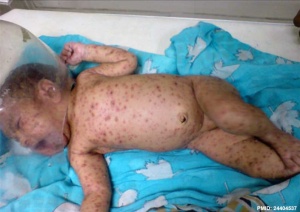
Risks are dependent on the infection timing.
- before 20 weeks (GA) - FVS can occur with an incidence of about 1%. The lesions can affect the skin, limbs, central and autonomous nervous systems, eyes, cause calcifications, and growth retardation; mortality is high. Lesions typically follow one or several nerve territories, suggesting that damage results from in utero zoster following primary fetal infection.
- during pregnancy - transmission can occur, but is usually asymptomatic; some infants develop zoster postnatally and a few have FVS.
- around delivery - often leads to disseminated neonatal varicella.
| Viral Links: viral infection | TORCH | cytomegalovirus | hepatitis | HIV | parvovirus | polio | rubella virus | chickenpox | Lymphocytic Choriomeningitis Virus | Zika virus | human papillomavirus | rotavirus | West Nile virus | varicella virus | vaccination | zoonotic infection | environment | ||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Abnormal Development Varicella Zoster Virus |
Taxonomy
The International Committee on Taxonomy of Viruses (ICTV) has developed a published code of classification for viruses (currently 2011 Release).
Order: Herpesvirales
- Subfamily: Alphaherpesvirinae
- Genus: Varicellovirus
- Species: Human herpesvirus 3
- Genus: Varicellovirus
USA Policy Statement
The 2011 Policy statement by the Committee on Infectious Diseases published in the journal Pediatrics as "Prevention of varicella: update of recommendations for use of quadrivalent and monovalent varicella vaccines in children"[4]
- "Two varicella-containing vaccines are licensed for use in the United States: monovalent varicella vaccine (Varivax [Merck & Co, Inc, West Point, PA]) and quadrivalent measles-mumps-rubella-varicella vaccine (MMRV) (ProQuad [Merck & Co, Inc]). It is estimated from postlicensure data that after vaccination at 12 through 23 months of age, 7 to 9 febrile seizures occur per 10,000 children who receive the MMRV, and 3 to 4 febrile seizures occur per 10,000 children who receive the measles-mumps-rubella (MMR) and varicella vaccines administered concurrently but at separate sites. Thus, 1 additional febrile seizure is expected to occur per approximately 2300 to 2600 children 12 to 23 months old vaccinated with the MMRV, when compared with separate MMR and varicella vaccine administration. The period of risk for febrile seizures is from 5 through 12 days after receipt of the vaccine(s). No increased risk of febrile seizures is seen among patients 4 to 6 years of age receiving MMRV. Febrile seizures do not predispose to epilepsy or neurodevelopmental delays later in life and are not associated with long-term health impairment. The American Academy of Pediatrics recommends that either MMR and varicella vaccines separately or the MMRV be used for the first dose of measles, mumps, rubella, and varicella vaccines administered at 12 through 47 months of age. For the first dose of measles, mumps, rubella, and varicella vaccines administered at ages 48 months and older, and for dose 2 at any age (15 months to 12 years), use of MMRV generally is preferred over separate injections of MMR and varicella vaccines."
- Links: CDC Factsheet
References
- ↑ LAFORET EG & LYNCH CL. (1947). Multiple congenital defects following maternal varicella; report of a case. N. Engl. J. Med. , 236, 534-7. PMID: 20293114 DOI.
- ↑ Sharma CM & Sharma D. (2013). A classical case of neonatal varicella. J Clin Neonatol , 2, 200. PMID: 24404537 DOI.
- ↑ Mandelbrot L. (2012). Fetal varicella - diagnosis, management, and outcome. Prenat. Diagn. , 32, 511-8. PMID: 22514124 DOI.
- ↑ Committee on Infectious Diseases. (2011). Policy statement—Prevention of varicella: update of recommendations for use of quadrivalent and monovalent varicella vaccines in children. Pediatrics , 128, 630-2. PMID: 21873692 DOI.
Textbooks
- Medical Microbiology. 4th edition. Baron S, editor. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 55 Togaviruses: Rubella Virus | Chapter 54Alphaviruses (Togaviridae) and Flaviviruses (Flaviviridae) | Table 55-1 Abnormalities Associated with Congenital Rubella Syndrome | Figure 55-3 Incidence rates of rubella USA 1966-1993
- Molecular Biology of the Cell. 4th edition. Alberts B, Johnson A, Lewis J, et al. New York: Garland Science; 2002. Viruses Exploit Host Cell Machinery for All Aspects of Their Multiplication
- Disease Control Priorities in Developing Countries. 2nd edition. Jamison DT, Breman JG, Measham AR, et al., editors. Washington (DC): World Bank; 2006. Chapter 20Vaccine-preventable Diseases
- Antenatal Care: Routine care for the healthy pregnant woman. NICE Clinical Guidelines, No. 62. National Collaborating Centre for Women's and Children's Health (UK). London: RCOG Press; 2008 Mar. 10.8. Rubella
Reviews
Javed S, Javed SA & Tyring SK. (2012). Varicella vaccines. Curr. Opin. Infect. Dis. , 25, 135-40. PMID: 22123665 DOI.
Andrei G & Snoeck R. (2011). Emerging drugs for varicella-zoster virus infections. Expert Opin Emerg Drugs , 16, 507-35. PMID: 21699441 DOI.
Lamont RF, Sobel JD, Carrington D, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E & Romero R. (2011). Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG , 118, 1155-62. PMID: 21585641 DOI.
Cohen A, Moschopoulos P, Maschopoulos P, Stiehm RE & Koren G. (2011). Congenital varicella syndrome: the evidence for secondary prevention with varicella-zoster immune globulin. CMAJ , 183, 204-8. PMID: 21262937 DOI.
Articles
Gnann JW. (2012). Varicella-zoster virus: Prevention through vaccination. Clin Obstet Gynecol , 55, 560-70. PMID: 22510639 DOI.
Piette JC, Wechsler B, Blétry O & Frances C. (1992). [Clinical approach to antiphospholipid syndrome: from facts to questions]. Presse Med , 21, 2081-4. PMID: 1297117
SIMPSON RE. (1954). Studies on shingles: is the virus ordinary chickenpox virus?. Lancet , 267, 1299-302. PMID: 13222825
Cole R & Kuttner AG. (1925). THE PROBLEM OF THE ETIOLOGY OF HERPES ZOSTER. J. Exp. Med. , 42, 799-820. PMID: 19869091
Search Pubmed
Search Pubmed: Varicella Zoster Virus | Congenital Varicella Syndrome | fetal varicella syndrome
| Environmental Links: Introduction | low folic acid | iodine deficiency | Nutrition | Drugs | Australian Drug Categories | USA Drug Categories | thalidomide | herbal drugs | Illegal Drugs | smoking | Fetal Alcohol Syndrome | TORCH | viral infection | bacterial infection | fungal infection | zoonotic infection | toxoplasmosis | Malaria | maternal diabetes | maternal hypertension | maternal hyperthermia | Maternal Inflammation | Maternal Obesity | hypoxia | biological toxins | chemicals | heavy metals | air pollution | radiation | Prenatal Diagnosis | Neonatal Diagnosis | International Classification of Diseases | Fetal Origins Hypothesis |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Department of Health and Ageing (Australia) Immunise Australia Program Website | The Australian Immunisation Handbook 9th Edition 2008
- Australia - Victoria Chickenpox (varicella zoster) in neonates
- World Health Organization (WHO) varicella
- CDC (USA) - Varicella (Chickenpox) Vaccination
- International Committee on Taxonomy of Viruses
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 1) Embryology Abnormal Development - Varicella Zoster Virus. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Varicella_Zoster_Virus
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G