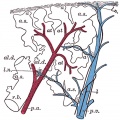
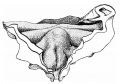
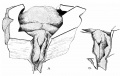
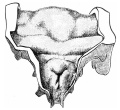
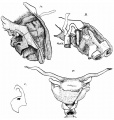
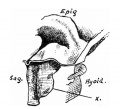
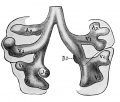
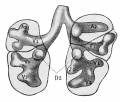
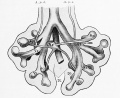
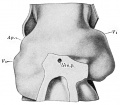
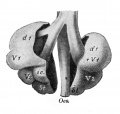
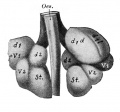
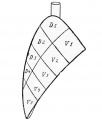
Introduction
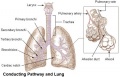
The respiratory system does not carry out its physiological function (of gas exchange) until after birth. The respiratory tract, diaphragm and lungs do form early in embryonic development. The respiratory tract is divided anatomically into 2 main parts:
- upper respiratory tract, consisting of the nose, nasal cavity and the pharynx
- lower respiratory tract consisting of the larynx, trachea, bronchi and the lungs.
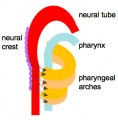
In the head/neck region, the pharynx forms a major arched cavity within the phrayngeal arches. The lungs go through 4 distinct histological phases of development and in late fetal development thyroid hormone, respiratory motions and amniotic fliud are thought to have a role in lung maturation. Branching is a key mechanism/process in lung development leading to alveolar saccules after about 23 branching generations (range of 18–30).
The two main respiratory cell types, squamous alveolar type 1 and alveolar type 2 (surfactant secreting), both arise from the same bi-potetial progenitor cell.[1] The third main cell type are macrophages (dust cells) that arise from blood monocyte cells.
Development of this system is not completed until the last weeks of Fetal development, just before birth. Therefore premature babies have difficulties associated with insufficient surfactant (end month 6 alveolar cells type 2 appear and begin to secrete surfactant).
Some Recent Findings
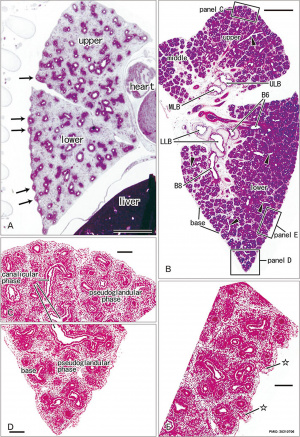
Pseudoglandular and canalicular stages
[2]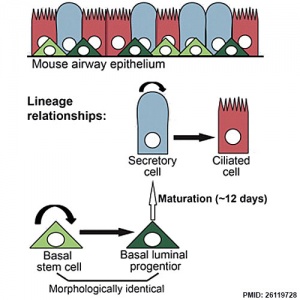
Respiratory epithelium cell development.
[3]
- Bronchial tree of the human embryo: Examination based on a mammalian model[4] "Imaging data of 41 human embryo specimens at Carnegie stages (CS) 16-23 (equivalent to 6-8 weeks after fertilization) belonging to the Kyoto collection were obtained using phase-contrast X-ray computed tomography. Three-dimensional bronchial trees were then reconstructed from these images."
- Transcriptional control of lung alveolar type 1 cell development and maintenance by NK homeobox 2-1[5] "The extraordinarily thin alveolar type 1 (AT1) cell constitutes nearly the entire gas exchange surface and allows passive diffusion of oxygen into the blood stream. Despite such an essential role, the transcriptional network controlling AT1 cells remains unclear. Using cell-specific knockout mouse models, genomic profiling, and 3D imaging, we found that NK homeobox 2-1 (Nkx2-1) is expressed in AT1 cells and is required for the development and maintenance of AT1 cells. Without Nkx2-1, developing AT1 cells lose 3 defining features-molecular markers, expansive morphology, and cellular quiescence-leading to alveolar simplification and lethality. NKX2-1 is also cell-autonomously required for the same 3 defining features in mature AT1 cells. Intriguingly, Nkx2-1 mutant AT1 cells activate gastrointestinal (GI) genes and form dense microvilli-like structures apically. Single-cell RNA-seq supports a linear transformation of Nkx2-1 mutant AT1 cells toward a GI fate. Whole lung ChIP-seq shows NKX2-1 binding to 68% of genes that are down-regulated upon Nkx2-1 deletion, including 93% of known AT1 genes, but near-background binding to up-regulated genes. Our results place NKX2-1 at the top of the AT1 cell transcriptional hierarchy and demonstrate remarkable plasticity of an otherwise terminally differentiated cell type. respiratory
- Complex Compound Inheritance of Lethal Lung Developmental Disorders Due to Disruption of the TBX-FGF Pathway[6] "Primary defects in lung branching morphogenesis, resulting in neonatal lethal pulmonary hypoplasias, are incompletely understood. To elucidate the pathogenetics of human lung development, we studied a unique collection of samples obtained from deceased individuals with clinically and histopathologically diagnosed interstitial neonatal lung disorders: acinar dysplasia (n = 14), congenital alveolar dysplasia (n = 2), and other lethal lung hypoplasias (n = 10). We identified rare heterozygous copy-number variant deletions or single-nucleotide variants (SNVs) involving TBX4 (n = 8 and n = 2, respectively) or FGF10 (n = 2 and n = 2, respectively) in 16/26 (61%) individuals. In addition to TBX4, the overlapping ∼2 Mb recurrent and nonrecurrent deletions at 17q23.1q23.2 identified in seven individuals with lung hypoplasia also remove a lung-specific enhancer region. Individuals with coding variants involving either TBX4 or FGF10 also harbored at least one non-coding SNV in the predicted lung-specific enhancer region, which was absent in 13 control individuals with the overlapping deletions but without any structural lung anomalies. The occurrence of rare coding variants involving TBX4 or FGF10 with the putative hypomorphic non-coding SNVs implies a complex compound inheritance of these pulmonary hypoplasias. Moreover, they support the importance of TBX4-FGF10-FGFR2 epithelial-mesenchymal signaling in human lung organogenesis and help to explain the histopathological continuum observed in these rare lethal developmental disorders of the lung."
- Review - How high resolution 3-dimensional imaging changes our understanding of postnatal lung development[7] "During the last 10 + years biologically and clinically significant questions about postnatal lung development could be answered due to the application of modern cutting-edge microscopic and quantitative histological techniques. These are in particular synchrotron radiation based X-ray tomographic microscopy (SRXTM), but also 3Helium Magnetic Resonance Imaging, as well as the stereological estimation of the number of alveoli and the length of the free septal edge. First, the most important new finding may be the following: alveolarization of the lung does not cease after the maturation of the alveolar microvasculature but continues until young adulthood and, even more important, maybe reactivated lifelong if needed to rescue structural damages of the lungs. Second, the pulmonary acinus represents the functional unit of the lung. Because the borders of the acini could not be detected in classical histological sections, any investigation of the acini requires 3-dimensional (imaging) methods. Based on SRXTM it was shown that in rat lungs the number of acini stays constant, meaning that their volume increases by a factor of ~ 11 after birth. The latter is very important for acinar ventilation and particle deposition."
- Development of the pulmonary pleura with special reference to the lung surface morphology: a study using human fetuses[2] "In and after the third trimester, the lung surface is likely to become smooth to facilitate respiratory movements. However, there are no detailed descriptions as to when and how the lung surface becomes regular. According to our observations of 33 fetuses at 9-16 weeks of gestation (crown-rump length [CRL], 39-125 mm), the lung surface, especially its lateral (costal) surface, was comparatively rough due to rapid branching and outward growing of bronchioli at the pseudoglandular stage of lung development. The pulmonary pleura was thin and, beneath the surface mesothelium, no or little mesenchymal tissue was detectable. Veins and lymphatic vessels reached the lung surface until 9 weeks and 16 weeks, respectively. In contrast, in 8 fetuses at 26-34 weeks of gestation (CRL, 210-290 mm), the lung surface was almost smooth because, instead of bronchioli, the developing alveoli faced the external surfaces of the lung. ... a smooth lung surface seemed to be established largely by the thick submesothelial tissue including veins and lymphatic vessels until 26 weeks."
- Review - Human lung development: recent progress and new challenges[8] "Recent studies have revealed biologically significant differences between human and mouse lung development, and have reported new in vitro systems that allow experimental manipulation of human lung models. At the same time, emerging clinical data suggest that the origins of some adult lung diseases are found in embryonic development and childhood. The convergence of these research themes has fuelled a resurgence of interest in human lung developmental biology. In this Review, we discuss our current understanding of human lung development, which has been profoundly influenced by studies in mice and, more recently, by experiments using in vitro human lung developmental models and RNA sequencing of human foetal lung tissue."
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Respiratory Development | Lung Embryology | Lung Development | Alveolar Embryology |
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- PDGF-A signaling is required for secondary alveolar septation and controls epithelial proliferation in the developing lung[9] "Platelet-derived growth factor A (PDGF-A) signaling through PDGF receptor α is essential for alveogenesis. Previous studies have shown that Pdgfa-/- mouse lungs have enlarged alveolar airspace with absence of secondary septation, both distinctive features of bronchopulmonary dysplasia. ...In the absence of PDGF-A, the number of PdgfraGFP+ cells was significantly decreased. In addition, proliferation of PdgfraGFP+ cells was reduced. During alveogenesis, PdgfraGFP+ myofibroblasts failed to form the α-smooth muscle actin rings necessary for alveolar secondary septation. These results indicate that PDGF-A signaling is involved in myofibroblast proliferation and migration."
- Review - In utero alcohol effects on foetal, neonatal and childhood lung disease[10] "Maternal alcohol use during pregnancy exposes both premature and term newborns to the toxicity of alcohol and its metabolites. Foetal alcohol exposure adversely effects the lung. In contrast to the adult "alcoholic lung" phenotype, an inability to identify the newborn exposed to alcohol in utero has limited our understanding of its effect on adverse pulmonary outcomes. This paper will review advances in biomarker development of in utero alcohol exposure. We will highlight the current understanding of in utero alcohol's toxicity to the developing lung and immune defense. Finally, we will present recent clinical evidence describing foetal alcohol's association with adverse pulmonary outcomes including bronchopulmonary dysplasia, viral infections such as respiratory syncytial virus and allergic asthma/atopy."
- Development and plasticity of alveolar type 1 cells[11] "Alveolar type 1 (AT1) cells cover >95% of the gas exchange surface and are extremely thin to facilitate passive gas diffusion. The development of these highly specialized cells and its coordination with the formation of the honeycomb-like alveolar structure are poorly understood. Using new marker-based stereology and single-cell imaging methods, we show that AT1 cells in the mouse lung form expansive thin cellular extensions via a non-proliferative two-step process while retaining cellular plasticity. In the flattening step, AT1 cells undergo molecular specification and remodel cell junctions while remaining connected to their epithelial neighbors. In the folding step, AT1 cells increase in size by more than 10-fold and undergo cellular morphogenesis that matches capillary and secondary septa formation, resulting in a single AT1 cell spanning multiple alveoli. Furthermore, AT1 cells are an unexpected source of VEGFA and their normal development is required for alveolar angiogenesis. Notably, a majority of AT1 cells proliferate upon ectopic SOX2 expression and undergo stage-dependent cell fate reprogramming."
- Clonal Dynamics Reveal Two Distinct Populations of Basal Cells in Slow-Turnover Airway Epithelium[3] "We investigated the mouse tracheal epithelial lineage at homeostasis by using long-term clonal analysis and mathematical modeling. This pseudostratified epithelium contains basal cells and secretory and multiciliated luminal cells. Our analysis revealed that basal cells are heterogeneous, comprising approximately equal numbers of multipotent stem cells and committed precursors, which persist in the basal layer for 11 days before differentiating to luminal fate. We confirmed the molecular and functional differences within the basal population by using single-cell qRT-PCR and further lineage labeling. Additionally, we show that self-renewal of short-lived secretory cells is a feature of homeostasis. We have thus revealed early luminal commitment of cells that are morphologically indistinguishable from stem cells."
- Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors[12] "Basal cells are multipotent airway progenitors that generate distinct epithelial cell phenotypes crucial for homeostasis and repair of the conducting airways. Little is known about how these progenitor cells expand and transition to differentiation to form the pseudostratified airway epithelium in the developing and adult lung. Here, we show by genetic and pharmacological approaches that endogenous activation of Notch3 signaling selectively controls the pool of undifferentiated progenitors of upper airways available for differentiation. This mechanism depends on the availability of Jag1 and Jag2, and is key to generating a population of parabasal cells that later activates Notch1 and Notch2 for secretory-multiciliated cell fate selection." Notch
- Alveolar progenitor and stem cells in lung development[1] "Alveoli are gas-exchange sacs lined by squamous alveolar type (AT) 1 cells and cuboidal, surfactant-secreting AT2 cells. Classical studies suggested that AT1 arise from AT2 cells, but recent studies propose other sources. Here we use molecular markers, lineage tracing and clonal analysis to map alveolar progenitors throughout the mouse lifespan. We show that, during development, AT1 and AT2 cells arise directly from a bipotent progenitor, whereas after birth new AT1 cells derive from rare, self-renewing, long-lived, mature AT2 cells that produce slowly expanding clonal foci of alveolar renewal."
- Lung epithelial branching program antagonizes alveolar differentiation[13] "Mammalian organs, including the lung and kidney, often adopt a branched structure to achieve high efficiency and capacity of their physiological functions. Formation of a functional lung requires two developmental processes: branching morphogenesis, which builds a tree-like tubular network, and alveolar differentiation, which generates specialized epithelial cells for gas exchange. ...We thus propose that lung epithelial progenitors continuously balance between branching morphogenesis and alveolar differentiation, and such a balance is mediated by dual-function regulators, including Kras and Sox9. The resulting temporal delay of differentiation by the branching program may provide new insights to lung immaturity in preterm neonates and the increase in organ complexity during evolution."
- Suppression of embryonic lung branching morphogenesis[14] "The role of HOM/C homeobox genes on rat embryonic lung branching morphogenesis was investigated using the lung bud explant culture system in an air/liquid interface. ...These results suggest a critical role for homeobox b3 and b4 genes in lung airway branching morphogenesis."
- Retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium[15]= "The developmental abnormalities associated with disruption of signaling by retinoic acid (RA), the biologically active form of vitamin A, have been known for decades from studies in animal models and humans. These include defects in the respiratory system, such as lung hypoplasia and agenesis. ....The data in this study suggest that disruption of Wnt/Tgfbeta/Fgf10 interactions represents the molecular basis for the classically reported failure to form lung buds in vitamin A deficiency."
Clinical
- Lung Function and Respiratory Symptoms at 11 Years in Extremely Preterm Children[16] "Following extremely preterm birth, impaired lung function and increased respiratory morbidity persist into middle childhood, especially those with bronchopulmonary dysplasia (BPD). Many of these children may not be receiving appropriate treatment."
- Pediatric lung transplantation.[17] "Lung transplantation is an accepted therapy for selected pediatric patients with severe end-stage vascular or parenchymal lung disease. Collaboration between the patients' primary care physicians, the lung transplant team, patients, and patients' families is essential. The challenges of this treatment include the limited availability of suitable donor organs, the toxicity of immunosuppressive medications needed to prevent rejection, the prevention and treatment of obliterative bronchiolitis, and maximizing growth, development, and quality of life of the recipients. This article describes the current status of pediatric lung transplantation, indications for listing, evaluation of recipient and donor, updates on the operative procedure,graft dysfunction, and the risk factors, outcomes, and future directions."
|
Textbooks
- Moore, K.L., Persaud, T.V.N. & Torchia, M.G. (2015). The developing human: clinically oriented embryology (10th ed.). Philadelphia: Saunders. Chapter 10 Respiratory System
- Schoenwolf, G.C., Bleyl, S.B., Brauer, P.R., Francis-West, P.H. & Philippa H. (2015). Larsen's human embryology (5th ed.). New York; Edinburgh: Churchill Livingstone. Chapter 11 Development of the Respiratory System and Body Cavities
- Before We Are Born (5th ed.) Moore and Persaud Chapter 13 p255-287
- Essentials of Human Embryology Larson Chapter 9 p123-146
- Human Embryology Fitzgerald and Fitzgerald Chapter 19,20 p119-123
- Anatomy of the Human Body 1918 Henry Gray The Respiratory Apparatus
Objectives
- Describe the development of the respiratory system from the endodermal and mesodermal components.
- Describe the main steps in the development of the lungs.
- Describe the development of the diaphragm and thoracic cavities.
- List the respiratory changes before and after birth.
- Describe the developmental aberrations responsible for the following malformations: tracheo - oesophageal fistula (T.O.F); oesphageal atresia; diaphragmatic hernia; lobar emphysema.
Development Overview
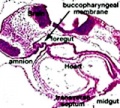
Week 4 - laryngotracheal groove forms on floor foregut.
Week 5 - left and right lung buds push into the pericardioperitoneal canals (primordia of pleural cavity)
Week 6 - descent of heart and lungs into thorax. Pleuroperitoneal foramen closes.
Week 7 - enlargement of liver stops descent of heart and lungs.
Month 3-6 - lungs appear glandular, end month 6 alveolar cells type 2 appear and begin to secrete surfactant.
Month 7 - respiratory bronchioles proliferate and end in alveolar ducts and sacs.
Mechanisms
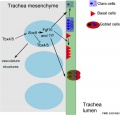
- Initiation - Budding of foregut endoderm to generate the trachea.
- Branching - A repeated mechanism of branching that is ongoing throughout development to form the conducting bronchioles then alveolar ducts.
- Surface area increase - Expansion of the surface area in late development generating eventually the thin air–blood barrier for gas exchange in the acini.
- Vascular development - Extension of a vascular capillary tree within the connective tissue and wall of the acini for gas exchange, and the lymphatic development for immunology of the lungs.
- Surfactant development - allows lung inflation and decreases the work of breathing and also related to immunology of the lungs.
- Musculoskeletal development - contributes the mechanical elements of ribs, intercostals and diaphragm required for breathing.
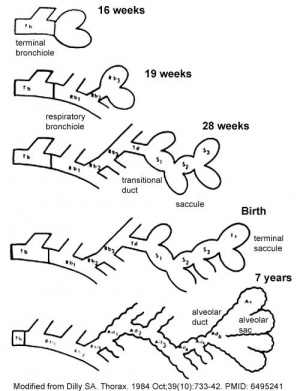
Lung Development Stages
The sequence is most important rather than the actual timing, which is variable both in development and in the existing literature.
Human Lung Stages
| Lung Stage
|
Human
|
Features
|
Vascular
|
| Embryonic
|
week 4 to 5
|
lung buds originate as an outgrowth from the ventral wall of the foregut where lobar division occurs
|
extra pulmonary artery then lobular artery
|
| Pseudoglandular
|
week 5 to 17
|
conducting epithelial tubes surrounded by thick mesenchyme are formed, extensive airway branching
|
Pre-acinar arteries
|
| Canalicular
|
week 16 to 25
|
bronchioles are produced, increasing number of capillaries in close contact with cuboidal epithelium and the beginning of alveolar epithelium development
|
Intra-acinar arteries
|
| Saccular
|
week 24 to 40
|
alveolar ducts and air sacs are developed
|
alveolar duct arteries
|
| Alveolar
|
late fetal to 8 years
|
secondary septation occurs, marked increase of the number and size of capillaries and alveoli
|
alveolar capillaries
|
| embryonic stage - pseudoglandular stage - canalicular stage - saccular stage - alveolar stage Links: Species Stage Comparison | respiratory
|
Species Comparison Timeline - Lung Stages
| Stage
|
Human
|
rabbit
|
sheep
|
mouse
|
rat
|
monkey
|
| Embryonic
|
4 to 7 weeks (E26 – E49)
|
n.d. – E18
|
E17 – E30
|
E9.5 – E12
|
E11 – E13
|
n.d. – E55
|
| Pseudoglandular
|
week 5 to 17
|
E18 – E24
|
E30 – E85
|
E12 – E16.5
|
E13 – E18.5
|
E55 – E85
|
| Canalicular
|
week 16 to 25
|
E23 – E27
|
E80 – E120
|
E16.5 – E17.5
|
E18.5 – E20
|
E75 – E115
|
| Saccular
|
week 24 to 38
|
E27 – E30
|
E110 – E140
|
E17.5 – P4
|
E21 – P4
|
E105 – term
|
| Alveolar (can be subdivided)
|
| Alveolar Phase 1
|
36 weeks (preterm) – 3 years
|
E30 – term (E31)
|
E120 – term (E145)
|
P4 – P21
|
P4 – P21
|
E125 – greater P180
|
| Alveolar Phase 2
|
term 3 – 21 years
|
term (E31) - n.d.
|
term (E145) - n.d.
|
P14 – young adulthood
|
P14 – young adulthood
|
P180 – young adulthood (7–8 years)
|
| Table Data[10] Links: Human stages table | respiratory
|
Embryonic stage
| Human Embryonic Lung Development
|
|
|
|
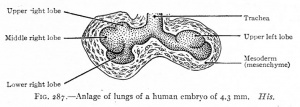
| CRL 4.3 mm, Week 4-5, Stage 12 to 13
|
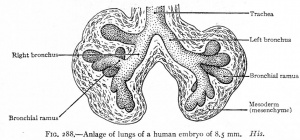
CRL 8.5 mm, Week 5, Stage 15 to 16
|
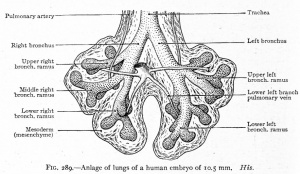
CRL 10.5 mm, Week 6 Stage 16 to 17
|
- Endoderm - tubular ventral growth from foregut pharynx.
- Mesoderm - mesenchyme of lung buds.
- Intraembryonic coelom - pleural cavities elongated spaces connecting pericardial and peritoneal spaces.
- Vascular - extra pulmonary artery then lobular artery.
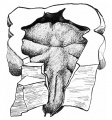
Pseudoglandular stage
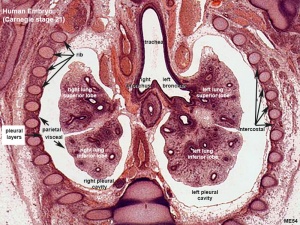
Respiratory histology (week 8)
- week 5 - 17
- tubular branching of the human lung airways continues
- by 2 months all segmental bronchi are present.
- lungs have appearance of a glandlike structure.
- stage is critical for the formation of all conducting airways.
- lined with tall columnar epithelium, the more distal structures are lined with cuboidal epithelium.
- Vascular - Pre-acinar arteries.
|
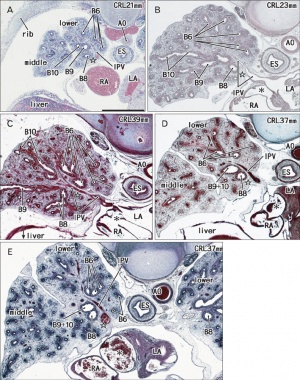
Human lung pseudoglandular stage[18]
|
Canalicular stage
- week 16 - 24
- canalicular phase of the bronchus can be mixed with the pseudoglandular phase.
- Lung morphology changes dramatically, beginning of alveolar epithelium development.
- differentiation of the pulmonary epithelium results in the formation of the future air-blood tissue barrier.
- Surfactant synthesis.
- future gas exchange regions can be distinguished from the future conducting airways of the lungs.
- Vascular - Intra-acinar arteries, canalization of the lung parenchyma by capillaries begins, increasing number of capillaries in close contact with cuboidal epithelium.
Saccular stage
- week 24 to near term.
- most peripheral airways form widened airspaces, termed saccules.
- saccules widen and lengthen the airspace (by the addition of new generations).
- future gas exchange region expands significantly.
- Fibroblastic cells also undergo differentiation, they produce extracellular matrix, collagen, and elastin. May have a role in epithelial differentiation and control of surfactant secretion
- Vascular - alveolar duct arteries, vascular tree also grows in length and diameter during this time.
|
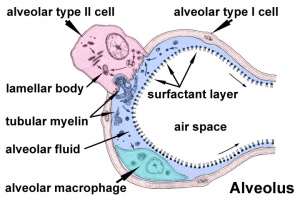
Alveolar sac structure
|
Alveolar stage
- near term through postnatal period.
- 1-3 years postnatally alveoli continue to form through a septation process increasing the gas exchange surface area.
- microvascular maturation occurs during this period.
- respiratory motions and amniotic fluid are thought to have a role in lung maturation.
- Vascular - alveolar capillaries.
Premature babies have difficulties associated with insufficient surfactant (end month 6 alveolar cells type 2 appear and begin to secrete surfactant). Lung growth continues postnatally to 20-23 years.
|
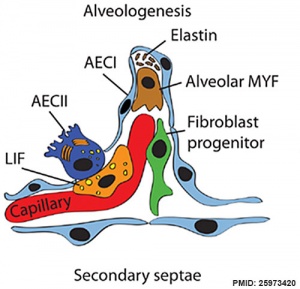
Respiratory secondary septum[19]
|
Surfactant
Pulmonary surfactant or simply surfactant (surface active agent) begins to be produced at the canalicular stage and both production and turnover are key for development. It is a mixture of lipids and proteins secreted by Type 2 alveolar cells between alveolar epithelium that reduces surface tension (detergent) at the air-liquid interface. The function of this secretion is to prevent collapse of the lung at the end of expiration. In humans, these cells and their secretion develop towards the very end of the third trimester, just before birth.
Surfactant is mainly phosphatidylcholine (PC) 80% in turn consisting of which dipalmitoyl-PC, palmitoyl-myristoyl-PC and palmitoyl-palmitoleoyl-PC together 75%, anionic phosphatidylglycerol and cholesterol are about 10% each.[20]
The surfactant proteins (SP) SP-A to -D comprise only about 2-5%. Surfactant protein D (SP-D) a multimeric collectin (collagen-containing C-type lectin) involved in innate immunity (anti-microbial)[21] and expressed in pulmonary and non-pulmonary epithelia.[22] Surfactant protein B (SP-B) and disaturated-phosphatidylcholine have both been shown to be higher in preterms with chorioamnionitis.[23]
Clinically surfactants used for surfactant replacement therapy are animal-derived preparations, commonly bovine (beractant, bovactant, BLES) or less common porcine (butantan, poractant-α and surfacen).[24]
Respiratory Species Comparison
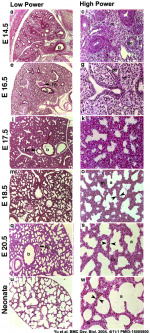
Mouse lung development
[25]Different species undergo lung development stages over different time courses[26] and some species ( mouse and rat) do not possess any respiratory bronchioles, the acini and the ventilatory units are therefore the same entity.
Mouse
Note that the model mouse respiratory system differs from human in size, distribution of cell types, and the time taken to develop. Additionally, the common laboratory mouse model (C57BL/6J} completely lacks respiratory breathing movements seen in the human fetus.[28]
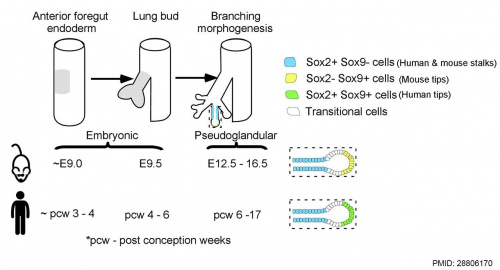
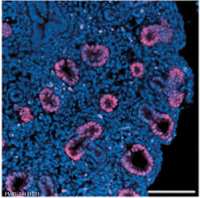
Human and mouse Sox expression[29]
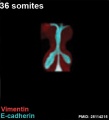
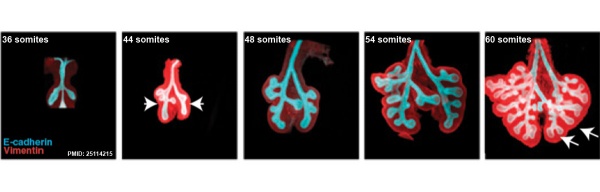
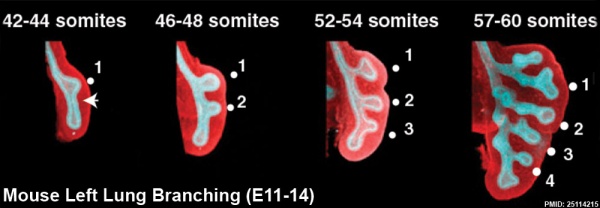
The following images are from a recent study of the development of bronchial branching in he mouse between E10 to E14.[30]
Mesenchyme (red) and epithelium (blue) the study used knockout mice to show the role of Wnt signalling in branching morphogenesis.
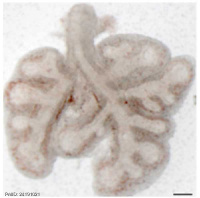
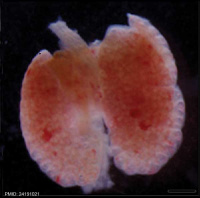
| Mouse lung E12.5 to E18.5
|
|
|
|
|
| E12.5 lungs
|
E14.5 lungs
|
E14.5 Sox9
|
E18.5 lungs
|
| Reference[31]
|
- Links: Wnt | Mouse Development
Embryonic Respiratory Development
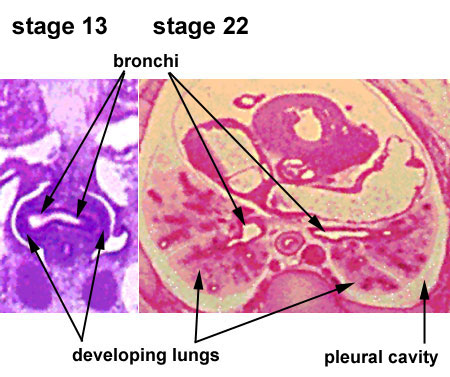
Pseudoglandular Respiratory Development
Pseudoglandular period identified in this paper (GA weeks 12 to 16)
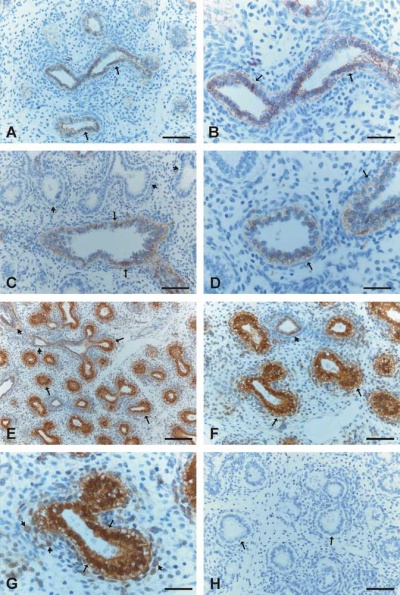
Human lung at pseudoglandular stage showing E- and N-cadherin and β-catenin localization.[32]
Endocrine Lung
| Neonatal Human
|
Fetal Rabbit
|
|
|
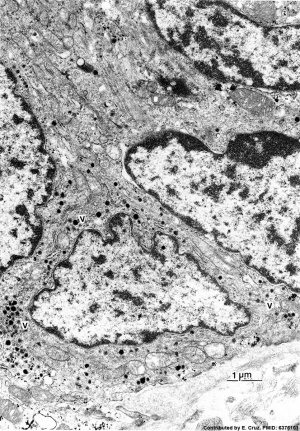
| Pulmonary neuroendocrine cell (EM)[33]
|
Neuroepithelial body[33]
|
Pulmonary neuroendocrine cells (PNECs)
- develop in late embryonic to early fetal period.[34][35]
- later in mid-fetal period clusters of these cells form neuroepithelial bodies (NEBs).
- first cell type to differentiate in the airway epithelium.
- differentiation regulated by proneural genes - mammalian homolog of the achaete-scute complex (Mash-1) and hairy and enhancer of split1 (Hes-1).[36]
- located in the fetal lung at bronchiole branching points.
- may stimulate mitosis to increase branching.
- secrete 2 peptides - gastrin-releasing peptide (GRP) and calcitonin gene related peptide (CGRP)
- Links: Endocrine - Other Tissues | OMIM - GRP | OMIM - CGRP
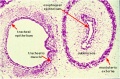
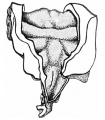
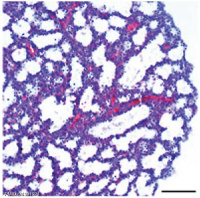
Lung Histology
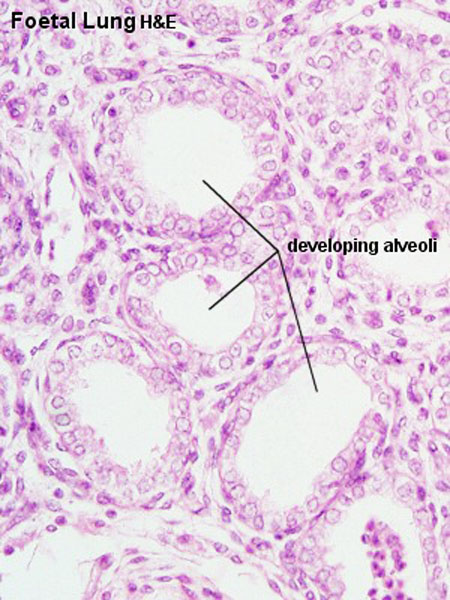
|
| Fetal lung histology
|
- Links: Respiratory System - Histology
Birth Changes
At birth the lung epithelium changes from a prenatal secretory to a postnatal absorptive function. Several factors have been identified as influencing this transport change including: epinephrine, oxygen, glucocorticoids, and thyroid hormones (for review see[37])
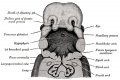
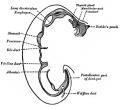
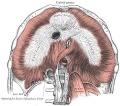
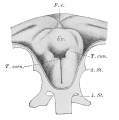
Upper Respiratory Tract
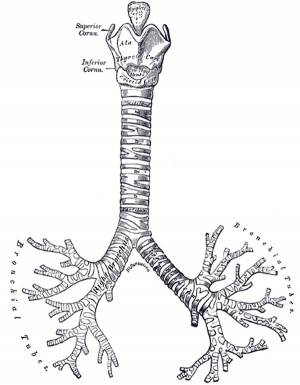
Adult upper respiratory tract conducting system
week 4 early respiratory endodermal bud
- part of foregut development
- anatomically the nose, nasal cavity and the pharynx
- the pharynx forms a major arched cavity within the pharyngeal arches
Movies
The animations below allow a comparison of early and late embryonic lung development. Compare the size and relative position of the respiratory structures and their anatomical relationship to the developing gastrointestinal tract.
Lung Cardiovascular
- Links: Cardiovascular System Development
Pulmonary Circulation
- pulmonary arteries and veins arise by vasculogenesis[38]
Pulmonary Veins
- vasculogenesis in the mesenchyme surrounding the terminal buds during the pseudoglandular stage.
- vasculogenesis - describes the formation of new blood vessels from pluripotent precursor cells.
- angiogenesis in the canalicular and alveolar stages.
- angiogenesis - describes the formation of new vessels from pre-existing vessels.
See also review[39]
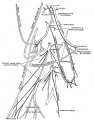
Bronchial Circulation
Bronchial Arteries
- vascularising the walls of the airways and the large pulmonary vessels providing giving oxygen and nutrients.
- extend within the bronchial tree to the periphery of the alveolar ducts.
- not found in the lungs until around 8 weeks of gestation.
- one or two small vessels extend from the dorsal aorta and run into the lung alongside the cartilage plates of the main bronchus.
Bronchial Veins
- small bronchial veins within the airway wall drain into the pulmonary veins.
- large bronchial veins seen close to the hilum and drain into the cardinal veins and the right atrium.
See review[39]
Molecular
TBX

|
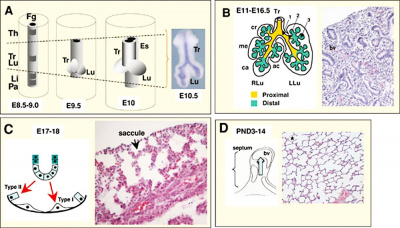
|
| Mouse respiratory Tbx4 and Tbx5 model[40]
|
Mouse respiratory development[41]]]
|
- Nkx2-1 (Titf1) - ventral wall of the anterior foregut, identifies the future trachea.
SOX
- Sox2 is essential for the initiation of lung development from the endodermal gut tube,
- Sox9 is essential for maintaining the tips and associated branching.
Several different Sox types are required for different stages of respiratory development. Wnt/β-catenin signaling does not regulate Sox9 expression in the lung.[31]

Human and mouse Sox expression[29]
FGF
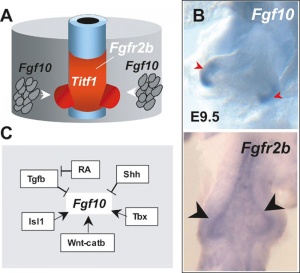
Fibroblast growth factor signaling
[41]- Localized Fgf10 expression not required for lung branching but prevents epithelial differentiation[42] "As the lung buds grow out, proximal epithelial cells become further and further displaced from the distal source of Fgf10 and differentiate into bronchial epithelial cells. Interestingly, our data presented here show that once epithelial cells are committed to the Sox2-positive airway epithelial cell fate, Fgf10 prevents ciliated cell differentiation and promotes basal cell differentiation."
- Opposing Fgf and Bmp activities regulate the specification of olfactory sensory and respiratory epithelial cell fates[43] " In this study, we provide evidence that in both chick and mouse, Bmp signals promote respiratory epithelial character, whereas Fgf signals are required for the generation of sensory epithelial cells. Moreover, olfactory placodal cells can switch between sensory and respiratory epithelial cell fates in response to Fgf and Bmp activity, respectively. Our results provide evidence that Fgf activity suppresses and restricts the ability of Bmp signals to induce respiratory cell fate in the nasal epithelium."
BMP
Bone morphogenic protein 4 (Bmp4) acts as in an autocrine signalling mechanism to limit bud outgrowth and is therefore involved in branching.
Nkx2-1
Transcriptional control of lung alveolar type 1 cell development and maintenance by NK homeobox 2-1 PNAS "Gas exchange in the lung relies on passive diffusion of oxygen and carbon dioxide across an extraordinarily thin epithelium that is nearly entirely made of the poorly understood alveolar type 1 (AT1) cell. Our study shows that all AT1 cells express and require NK homeobox 2-1 (Nkx2-1) for their development and maintenance. Nkx2-1 mutant AT1 cells lose their characteristic molecular and cellular features and transform toward a gastrointestinal fate, highlighting remarkable plasticity of an otherwise terminally differentiated cell type. This work establishes NKX2-1 as the first overarching factor in the AT1 cell transcriptional hierarchy and paves the way for unraveling not only the unique biology of AT1 cells but also cell type-specific roles of a lineage transcription factor." See also 2012 review paper Signaling Networks Regulating Development of the Lower Respiratory Tract
The NKX2-1 gene encodes a transcription factor that is expressed during early development of thyroid, lung, and neural (forebrain regions including basal ganglia and hypothalamus). NKX2-1 was originally called thyroid transcription factor-1 (TTF1) due to the fact that it mediated thyroid-specific gene transcription.
Links: OMIM Nkx2-1
Other
- Heparan sulfate in lung morphogenesis[44] "Heparan sulfate (HS) is a structurally complex polysaccharide located on the cell surface and in the extracellular matrix, where it participates in numerous biological processes through interactions with a vast number of regulatory proteins such as growth factors and morphogens. ...he potential contribution of HS to abnormalities of lung development has yet to be explored to any significant extent, which is somewhat surprising given the abnormal lung phenotype exhibited by mutant mice synthesizing abnormal HS."
- Signaling via Alk5 controls the ontogeny of lung Clara cells[45] "Clara cells, together with ciliated and pulmonary neuroendocrine cells, make up the epithelium of the bronchioles along the conducting airways. Clara cells are also known as progenitor or stem cells during lung regeneration after injury. ...Using lung epithelial cells, we show that Alk5-regulated Hes1 expression is stimulated through Pten and the MEK/ERK and PI3K/AKT pathways. Thus, the signaling pathway by which TGFbeta/ALK5 regulates Clara cell differentiation may entail inhibition of Pten expression, which in turn activates ERK and AKT phosphorylation."
- Wt1 and retinoic acid signaling in the subcoelomic mesenchyme control the development of the pleuropericardial membranes and the sinus horns[46] "Pericardium and sinus horn formation are coupled and depend on the expansion and correct temporal release of pleuropericardial membranes from the underlying subcoelomic mesenchyme. Wt1 and downstream Raldh2/retinoic acid signaling are crucial regulators of this process."
- Links: Sox | StemBook - Specification and patterning of the respiratory system
References
- ↑ 1.0 1.1 Desai TJ, Brownfield DG & Krasnow MA. (2014). Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature , 507, 190-4. PMID: 24499815 DOI.
- ↑ 2.0 2.1 Yamamoto M, Wilting J, Abe H, Murakami G, Rodríguez-Vázquez JF & Abe SI. (2018). Development of the pulmonary pleura with special reference to the lung surface morphology: a study using human fetuses. Anat Cell Biol , 51, 150-157. PMID: 30310706 DOI.
- ↑ 3.0 3.1 Watson JK, Rulands S, Wilkinson AC, Wuidart A, Ousset M, Van Keymeulen A, Göttgens B, Blanpain C, Simons BD & Rawlins EL. (2015). Clonal Dynamics Reveal Two Distinct Populations of Basal Cells in Slow-Turnover Airway Epithelium. Cell Rep , 12, 90-101. PMID: 26119728 DOI.
- ↑ Fujii S, Muranaka T, Matsubayashi J, Yamada S, Yoneyama A & Takakuwa T. (2023). Bronchial tree of the human embryo: Examination based on a mammalian model. J Anat , , . PMID: 37602519 DOI.
- ↑ Little DR, Gerner-Mauro KN, Flodby P, Crandall ED, Borok Z, Akiyama H, Kimura S, Ostrin EJ & Chen J. (2019). Transcriptional control of lung alveolar type 1 cell development and maintenance by NK homeobox 2-1. Proc. Natl. Acad. Sci. U.S.A. , 116, 20545-20555. PMID: 31548395 DOI.
- ↑ Karolak JA, Vincent M, Deutsch G, Gambin T, Cogné B, Pichon O, Vetrini F, Mefford HC, Dines JN, Golden-Grant K, Dipple K, Freed AS, Leppig KA, Dishop M, Mowat D, Bennetts B, Gifford AJ, Weber MA, Lee AF, Boerkoel CF, Bartell TM, Ward-Melver C, Besnard T, Petit F, Bache I, Tümer Z, Denis-Musquer M, Joubert M, Martinovic J, Bénéteau C, Molin A, Carles D, André G, Bieth E, Chassaing N, Devisme L, Chalabreysse L, Pasquier L, Secq V, Don M, Orsaria M, Missirian C, Mortreux J, Sanlaville D, Pons L, Küry S, Bézieau S, Liet JM, Joram N, Bihouée T, Scott DA, Brown CW, Scaglia F, Tsai AC, Grange DK, Phillips JA, Pfotenhauer JP, Jhangiani SN, Gonzaga-Jauregui CG, Chung WK, Schauer GM, Lipson MH, Mercer CL, van Haeringen A, Liu Q, Popek E, Coban Akdemir ZH, Lupski JR, Szafranski P, Isidor B, Le Caignec C & Stankiewicz P. (2019). Complex Compound Inheritance of Lethal Lung Developmental Disorders due to Disruption of the TBX-FGF Pathway. Am. J. Hum. Genet. , , . PMID: 30639323 DOI.
- ↑ Schittny JC. (2018). How high resolution 3-dimensional imaging changes our understanding of postnatal lung development. Histochem. Cell Biol. , 150, 677-691. PMID: 30390117 DOI.
- ↑ Nikolić MZ, Sun D & Rawlins EL. (2018). Human lung development: recent progress and new challenges. Development , 145, . PMID: 30111617 DOI.
- ↑ Gouveia L, Betsholtz C & Andrae J. (2018). PDGF-A signaling is required for secondary alveolar septation and controls epithelial proliferation in the developing lung. Development , 145, . PMID: 29636361 DOI.
- ↑ 10.0 10.1 Gauthier TW & Brown LA. (2017). In utero alcohol effects on foetal, neonatal and childhood lung disease. Paediatr Respir Rev , 21, 34-37. PMID: 27613232 DOI.
- ↑ Yang J, Hernandez BJ, Martinez Alanis D, Narvaez del Pilar O, Vila-Ellis L, Akiyama H, Evans SE, Ostrin EJ & Chen J. (2016). The development and plasticity of alveolar type 1 cells. Development , 143, 54-65. PMID: 26586225 DOI.
- ↑ Mori M, Mahoney JE, Stupnikov MR, Paez-Cortez JR, Szymaniak AD, Varelas X, Herrick DB, Schwob J, Zhang H & Cardoso WV. (2015). Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development , 142, 258-67. PMID: 25564622 DOI.
- ↑ Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD & Chen J. (2013). Lung epithelial branching program antagonizes alveolar differentiation. Proc. Natl. Acad. Sci. U.S.A. , 110, 18042-51. PMID: 24058167 DOI.
- ↑ Yoshimi T, Hashimoto F, Takahashi S & Takahashi Y. (2010). Suppression of embryonic lung branching morphogenesis by antisense oligonucleotides against HOM/C homeobox factors. In Vitro Cell. Dev. Biol. Anim. , 46, 664-72. PMID: 20535580 DOI.
- ↑ Chen F, Cao Y, Qian J, Shao F, Niederreither K & Cardoso WV. (2010). A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J. Clin. Invest. , 120, 2040-8. PMID: 20484817 DOI.
- ↑ Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S & Stocks J. (2010). Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am. J. Respir. Crit. Care Med. , 182, 237-45. PMID: 20378729 DOI.
- ↑ Solomon M, Grasemann H & Keshavjee S. (2010). Pediatric lung transplantation. Pediatr. Clin. North Am. , 57, 375-91, table of contents. PMID: 20371042 DOI.
- ↑ Abe S, Yamamoto M, Noguchi T, Yoshimoto T, Kinoshita H, Matsunaga S, Murakami G & Rodríguez-Vázquez JF. (2014). Fetal development of the minor lung segment. Anat Cell Biol , 47, 12-7. PMID: 24693478 DOI.
- ↑ Chao CM, El Agha E, Tiozzo C, Minoo P & Bellusci S. (2015). A breath of fresh air on the mesenchyme: impact of impaired mesenchymal development on the pathogenesis of bronchopulmonary dysplasia. Front Med (Lausanne) , 2, 27. PMID: 25973420 DOI.
- ↑ Bernhard W. (2016). Lung surfactant: Function and composition in the context of development and respiratory physiology. Ann. Anat. , 208, 146-150. PMID: 27693601 DOI.
- ↑ Sorensen GL. (2018). Surfactant Protein D in Respiratory and Non-Respiratory Diseases. Front Med (Lausanne) , 5, 18. PMID: 29473039 DOI.
- ↑ Vieira F, Kung JW & Bhatti F. (2017). Structure, genetics and function of the pulmonary associated surfactant proteins A and D: The extra-pulmonary role of these C type lectins. Ann. Anat. , 211, 184-201. PMID: 28351530 DOI.
- ↑ Verlato G, Simonato M, Giambelluca S, Fantinato M, Correani A, Cavicchiolo ME, Priante E, Carnielli V & Cogo P. (2018). Surfactant Components and Tracheal Aspirate Inflammatory Markers in Preterm Infants with Respiratory Distress Syndrome. J. Pediatr. , 203, 442-446. PMID: 30270169 DOI.
- ↑ Tridente A, De Martino L & De Luca D. (2019). Porcine vs bovine surfactant therapy for preterm neonates with RDS: systematic review with biological plausibility and pragmatic meta-analysis of respiratory outcomes. Respir. Res. , 20, 28. PMID: 30728009 DOI.
- ↑ Yu H, Wessels A, Chen J, Phelps AL, Oatis J, Tint GS & Patel SB. (2004). Late gestational lung hypoplasia in a mouse model of the Smith-Lemli-Opitz syndrome. BMC Dev. Biol. , 4, 1. PMID: 15005800 DOI.
- ↑ Lewin G & Hurtt ME. (2017). Pre- and Postnatal Lung Development: An Updated Species Comparison. Birth Defects Res , 109, 1519-1539. PMID: 28876535 DOI.
- ↑ Pinkerton KE & Joad JP. (2000). The mammalian respiratory system and critical windows of exposure for children's health. Environ. Health Perspect. , 108 Suppl 3, 457-62. PMID: 10852845
- ↑ Kleven GA & Ronca AE. (2009). Prenatal behavior of the C57BL/6J mouse: a promising model for human fetal movement during early to mid-gestation. Dev Psychobiol , 51, 84-94. PMID: 18980217 DOI.
- ↑ 29.0 29.1 Waghray A & Rajagopal J. (2017). Tips from the embryonic lung. Elife , 6, . PMID: 28806170 DOI.
- ↑ Kadzik RS, Cohen ED, Morley MP, Stewart KM, Lu MM & Morrisey EE. (2014). Wnt ligand/Frizzled 2 receptor signaling regulates tube shape and branch-point formation in the lung through control of epithelial cell shape. Proc. Natl. Acad. Sci. U.S.A. , 111, 12444-9. PMID: 25114215 DOI.
- ↑ 31.0 31.1 Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MA, Kopp JL, Sander M, Wellik DM & Spence JR. (2013). Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc. Natl. Acad. Sci. U.S.A. , 110, E4456-64. PMID: 24191021 DOI.
- ↑ Kaarteenaho R, Lappi-Blanco E & Lehtonen S. (2010). Epithelial N-cadherin and nuclear β-catenin are up-regulated during early development of human lung. BMC Dev. Biol. , 10, 113. PMID: 21080917 DOI.
- ↑ 33.0 33.1 DiAugustine RP & Sonstegard KS. (1984). Neuroendocrinelike (small granule) epithelial cells of the lung. Environ. Health Perspect. , 55, 271-95. PMID: 6376101
- ↑ Cutz E. (1982). Neuroendocrine cells of the lung. An overview of morphologic characteristics and development. Exp. Lung Res. , 3, 185-208. PMID: 6188605
- ↑ Cutz E, Gillan JE & Bryan AC. (1985). Neuroendocrine cells in the developing human lung: morphologic and functional considerations. Pediatr. Pulmonol. , 1, S21-9. PMID: 3906540
- ↑ McGovern S, Pan J, Oliver G, Cutz E & Yeger H. (2010). The role of hypoxia and neurogenic genes (Mash-1 and Prox-1) in the developmental programming and maturation of pulmonary neuroendocrine cells in fetal mouse lung. Lab. Invest. , 90, 180-95. PMID: 20027181 DOI.
- ↑ Barker PM & Olver RE. (2002). Invited review: Clearance of lung liquid during the perinatal period. J. Appl. Physiol. , 93, 1542-8. PMID: 12235057 DOI.
- ↑ Hall SM, Hislop AA & Haworth SG. (2002). Origin, differentiation, and maturation of human pulmonary veins. Am. J. Respir. Cell Mol. Biol. , 26, 333-40. PMID: 11867341 DOI.
- ↑ 39.0 39.1 Hislop AA. (2002). Airway and blood vessel interaction during lung development. J. Anat. , 201, 325-34. PMID: 12430957
- ↑ Arora R, Metzger RJ & Papaioannou VE. (2012). Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. , 8, e1002866. PMID: 22876201 DOI.
- ↑ 41.0 41.1 Cardoso WV & Kotton DN. (2008). Specification and patterning of the respiratory system. , , . PMID: 20614584 DOI.
- ↑ Volckaert T, Campbell A, Dill E, Li C, Minoo P & De Langhe S. (2013). Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development , 140, 3731-42. PMID: 23924632 DOI.
- ↑ Maier E, von Hofsten J, Nord H, Fernandes M, Paek H, Hébert JM & Gunhaga L. (2010). Opposing Fgf and Bmp activities regulate the specification of olfactory sensory and respiratory epithelial cell fates. Development , 137, 1601-11. PMID: 20392740 DOI.
- ↑ Thompson SM, Jesudason EC, Turnbull JE & Fernig DG. (2010). Heparan sulfate in lung morphogenesis: The elephant in the room. Birth Defects Res. C Embryo Today , 90, 32-44. PMID: 20301217 DOI.
- ↑ Xing Y, Li C, Li A, Sridurongrit S, Tiozzo C, Bellusci S, Borok Z, Kaartinen V & Minoo P. (2010). Signaling via Alk5 controls the ontogeny of lung Clara cells. Development , 137, 825-33. PMID: 20147383 DOI.
- ↑ Norden J, Grieskamp T, Lausch E, van Wijk B, van den Hoff MJ, Englert C, Petry M, Mommersteeg MT, Christoffels VM, Niederreither K & Kispert A. (2010). Wt1 and retinoic acid signaling in the subcoelomic mesenchyme control the development of the pleuropericardial membranes and the sinus horns. Circ. Res. , 106, 1212-20. PMID: 20185795 DOI.
Reviews
Nikolić MZ, Sun D & Rawlins EL. (2018). Human lung development: recent progress and new challenges. Development , 145, . PMID: 30111617 DOI.
Furlow PW & Mathisen DJ. (2018). Surgical anatomy of the trachea. Ann Cardiothorac Surg , 7, 255-260. PMID: 29707503 DOI.
Mecham RP. (2018). Elastin in lung development and disease pathogenesis. Matrix Biol. , , . PMID: 29331337 DOI.
Schittny JC. (2017). Development of the lung. Cell Tissue Res. , 367, 427-444. PMID: 28144783 DOI.
Stabler CT & Morrisey EE. (2017). Developmental pathways in lung regeneration. Cell Tissue Res. , 367, 677-685. PMID: 27957616 DOI.
Herriges M & Morrisey EE. (2014). Lung development: orchestrating the generation and regeneration of a complex organ. Development , 141, 502-13. PMID: 24449833 DOI.
Stocks J, Hislop A & Sonnappa S. (2013). Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med , 1, 728-42. PMID: 24429276 DOI.
Gauthier TW & Brown LA. (2017). In utero alcohol effects on foetal, neonatal and childhood lung disease. Paediatr Respir Rev , 21, 34-37. PMID: 27613232 DOI.
Rackley CR & Stripp BR. (2012). Building and maintaining the epithelium of the lung. J. Clin. Invest. , 122, 2724-30. PMID: 22850882 DOI.
Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR & Jesudason E. (2010). Lung organogenesis. Curr. Top. Dev. Biol. , 90, 73-158. PMID: 20691848 DOI.
Morrisey EE & Hogan BL. (2010). Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell , 18, 8-23. PMID: 20152174 DOI.
Burri PH. (2006). Structural aspects of postnatal lung development - alveolar formation and growth. Biol. Neonate , 89, 313-22. PMID: 16770071 DOI.
Merkus PJ. (2003). Effects of childhood respiratory diseases on the anatomical and functional development of the respiratory system. Paediatr Respir Rev , 4, 28-39. PMID: 12615030
Chinoy MR. (2003). Lung growth and development. Front. Biosci. , 8, d392-415. PMID: 12456356
Burri PH. (1984). Fetal and postnatal development of the lung. Annu. Rev. Physiol. , 46, 617-28. PMID: 6370120 DOI.
Articles
Mund SI, Stampanoni M & Schittny JC. (2008). Developmental alveolarization of the mouse lung. Dev. Dyn. , 237, 2108-16. PMID: 18651668 DOI.
Burri PH. (2006). Structural aspects of postnatal lung development - alveolar formation and growth. Biol. Neonate , 89, 313-22. PMID: 16770071 DOI.
Hall SM, Hislop AA & Haworth SG. (2002). Origin, differentiation, and maturation of human pulmonary veins. Am. J. Respir. Cell Mol. Biol. , 26, 333-40. PMID: 11867341 DOI.
Hall SM, Hislop AA, Pierce CM & Haworth SG. (2000). Prenatal origins of human intrapulmonary arteries: formation and smooth muscle maturation. Am. J. Respir. Cell Mol. Biol. , 23, 194-203. PMID: 10919986 DOI.
Sparrow MP, Weichselbaum M & McCray PB. (1999). Development of the innervation and airway smooth muscle in human fetal lung. Am. J. Respir. Cell Mol. Biol. , 20, 550-60. PMID: 10100986 DOI.
Search PubMed
Search April 2010
- Respiratory System Development - All (30795) Review (3706) Free Full Text (7943)
- Respiratory Development - All (28939) Review (5876) Free Full Text (7203)
Search Pubmed: Respiratory System Development | Respiratory Development
Terms
| Respiratory Terms (expand to view)
|
- adenovirus - A Class I virus containing a single double-stranded DNA (dsDNA), which can cause infections in the upper respiratory tract in many animals. (More? viral infection)
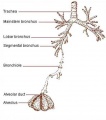
- alveolar duct - Anatomical short region lying between the end of the respiratory bronchioles and the final alveolar sacs. Term is also used in the mammary gland, to describe the smallest of the intralobular ducts into which the secretory alveoli open.
- alveolar sac - (alveolus), Latin alveolus = little cavity) Anatomical and functional end of the mammalian lung respiratory tree where gas exchange occurs. In humans, during lung development these are the last features to form from 7 months onwards.
- alveolar - Term used in relation to the alveoli of the lungs. The final functional sac of the respiratory tree where gas exchange occurs between the alveolar space and the pulmonary capillaries.
- alveolar stage - Term used to describe lung development, the final histological/developmental stage (Pseudoglandular, Fetal Canalicular, Terminal sac, Alveolar). This stage occurs from late fetal/neonate with alveoli formation, the final functional sac of the respiratory tree exists, where gas exchange occurs between the alveolar space and the pulmonary capillaries. (embryonic stage - pseudoglandular stage - canalicular stage - saccular stage - alveolar stage)
- alveolus - (alveolar sacs, plural alveoli, Latin alveolus = little cavity) Anatomical and functional end of the mammalian lung respiratory tree where gas exchange occurs. In humans, during lung development these are the last features to form from 7 months onwards. The acinus starts approximately 3 to 4 generations proximal of the bronchioalveolar duct junction and ends about 4 generations of alveolar ducts distal of the bronchioalveolar duct junction.
- angiogenesis - vascular growth by direct extension from pre-existing blood vessels. (see also vasculogenesis).
- apgar - Non-invasive clinical test designed by Dr Virginia Apgar (1953) carried out immediately on newborn. The name is also an acronym for: Activity (Muscle Tone), Pulse, Grimace (Reflex Irritability), Appearance (Skin Color), Respiration. A score is given for each sign at one minute and five minutes after the birth. (More? Apgar test)
- apnea - Respiratory term meaning the cessation of breathing.
- assisted ventilation - Clinical term referring to newborn (perinatal) respiration assistance required immediately following delivery, the infant given minimal breaths for any duration with bag and mask or bag and endotracheal tube within the first several minutes from birth. Excludes free flow oxygen only and laryngoscopy for aspiration of meconium.
- asthma - Flow limitation during tidal expiration in early life significantly associated with the development of physician-diagnosed asthma by the age of 2 years. Infants with abnormal lung function soon after birth may have a genetic predisposition to asthma or other airway abnormalities that predict the risk of subsequent lower respiratory tract illness. Asthma phenotypes have a number of different classifications; allergic asthma, intrinsic or nonallergic asthma, infectious asthma, and aspirin-exacerbated asthma, and environmental exposures (occupational agents, smoking, air pollution, cold dry air) (More? PMID 5439356)
- azygos lobe - Common condition (0.5% of population). The right lung upper lobe expands either side of the posterior cardinal. There is also some course variability of the phrenic nerve in the presence of an azygos lobe.
- Bochdalek hernia - The most common form (80-85%) of the Congenital Diaphragmatic Hernia (CDH) types occurring mainly on the postero-lateral (left) side of the respiratory diaphragm. (More? congenital diaphragmatic hernia)
- bronchi - (Latin, bronchos = windpipe) Plural of bronchus, the two subdivisions of the trachea carrying air to the lungs. Embryologically form as an endodermal outpocket of the foregut which branch (bronchiole, subdivision of the bronchus) as they grow. Airway: trachea - bronchi - lobar bronchi - segmental bronchi - bronchioles - conducting bronchioles - terminal bronchioles - respiratory bronchioles - alveolar ducts.
- bronchiole - A smaller branch subdivision of the respiratory tract bronchus, lack supporting cartilage skeletons and have a diameter of about 1 mm. Epithelium is initially ciliated and graduates to simple columnar epithelium and lining no longer contain mucous-producing cells.
- bronchopulmonary dysplasia - (chronic lung disease in preterm infants) Clinical term for a heterogeneous lung disease seen in preterm (premature) infants and diagnosed within the first months of life. Condition was first described in 1967. (More? preterm birth American Lung Association)
- canalicular stage - (fetal canalicular, canalicular phase) Term used to describe lung development, after early embryonic the second of the histological/developmental stages (pseudoglandular, fetal canalicular, terminal sac, alveolar). This stage occurs during the fetal period from week 16 to 24. During this stage there is lung bud mesenchymal angiogenesis and cellular differentiation into different stromal cell types (fibroblasts, myoblasts and chondrocytes). (embryonic stage - pseudoglandular stage - canalicular stage - saccular stage - alveolar stage)
- carbon monoxide - (CO) A colourless and odorless gas formed mainly as a by-product of incomplete combustion of hydrocarbons and can cause cytotoxicity by tissue hypoxia. Carbon monoxide enters circulation though the respiratory system, binding to haemoglobin to form carboxy-haemoglobin (COHb), with fetal haemoglobin binding with a greater affinity.
- CDH - Acronym for Congenital Diaphragmatic Hernia, a musculoskeletal abnormality of the respiratory diaphragm. The most common form being the Bochdalek hernia.
- chorioamnionitis - (amnionitis, intra-amniotic infection) intrauterine bacterial infection/inflammation that can cause preterm birth and affect respiratory development directly as well as thought the underdeveloped brainstem, resulting in reduced respiratory drive.
- chronic lung disease - (CLD) Clinical term, a neonatal chronic lung disease can be caused by prolonged mechanical ventilation (MV) and oxygen-rich gas with premature infants.
- Clara cells - Respiratory tract epithelial cells on the luminal surface of airways. These cells have a dome shaped cytoplasmic protrusion and no cilia and their function is secretory and xenobiotic. Clara cells can act as progenitor cell in small airways replacing injured terminally differentiated epithelial cells.
- Clara cell secretory protein - (CCSP) A protective lung protein secreted from non-ciliated bronchiolar epithelial cells in the conducting airways of mammals. The protein increases in expression level post-natally and is thought to have antioxidant, immunomodulatory, and anticarcinogenic properties.
- connective tissue fibers - form a continuous alveolar support with axial, peripheral and septal fibers.
- congenital diaphragmatic hernia - Abnormality due to failure of the pleuroperitoneal foramen (foramen of Bochdalek) to close (left side), allows viscera into thorax Intestine, stomach or spleen can enter the pleural cavity, compressing the lung. Rarer (Morgagni hernia) is an opening in the front of the diaphragm. (More? congenital diaphragmatic hernia | GeneReviews
- congenital laryngeal webs - Laryngeal abnormality due to embryonic (week 10) incomplete recanalization of the laryngotracheal tube during the fetal period. Rare abnormality occuring mainly at the level of the vocal folds (glottis).
- corticosteroid - An endocrine steroidal hormone produced by the adrenal cortex. Clinically, corticosteroids are also used for lung maturation of the premature neonate.
- cystic fibrosis - Inherited disease of the mucus and sweat glands, causes mucus to be thick and sticky. Clogging the lungs, causing breathing problems and encouraging bacterial grow. (Covered elsewhere in the course)
- diaphragm - A general term for a membranous sheet, used to describe the respiratory diaphragm. The muscular sheet separating chest from abdomen with several different embryonic origins. Regular contraction of the diaphragm is required in respiration. The diaphragm forms initially at the lower end of the pleuroperitoneal canal. (Embryonic origins: transverse septum (septum transversum) - tendon of the diaphragm, 3rd to 5th somite pairs - musculature of diaphragm, ventral pleural sac - connective tissue, mesentry of oesophagus - connective tissue around oesophasus and inferior vena cava, and pleuroperitoneal membranes - connective tissue around central tendon)
- endoderm - (Greek, endo = inside + derma = skin) One of the initial 3 germ cell layers (ectoderm, mesoderm, endoderm) formed by the process of gastrulation. The endoderm forms the epithelial lining glands and of the respiratory tract.
- epaxial muscle - Anatomical term describing skeletal muscles which lie dorsal (posterior) to the vertebral column developing from the somite myotome. At the ribcage level the levatores costarum muscles involved with rib elevation during respiration.
- epiglottis - (Greek, epi = above, upon) cartilaginous part of the larynx above the glottis, which in infancy directs food into the esophagus and not the trachea . Embryologically it develops in the foregut from the hypobranchial eminence, behind the undeveloped tongue, from which it separates at about 7 weeks. Postnatal anatomical development in humans involves a maturational descent in infancy (4 and 6 months of age). Contains lymphoid tissue (larynx-associated lymphoid tissue, LALT and Bronchus-associated lymphoid tissue, BALT).
- Extracorporeal Membrane Oxygenation - (ECMO) an invasive therapy that has been investigated and utilized in newborn infants with cardiorespiratory failure.
- fetal breathing movements - (FBM) Occur in the third trimester preparing both the skeletomuscular and neural system, and lungs mechanically and the amount of liquid within the developing lungs.
- fistula - An abnormal communication between 2 structures (organs, vessels, cavities) that do not normally connect, can occur between the trachea and oesophagus.
- foregut - The first of the three part/division (foregut - midgut - hindgut) of the early forming gastrointestinal tract. The foregut runs from the buccopharyngeal membrane to the midgut and forms all the tract (esophagus and stomach) from the oral cavity to beneath the stomach. In addition, a ventral bifurcation of the foregut will also form the respiratory tract epithelium.
- glottis - (Greek, = larynx) the boundary between pharynx to the larynx and consists of the vocal folds and their associated intervening space.
- HIF-1 - A transcription factor that is one of the main regulators of homeostasis in human tissues exposed to hypoxia, due to inflammation and/or insufficient circulation.
- hyaline membrane disease - (Newborn Respiratory Distress Syndrome) Abnormality due to a membrane-like substance from damaged pulmonary cells.
- hypopharynx - connects the oropharynx to the oesophagus and the larynx, the region of pharynx below the hyoid bone.
- laryngeal cleft - (LC, laryngeal-tracheo-oesophageal cleft) A rare foregut abnormality allowing digestive tract and the airway to communicate causing chronic cough, aspiration and respiratory distress. The downward extension of the cleft determines the classification of the abnormality.
- laryngeal webs - (congenital laryngeal webs) Laryngeal abnormality due to embryonic (week 10) incomplete recanalization of the laryngotracheal tube. Rare abnormality occuring mainly at the level of the vocal folds (glottis).
- laryngotracheal groove - Early embryonic foregut developmental feature, forms on the anterior (ventral) wall of the pharynx and gives rise to larynx, trachea and entire respiratory tree. In humans, this feature is the first indication of respiratory development and appears during week 4.
- larynx - Site of the the vocal folds in the neck. Embryologically develops from the foregut with the lining derived from endoderm and the cartilage from pharyngeal arch 4 and 6. Beginning as a simple foregut groove, the laryngotracheal groove which folds to form the laryngotracheal bud, then the larynx and trachea.
- late-gestation lung protein 1 - (LGL1) A glycoprotein secreted by fetal lung mesenchyme and fetal kidney, involved in retinoic acid stimulated branching morphogenesis.
- lipofibroblast - (lipid interstitial cell, pulmonary lipofibroblast) Cell involved in secondary septum formation during the alveolar stage of lung development (late fetal to postnatal). Cell is recognizable by a number of characteristic lipid droplets and contains cortical contractile filaments.
- lobar emphysema - (overinflated lung) Abnormality of an overinflated left upper lobe There is a collapsed lower lobe The left lung is herniating across the mediastinum.
- measles - (paramyxovirus) Measles (rubeola) is mainly a respiratory viral infection, clinically different from Rubella.
- meconium aspiration syndrome - (MAS) Fetal stress in the third trimester, prior to/at/ or during parturition can lead to premature meconium discharge into the amniotic fluid and sunsequent ingestion by the fetus and damage to respiratory function.
- medullary respiratory centres - medulla oblongata collection of nuclei organised into ventral and dorsal respiratory groups. The ventral respiratory nuclei pre-Bötzinger complex (pBÖTC) required for respiratory rhythmogenesis.
- mitochondria - Double membraned cell organelle located in the cytoplasm, a cell may contain 100's or more mitochondria, the number can relate to the metabolic activity of that cell. Functions in cell respiration, providing energy to the cell and also has a role in the process of apoptosis (programmed cell death).
- newborn respiratory distress syndrome - (respiratory distress syndrome, RDS, hyaline membrane syndrome) - surfactant deficiency at birth more common in preterm birth. RDS Info
- nitrofen - A diphenyl ether herbicide teratogen used in rodent development to generate a range of developmental abnormalities, including congenital diaphragmatic hernia.
- oropharynx - The second portion of the pharynx (throat) connecting the nasopharynx and laryngopharynx (hypopharynx). Region between the palate and the hyoid bone, anteriorly divided from the oral cavity by the tonsillar arch.
- parathyroid hormone-related protein - (PTHrP) A protein named for its evolutionary and structural relationship to parathyroid hormone (PTH). A protein hormone produced by many fetal tissues and with a number of different functions including a possible autocrine role in lung development.
- parietal pleura - Serous membrane which forms the outer lining of pleural cavity. mesoderm of the thoracic cavity body wall and derived from epithelia of pericardioperitoneal canals from intra-embryonic coelom. The inner pleural layer, visceral pleura, is splanchnic mesoderm in origin.
- Pentalogy of Cantrell - A developmental abnormality of the anterior diaphragm, diaphragmatic pericardium, abdominal wall, cardiovascular and lower sternum.
- Persistent Pulmonary Hypertension of the Newborn - (PPHN) A serious newborn condition due to due to the failure of closure one of the prenatal circulatory shunts, the ductus arteriosus. Occurs in about 1-2 newborns per 1000 live births and results in hypoxemia.
- pharynx - (throat) embryo uppermost end of the combined gastrointestinal and respiratory tract beginning at the buccopharyngeal membrane and forms a major arched cavity within the phrayngeal arches. Also used as a respiratory term describing the initial segment of the upper respiratory tract divided anatomically into three regions: nasopharynx, oropharynx, and laryngopharynx (hypopharynx). Anatomically extends from the base of the skull to the level of the sixth cervical vertebra.
- pleural cavity - Anatomical body cavity in which the lungs develop and lie. Forms in the lateral plate mesoderm as part of the early single intra-embryonic coelom, the pleural cavities are initially two narrow canals.
- pleuropericardial fold - (pleuropericardial membrane) An early embryonic fold which restricts the communication between pleural cavity and pericardiac cavity, contains both the |cardinal vein and phrenic nerve.
- pleuroperitoneal foramen - The developmental opening occurring in the intra-embryonic coelom before formation of the pleuroperitoneal membrane.
- PLUNC - Acronym for Palate, LUng, Nasal epithelium Clone protein, related to the lipid transfer/lipopolysaccharide binding protein (LT/LBP) family. This protein is secreted by the airway conducting epithelia and acts as a surfactant that may interfere with biofilm formation by airway pathogens.
- pulmonary acini (singular - acinus) region of the lung supplied with air from one of the terminal bronchioles, anatomical and functional end of the mammalian lung respiratory tree where gas exchange occurs. Starts approximately 3 to 4 generations proximal of the bronchio-alveolar duct junction and ends about 4 generations of alveolar ducts distal of the bronchioalveolar duct junction.
- pulmonary arterial hypertension - (PAH) a progressive disease characterized by abnormalities of vascular tone and reactivity, vessel wall structure, growth, and thrombosis that in newborns, infants, and children can contribute to poor outcomes in cardiac, pulmonary, and systemic diseases.
- respiratory - Term used in relation to breathing (in and out) or associated with the lungs. Anatomically used to describe the lungs, air pathways and associated muscles. In cell biology used in relation to mitochondrial use of oxygen to produce energy and carbon dioxide waste.
- respiratory bronchioles - may contain alveoli and have surface surfactant-producing Respiratory bronchioles can contain alveoli and surfactant-producing cells, and give rise to between 2 to 11 alveolar ducts.
- respiratory sinus arrhythmia - (RSA) Clinically used as an index of cardiac vagal activity, measured breath-by-breath by subtracting the minimum heart rate (HR) during expiration from the maximum HR during inspiration.
- respiratory tree - Anatomical term to describe the components of the respiratory system (lungs) as they branch again and again ending in the functional units, the alveolar sacs (alveolus).
- saccular stage - (terminal sac stage) process of lung epithelial cell differentiation, vascular remodeling and thinning of the mesenchyme. This process leads to enlargement of the diameter and surface area of the alveolar sacs. Distal epithelial cells form 2 populations: 1. cells flattens, thins, and spreads to form type I cells; 2. cells remain cuboidal, acquire surfactant filled lamellar bodies and differentiate into type II cells. Sacculation is a general anatomical term meaning to formed a series of sac-like expansions. (embryonic stage - pseudoglandular stage - canalicular stage - saccular stage - alveolar stage)
- secondary septa - (secondary septa) process in the lung alveolar stage of development (postnatally) where the double capillary network in the immature gas-exchange region fuses to form a single capillary layer.
- septum transversum - (transverse septum) A mesodermal region in the early embryo. Identified externally as the junctional site between amnion and yolk sacs, and internally (within the embryo) lying directly beneath the heart and at the foregut/midgut junction. This ventro-dorsal "plate" of mesoderm contributes several structures including: the central tendon of diaphragm and some of the liver.
- stenosis - Term used to describe an abnormal narrowing, usually in relation to a tube for example: respiratory tract, blood vessel, gastrointestinal tract.
- stomodeum - (stomadeum, stomatodeum) The primordial mouth region of the developing embryonic head.
- surfactant - (surface active agent ; pulmonary surfactant) A mixture of lipids and proteins secreted by Type 2 alveolar cells between alveolar epithelium that reduces surface tension (detergent) at the air-liquid interface. The function is to prevent collapse of the lung at the end of expiration. In humans, these cells and their secretion develop towards the very end of the third trimester, just before birth. Clinical surfactants used for surfactant replacement therapy are animal-derived preparations, commonly bovine (beractant, bovactant, BLES) or less common porcine (butantan, poractant-α and surfacen) PMID30728009.
- surfactant protein D - (SP-D) a multimeric collectin (collagen-containing C-type lectin) involved in innate immunity (anti-microbial) and expressed in pulmonary and non-pulmonary epithelia. PMID 29473039
- surfactant replacement therapy - (surfactant therapy) A clinical birth term referring to the endotracheal instillation of a surface-active suspension for treating surfactant deficiency due to either preterm birth or pulmonary injury resulting in respiratory distress (newborn respiratory distress syndrome).
- tachypnea - (Greek, tachypnea = rapid breathing) Clinical term describing an increased respiratory rate of greater than 60 breaths/minute in a quiet resting baby.
- terminal sac stage - (saccular stage, terminal sac phase, immature alveoli) Term used to describe the second last histological/developmental stage (pseudoglandular stage, Fetal Canalicular, saccular stage, Alveolar) of lung development. This stage occurs from late fetal week 24 to 36. During this stage branching and growth of the terminal sacs occurs, with cellular differentiation of the type -II pneumonocytes and type - I pneumonocytes The final functional sac of the respiratory tree occurs at the next neonatal period, where gas exchange occurs between the alveolar space and the pulmonary capillaries. (embryonic stage - pseudoglandular stage - canalicular stage - saccular stage - alveolar stage)
- trachea - (windpipe) In the embryo, a ventral out-pocket of pharynx endoderm that branches in week 4 stage 13 into the right and left bronchi within the lung buds. The endoderm has associated mesoderm that later differentiates to form most structures outside the respiratory epithelium. In the adult, the trachea forms the functional connection between the pharynx and larynx to the lungs. Adult trachea is a ciliated pseudostratified columnar epithelium supported by C-shaped rings of hyaline cartilage.
- tracheoesophageal fistula - Abnormal connection between the trachea and oesophagus.
- Trisomy 21 - (Down syndrome) associated with significant cardiovascular and pulmonary mortality and morbidity of neonates, infants, and children. Infant lung histology may share features of decreased lung vascular and alveolar growth. (More? Trisomy 21 | PMID 25621156)
- vagus - (Latin, vagus = wandering) cranial nerve X (CN X) A mixed nerve that leaves the head and neck to innervate respiratory tract (larynx, lungs), gastrointestinal tract (pharynx, esophagus, stomach), cardiac (heart) and abdominal viscera. This mixed nerve has sensory, motor and autonomic functions of viscera (glands, digestion, heart rate).
- vasculogenesis - the formation of new blood vessels from angioblasts or endothelial progenitor cells (EPCs) that migrate, differentiate, and grow into tubes in response to signals from surrounding cells. (see also angiogenesis).
- VEGF - (vascular endothelial growth factor) a specific mitogen and survival factor required for endothelium growth and development also required in lung vasculature development and remodelling.
- ventilatory unit - region from a respiratory bronchiole extending to the supported alveolar ducts and alveoli.
- visceral pleura - Serous membrane which forms the inner lining of pleural cavity, both covering and attached to the lungs. Embryonically derived from the splanchnic mesoderm. The outer pleural layer, parietal pleura, is derived from mesoderm of the thoracic cavity body wall.
|
Additional Images
Upper Respiratory Tract
Lower Respiratory Tract
Model Sox9 lung development PMID 24274029
Human right lung 7-8 weeks PMID 24693478
Mouse 36 somites PMID 25114215
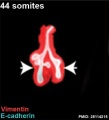
Mouse 44 somites PMID 25114215
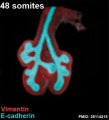
Mouse 48 somites PMID 25114215
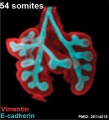
Mouse 54 somites PMID 25114215
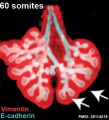
Mouse 60 somites PMID 25114215
Historic Images
| Historic Disclaimer - information about historic embryology pages
|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers)
|
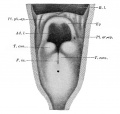
week 4 early respiratory endodermal bud
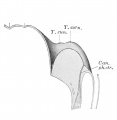
week 4 later ventral endoderm growth
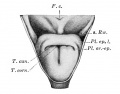
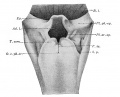
conducting system bronchi to lungs
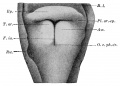
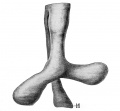
secondary lobule from the human lung
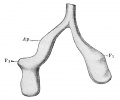
longitudinal section of a primary lung lobule
transverse section of the thorax showing the middle and posterior mediastinum
Adult cervical plexus (phrenic nerve shown lower right)
Frazer JE. Development of the larynx. (1910) J Anat. 44: 156-191. PMID 17232839
Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
- “Respiratory
embryo 2.5 mm (Rob. Meyer No. 300)
larynx entrance embryo 8 mm
larynx entrance embryo 8-9 mm (28-29 days)
larynx embryo 8-9 mm (28-29 days)
larynx entrance embryo 15-16 mm (40-42 days)
larynx entrance embryo 30 mm
larynx entrance embryo of 16/23 cm
larynx entrance embryo of 29/43 cm
Epithelial lung embryo 18.5 mm (Rob. Meyer No. 338)
Epithelial lung embryo 28.7 mm (Chr. 1)
ight lung fetus of 100 mm
mesodermal lungs embryo of 5 mm
Mesodermal lung embryo 7 mm (Chr. 1)
Mesodermal lung embryo 15 mm
Mesodermal lung embryo 15 mm
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 26) Embryology Respiratory System Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Respiratory_System_Development
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G