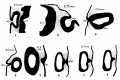Introduction
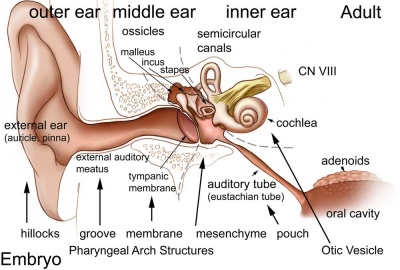
Adult hearing embryonic origins.
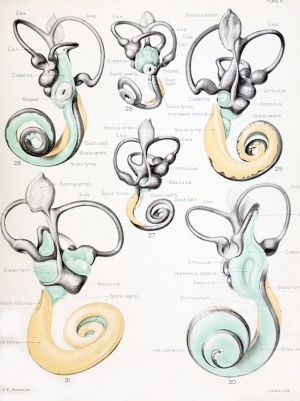
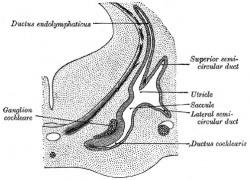
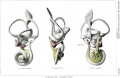
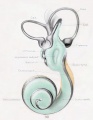
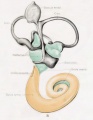
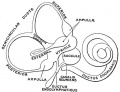
Historic images - The membranous labyrinth
The inner ear is derived from a pair of surface sensory placodes (otic placodes) that appear in human development during week 4 (GA week 6) in the head region lying behind the second pharyngeal arch.
These otic placodes fold inwards forming initially a depression, then pinch off entirely from the surface forming an epithelium surrounding a fluid-filled sac or vesicle (otic vesicle, otocyst, auditory vesicle). The vesicle sinks into the head mesenchyme some of which closely surrounds the otocyst forming the otic capsule.
The otocyst finally lies close to the early developing hindbrain (rhombencephalon) and the developing vestibulo-cochlear-facial ganglion complex.
The otocyst epithelium then undergoes a series of morphological changes, forming the primitive membranous labyrinth. During the human fetal period this will differentiate into the inner ear components for hearing (cochlea) and balance (semi-circular canals). Cochlear development involving a transcription factor spatiotemporal gradient continues through to GA week 16.[1]
The adult cochlear has a "snail-shell" appearance, with the total number of turns differing between species. The adult human cochlear is typically described as having 2.5 turns, but this can vary up to 2.75 or even 3 turns.[2]
The "organ of corti" that develops within the cochlea was first identified by Alfonso Giacomo Gaspare Corti (1822–1876), an Italian anatomist, in 1851.
Some Recent Findings
- Characterization of the development of the mouse cochlear epithelium at the single cell level[3] "Mammalian hearing requires the development of the organ of Corti, a sensory epithelium comprising unique cell types. The limited number of each of these cell types, combined with their close proximity, has prevented characterization of individual cell types and/or their developmental progression. To examine cochlear development more closely, we transcriptionally profile approximately 30,000 isolated mouse cochlear cells collected at four developmental time points. Here we report on the analysis of those cells including the identification of both known and unknown cell types. Trajectory analysis for OHCs indicates four phases of gene expression while fate mapping of progenitor cells suggests that OHCs and their surrounding supporting cells arise from a distinct (lateral) progenitor pool. Tgfβr1 is identified as being expressed in lateral progenitor cells and a Tgfβr1 antagonist inhibits OHC development."
- β-Catenin is required for radial cell patterning and identity in the developing mouse cochlea[4] "Development of multicellular organs requires the coordination of cell differentiation and patterning. Critical for sound detection, the mammalian organ of Corti contains functional units arranged tonotopically along the cochlear turns. Each unit consists of sensory hair cells intercalated by nonsensory supporting cells, both specified and radially patterned with exquisite precision during embryonic development. However, how cell identity and radial patterning are jointly controlled is poorly understood. Here we show that β-catenin is required for specification of hair cell and supporting cell subtypes and radial patterning of the cochlea in vivo. In 2 mouse models of conditional β-catenin deletion, early specification of Myosin7-expressing hair cells and Prox1-positive supporting cells was preserved. While β-catenin-deficient cochleae expressed FGF8 and FGFR3, both of which are essential for pillar cell specification, the radial patterning of organ of Corti was disrupted, revealed by aberrant expression of cadherins and the pillar cell markers P75 and Lgr6. Moreover, β-catenin ablation caused duplication of FGF8-positive inner hair cells and reduction of outer hair cells without affecting the overall hair cell density. In contrast, in another transgenic model with suppressed transcriptional activity of β-catenin but preserved cell adhesion function, both specification and radial patterning of the organ of Corti were intact. Our study reveals specific functions of β-catenin in governing cell identity and patterning mediated through cell adhesion in the developing cochlea.
- Formation of the Periotic Space During the Early Fetal Period in Humans[5] "Digital data sets from magnetic resonance images and phase-contrast X-ray tomography images of 24 inner ear organs from 24 human fetuses from the Kyoto Collection (fetuses in trimesters 1 and 2; crown-rump length: 14.4-197 mm) were analyzed. The membranous labyrinth was morphologically differentiated in samples at the end of the embryonic period (Carnegie stage 23), and had grown linearly to more than eight times in size during the observation period. The periotic space was first detected at the 35-mm samples, around the vestibule and basal turn of the cochlea, which elongated rapidly to the tip of the cochlea and semicircular ducts, successively, and almost covered the membranous labyrinth at the 115-mm CRL stage or later. In those samples, several ossification centers were detected around the space."
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Inner Ear Development | Inner Ear Embryology | Organ of Corti Development |
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- Developmental profiling of microRNAs in the human embryonic inner ear[6] "Due to the extreme inaccessibility of fetal human inner ear tissue, defining of the microRNAs (miRNAs) that regulate development of the inner ear has relied on animal tissue. In the present study, we performed the first miRNA sequencing of otic precursors in human specimens. Using HTG miRNA Whole Transcriptome assays, we examined miRNA expression in the cochleovestibular ganglion (CVG), neural crest (NC), and otic vesicle (OV) from paraffin embedded (FFPE) human specimens in the Carnegie developmental stages 13, 14 and 15. We found that in human embryonic tissues, there are different patterns of miRNA expression in the CVG, NC and OV. In particular, members of the miR-183 family (miR-96, miR-182, and miR-183) are differentially expressed in the CVG compared to NC and OV at Carnegie developmental stage 13. We further identified transcription factors that are differentially targeted in the CVG compared to the other tissues from stages 13-15, and we performed gene set enrichment analyses to determine differentially regulated pathways that are relevant to CVG development in humans. These findings not only provide insight into the mechanisms governing the development of the human inner ear, but also identify potential signaling pathways for promoting regeneration of the spiral ganglion and other components of the inner ear." MicroRNA
- Role of BDNF and neurotrophic receptors in human inner ear development[7] "The expression patterns of the neurotrophin, brain-derived neurotrophic factor, BDNF, and the neurotrophic receptors-p75NTR and Trk receptors-in the developing human fetal inner ear between the gestational weeks (GA) 9 to 12 are examined via in situ hybridization and immunohistochemistry. BDNF mRNA expression was highest in the cochlea at GA 9 but declined in the course of development. In contrast to embryonic murine specimens, a decline in BDNF expression from the apical to the basal turn of the cochlea could not be observed. ...Our findings suggest that BDNF and neurotrophin receptors are important players during early human inner ear development. In particular, they seem to be important for the survival of the afferent sensory neurons."
- Lineage tracing of Sox2-expressing progenitor cells in the mouse inner ear reveals a broad contribution to non-sensory tissues and insights into the origin of the organ of Corti[8] "The transcription factor Sox2 is both necessary and sufficient for the generation of sensory regions of the inner ear. ...We find that Sox2-expressing cells in the early otocyst give rise to large numbers of non-sensory structures throughout the inner ear, and that Sox2 only becomes a truly prosensory marker at embryonic day (E)11.5. Our fate map reveals the organ of Corti derives from a central domain on the medial side of the otocyst and shows that a significant amount of the organ of Corti derives from a Sox2-negative population in this region." Sox | mouse
- Development of the stria vascularis and potassium regulation in the human fetal cochlea[9] "We present an investigation on the development of the stria vascularis in the human fetal cochlea between 9 and 18 weeks of gestation (W9-W18) and show the cochlear expression dynamics of key potassium-regulating proteins. At W12, MITF+/SOX10+/KIT+ neural-crest-derived melanocytes migrated into the cochlea and penetrated the basement membrane of the lateral wall epithelium, developing into the intermediate cells of the stria vascularis. These melanocytes tightly integrated with Na+ /K+ -ATPase-positive marginal cells, which started to express KCNQ1 in their apical membrane at W16. At W18, KCNJ10 and gap junction proteins GJB2/CX26 and GJB6/CX30 were expressed in the cells in the outer sulcus, but not in the spiral ligament."
- Distribution and development of peripheral glial cells in the human fetal cochlea[10] "The adult human cochlea contains various types of peripheral glial cells that envelop or myelinate the three different domains of the spiral ganglion neurons: the central processes in the cochlear nerve, the cell bodies in the spiral ganglia, and the peripheral processes in the osseous spiral lamina. ...The developmental dynamics of the peripheral glial cells in the human fetal cochlea is in support of a neural crest origin. Our study provides the first overview of the distribution and maturation of peripheral glial cells in the human fetal cochlea from W9 to W22." Neural Crest Development
- Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells[11] "Neural precursor cells of the central nervous system undergo successive temporal waves of terminal division, each of which is soon followed by the onset of cell differentiation. The organ of Corti in the mammalian cochlea develops differently, such that precursors at the apex are the first to exit from the cell cycle but the last to begin differentiating as mechanosensory hair cells. ...The dynamic relationship between the restriction of Shh expression in the developing spiral ganglion and its proximity to regions of the growing cochlear duct dictates the timing of terminal mitosis of hair cell precursors and their subsequent differentiation." Sonic hedgehog
- Tbx1 and Brn4 regulate retinoic acid metabolic genes during cochlear morphogenesis.[12] "These results indicate that Tbx1 expression in the POM regulates cochlear outgrowth potentially via control of local retinoic acid activity."
|
Otic Placode
The embryonic surface sensory placode associated with hearing and balance. This will be lost from the embryo surface to form the otocyst or otic vesicle.
- Stage 11 - single layer of ectodermal cells organized in a columnar epithelium, which differs in cell shape from the surrounding cuboidal epithelia that will contribute the epithelia of the skin.
- zebrafish model - a number of specific genes are involved in initial induction of the otic placode including both growth factors (fgf3 and fgf8) and transcription factors (dlx3b, dlx4b, and foxi1).[13]
- Proliferation of the otic placode cells leads to an inward folding, or invagination, giving the external appearance of a depression on the lateral sides of the early developing neck region.
- The epithelium is still a single layer of cells, which continues to invaginate until the edges of the disc of cells come into apposition on the embryo surface.
- mouse model - placodal invagination but not specification requires placodal expression of the transcription factor Sox9.[14]
Sensory Placodes
- Week 4 a series of thickened surface ectodermal patches form in pairs rostro-caudally in the head region.
- Recent research suggests that all sensory placodes may arise from common panplacodal primordium origin around the neural plate, and then differentiate to eventually have different developmental fates.
- Each pair of sensory placodes will later contribute key components of each of our special senses (hearing, vision, smell and taste).
- Otic Placode - one of the first to form and contributes inner ear structures.
- Optic (Lens) Placode - lies on the surface, adjacent to the outpocketing of the nervous system (which will for the retina) and will form the lens.
- Nasal Placode - 2 components (medial and lateral) and will form the nose olefactory epithelium.
- Other species have a number of additional placodes which form other sensory structures (fish, lateral line receptor).
- Note that their initial postion on the developing head is significantly different to their final position in the future sensory system.
- Links: Placodes | Week 4
Cochlea Timeline
The otocyst will differentiate to form all components of the membranous labyrinth. The organ of Corti forms initially from the central domain on the medial side of the otocyst.[8] Sox2 regulates organ of Corti prosensory progenitor development, through Jag1 (Notch ligand) signaling.
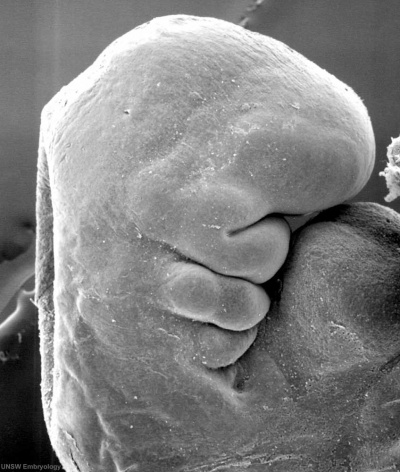
|
Stage 13 otic vesicle now lies beneath the embryo surface.
- Stage 13 - the otic placode has sunk from the surface ectoderm to form a hollow epithelial ball, the otocyst (otic vesicle), which now lies beneath the surface surrounded by mesenchyme (mesoderm and neural crest).
- The epithelia of this ball varies in thickness and has begun to distort, it will eventually form the inner ear membranous labyrinth.
- forms otocyst
- branches form and generate endolymphatic duct and sac
- forms vestibular (dorsal) and cochlear (ventral) regions
- differentiation of otic vesicle to membranous labyrinth
|
- Otocyst historic drawings
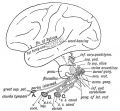
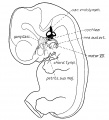
Section human embryo 12 days hind-brain
Week 5
Stage 13 embryo (week 5) showing otocyst that will form the inner ear.

| A. Ventrolateral view of the whole embryo with 5-mm scale bar. At this stage of development no middle or external ear structures are apparent and will be derived later from pharyngeal arches one and two (labeled).
|
B. The gray bar through the head indicates the plane of cross-section, which is a cross-section of the head showing the size and position of the otic vesicles. At this stage of development they lie within the head mesenchyme behind pharyngeal arch one and two and in close apposition to the developing hindbrain. Note the close position of the otic vesicle to the rhombomeres, hindbrain folds that represent the initial segmentation of the hindbrain. Also shown are developing cranial ganglia and blood vessel lying adjacent to the otic vesicles. The wall of the otic vesicle at this stage is a simple epithelium.
|
Week 7
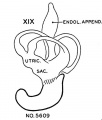
Based upon Streeter.[15]
- Carnegie stage 18 - L-shaped cochlear duct.
- Carnegie stage 19 - Tip of cochlea turns up; short tip.

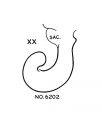
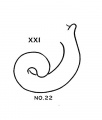
Week 8
Based upon Streeter.[15]
- Carnegie stage 20 - Long tip; transitional stage.
- Carnegie stage 21 - Return down curve (tip turns down).
- Carnegie stage 22 - Transitional stage.
- Carnegie stage 23 - Tip turns up second time. Tip turns down second time (second return down curve).
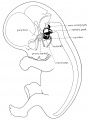
Stage 22 embryo (week 8) showing the embryo near the end of the embryonic period.
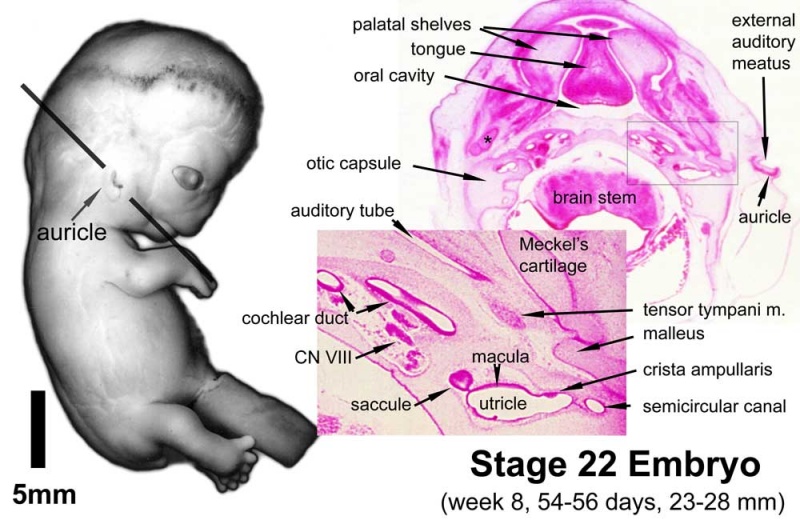
| A. Lateral view of the whole embryo with 5 mm scale bar. Note the well developed external ear with simplified adult structure and narrower meatal opening. The grey bar through the head indicates the plane of cross-section for (B) and (C).
|
B. Cross-section of the head at the plane of the skull base and oral cavity to the top. The otic capsule is well formed by this stage containing all the membranous labyrinth structures. It is still a cartilaginous structure ventral to the brainstem and lying behind the oral cavity. The tongue occupies the floor of the oral cavity with the unfused palatal shelves lying lateral and the auditory tubes clearly shown on the posterior wall. The external ear is visible on the right hand side of the head with a band of cartilage (dark stain) within the auricle.
C. The gray box indicates this region: detail of inner and middle ear development. The middle ear cavity has not yet formed and the ossicles (malleus shown) are embedded in mesenchyme that is being lost. The tensor tympani muscle is differentiating in the adjacent mesenchyme. The inner ear membranous labyrinth has formed its adult external structure. The section through the turns of the cochlear duct shows the internal cochlea structure is still underdeveloped; in contrast, the balance region is more developed.
|
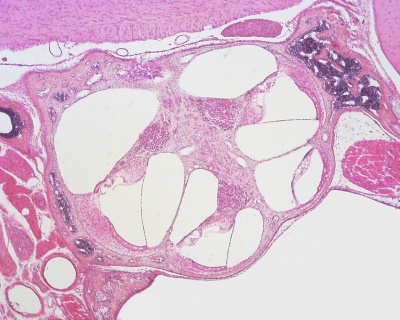
The Membranous Labyrinth
Periotic space first appears in week 8 embryos 35 mm CRL around the vestibule and basal turn of the cochlea.[5]
Fetal
Week 8.4
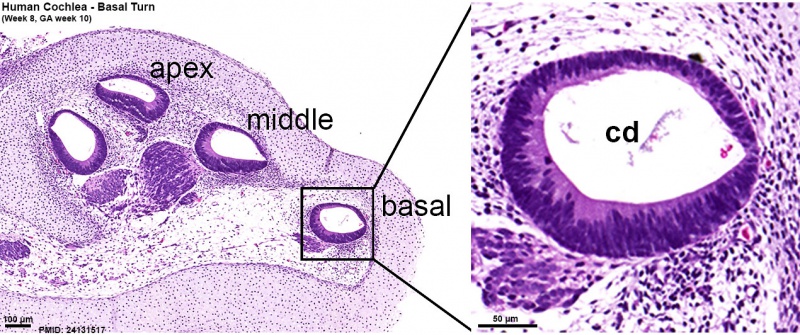
Human fetal cochlea basal turn week 8.4 Gestational Week GA 10.4 (Stain - Haematoxylin Eosin)[16]
Week 10
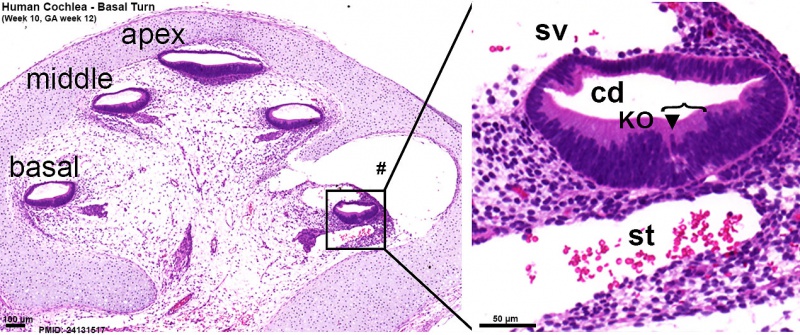
Human fetal cochlea basal turn week 10 Gestational Week GA 12 (Stain - Haematoxylin Eosin)[16]
Week 14
Periotic space almost covers the membranous labyrinth in the fetus 115 mm CRL week 14 (GA week 16).[5]
Week 18 - 22
Developmental data from an EM study of the human organ of corti.[17] Original GA data has been recalculated as fertilisation ages.
| Week
|
Event
|
| 18
|
stria vascularis, tectorial membrane, afferent nerve ending
|
| 20
|
Nuel space, stereocilia maturation, efferent nerve ending
|
| 22
|
tunnel of corti, kinocilium, space between hair and supporting cell
|
| Week 18
|
Week 20
|
Week 22
|
- stria vascularis - 18-20 weeks (GA 20 week 3 days - 22 week)
- tectorial membrane - 18 weeks (GA 20 week 3 days)
- Afferent nerve ending - 18 weeks (GA 20 week 3 days)
|
- Nuel space - 20 weeks (GA 22 week 0 days - 24 week)
- Efferent nerve ending - 20 weeks (GA 22 week 0 days)
- Maturation of stereocilia - 20 weeks (GA 22 week 0 days - 24 week 0 days)
|
- tunnel of corti - GA 24 week
- Space between hair and supporting cell - 24 week
- Presence of kinocilium - GA 24 week 0 days
|
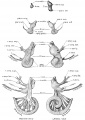
Historic
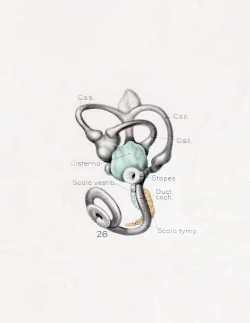
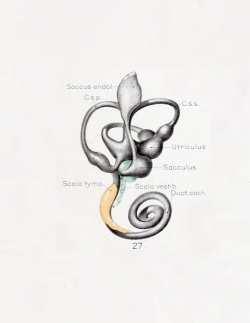
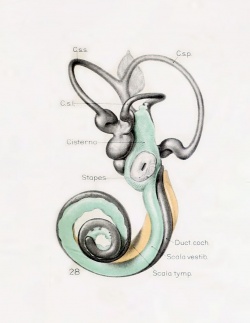
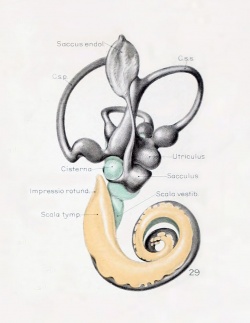
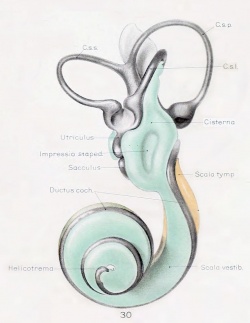
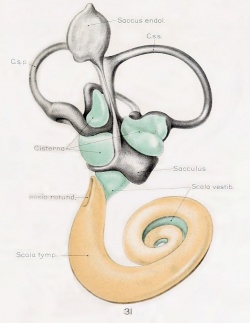
A series of historic wax-plate reconstructions of the membranous labyrinth and the surrounding periotic tissue-spaces showing development stages (median and lateral views) of these spaces at the same scale (ages are only approximations calculated on CRL).
| Human Fetal Membranous Labyrinth Development
|
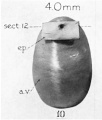
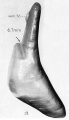
| Fetus 50 mm CRL 10 weeks (GA 12)
|
|
|
| lateral view
|
median view
|
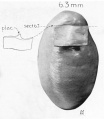
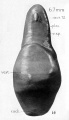
| Fetus 85 mm CRL 14 weeks (GA 16)
|
|
|
| lateral view
|
median view
|
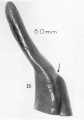
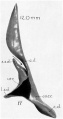
| Fetus 130 mm CRL 15 weeks (GA 17)
|
|
|
| lateral view
|
median view
|
- Links: Growth of the Otic Capsule (1918) | Fetal CRL | Fetal Development
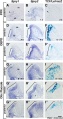
Fetal Cochlea Molecular
Human fetal cochlea basal turn by Gestational Week GA[16]
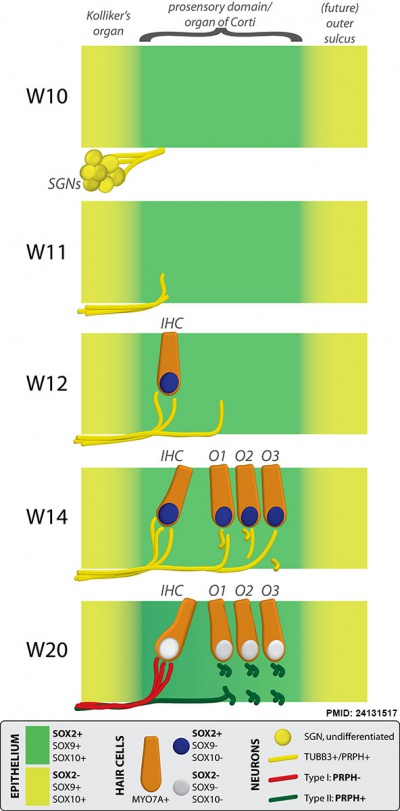
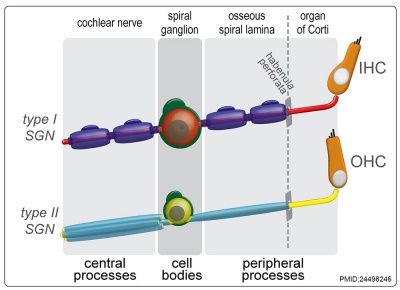
Abbreviations
- SGN - spiral ganglion neuron
- IHC - inner hair cell
- O1 - first row of outer hair cells
- O2 - second row of outer hair cells
- O3 - third row of outer hair cells
- OHC - outer hair cell.
|
Gestational Week GA
Week 10 (W10) - SOX2 identifies the prosensory domain within the SOX9/SOX10+ cochlear duct epithelium. Neurites from the adjoining TUBB3+/PRPH + SGNs do not yet penetrate into the epithelium.
Week 11 (W11) - Penetration starts prior to hair cell differentiation.
Week 12 (W12) - The first MYO7A+/SOX9-/SOX10-/SOX2+ (inner) hair cell can be seen, and is contacted by multiple TUBB3+ and PRPH + neurites. Penetrating neurites are also found at the location of the future OHCs.
Week 14 (W14) - Both the IHCs and OHCs have differentiated, and neurites underneath the OHCs start to run in a spiral direction. At this stage, hair cells still express SOX2.
Week 20 (W20) - SOX2 is downregulated in all hair cells, as opposed to the other cells in the organ of Corti. PRPH expression distinguishes between type I (PRPH-) and type II (PRPH+) neurites.
|
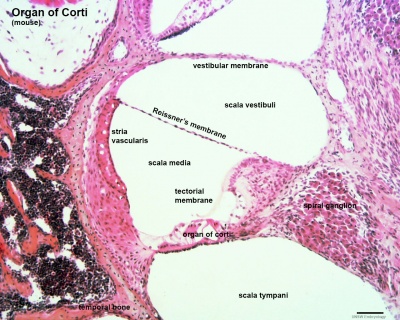
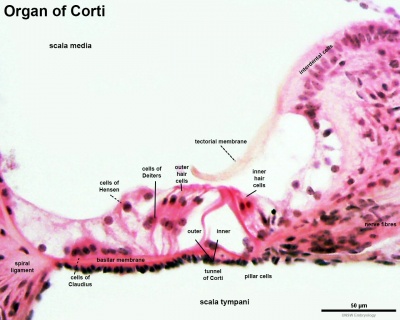
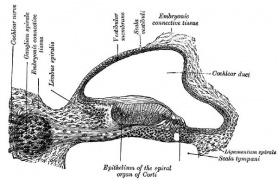
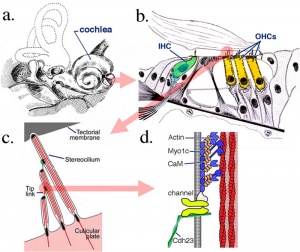
Organ of Corti
Within the cochlea, the specialised structure required for converting mechanical vibration into an electrical signal occurs at the organ of Corti (spiral organ of Corti).
The images (mouse) below show the detail of the specialised structure, the organ of Corti, that develops through the fetal period.
Endolymphatic Sac
- the adult endolymphatic sac is filled with endolymphatic fluid
- with a unique composition of high potassium and low sodium ions
- it is not known in humans at what stage of development this ionic status is achieved
- In the rat, adult sodium levels are seen in the first week after birth, while both potassium and chloride levels were below the normal adult levels.
|
|
Vestibular Sac
- generates 3 expansions - form semicircular ducts
- remainder forms utricle
- epithelia lining generates - hair cells, ampullary cristae, utricular macula
- Vestibular - Otoconia, otoconin- inner ear biominerals
Cochlear Sac
- generates coiled cochlear duct (humans 2 1/2 turns)
- remainder forms saccule
- epithelia lining generates
- hair cells
- structures of organ of corti
- saccular macula
Scala Media
three spiral passages of cochlea
(Latin, medius = middle) spiral of middle cochlear duct lying between scala vestibuli and scala tympani, containing endolymph.
Scala Tympani
(Latin, tympanon = drum) the spiralling cochlear duct below spiral lamina, containing perilymph and ending at round window near tympanic membrane.
Scala Vestibuli
(Latin, vestibulum = cavity at beginning of canal) the spiralling cochlear duct above spiral lamina, containing perilymph, beginning near the vestbule and ending where it communicates with the scala tympani at the helicotrema.
Endolymph
- extracellular fluid secreted by the stria vascularis.
- potassium is the main cation required for depolarizing electrical current in the hair cells.
Perilymph
- extracellular fluid similar in composition to either plasma or cerebrospinal fluid.
- sodium is the main cation.
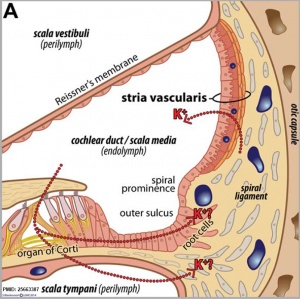
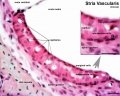
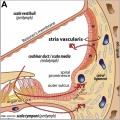
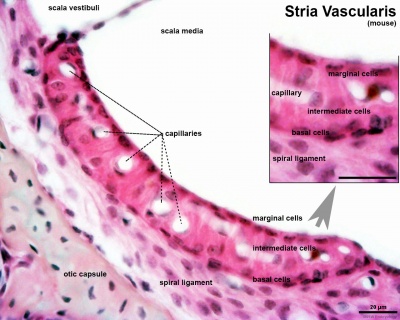
Stria Vascularis
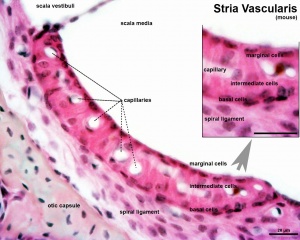
|
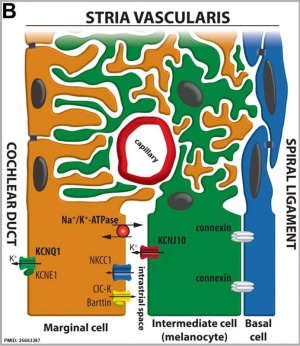
|
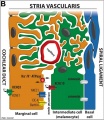
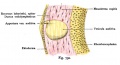
| In the adult the stria vascularis functions to synthesise and secretes endolymph. The three main cell types within the stria that have different embryological origins and are connected by different forms of cell junctions.
|
Anatomical (upper half) and compartmental (lower half) model of the adult stria vascularis showing the three cellular layers and depicting the location of potassium regulating channels. The stria vascularis is electrochemically isolated from neighboring structures by tight junctions (black bars).[9]
|
| Marginal cells
|
Intermediate cells
|
Basal cells
|
| line the lumen of the cochlear duct
|
melanocyte-like cells lie between the marginal and basal cell layers
|
mesenchymal spiral ligament fibrocytes
|
| derived from epithelia.
|
derived from the neural crest.
|
derived from otic mesenchyme.[18]
|
- Links: Neural Crest - Inner Ear | Neural Crest - Melanocytes
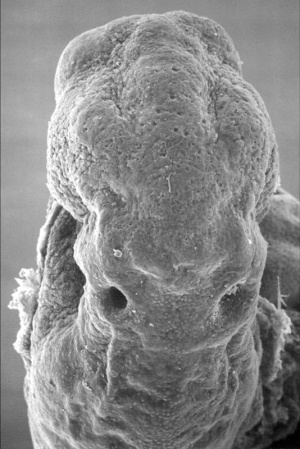
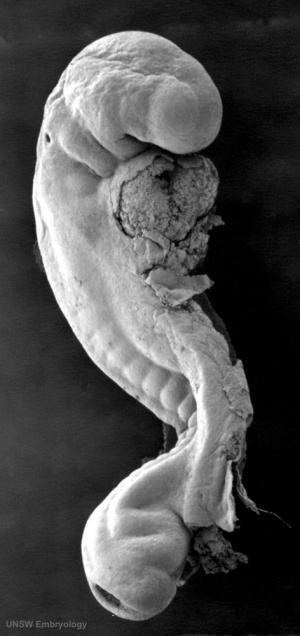
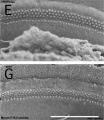
Mouse SEM
The gallery below shows scanning electron micrographs of the developing mouse (E18.5) cochlea.[19]
Adult Cochlea
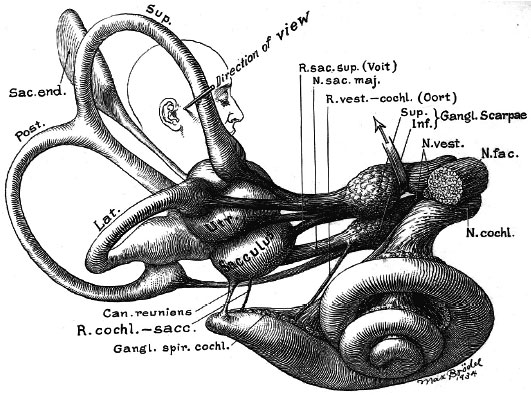
Adult inner ear (Max Brödel 1934)
| Magnetic Resonance Images of the Adult Cochlea[20]
|
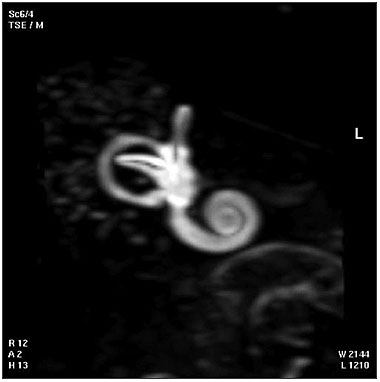
|
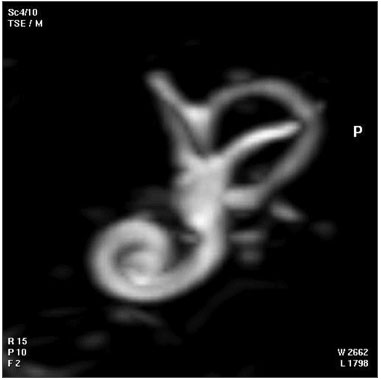
|
The investigators used a method to obtain magnetic resonance imaging (MRI) to measure cochlear length from the temporal bones of 6 cadavers. By overlapping digitalized rulers on these images it was possible to measure cochlear length. Adult cochlear length varied between 17 and 26.5 millimeters.[20]
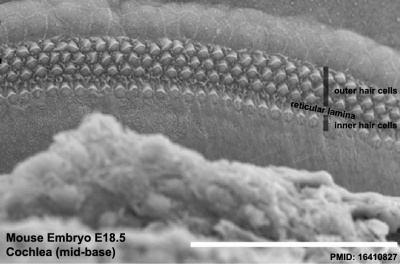
Mouse cochlea (SEM) showing organization of the hair cells.[19]
- Mouse Cochlea Links: Cochlea overview SEM | Base region SEM | Mid-base and Apex region SEM | Mid-base region SEM | Mid-base hair cells SEM | Mouse Development
Bony Labyrinth
- formed from chrondified mesoderm
- Periotic Capsule
- mesenchyme within capsule degenerates to form space filled with perilymph
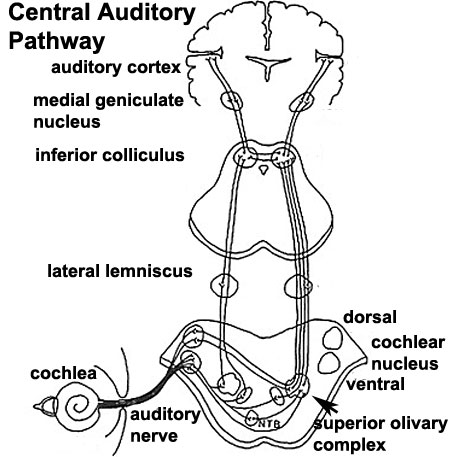
Auditory Neural Pathway
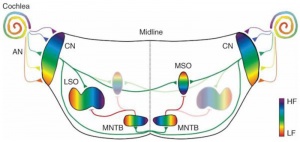
Hearing sound localization circuits brainstem
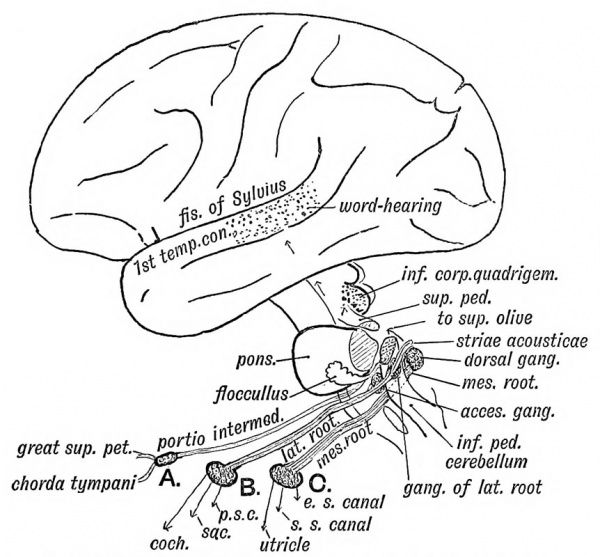
Vestibulocochlear Nerve
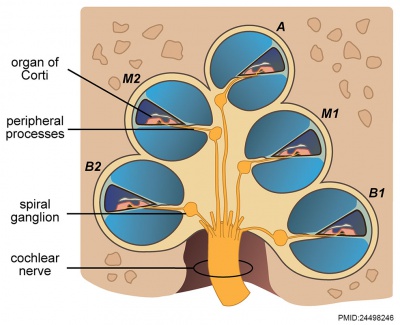
|
| Adult cochlea nerve glia cartoon[10]
|
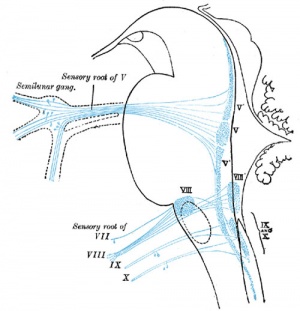
Afferent (sensory) cranial nerve brainstem primary terminal nuclei
- forms beside otocyst
- from wall of otocyst and neural crest cells
- bipolar neurons
Vestibular Neurons
- outer end of internal acoustic meatus
- innervate hair cells in membranous labyrinth
- axons project to brain stem and synapse in vestibular nucleus
Cochlear Neurons
- cell bodies lie in modiolus
- central pillar of cochlear
- innervate hair cells of spiral organ
- axons project to cochlear nucleus
- Links: Hearing - Neural Pathway
Cochlea Glial
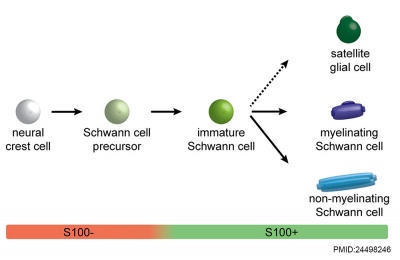
|
|
| Cochlea glial lineage [10]
|
Adult cochlea nerve glia cartoon[10]
|
- Links: Hearing - Neural Pathway
Inner Ear Genes
- hindbrain segmentation occurs at same time placode arises
- otocyst adjacent to rhombomere 5
- may influence development
- Hoxa1, kreisler, Fgf3
- genes regulating neural crest cells (neural genes)
- Pax2 Ko affects cochlear and spiral ganglion, but not vestibular apparatus
- nerogenin 1 affects both ganglia
- LIN28B - RNA-binding protein times auditory prosensory cell cycle withdrawal and differentiation through both let-7–dependent and independent mechanisms.[21]
From a recent study of human fetal tissue.[1]
- Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) - apical poles of the sensory epithelium of the cochlear duct and the vestibular end organs GA week 11. Limited to hair cells of the organ of Corti by GA week 12.
- transforming growth factor-β-activated kinase-1 (TAK1) - inner hair cells GA week 12 and colocalized with p75 neurotrophic receptor expression.
- SRY (sex-determining region Y)-box 2 (SOX2) - supporting cells of utricle at the earliest stage examined at GA week 9.
- GATA binding protein 3 (GATA3) - cochlear sensory epithelium and spiral ganglia at GA week 9. Midline of both the utricle and saccule in the zone corresponding to the striolar reversal zone where the hair cell phenotype switches from type I to type II.
Tsukushi (TSK) - mouse study[22]
- a small, secreted, leucine-rich-repeat proteoglycan-interacts with and regulates cellular signaling cascades.
- interacts with Sox2 and BMP4 to control stereocilia formation in the inner hair cells.
- early embryonic stages - accumulates in nonprosensory regions
- late embryonic stages - and in both nonprosensory and prosensory.
- adult mice - localized in the organ of Corti, spiral ganglion cells, and the stria vascularis.
Semicircular canal
- Otx1- cochlear and vestibular normal
Sensory Organs
- thyroid hormone receptor beta
- Zebrafish-mindbomb mutant has excess hair cells but not supporting cells, Notch-Delta signaling
- Gene Expression-inner ear
- Brn-3c and Hair cell development
- Supporting Cells- p27kip
- Thyroid Hormone
- Ganglion neurons require growth factors
- vestibular neurons- BDNF, NT3
Sox9 20346939
Sox2 20071536
Other Species Overview
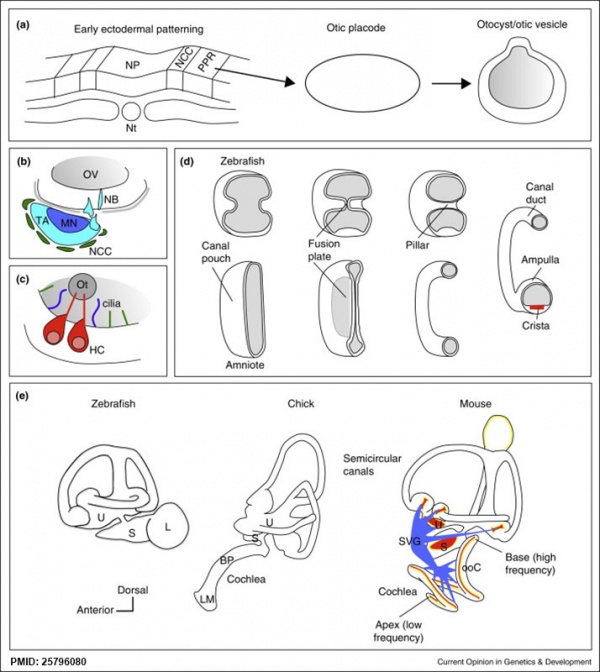
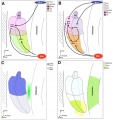
Comparison of zebrafish and late stage chick and mouse embryos[23]
Zebrafish Development Chicken Development | Mouse Development
|
(a) Formation of the pre-placodal region (PPR), otic placode and otocyst (otic vesicle) from cranial ectoderm. The otocyst is the source of nearly all cell types of the mature ear.
(b) Otic neurogenesis: neuroblasts are specified from otic vesicle epithelium, but delaminate from it and accumulate beneath the ear in a transit amplifying population (light blue). Neurons (dark blue) differentiate from this population, and innervate sensory hair cells in the overlying otic epithelium. The ganglion develops in close association with neural crest cells (green), which give rise to glia.
(c) Early otolith formation in the zebrafish otic vesicle. At least three distinct populations of cilia can be distinguished: immotile hair cell kinocilia (red), which tether the otolith at early stages; motile cilia (blue) in the vicinity of the sensory hair cells, which do not bind otolithic material, and shorter immotile cilia (green).
(d) Schematic comparison of semicircular canal formation in the zebrafish ear (top row) and a generalised amniote ear (bottom row). A single canal is illustrated for clarity. Epithelia adhere at a fusion plate, from which cells are cleared to make the duct. The end result of both events is the same (right hand image), but the fusion plate is much smaller in the zebrafish.
(e) Comparative sketches of inner ears from adult zebrafish and late stage chick and mouse embryos. Sensory (red), neuronal (blue) and endolymph-regulating (yellow) cells are shown for the mouse ear.
Abbreviations: A, ampulla; BP, basilar papilla; HC, hair cell; L, lagena; LM, lagenar macula; MN, maturing neurons; NB, neuroblasts; NCC, neural crest cells; NP, neural plate; Nt, notochord; ooC, organ of Corti; Ot, otolith; OV, otic vesicle; PPR, preplacodal region; S, saccule; SVG, spiral and vestibular ganglion; TA, transit amplifying population of neuroblasts; U, utricle.
(text from original figure legend)
|
Mouse
- Dual embryonic origin of the mammalian otic vesicle forming the inner ear[24] "The inner ear and cochleovestibular ganglion (CVG) derive from a specialized region of head ectoderm termed the otic placode. During embryogenesis, the otic placode invaginates into the head to form the otic vesicle (OV), the primordium of the inner ear and CVG. Non-autonomous cell signaling from the hindbrain to the OV is required for inner ear morphogenesis and neurogenesis. In this study, we show that neuroepithelial cells (NECs), including neural crest cells (NCCs), can contribute directly to the OV from the neural tube. ...This study defines a dual cellular origin of the inner ear from sensory placode ectoderm and NECs, and changes the current paradigm of inner ear neurosensory development."
References
- ↑ 1.0 1.1 Johnson Chacko L, Sergi C, Eberharter T, Dudas J, Rask-Andersen H, Hoermann R, Fritsch H, Fischer N, Glueckert R & Schrott-Fischer A. (2020). Early appearance of key transcription factors influence the spatiotemporal development of the human inner ear. Cell Tissue Res. , 379, 459-471. PMID: 31788757 DOI.
- ↑ Biedron S, Westhofen M & Ilgner J. (2009). On the number of turns in human cochleae. Otol. Neurotol. , 30, 414-7. PMID: 19225438 DOI.
- ↑ Kolla L, Kelly MC, Mann ZF, Anaya-Rocha A, Ellis K, Lemons A, Palermo AT, So KS, Mays JC, Orvis J, Burns JC, Hertzano R, Driver EC & Kelley MW. (2020). Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat Commun , 11, 2389. PMID: 32404924 DOI.
- ↑ 4.0 4.1 Jansson L, Ebeid M, Shen JW, Mokhtari TE, Quiruz LA, Ornitz DM, Huh SH & Cheng AG. (2019). β-Catenin is required for radial cell patterning and identity in the developing mouse cochlea. Proc. Natl. Acad. Sci. U.S.A. , 116, 21054-21060. PMID: 31570588 DOI.
- ↑ 5.0 5.1 5.2 Ishikawa A, Ohtsuki S, Yamada S, Uwabe C, Imai H, Matsuda T & Takakuwa T. (2018). Formation of the Periotic Space During the Early Fetal Period in Humans. Anat Rec (Hoboken) , 301, 563-570. PMID: 29293291 DOI.
- ↑ Chadly DM, Best J, Ran C, Bruska M, Woźniak W, Kempisty B, Schwartz M, LaFleur B, Kerns BJ, Kessler JA & Matsuoka AJ. (2018). Developmental profiling of microRNAs in the human embryonic inner ear. PLoS ONE , 13, e0191452. PMID: 29373586 DOI.
- ↑ Johnson Chacko L, Blumer MJF, Pechriggl E, Rask-Andersen H, Dietl W, Haim A, Fritsch H, Glueckert R, Dudas J & Schrott-Fischer A. (2017). Role of BDNF and neurotrophic receptors in human inner ear development. Cell Tissue Res. , 370, 347-363. PMID: 28924861 DOI.
- ↑ 8.0 8.1 Gu R, Brown RM, Hsu CW, Cai T, Crowder AL, Piazza VG, Vadakkan TJ, Dickinson ME & Groves AK. (2016). Lineage tracing of Sox2-expressing progenitor cells in the mouse inner ear reveals a broad contribution to non-sensory tissues and insights into the origin of the organ of Corti. Dev. Biol. , 414, 72-84. PMID: 27090805 DOI.
- ↑ 9.0 9.1 9.2 Locher H, de Groot JC, van Iperen L, Huisman MA, Frijns JH & Chuva de Sousa Lopes SM. (2015). Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss. Dev Neurobiol , 75, 1219-40. PMID: 25663387 DOI.
- ↑ 10.0 10.1 10.2 10.3 Locher H, de Groot JC, van Iperen L, Huisman MA, Frijns JH & Chuva de Sousa Lopes SM. (2014). Distribution and development of peripheral glial cells in the human fetal cochlea. PLoS ONE , 9, e88066. PMID: 24498246 DOI.
- ↑ Bok J, Zenczak C, Hwang CH & Wu DK. (2013). Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells. Proc. Natl. Acad. Sci. U.S.A. , 110, 13869-74. PMID: 23918393 DOI.
- ↑ Braunstein EM, Monks DC, Aggarwal VS, Arnold JS & Morrow BE. (2009). Tbx1 and Brn4 regulate retinoic acid metabolic genes during cochlear morphogenesis. BMC Dev. Biol. , 9, 31. PMID: 19476657 DOI.
- ↑ Solomon KS, Kwak SJ & Fritz A. (2004). Genetic interactions underlying otic placode induction and formation. Dev. Dyn. , 230, 419-33. PMID: 15188428 DOI.
- ↑ Barrionuevo F, Naumann A, Bagheri-Fam S, Speth V, Taketo MM, Scherer G & Neubüser A. (2008). Sox9 is required for invagination of the otic placode in mice. Dev. Biol. , 317, 213-24. PMID: 18377888 DOI.
- ↑ 15.0 15.1 Streeter GL. Developmental Horizons In Human Embryos Description Or Age Groups XIX, XX, XXI, XXII, And XXIII, Being The Fifth Issue Of A Survey Of The Carnegie Collection. (1957) Carnegie Instn. Wash. Publ. 611, Contrib. Embryol., 36: 167-196.
- ↑ 16.0 16.1 16.2 Locher H, Frijns JH, van Iperen L, de Groot JC, Huisman MA & Chuva de Sousa Lopes SM. (2013). Neurosensory development and cell fate determination in the human cochlea. Neural Dev , 8, 20. PMID: 24131517 DOI.
- ↑ Igarashi Y & Ishii T. (1980). Embryonic development of the human organ of Corti: electron microscopic study. Int. J. Pediatr. Otorhinolaryngol. , 2, 51-62. PMID: 7188054
- ↑ Trowe MO, Maier H, Petry M, Schweizer M, Schuster-Gossler K & Kispert A. (2011). Impaired stria vascularis integrity upon loss of E-cadherin in basal cells. Dev. Biol. , 359, 95-107. PMID: 21925491 DOI.
- ↑ 19.0 19.1 Kiernan AE, Xu J & Gridley T. (2006). The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. , 2, e4. PMID: 16410827 DOI.
- ↑ 20.0 20.1 Pochini Sobrinho F, Lazarini PR, Yoo HJ, Abreu Júnior Ld & Meira Ade S. (2009). A method for measuring the length of the cochlea through magnetic resonance imaging. Braz J Otorhinolaryngol , 75, 261-7. PMID: 19575114
- ↑ Golden EJ, Benito-Gonzalez A & Doetzlhofer A. (2015). The RNA-binding protein LIN28B regulates developmental timing in the mammalian cochlea. Proc. Natl. Acad. Sci. U.S.A. , 112, E3864-73. PMID: 26139524 DOI.
- ↑ Miwa T, Ohta K, Ito N, Hattori S, Miyakawa T, Takeo T, Nakagata N, Song WJ & Minoda R. (2020). Tsukushi is essential for the development of the inner ear. Mol Brain , 13, 29. PMID: 32127020 DOI.
- ↑ Whitfield TT. (2015). Development of the inner ear. Curr. Opin. Genet. Dev. , 32, 112-8. PMID: 25796080 DOI.
- ↑ Freyer L, Aggarwal V & Morrow BE. (2011). Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development , 138, 5403-14. PMID: 22110056 DOI.
Reviews
Magariños M, Pulido S, Aburto MR, de Iriarte Rodríguez R & Varela-Nieto I. (2017). Autophagy in the Vertebrate Inner Ear. Front Cell Dev Biol , 5, 56. PMID: 28603711 DOI.
Nakajima Y. (2015). Signaling regulating inner ear development: cell fate determination, patterning, morphogenesis, and defects. Congenit Anom (Kyoto) , 55, 17-25. PMID: 25040109 DOI.
Basch ML, Brown RM, Jen HI & Groves AK. (2016). Where hearing starts: the development of the mammalian cochlea. J. Anat. , 228, 233-54. PMID: 26052920 DOI.
Chatterjee S, Kraus P & Lufkin T. (2010). A symphony of inner ear developmental control genes. BMC Genet. , 11, 68. PMID: 20637105 DOI.
Driver EC & Kelley MW. (2009). Specification of cell fate in the mammalian cochlea. Birth Defects Res. C Embryo Today , 87, 212-21. PMID: 19750520 DOI.
Articles
Schade-Mann T, Münkner S, Eckrich T & Engel J. (2020). Calcium signaling in interdental cells during the critical developmental period of the mouse cochlea. Hear. Res. , 389, 107913. PMID: 32120242 DOI.
Miwa T, Ohta K, Ito N, Hattori S, Miyakawa T, Takeo T, Nakagata N, Song WJ & Minoda R. (2020). Tsukushi is essential for the development of the inner ear. Mol Brain , 13, 29. PMID: 32127020 DOI.
Johnson Chacko L, Sergi C, Eberharter T, Dudas J, Rask-Andersen H, Hoermann R, Fritsch H, Fischer N, Glueckert R & Schrott-Fischer A. (2020). Early appearance of key transcription factors influence the spatiotemporal development of the human inner ear. Cell Tissue Res. , 379, 459-471. PMID: 31788757 DOI.
Gnedeva K & Hudspeth AJ. (2015). SoxC transcription factors are essential for the development of the inner ear. Proc. Natl. Acad. Sci. U.S.A. , 112, 14066-71. PMID: 26504244 DOI.
Santi PA, Rapson I & Voie A. (2008). Development of the mouse cochlea database (MCD). Hear. Res. , 243, 11-7. PMID: 18603386 DOI.
Search PubMed
May 2010 "Inner Ear Development" All (4027) Review (452) Free Full Text (750)
Search Pubmed: Inner Ear Development Cochlea Development
Historic
Keith A. Human Embryology and Morphology. (1902) London: Edward Arnold.
Van der Stricht O. The development of the pillar cells, tunnel space, and Nuel's spaces in the organ of Corti. (1919) J Comp. Neurol. 30: 283-.
Additional Images
human vascularis development
Mouse cochlea gene expression
Cochlear and vestibular specification
Mouse cochlea development cartoon
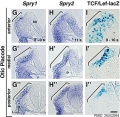
Mouse otic placode Spry1, Spry2, and Wnt
Mouse otic placode Spry1, Spry2, and Wnt
Mouse - inner ear cartoon
Human inner ear MicroCT PMID 30108474
Cat inner ear MicroCT PMID 30108474
Historic Images
| Historic Disclaimer - information about historic embryology pages
|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers)
|
Van der Stricht O. The development of the pillar cells, tunnel space, and Nuel's spaces in the organ of Corti. (1919) J Comp. Neurol. 30: 283-.
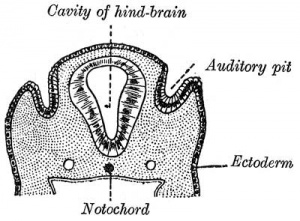
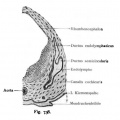
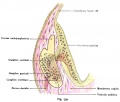
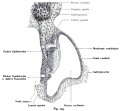
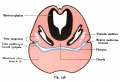
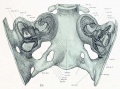
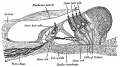
Lewis Fig. 16 Dorsal view of temporal and occipital cartilages, showing the relation of the inner ear to the otic capsule.
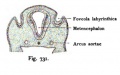
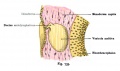
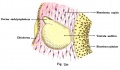
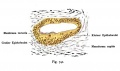
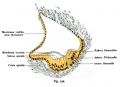
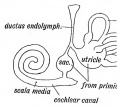
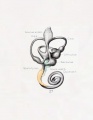
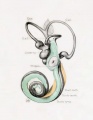
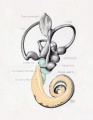
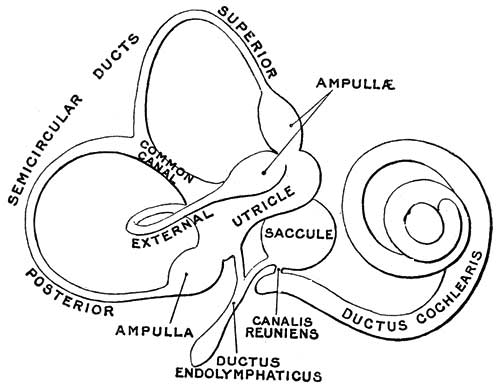
Fig. 44. Diagram of the Membranous Labyrinth.
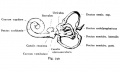
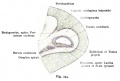
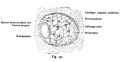
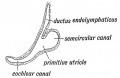
Fig. 45. The Otocyst in an Embryo of five weeks ; it shows a demarcation into the various parts of the Membranous Labyrinth.
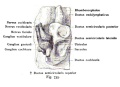
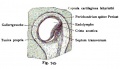
Fig. 46. Showing the Nerve Structures concerned in the Sense of Hearing.
Anson BJ. The early development of the membranous labyrinth in mammalian embryos, with special reference to the endolymphatic duct and the utriculo—endolymphatic duct. (1934) Anat. Rec. 59: 15-25.
Fig. 1-8. Transverse sections of embryos in the Harvard Embryological Collection
Template:Ref-Streeter1906
Fetus 50 mm CRL (lateral)
Fetus 85 mm CRL (lateral)
Fetus 130 mm CRL (lateral)
Fetus 130 mm CRL (median)
Streeter GL. Developmental Horizons In Human Embryos Description Or Age Groups XIX, XX, XXI, XXII, And XXIII, Being The Fifth Issue Of A Survey Of The Carnegie Collection. (1957) Carnegie Instn. Wash. Publ. 611, Contrib. Embryol., 36: 167-196.
Terms
| Hearing Terms
|
Hearing and Balance Development
- altricial animal - Term used to describe an animal born in a helpless state, with incomplete development of sensory systems at birth. For example rats and mice are born with incomplete development of visual and auditory systems. (More? Animal Development)
- ampulla - Term used to describe an anatomical dilation of a tube or canal lumen. Anatomical description of the opening end of the uterine tube lying above the ovary and the enlarged initial segmeny of the semicircular canals of the inner ear vestibular system. (More? inner ear)
- aneurism - (Greek, aneurysma = a widening, aneurysm) A term used to describe an abnormal widening of a vessel or anatomical tubal structure.
- aquaeductus vestibuli - see vestibular aqueduct (More? inner ear)
- auditory neuropathy - (AN) abnormality of transmission of sound information to the brain.
- auditory tube - (eustachian tube) between the middle ear and oral cavity, has a bony (tympanic 1/3) and cartilaginous (pharyngeal 2/3) portion. The main role is equalization of pressure and fluid drainage in the middle ear. (More? middle ear)
- auricular hillock - see hillock (More? middle ear)
- atresia - narrowing, usually of an anatomical tube or cavity.
- autophagocytosis - (Greek, auto = self, phagy = eating, also called autophagy) a cell death mechanism that uses the cell's own lysosomes to self digest.
- border cells - columnar cells within the organ of Corti on the medial portion of the basilar membrane. (More? inner ear)
- canalis reuniens - (ductus reuniens, canaliculus reuniens, canalis reuniens, Hensen's canal, Hensen's duct, uniting canal, canalis reuniens of Hensen) short narrow canal connecting the cochlea duct to the saccule. (Victor Hensen, 1835-1924) (More? inner ear)
- cerumen - (ear wax) produced by glands in the skin of the outer portion of the ear canal. (More? Outer Ear)
- chondrified - the developmental differentiation of cartilage from mesenchye, an embryonic connective tissue.
- cristae ampullaris - located in the ampulla of the membranous semicircular canals a region with both supporting and hair cells. The hair cell cilia are embedded in the gelatinous cupula. (More? inner ear)
- claudius cells - (cells of Claudius) columnar cells with microvilli overlying the basilar membrane and extend from Hensen's cells to the spiral prominence. Barrier cells that lie external to the organ of corti in endolymph. (More? inner ear)
- cochlear sac - embryonic structure, which will form the coiled cochlear duct and contribute to the saccule. (More? inner ear)
- cochlear aqueduct - a bony channel containing the fibrous periotic duct. It connects the basal turn of the cochlea perilymphatic space with the subarachnoid space of the posterior cranial cavity. (More? inner ear)
- cochlin - major constituent of the inner ear extracellular matrix. (More? inner ear)
- collagen type II - major constituent of the inner ear extracellular matrix. (More? inner ear)
- conductive loss - term used to describe one of the two major classes of hearing loss involving external and middle ear abnormalities (other form is Sensorineural loss).
- connexins - channel proteins of the gap junctions that allow rapid communication between adjacent cells. The two connexins Cx26 and Cx30 are the major proteins of cochlear gap junctions.
- connexin 26 - A strikingly high proportion (50%) of congenital bilateral nonsyndromic sensorineural deafness cases have been linked to mutations in the GJB2 coding for the connexin26
- cupular deposits - basophilic material on the cupulae of the semicircular ducts, an postnatal ageing phenomenon seen in some vestibular labyrinth. (More? inner ear)
- clinical weeks - taken from last menstrual period (LMP) and therefore approximately two weeks before fertilization occurs.
- Deiters' cells - (outer phalangeal cells)
- discoidin domain receptor 1 - (DDR1) a tyrosine kinase receptor activated by native collagen, expressed in the basement membrane and with fibrillar collagens. Found in basal cells of the stria vascularis, type III fibrocytes, and cells lining the basilar membrane of the organ of Corti.
- ductus utriculosaccularis - inner ear structure, a canal from the utricle that joins the ductus endolymphaticus from the saccule posterior wall, and then passes along the aquaductus vestibuli and ends in a blind pouch (saccus endolymphaticus) in the petrous portion of the temporal bone, also in contact with the dura mater. (More? image | balance | inner ear)
- endochondral ossification - the process of bone formation from a pre-existing cartilage template. (More? middle ear)
- endoderm - One of the initial 3 germ cell layers (ectoderm, mesoderm and endoderm) formed by the process of gastrulation. The endoderm forms as a cuboidal epithelium and contributes not only to the trilaminar embryo, but also lines the yolk sac. It will form the entire epithelial lining of the gastrointestinal tract (GIT), contribute to the accessory organs of GIT and also forms the epithelial lining of the respiratory tract.
- endolymphatic fluid - (endolymph, Scarpa's fluid) fluid that fills all the membranous labyrinth of the inner ear, except for the cochlea scala tympani and scala vestibuli which are filled with perilymph.
- endolymphatic sac - inner ear structure that has anatomically both an intraosseous and extraosseous component. Th e sac has functions regulating endolymph that are both secretory and absorptive. Also the site of endolymphatic sac tumors either sporadical occurring or associated with the autosomal-dominant von Hippel-Lindau (VHL) disease, due to a germ line mutation. (More? inner ear)
- embryological weeks - taken from the time of fertilization which typically occurs around the middle (day 14), or just after, of the typical 28 day menstrual cycle. (More? Embryonic Development)
- Emx2 - homeobox gene affecting middle ear and inner ear development.
- eustachian tube - (auditory tube) A cavity linking the pharynx to the middle ear, which develops from the first pharyngeal pouch. Named after Bartolomeo Eustachi (1500 - 1574) an Italian anatomist. (More? middle ear)
- external auditory meatus - (ear canal) develops from the first pharyngeal cleft. (More? outer ear)
- espins - calcium-resistant actin-bundling proteins enriched in hair cell stereocilia and sensory cell microvilli and spiral ganglion neurons (SGNs)
- eustachian tube - (auditory tube) between the middle ear and oral cavity, equalization of pressure in the middle ear. (More? middle ear)
- external auditory meatus - (EAM, ear canal) cavity connecting the external ear to the tympanic membrane. The adult human ear canal is about 2.5 cm long and 0.7 cm in diameter. (More? Outer Ear)
- fenestra ovalis - (oval window) separates the tympanic cavity from the vestibule of the osseous labyrinth. (More? inner ear)
- fenestra rotunda - (round window) separates the tympanic cavity from the scala tympani of the cochlea. (More? inner ear)
- fetus - (foetus) term used to describe human development after the 8th week (10th clinical week, LPM) and covers the developmental periods of second and third trimester.
- fibroblast growth factor 1 - (Fgf-1) a growth factor released from cochlea sensory epithelium which stimulates spiral ganglion neurite branching.
- fibroblast growth factor 8 - (Fgf-8) a growth factor released by inner hair cells which regulates pillar cell number, position and rate of development.
- fibroblast growth factor receptor 3 - (Fgfr-3) a tyrosine kinase receptor with a role in the commitment, differentiation and position of pillar cells in the organ of corti
- fundamental frequency - (natural frequency) the lowest frequency in a harmonic series, for the female voice this is about 225 Hz.
- Glaserian fissure - (petrotympanic fissure, squamotympanic fissure) the fissure in the temporal bone that runs between the temporomandibular joint to the tympanic cavity. Named after Johann Glaser (1629–1675) a Swiss anatomist.
- helicotrema - term used to describe the cochlear apex. (More? inner ear)
- Hensen’s stripe - within the cochlea tectorial membrane a ridge that runs longitudinally along the lower surface immediately adjacent to the hair bundles of the inner hair cells.
- Hes - (hairy and enhancer of split) family of factors, which has been shown to be a general negative regulator of neurogenesis (Zheng, 2000).
- hillock - a small hill, used to describe the six surface elevations on pharyngeal arch one and two. (More? outer ear)
- Incus - (anvil) auditory ossicle (More? middle ear)
- inner phalangeal cells - in the cochlea a single row of cells, that along with and three rows of outer phalangeal cells (Deiter's cells), are the hair cell supporting cells. (More? inner ear)
- inner pillar cells - organ of Corti cells arranged in rows and form a boundary between the single row of inner hair cells and three rows of outer hair cells. These cells have surface-associated microtubule bundles. (More? inner ear)
- inner sulcus - area of the cochlear duct. (More? inner ear)
- internal auditory meatus - (internal acoustic meatus, IAM) Anatomical canal in which CN VII and CN VIII ganglia reside and pass through to the brainstem. This bony canal lies between the posterior surface of the petrous pyramid and the bony labyrinth within the dense petrous bone. Also associated clinically with the site where acoustic neuromas may occur. (More? inner ear)
- kimura membrane - (Kimura’s membrane) within the cochlea tectorial membrane a thickening of the lower surface into which the hair bundles of the outer hair cells are imbedded.
- kinocilium - inner ear hair cell specialised type of cilium on the cell apex.
- Kolliker's organ - (Kollicker's organ, greater epithelial ridge) Developing cochlear structure consisting of columnar-shaped supporting cells filling the inner sulcus and lying directly under the tectorial membrane. This transient organ regresses and generates the space of the inner sulcus. Rudolph Albert von Kolliker (1817-1905)?? (More? inner ear
- lateral semicircular duct - (external) (More? image)
- LMP - acronym for last menstrual period, used to clinically measure gestation.
- malleus - (hammer) auditory ossicle (More? middle ear)
- mastoid process - of temporal bone (More? middle ear)
- Math1 - homolog of the Drosophila proneural gene atonal, necessary and sufficient for the production of hair cells in the mouse inner ear. Negatively regulated by Hes1 and Hes5
- meatal plug - temporary blockage of the external auditory meatus which forms at the end of the embryonic period and remains present until the seventh month.
- meatus - anatomical opening, cavity or space (external acoustic meatus, internal auditory meatus)
- mechano-electrical transduction - (MET) occurs within the cochlear hair cells hair bundle. A mechanical stimulus of the hair bundle causes the tip-links to be tensioned, opening ion channels, resulting in the generation of the cell receptor potential. (More? inner ear)
- Meckel's cartilage - first pharyngeal ach cartilage, located within the mandibular prominence. This cartilage first appears at stage 16, stage 20 the beginning of membranous ossification. Named after Johann Friedrich Meckel, (1781 - 1833) a German anatomist. (http://www.whonamedit.com/doctor.cfm/1840.html) (More? middle ear)
- mucopolysaccharidosis - (MPS IIIB, Sanfilippo Syndrome type B) abnormality caused by a deficiency in the lysosomal enzyme N-acetyl-glucosaminidase (Naglu). Children with MPS IIIB develop abnormal hearing, and mental functioning culminating in early death.
- netrin-1 - secreted growth factor, expressed in the organ of Corti and spiral ganglion cells, role in process outgrowth. (More? inner ear)
- otoacoustic emissions testing - (OET) hearing test measures sounds generated by the outer hair cells of the cochlea in response to clicks or tone bursts emitted and recorded by a tiny microphone placed in the infant’s external ear canal. (More? Hearing test)
- olivocochlear - brainstem cholinergic and GABAergic efferent system that innervates sensory cells and sensory neurons of the inner ear.
- organ of Corti - (spiral organ) cochlea component required for converting vibration into neural signals. (More? inner ear)
- organ of Corti protein II - (OCP-II) cytosolic protein or transcription factor? (More? inner ear)
- otolithic membrane - extracellular matrix that cover the sensory epithelia of the inner ear. (More? inner ear)
- ossicle - (small bone, auditory ossicles) the individual bone of the three middle ear bones (malleus, incus, stapes), which reduce vibrational amplitude but increase force to drive fluid-filled inner ear. (More? middle ear)
- otitis media - ICD-11 [1] middle ear infection, peak age prevalence is 6 to 18 months old with many children (75%) have at least one episode by school age. Forms include on-suppurative, suppurative, acute otitis media, chronic otitis media. (More? hearing abnormalities, hearing test)
- otic cup - the early embryonic transient structure formed by the invagination (folding inward) of the otic placode, to cup, then vesicle stage. This will eventually form inner ear structures.
- otic placode - Embryonic ectodermal epithelium giving rise to inner ear structures. (More? inner ear | Placodes)
- otic vesicle - the early embryonic transient structure formed by the invagination (folding inward) and fusion of the otic cup, separating this structure from the embryo surface. This will eventually form inner ear structures.(More? inner ear)
- otoconin - inner ear biominerals required for vestibular apparatus function. (More? inner ear)
- otogelin - (Otog) an inner ear specific glycoprotein expressed in cochlea cells at different developmental times. (More? inner ear)
- otolithic membrane - a membrane within the utricle and saccule containing embedded hair cell cilia and small crystalline bodies of calcium carbonate (otoliths). Functions to detect head motion.
- otoliths - small crystalline bodies of calcium carbonate found within the otolitic membrane of the utricle and saccule. (More? inner ear)
- ototoxic - compound or drug causing temporary or permanent hearing loss. (More? {{hearing abnormalities)
- outer hair cells - (OHCs) three rows of hair cells that function to increase basilar membrane motion through a local mechanical feedback process within the cochlea, the " cochlear amplifier".
- outer pillar cells - arranged in rows and form a boundary between the single row of inner hair cells and three rows of outer hair cells. (More? inner ear)
- paratubal musculature - muscles lying beside the auditory (Eustachian) tube. The tensor veli, palatini (TVP) and tensor tympani muscles. (More? middle ear)
- perilymph - the fluid between the membraneous labyrinth of the ear and the bone which encloses it.
- perilymphatic space - the space between the outer wall of the membranous labyrinth and the wall of the bony labyrinth that contains the perilymph.
- petrous portion of temporal bone -
- pejvakin gene - in humans, two missense mutations in this gene cause non-syndromic recessive deafness (DFNB59) by affecting the function of auditory neurons.
- petrotympanic fissure - (Glaserian fissure, squamotympanic fissure) the fissure in the temporal bone that runs between the temporomandibular joint to the tympanic cavity.
- pharyngeal arch - (More? Outer Ear) pharyngeal pouch pharyngeal membrane Pharynx
- pillar cells - (PC) form an inner and outer row of support cells that form a boundary between inner and outer hair cells. (More? inner ear)
- preyer reflex - ear flick in mouse in response to sound.
- presbyacusis - age-related hearing loss, is the cumulative effect of aging on hearing.
- prestin - a motor protein structurally similar to the anion transporter family expressed in cochlear outer hair cells. (More? inner ear)
- preauricular tag - skin tags located in front of the external ear opening, are common in neonates and in most cases are normal, though in some cases are indicative of other associated abnormalities.
- protocadherin 15 - (Pcdh15) required for initial formation of stereocilia bundles and changes in the actin meshwork within hair cells. The Ames waltzer (av) mouse mutant has both auditory and vestibular abnormalities from a mutation in this gene.
- Reichert's cartilage - pharyngeal arch 2 cartilage, named after Karl Bogislaus Reichert (1811 - 1883) a German anatomist. (More? middle ear | pharyngeal arch)
- Reissner's membrane - (vestibular membrane, vestibular wall) is a membrane located inside the cochlea separating the scala media from scala vestibuli. Named after Ernst Reissner (1824-1878) a German anatomist. It primarily functions as a diffusion barrier, allowing nutrients to travel from the perilymph to the endolymph of the membranous labyrinth.
- rhombomere - hindbrain rostrocaudal segmentation established by expression of Hox homeodomain transcription factors. Histologically rhombomeres are visible as undulating folds (scalloping) of the neural tube in the hindbrain region and have associated cranial nerves.
- saccular macula - (macula of saccule) thickened anterior part of the saccule containing the saccular filaments of the acoustic nerve. (More? balance)
- Saccule - (Latin, sacculus = a small pouch) sensory cells in the inner ear that translate head movements into neural signals for the brain to interpret. (More? balance)
- sacculocollic reflex - thought to have a role in maintaining the relative position of the head and the body against the vertical linear acceleration of gravity.
- scala tympani - one of the three cochlea cavities, it is filled with perilymph.
- Scarpa's ganglion - (vestibular ganglion) primary afferent vestibular neuron ganglion of the vestibular nerve. Located within the internal auditory meatus. (More? inner ear)
- semicircular canals - series of fluid-filled loops of the inner ear required for balance and sensing acceleration. (More? inner ear)
- sensorineural - term used to describe one of the two major classes of hearing loss involving the central pathway from the cochlear (other form is conductive loss).
- space of Nuel - within the cochlea, an organ of Corti space between the outer pillar cells and the phalangeal and hair cells. Named after Jean-Pierre Nuel (1847-1920) a Belgian ophthalmologist. (More? inner ear)
- spiral ganglion neurons - (SGN) innervate the inner (Type I) and outer (Type II) hair cells of the cochlea. (More? inner ear)
- stapedius muscle - (innervated by CN VII tympanic branch) one of the two muscles in the middle ear, contraction of this muscle pulls the stapes and dampens auditory ossicle movement. (More? middle ear)
- startle response - {Moro reflex)
- stereocilia -finger-like projections from the apical surface of sensory hair cells forming the hair bundle in the cochlea. Formed by tightly cross-linked parallel actin filaments in a paracrystalline array with cell surface specializations (tip links, horizontal top connectors, and tectorial membrane attachment crowns).
- stratified squamous epithelia - classification of epithelium which transiently forms a plug in external ear canal to the outer eardrum.
- stria vascularis - forms the outer wall of the cochlear duct of the mammalian cochlea is composed primarily of three types of cells. Marginal cells line the lumen of the cochlear duct and are of epithelial origin. Basal cells also form a continuous layer and they may be mesodermal or derived from the neural crest. Intermediate cells are melanocyte-like cells, presumably derived from the neural crest, and are scattered between the marginal and basal cell layers. The stria forms endolymph and also contains a rich supply of blood vessels. (More? inner ear)
- synostotically - anatomically normally separate skeletal bones fused together. (More? middle ear)
- tectorial membrane - within the cochlea an extracellular matrix produced by interdental cells, that covers the sensory epithelial hair cells of the organ of corti. (More? inner ear)
- alpha-tectorin and beta- (TECTA, TECTB) major non-collagenous protein component of the tectorial membrane forming a striated-sheet matrix. Synthesized as glycosylphosphatidylinositol-linked, membrane bound precursors.
- tensor tympani - (innervated by CN V mandibular nerve) one of the two muscles in the middle ear, contraction of this muscle pulls the malleus and tenses the tympanic membrane, dampening auditory ossicle movement. The muscle arises from auditory tube (cartilaginous portion) and is inserted into the malleus (manubrium near the root).
- tonotopy - term describing the mapping along the tectorial membrane within the cochlea of the different sound frequencies. (More? inner ear)
- tympanic membrane - (ear drum)
- tympanic ring - an incomplete circle, in the concavity of which is a groove, the tympanic sulcus, for the attachment of the circumference of the tympanic membrane. In the newborn the ring is still open and expands laterally to form the tympanic part of temporal bone.
- vestibular evoked myogenic potential - (VEMP) a test for vestibular disorders (utricle and superior nerve) response elicited by loud clicks or tone bursts recorded from the tonically contracted sternocleidomastoid muscle.
- vestibular ganglion - (Scarpa's ganglion) primary afferent vestibular neuron ganglion of the vestibular nerve. Located within the internal auditory meatus. (More? inner ear)
- vestibular membrane - (Reissner's) extends from the spiral lamina to the outer wall and divides the cochlea into an upper scala vestibuli, a lower scala tympani. (More? inner ear)
- Vestibulocochlear Nerve - Cranial Nerve VIII CN VIII
- Whirlin - A PDZ scaffold protein expressed in hair cells at the stereocilia tips, essential for the stereocilia elongation process. The DFNB31 gene mutations cause hearing loss in human and mouse. This protein can interact with membrane-associated guanylate kinase (MAGUK) protein, erythrocyte protein p55 (p55). (More? inner ear)
- Wnt7a - signaling through the Wnt pathway regulates the development of hair cell unidirectional stereociliary bundle orientation. (More? inner ear)
|
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Hearing - Inner Ear Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Hearing_-_Inner_Ear_Development
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G