Sensory - Hearing and Balance Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
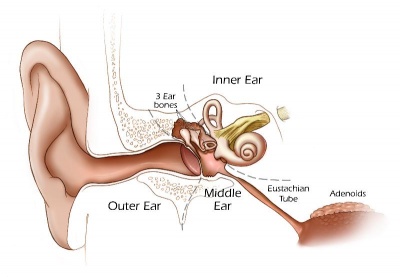
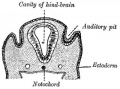
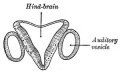
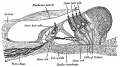
We use the sense of balance and hearing to position ourselves in space, sense our surrounding environment, and to communicate. Portions of the ear appear very early in development as specialized region (otic placode) on the embryo surface that sinks into the mesenchyme to form a vesicle (otic vesicle = otocyst) that form the inner ear.
This region connects centrally to the nervous system and peripherally through specialized bones to the external ear (auricle). This organisation develops different sources forming the 3 ear parts: inner ear (otic placode, otocyst), middle ear (1st pharyngeal pouch and 1st and 2nd arch mesenchyme), and outer ear (1st pharyngeal cleft and 6 surface hillocks).
This complex origin, organisation, and timecourse means that abnormal development of any one system can impact upon the development of hearing.
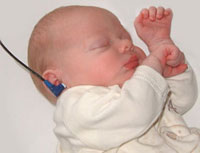
In Australia, there is now an early postnatal screening of neonatal hearing as part of a NSW State Wide Infant Screening Hearing (SWISH) Program using Automated Auditory Brainstem Response (AABR).
Use the hearing links below to see more detailed information about development of the three hearing divisions and abnormalities.
| Senses Links: Introduction | placode | Hearing and Balance hearing | balance | vision | smell | taste | touch | Stage 22 | Category:Sensory |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Hearing Development |
Textbooks
- Larsen's Human Embryology (4th ed.) Schoenwolf, Larsen, Bleyl, Brauer and Francis-West Chapter 17 Development of the Ears and Eyes
- The Developing Human: Clinically Oriented Embryology (6th ed.) Moore and Persaud Chapter 19: p491-511
- Essentials of Human Embryology Larsen Chapter 12: p252-272
- Before We Are Born (5th ed.) Moore and Persaud Chapter 20: p460-479
- Journal of Cell Biology The Cell Biology of the Senses - The cell biology of hearing July 12, 2010 .
Timeline
Embryonic Development
Based on data from the Carnegie embryo collection.[3][4]
| Week: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Carnegie stage: | 1 2 3 4 | 5 6 | 7 8 9 | 10 11 12 13 | 14 15 | 16 17 | 18 19 | 20 21 22 23 |
Week 3
GA week 5
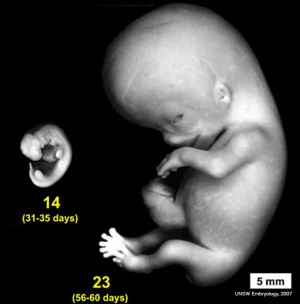
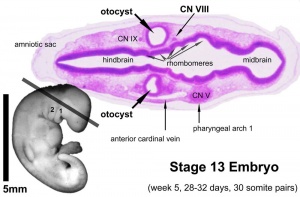
- Carnegie stage 9 - otic placode (disc or otic zone) appears opposite the rhombencefhalic fold.[5][6]
Week 4
GA week 6
- Carnegie stage 10 - 10 somites first indication of placode invagination[7] 12 somites cells migrate from the otic disc.[8]
- Carnegie stage 11 - 16 somites otic pit is formed and lies dorsal to the second pharyngeal groove (cleft).[8]
- Carnegie stage 12 - otic vesicle (otocyst) is forming and connects by a narrow pore with the surface[9] Otocyst ventral wall contributes to the vestibulocochlear crest.
- Carnegie stage 13 - otic vesicle closes from surface and endolymphatic appendage apparent. A capillary network forms around the otic vesicle with esoderm becomes condensed as the otic capsule.[10] Vestibulocochlear ganglion (vestibular part) and vestibular nerve fires present.
Week 5
GA week 7
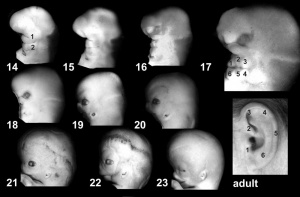
- Carnegie stage 14 - otic vesicle ventral portion elongates to form the cochlear duct and endolymphatic appendage becomes tapered.[10]
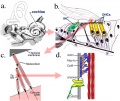
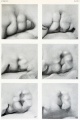
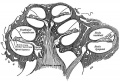
- Carnegie stage 15 - otic capsule formed by condensed mesenchyme. Ganglion vestibular nerve fibres extend to the otocyst epithelium. External ear auricular hillocks appear.
Week 6
GA week 8
- Carnegie stage 16 - otic vesicle wall thickens prior to appearance of the semicircular ducts.[11] A utriculosaccular diverticulum and spiral ganglion appears. Auricular hillocks present (tragus, crus helicis, helix, and antitragus).
- Carnegie stage 17 - otic capsule now dense mesenchyme. Otic vesicle vestibular part wall thins prior to semicircular duct appearing. Geniculate ganglion forms. Auditory ossicles, tubotympanic recess and chorda tympani appear. First pharyngeal groove (cleft or hyomandibular groove) begins to form the concha and the external acoustic meatus. Six auricular hillocks present (1 tragus, 2 and 3 crus helicis, 4 and 5 helix, and 6 antitragus).
Week 7
GA week 9
- Carnegie stage 18 - otic capsule precartilaginous. Semicircular ducts form in the order anterior, posterior, and lateral from thickened epithelial areas and adjacent epithelial layers fuse. Cochlear duct is now L-shaped. First pharyngeal arch bar begins to chondrify (Meckel’s cartilage), second arch may chondrify (Reichert’s cartilage), stapes and stapedius commence. Auricular hillocks merge to form auricle primordia.
- Carnegie stage 19 - otic capsule now cartilaginous. Cochlea tip becomes curled. Malleus and incus present.
Week 8
GA week 10
- Carnegie stage 20 - otic capsule connected with the basal plate and with the future exoccipitals. Tip of the cochlea is elongated and curled. Tensor tympani and stapedius present.
- Carnegie stage 21 - tip of the cochlea is recurved.
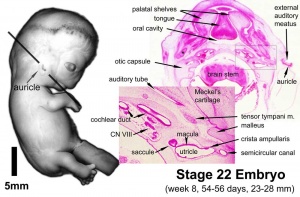
- Carnegie stage 22 - cochlea continues spiral growth.
- Carnegie stage 23 - cochlea shows nearly 21/2 turns. otic capsule cartilage separated from the semicircular ducts by a pre cartilaginous zone. Labyrinth has practically completed its gross development and ductus reuniens is well defined.[12]
Fetal
- Week 9 - Mesenchyme surrounding membranous labryinth (otic capsule) chondrifies
- Week 12 - 16 - Capsule adjacent to membranous labryinth undegoes vacuolization to form a cavity (perilymphatic space) around membranous labrynth and fills with perilymph.
- 2nd Trimester - (week 16 - 24) Centres of ossification appear in remaining cartilage of otic capsule form petrous portion of temporal bone. Continues to ossify to form mastoid process of temporal bone.
- 3rd Trimester - Vibration acoustically of maternal abdominal wall induces startle response in fetus.
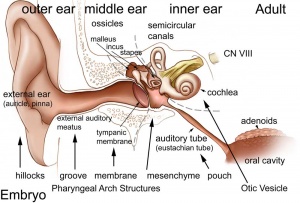
Embryonic Origin Overview
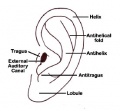
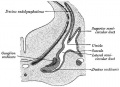
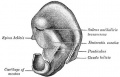
External Ear
- Auricle - Pharyngeal Arches 1 and 2 (ectoderm, mesoderm)
- External Auditory Meatus - Pharyngeal Arch 1 groove or cleft (ectoderm)
- Tympanic Membrane - Pharyngeal Arch 1 membrane (ectoderm, mesoderm, endoderm)
Middle Ear
- Middle Ear Ossicles
- Middle Ear Muscles
- Tensor tympani - Pharyngeal Arch 1 (mesoderm)
- Stapedius - Pharyngeal Arch 2 (mesoderm)
- Middle ear cavity - Pharyngeal Arch 1 pouch (endoderm)
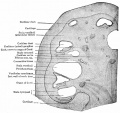
Inner Ear
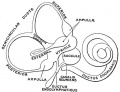
- Inner Ear Labyrinth
- Cochlea - Otic vesicle - Otic placode (ectoderm)
- Semicircular canals - Otic vesicle - Otic placode (ectoderm)
- Saccule and utricle - Otic vesicle - Otic placode (ectoderm)
- Cranial Nerve VIII
- Auditory component - Otic vesicle and neural crest (ectoderm)
- Vestibular component - Otic vesicle and neural crest (ectoderm)
Inner Ear
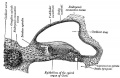
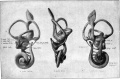
- The inner ear is derived from a pair of surface sensory placodes (otic placodes) in the head region.
- These placodes fold inwards forming a depression, then pinch off entirely from the surface forming a fluid-filled sac or vesicle (otic vesicle, otocyst).
- The vesicle sinks into the head mesenchyme some of which closely surrounds the otocyst forming the otic capsule.
- The otocyst finally lies close to the early developing hindbrain (rhombencephalon) and the developing vestibulo-cochlear-facial ganglion complex.
Links: Inner Ear
Middle Ear
- The middle ear ossicles (bones) are derived from 1st and 2nd arch mesenchyme.
- The space in which these bones sit is derived from the 1st pharyngeal pouch.
Links: Middle Ear
Outer Ear
- The external ear is derived from 6 surface hillocks, 3 on each of pharyngeal arch 1 and 2.
- The external auditory meatus is derived from the 1st pharyngeal cleft.
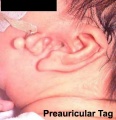
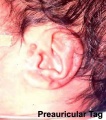
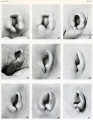
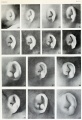
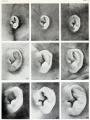
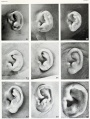
- The newborn external ear structure and position is an easily accessible diagnostic tool for potential abnormalities or further clinical screening.
Links: Outer Ear
Postnatal Changes
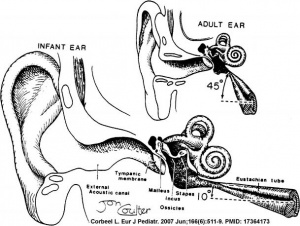
There are a number of postnatal changes associated with growth of the head that affect the newborn to adult auditory tube and its functions. The auditory tube (Eustachian, otopharyngeal or pharyngotympanic) space connects the middle ear cavity to nasopharynx portion of pharynx.
Auditory Tube Functions
- Ventilation - pressure equalization in the middle ear
- Clearance - allow fluid drainage from the middle ear, tube is normally closed and opened by muscles
Auditory Tube Postnatal
- Birth - (neonatal to early childhood) the tube is initially short (17-18 mm), narrower and runs almost horizontal. The tube is opened by a single muscle, tensor palati muscle.
- Adult - the tube is longer (twice as long), wider and runs at approximately 45 degrees to the horizontal. Tube is opened by two separate muscles, tensor palati and levator palati.
Abnormalities
There are many different abnormalities of hearing development that can result in hearing loss and can broadly be divided into either conductive or sensorineural loss. These abnormalities can have genetic, environmental or unknown origins. In addition, abnormalities of the external ear (position and structure) is used as a clinical diagnostic tool for developmental abnormalities in other systems.
- Inner ear - common cavity, severe cochlear hypoplasia
- Middle ear - rare and can be part of first arch syndrome, Malleus, Incus and Stapes Fixation
- Cholesteatoma- Epithelium trapped within skull base in development, erosion of bones: temporal bone, middle ear, mastoid
- Outer ear - Several genetic effects and syndromes, Environmental Effects
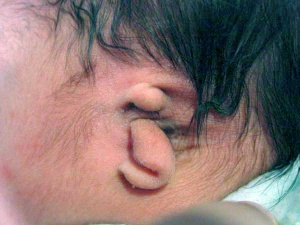
- Microtia - abnormally small external ear
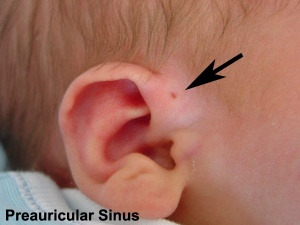
- Preauricular sinus - occurs in 0.25% births, bilateral (hereditary) 25-50%, unilateral (mainly the left), duct runs inward can extend into the parotid gland, Postnatally sites for infection
Links: Hearing Abnormalities
Congenital Deafness
Sensorineural - cochlear or central auditory pathway Conductive - disease of outer and middle ear
Sensorineural
- Hereditary
- recessive- severe
- dominant- mild
- can be associated with abnormal pigmentation (hair and irises)
- Acquired
- rubella (German measles), maternal infection during 2nd month of pregnancy, vaccination of young girls
- cytomegalovirus[13]
- streptomycin
- antibiotic
- thalidomide
Conductive
- disease of outer and middle ear
- can be produced by otitis media with effusion, that is widespread in young children.
- temporary blockage of outer or middle ear
Fetal Alcohol Syndrome
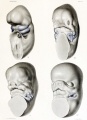
- Postion- Lower or uneven height, "railroad track” appearance, curve at top part of outer ear is under-developed, folded over parallel to curve beneath
Newborn Hearing Screening
In Australia, there is now an early postnatal screening of neonatal hearing as part of a NSW State Wide Infant Screening Hearing (SWISH) Program using Automated Auditory Brainstem Response (AABR).
- Very low birthweight infants and universal newborn hearing screening in a developing country[14]
Links: NSW Statewide Infant Screening - Hearing (SWISH) Program
Bionic Ear
The "Cochlear Implant" was pioneered in development by Professor Graeme Clark (1960s, Australia).[15] It consists of an array of electrodes implanted within cochlea, that directly electrically stimulate the auditory nerve fibres.
- Young children with cochlear implants compared with children with normal hearing.[16]
References
- ↑ 1.0 1.1 Schrauwen I & Van Camp G. (2010). The etiology of otosclerosis: a combination of genes and environment. Laryngoscope , 120, 1195-202. PMID: 20513039 DOI.
- ↑ Dorrn AL, Yuan K, Barker AJ, Schreiner CE & Froemke RC. (2010). Developmental sensory experience balances cortical excitation and inhibition. Nature , 465, 932-6. PMID: 20559387 DOI.
- ↑ O'Rahilly R. The early development of the otic vesicle in staged human embryos. (1963) J Embryol Exp Morphol., 11: 741-55 PMID 14081992
- ↑ O'Rahilly R. (1983). The timing and sequence of events in the development of the human eye and ear during the embryonic period proper. Anat. Embryol. , 168, 87-99. PMID: 6650859
- ↑ Bartelmez GW. The origin of the otic and optic primordia in man. (1922) J. Comp. Neural., 34: 201-232.
- ↑ Ingalls NW. A human embryo at the beginning of segmentation, with special reference to the vascular system. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. 274, 11: 61-90.
- ↑ Corner GW. A well-preserved human embryo of 10 somites. (1929) Carnegie Instn. Wash. Publ. 394, Contrib. Embryol., Carnegie Inst. Wash. 20: 81-102.
- ↑ 8.0 8.1 Bartelmez GW. and Evans HM. Development of the human embryo during the period of somite formation, including embryos with 2 to 16 pairs of somites. (1926) Contrib. Embryol., Carnegie Inst. Wash. Publ. 362, 17: 1-67.
- ↑ Streeter GL. Developmental horizons in human embryos. Description of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites. (1942) Contrib. Embryol., Carnegie Inst. Wash. Publ. 541, 30: 211-245.
- ↑ 10.0 10.1 Streeter GL. Developmental horizons in human embryos. Description of age group XIII, embryos about 4 or 5 millimeters long, and age group XIV, period of indentation of the lens vesicle. (1945) Carnegie Instn. Wash. Publ. 557, Contrib. Embryol., Carnegie Inst. Wash., 31: 27-63.
- ↑ Streeter GL. Developmental horizons in human embryos. Description of age groups XV, XVI, XVII, and XVIII, being the third issue of a survey of the Carnegie collection. (1948) Contrib. Embryol., Carnegie Inst. Wash. 575, 32: 133-203.
- ↑ Streeter GL. On the development of the membranous labyrinth and the acoustic and facial nerves in the human embryo. (1906) Amer. J Anat. 6:139-165.
- ↑ Yinon Y, Farine D & Yudin MH. (2010). Cytomegalovirus infection in pregnancy. J Obstet Gynaecol Can , 32, 348-354. PMID: 20500943 DOI.
- ↑ Olusanya BO. (2010). Perinatal profile of very low birthweight infants under a universal newborn hearing screening programme in a developing country: a case-control study. Dev Neurorehabil , 13, 156-63. PMID: 20450464 DOI.
- ↑ Clark GM. (2008). Personal reflections on the multichannel cochlear implant and a view of the future. J Rehabil Res Dev , 45, 651-93. PMID: 18816421
- ↑ Schramm B, Bohnert A & Keilmann A. (2010). Auditory, speech and language development in young children with cochlear implants compared with children with normal hearing. Int. J. Pediatr. Otorhinolaryngol. , 74, 812-9. PMID: 20452685 DOI.
Online Textbooks
- Developmental Biology (6th ed.) Gilbert, Scott F. Sunderland (MA): Sinauer Associates, Inc.; c2000. Evolution of the mammalian middle ear bones from the reptilian jaw | Chick embryo rhombomere neural crest cells | Some derivatives of the pharyngeal arches | Formation of the Neural Tube | Differentiation of the Neural Tube | Tissue Architecture of the Central Nervous System | Neuronal Types | Snapshot Summary: Central Nervous System and Epidermis
- Neuroscience Purves, Dale; Augustine, George J.; Fitzpatrick, David; Katz, Lawrence C.; LaMantia, Anthony-Samuel; McNamara, James O.; Williams, S. Mark. Sunderland (MA): Sinauer Associates, Inc. ; c2001 The Auditory System | The Inner Ear | The Middle Ear | The External Ear | Early Brain Development | Construction of Neural Circuits | Modification of Brain Circuits as a Result of Experience
- Molecular Biology of the Cell (4th Edn) Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter. New York: Garland Publishing; 2002. Neural Development | The three phases of neural development
- Clinical Methods 63. Cranial Nerves IX and X: The Glossopharyngeal and Vagus Nerves | The Tongue | 126. The Ear and Auditory System | An Overview of the Head and Neck - Ears and Hearing | Audiometry
- Health Services/Technology Assessment Text (HSTAT) Bethesda (MD): National Library of Medicine (US), 2003 Oct. Developmental Disorders Associated with Failure to Thrive
- Eurekah Bioscience CollectionCranial Neural Crest and Development of the Head Skeleton
Reviews
Powles-Glover N & Maconochie M. (2018). Prenatal and postnatal development of the mammalian ear. Birth Defects Res , 110, 228-245. PMID: 29193857 DOI.
Schwander M, Kachar B & Müller U. (2010). Review series: The cell biology of hearing. J. Cell Biol. , 190, 9-20. PMID: 20624897 DOI.
The International Journal of Developmental Biology Vol. 51 Nos. 6/7 (2007) Ear Development
Search
Bookshelf: hearing development
Search Pubmed: hearing development
Search Entrez: hearing development
Additional Images
Historic Images
See also Category:Hearing
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Embryo Images - Hearing
- NIDCD - Balance Disorders
- NSW Health - NSW Statewide Infant Screening - Hearing (SWISH) Program
- American Academy of Audiology - American Academy of Audiology | In Memoriam: Judy Gravel
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Terms
| Hearing Terms | ||
|---|---|---|
Hearing and Balance Development
|
Cite this page: Hill, M.A. (2024, April 27) Embryology Sensory - Hearing and Balance Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Sensory_-_Hearing_and_Balance_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G