SH Lecture - Respiratory System Development
| Embryology - 1 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
- Current research suggests that both genetic and the developmental environment (fetal and postnatal) can influence the growth, differentiation and function of the respiratory system.
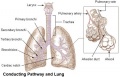
| Lower respiratory tract | Respiratory tree |
|---|---|
2019 Respiratory Development Lecture PDF
| Lecture Archive |
|---|
| 2018 PDF | 2017 | 2017 PDF | 2016 | 2016 PDF | 2015 | 2015 PDF | 2014 | Lecture 2014 PDF | 2013 PDF | 2013 | 2012 | 2012 PDF (10 pages) | eMed Link to Learning Activity - Respiratory System Development |
- SH Links: Lymphatic Lecture | Lymphatics Practical Support | Respiratory Lecture | Respiratory Practical Support | Medicine
The respiratory system does not carry out its physiological function (of gas exchange) until after birth, though the respiratory tract, diaphragm and lungs do begin to form early in embryonic development and continue through fetal development, only functionally maturing just before birth. The lungs continue to grow postnatally through childhood and some research finding suggest that there remains potential for growth in the adult.
The respiratory tract is divided anatomically into 2 main parts:
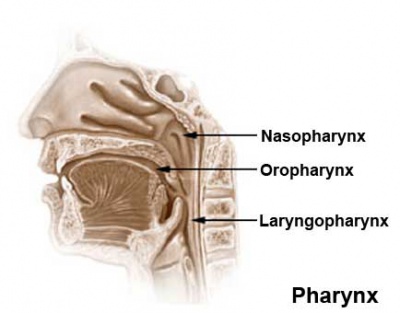
- upper respiratory tract - consisting of the nose, nasal cavity and the pharynx.
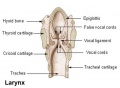
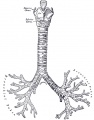
- lower respiratory tract - consisting of the larynx, trachea, bronchi and the lungs.
The respiratory "system" usually includes descriptions of not only the functional development of the lungs, but also related musculoskeletal (diaphragm) and vascular (pulmonary) development.
AimTo understand the prenatal and postnatal developmental anatomy of human respiratory organs.
|
Key Concepts
| ||||||||||||
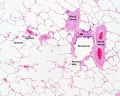
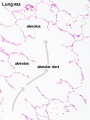
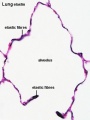
Respiratory Functional Unit
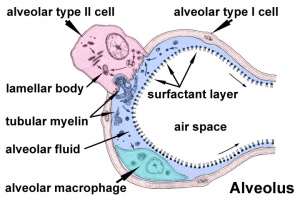
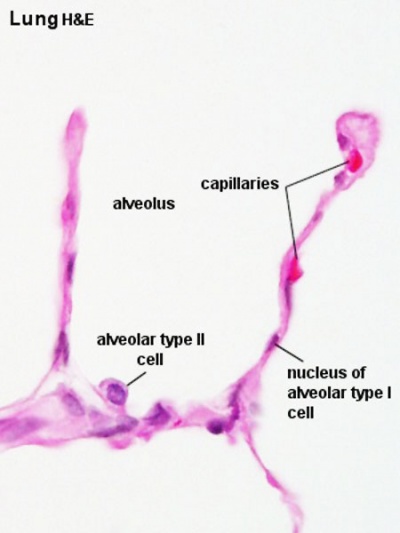
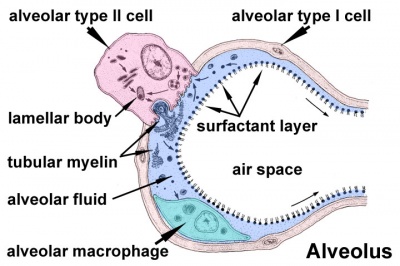
Alveolus
Alveolus (Latin alveolus = "little cavity", plural is alveoli)
|
|
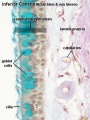
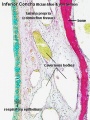
| Alveolus histology | Alveolus structure |
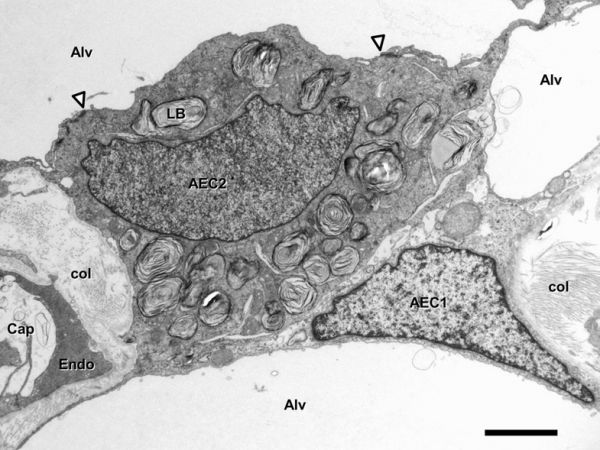
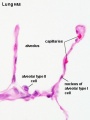
Inter-Alveolar Septum[6]
Septum containing Type I (AEC1) and type II (AEC2) alveolar epithelial cell, alveolar lumen (Alv), capillary lumen (Cap), capillary endothelial cell (Endo ). Lamellar bodies (LB) in type II cell. Arrowheads mark tight junctions between type II and type I cell. Collagen fibrils (col) are present in the interstitium. Transmission electron microscopy. Scale bar 2 µm
|
|
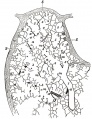
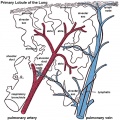
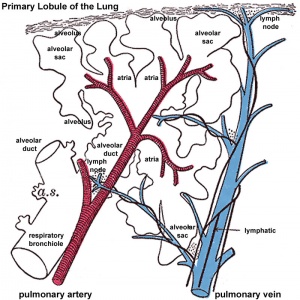
Primary Lobule
|
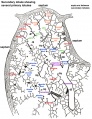
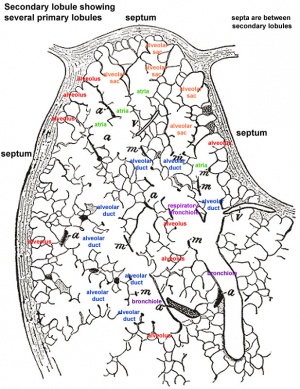
Secondary Lobule
|
Developmental Overview
Germ Layers
- endoderm and splanchnic mesoderm form majority of conducting and alveoli.
- ectoderm will contribute the neural innervation.
- mesoderm also contributes the supporting musculoskeletal components.
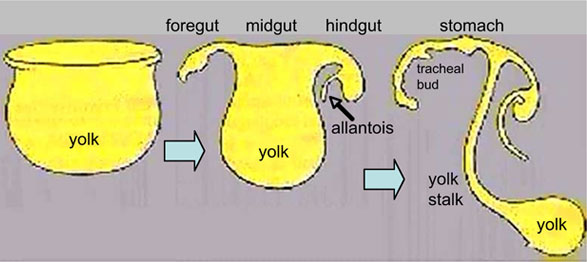
Week 4 - laryngotracheal groove forms on floor foregut.
Week 5 - left and right lung buds push into the pericardioperitoneal canals (primordia of pleural cavity)
Week 6 - descent of heart and lungs into thorax. Pleuroperitoneal foramen closes.
Week 7 - enlargement of liver stops descent of heart and lungs.
Month 3-6 - lungs appear glandular, end month 6 alveolar cells type 2 appear and begin to secrete surfactant.
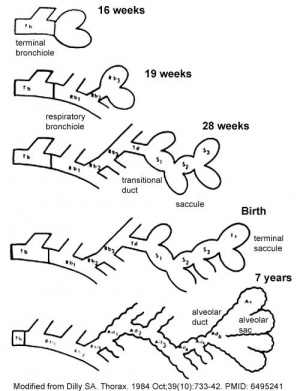
Month 7 - respiratory bronchioles proliferate and end in alveolar ducts and sacs.
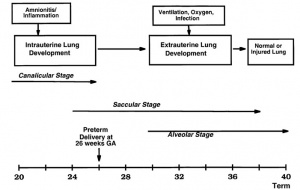
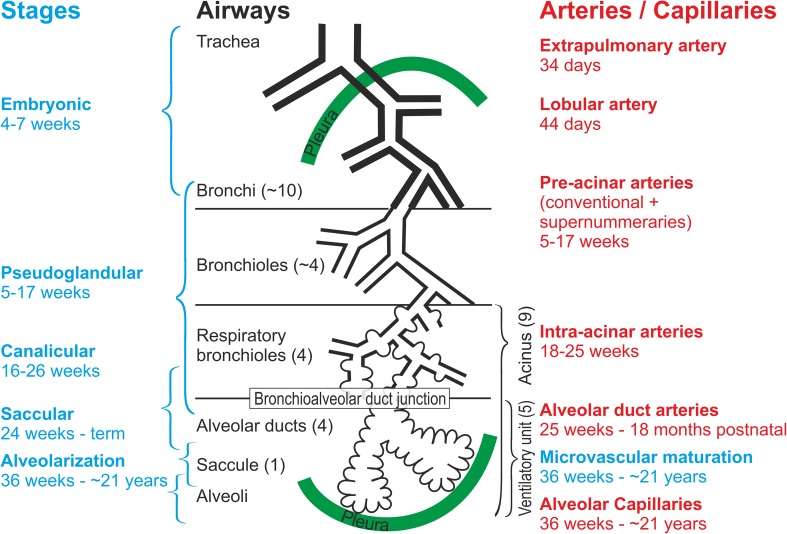
Development Stages
Note - the sequence is important rather than the actual timing, which is variable in the existing literature.
| Lung Stage | Human | Features | Vascular | |
|---|---|---|---|---|
| Embryonic | week 4 to 5 | lung buds originate as an outgrowth from the ventral wall of the foregut where lobar division occurs | extra pulmonary artery then lobular artery | |
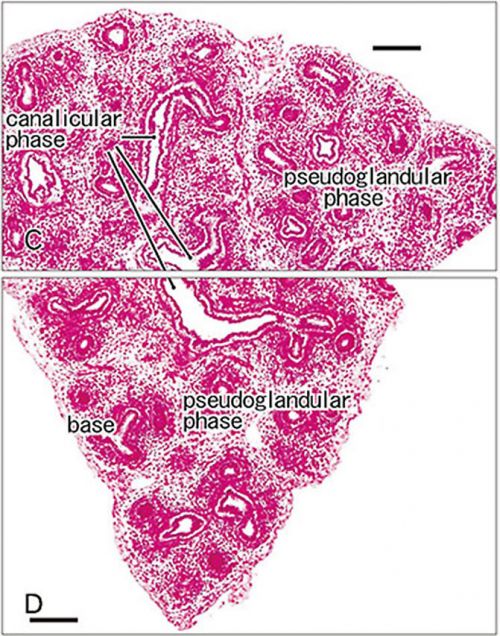
| Pseudoglandular | week 5 to 17 | conducting epithelial tubes surrounded by thick mesenchyme are formed, extensive airway branching | Pre-acinar arteries | |
| Canalicular | week 16 to 25 | bronchioles are produced, increasing number of capillaries in close contact with cuboidal epithelium and the beginning of alveolar epithelium development | Intra-acinar arteries | |
| Saccular | week 24 to 40 | alveolar ducts and air sacs are developed | alveolar duct arteries | |
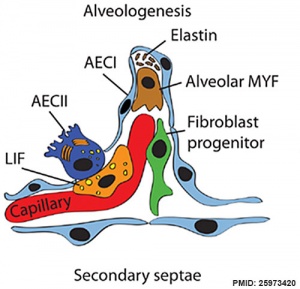
| Alveolar | late fetal to 8 years | secondary septation occurs, marked increase of the number and size of capillaries and alveoli | alveolar capillaries | |
| embryonic stage - pseudoglandular stage - canalicular stage - saccular stage - alveolar stage Links: Species Stage Comparison | respiratory | ||||
| Lung Stages - timing, airway and vascular development[7] |
|---|
|
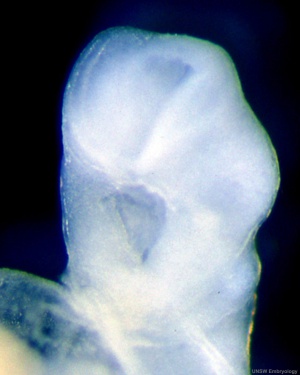
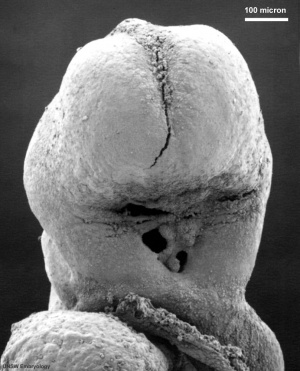
Embryonic
Week 4 to 5 - lung buds originate as an outgrowth from the ventral wall of the foregut where lobar division occurs.
|
||
|
| |
| Stomodeum (Week 4, stage 11, GA week 6) | Buccopharyngeal membrane (Week 4, stage 11, GA week 6) | |
(Week 5, stage 14, GA week 7)
- week 4 - 5
- Endoderm - tubular ventral growth from foregut pharynx.
- Mesoderm - mesenchyme of lung buds.
- Intraembryonic coelom - pleural cavities elongated spaces connecting pericardial and peritoneal spaces.
Stage 13 - Trachea and Lung buds (MRI sagittal sections)
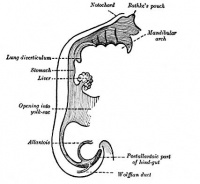
|
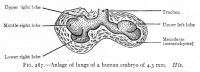
|
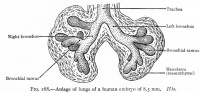
|
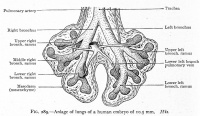
|
| Week 4 | Week 4-5 (Stage 12 to 13) | Week 5 (Stage 15 to 16) | Week 6 (Stage 16 to 17) |
Pseudoglandular stage
Week 8 |
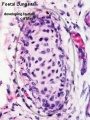
Fetal lung histology |
(This is what a gland looks like.)
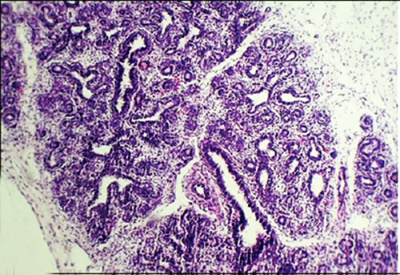
| Pseudoglandular and Canalicular Stages |
|---|
| Canalicular phase of bronchus can be mixed with the pseudoglandular phase.
Human Lung showing a mix of pseudoglandular and canalicular stages[8] |
Canalicular stage
|
|
Saccular stage
- week 24 to near term.
- most peripheral airways form widened "airspaces", termed saccules.
- saccules widen and lengthen the airspace (by the addition of new generations).
- future gas exchange region expands significantly.
- Fibroblastic cells also undergo differentiation, they produce extracellular matrix, collagen, and elastin.
- May have a role in epithelial differentiation and control of surfactant secretion.
- Alveolar Cells Type II (Type II pneumocytes)
- begin to secrete surfactant, levels of secretion gradually increase to term.
- allows alveoli to remain inflated
- Vascular tree - also grows in length and diameter during this time.
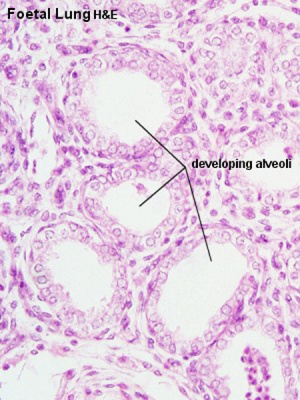
Alveolar stage
|
|
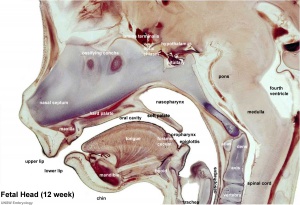
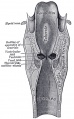
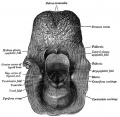
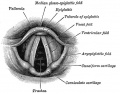
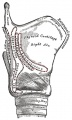
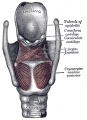
Upper Respiratory Tract
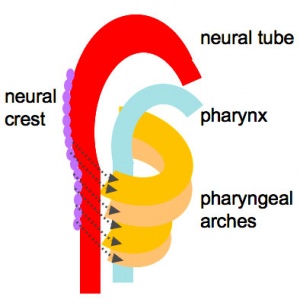
Foregut Development - from the oral cavity the next portion of the foregut is initially a single gastrointestinal (oesophagus) and respiratory (trachea) common tube, the pharynx which lies behind the heart. Note that the respiratory tract will form from a ventral bud arising at this level.
|
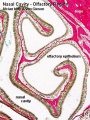
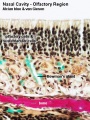
Note - Specialised olfactory epithelium for smell, a small region located in roof of nasal cavity. |
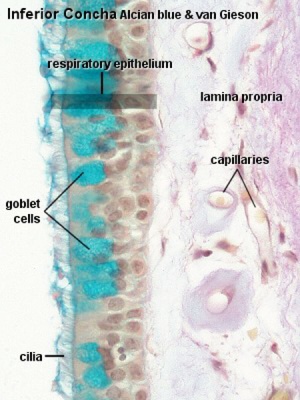
|
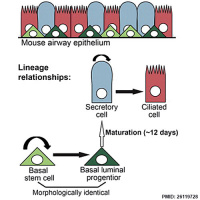
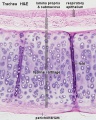
Respiratory epithelium
Respiratory epithelium development |
| Additional Information - Histology | ||
|---|---|---|
This will be covered in detail in your associated SH Practical class.
|
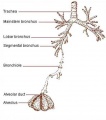
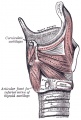
Lower Respiratory Tract
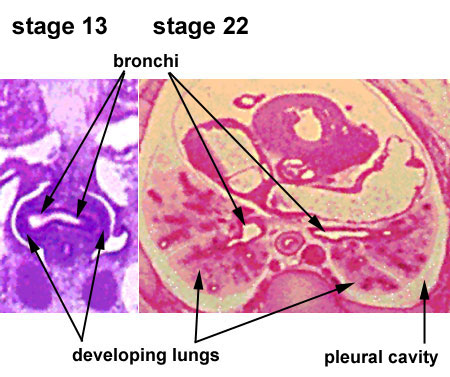
|
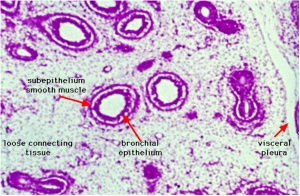
|
| Stage 13 (Week 4-5) | Stage 22 (Week 8) |
- lung buds ( endoderm epithelial tubes) grow/push into mesenchyme covered with pleural cells (lung border)
- generates a tree-like network by repeated:
- elongation
- terminal bifurcation
- lateral budding
Growth initially of branched "conducting" system of bronchial tree, followed by later development of the "functional units" of the alveoli.
| Additional Information - Histology | ||
|---|---|---|
| This will be covered in detail in your associated SH Practical class.
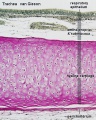
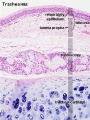
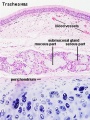
Respiratory Trachea Mucosa - formed by epithelium and underlying lamina propria.
Submucosa - connective tissue and submucosal glands
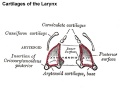
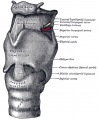
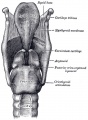
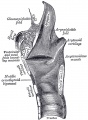
Cartilage
Hyaline Cartilage Development
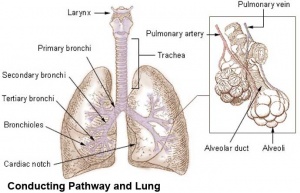
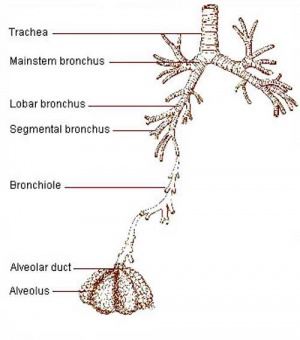
Bronchi Branching main bronchi -> lobar bronchi -> segmental bronchi (supply lung bronchopulmonary segments) -> bronchi -> bronchioles (smaller than 1 mm) -> respiratory bronchioles.
Bronchioles
Respiratory Bronchioles
|
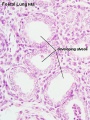
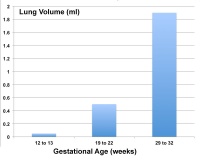
Fetal Lung Volume
Each human lung volume as determined by ultrasound and matched to gestational age[9]
|
|
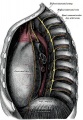
Pleural Cavity
- anatomical body cavity in which the lungs develop and lie.
- pleural cavity forms in the lateral plate mesoderm as part of the early single intraembryonic coelom.
- This cavity is initially continuous with pericardial and peritoneal cavities and form initially as two narrow canals.
- later becomes separated by folding (pleuropericardial fold, pleuroperitoneal membrane) and the later formation of the diaphragm.
- pleuropericardial fold - (pleuropericardial membrane) An early embryonic fold which restricts the communication between pleural cavity and pericardiac cavity, contains both the cardinal vein and phrenic nerve.
- pleuroperitoneal membrane - An early embryonic membrane that forms inferiorly at the septum transversum to separate peritoneal cavity from pleural cavity.
Pleura
- serous membrane covers the surface of the lung and the spaces between the lobes.
- arranged as a closed invaginated sac.
- two layers (pulmonary, parietal) continuous with each other, the potential space between them is the pleural cavity.
- filled with pleural fluid produced by parietal pleura and reabsorbed by parietal pleural lymphatics.[10]
- excess fluid - pleural effusion.
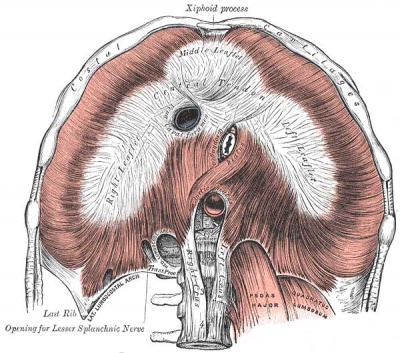
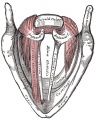
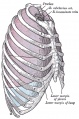
Diaphragm
Adult Diaphragm.
- Not respiratory tract but musculoskeletal development, there are 5 embryonic elements that contribute to the diaphragm.
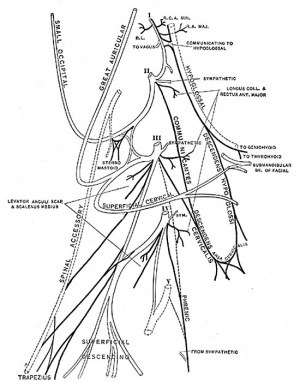
- Innervation of the human diaphragm is by the phrenic nerves
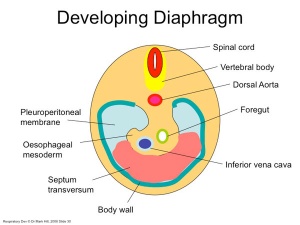
|
|
- Phrenic Nerves - arising from the same segmental levels as the diaphragm skeletal muscles, segmental levels C3 to C5.
- The paired phrenic nerves are mixed nerves
- motor neurons for the diaphragm
- sensory nerves for other abdominal structures (mediastinum, pleura, liver, gall bladder).
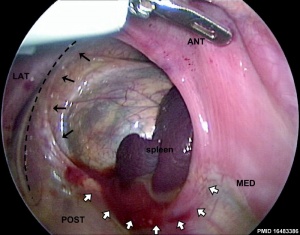
Bochdalek hernia - most common on the posterior left side (85%). Failure of the pleuroperitoneal foramen (foramen of Bochdalek) to close allows viscera into thorax. Intestine, stomach or spleen can enter the pleural cavity, compressing the lung.
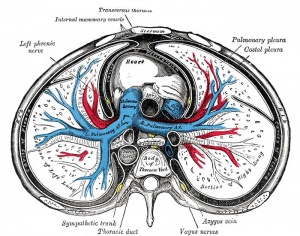
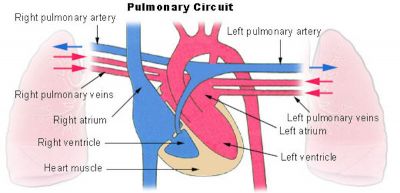
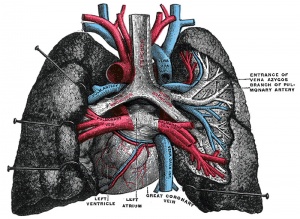
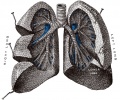
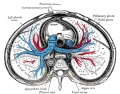
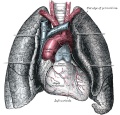
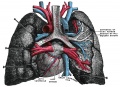
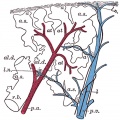
Pulmonary Circulation
- the pulmonary system not "functional" until after birth
- pulmonary arteries - 6th aortic arch arteries
- pulmonary veins - are incorporated into the left atrium wall
- bronchial arteries - branches from dorsal aorta
Pulmonary circulation
Fetal
Fetal Respiratory Movements
- Fetal respiratory movements (FRM) or Fetal breathing movements (FBM) are regular muscular contrations occurring in the third trimester.
- preparing the respiratory muscular system for neonatal function.
- may also have a role in late lung development.
Postnatal
The First Breath
|

Alveolar sac structure |
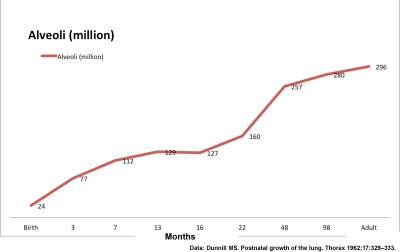
Alveoli
|

Human Alveoli Number |
Respiratory Rate
- neonatal rate is higher (30-60 breaths/minute) than adult (12-20 breaths/minute).
- tachypnea - (Greek, rapid breathing) an increased respiratory rate of greater than 60 breaths/minute in a quiet resting baby
| Age | Rate (breaths/minute) |
| Infant (birth - 1 year) | 30 - 60 |
| Toddler (1 - 3 years) | 24 - 40 |
| Preschool (3 - 6 years) | 22 - 34 |
| School age (6 - 12 years) | 18 - 30 |
| Adolescent (12 - 18 years) | 12 - 16 |
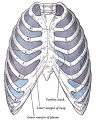
Rib Orientation
|
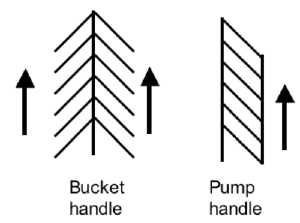
Rib orientation |
Respiratory Tract Abnormalities
Respiratory System - Abnormalities
- Meconium Aspiration Syndrome - (MAS) Meconium is the gastrointestinal contents that accumulate in the intestines during the fetal period. Fetal stress in the third trimester, prior to/at/ or during parturition can lead to premature meconium discharge into the amniotic fluid and sunsequent ingestion by the fetus and damage to respiratory function. Damage to placental vessels meconium myonecrosis may also occur.
- Newborn Respiratory Distress Syndrome - (Hyaline Membrane Disease) membrane-like substance from damaged pulmonary cells, absence of surfactant, if prolonged can be irreversible, intrauterine asphyxia, prematurity and maternal diabetes medline plus | eMedicine
- Tracheoesophageal Fistula - Tracheo-Oesophageal Fistula, Oesophageal Atresia - Oesophageal Atresia with or without tracheo-oesophageal fistula Fistula - an abnormal communication between 2 structures (organs, vessels, cavities) that do not normally connect.
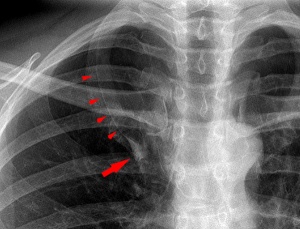
- Lobar Emphysema (Overinflated Lung) - There is an overinflated left upper lobe There is a collapsed lower lobe The left lung is herniating across the mediastinum
- Congenital Diaphragmatic Hernia - (1 in 3,000 live births) Failure of the pleuroperitoneal foramen (foramen of Bochdalek) to close (left side), allows viscera into thorax -iIntestine, stomach or spleen can enter the pleural cavity, compressing the lung. rare (Morgagni hernia) -an opening in the front of the diaphragm. Congenital Diaphragmatic Hernia | GeneReviews
- Azygos Lobe - Common condition (0.5% of population). The right lung upper lobe expands either side of the posterior cardinal. There is also some course variability of the phrenic nerve in the presence of an azygos lobe.
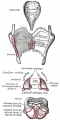
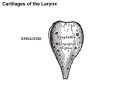
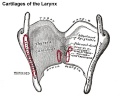
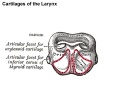
- Congenital Laryngeal Webs - Laryngeal abnormality due to embryonic (week 10) incomplete recanalization of the laryngotracheal tube during the fetal period. Rare abnormality occuring mainly at the level of the vocal folds (glottis).
- Hyaline Membrane Disease - (Newborn Respiratory Distress Syndrome) historic terminology, a membrane-like substance from damaged pulmonary cells.
- Bronchopulmonary Dysplasia - A chronic lung disease which can occur following premature birth and related lung injury. Most infants who develop BPD are born more than 10 weeks before their due dates, weigh less than 1,000 grams (about 2 pounds) at birth, and have breathing problems.
- Asthma - Flow limitation during tidal expiration in early life significantly associated with the development of physician-diagnosed asthma by the age of 2 years. Infants with abnormal lung function soon after birth may have a genetic predisposition to asthma or other airway abnormalities that predict the risk of subsequent lower respiratory tract illness.
- Cystic Fibrosis - Inherited disease of the mucus and sweat glands, causes mucus to be thick and sticky. Clogging the lungs, causing breathing problems and encouraging bacterial grow. (Covered elsewhere in the course)
- Vascular development - During both the prenatal and postnatal development, impaired angiogenesis from a number of causes can decrease alveolarization of the lung. (decreased VEGF expression, increased oxygen tension, inflammatory cytokines, other adverse stimuli)
Environmental Factors
The lung is most sensitive to environmental effects given the long timecourse of development, including postnatal, multi-system origins, immune interactions, and our growing understanding of the effects of the prenatal environment on adult health (DOHAD). Below are some recent reviews of related topics.(not part of today's lecture presentation)
- Maternal alcohol[4]
- Maternal obesity[11]
- Maternal diabetes[12]
- Maternal smoking[13]
- Chronic hypoxaemia[14]
- Environmental chemicals[15]
Respiratory Terms
| Respiratory Terms (expand to view) |
|---|
|
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
Additional Information
| Additional Information - Content shown under this heading is not part of the material covered in this class. It is provided for those students who would like to know about some concepts or current research in topics related to the current class page. |
| Developing Rat Lung (3D view) |
|---|
| From transitional bronchiole to alveolus!
<html5media height="500" width="856">File:Rat respiratory 01.mp4</html5media> |
| The flight starts by entering a transitional bronchiole.
Domes of Club cell are visible on the surface of the bronchiole. Turning left an alveolar duct is entered. Various alveoli and the entrance of few alveolar ducts are visible. Shortly before the end of first alveolar duct, the fight turns left again and flies down another alveolar duct. After a short distance it ends in front of an alveolus which is subdivided by a low ridge representing a still forming new alveolar septum. Rat lung at postnatal day 36. Surface rendering of a sample scanned by SRXTM for Schittny et al. (Schittny et al. 2008) using the software Imaris 4.1 (Bitplane, Zürich, Switzerland). Because the magnification is changing during the flight a scale bar could not be easily shown. However, the entrance of the bronchiole has a diameter of ~100 µm (text from suppl. information) Schittny JC. (2018). How high resolution 3-dimensional imaging changes our understanding of postnatal lung development. Histochem. Cell Biol. , 150, 677-691. PMID: 30390117 DOI.
|
Lung Development - Respiratory Health and Disease
Nikolić MZ, Sun D & Rawlins EL. (2018). Human lung development: recent progress and new challenges. Development , 145, . PMID: 30111617 DOI.
Arigliani M, Spinelli AM, Liguoro I & Cogo P. (2018). Nutrition and Lung Growth. Nutrients , 10, . PMID: 30021997 DOI.
Finke I, de Jongste JC, Smit HA, Wijga AH, Koppelman GH, Vonk J, Brunekreef B & Gehring U. (2018). Air pollution and airway resistance at age 8 years - the PIAMA birth cohort study. Environ Health , 17, 61. PMID: 30016982 DOI.
Stocks J, Hislop A & Sonnappa S. (2013). Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med , 1, 728-42. PMID: 24429276 DOI.
Merkus PJ. (2003). Effects of childhood respiratory diseases on the anatomical and functional development of the respiratory system. Paediatr Respir Rev , 4, 28-39. PMID: 12615030
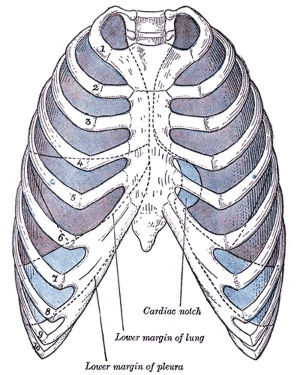
| Grays - Respiratory Images |
|---|
|
|
| Respiratory Histology |
|---|
|
Histology will be covered in more detail in your associated practical class. Fetal Histology
Adult Histology
|
References
- ↑ Ardini-Poleske ME, Clark RF, Ansong C, Carson JP, Corley RA, Deutsch GH, Hagood JS, Kaminski N, Mariani TJ, Potter SS, Pryhuber GS, Warburton D, Whitsett JA, Palmer SM & Ambalavanan N. (2017). LungMAP: The Molecular Atlas of Lung Development Program. Am. J. Physiol. Lung Cell Mol. Physiol. , 313, L733-L740. PMID: 28798251 DOI.
- ↑ Schittny JC. (2017). Development of the lung. Cell Tissue Res. , 367, 427-444. PMID: 28144783 DOI.
- ↑ Stabler CT & Morrisey EE. (2017). Developmental pathways in lung regeneration. Cell Tissue Res. , 367, 677-685. PMID: 27957616 DOI.
- ↑ 4.0 4.1 Gauthier TW & Brown LA. (2017). In utero alcohol effects on foetal, neonatal and childhood lung disease. Paediatr Respir Rev , 21, 34-37. PMID: 27613232 DOI.
- ↑ Herriges M & Morrisey EE. (2014). Lung development: orchestrating the generation and regeneration of a complex organ. Development , 141, 502-13. PMID: 24449833 DOI.
- ↑ Knudsen L & Ochs M. (2018). The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem. Cell Biol. , 150, 661-676. PMID: 30390118 DOI.
- ↑ Schittny JC. (2018). How high resolution 3-dimensional imaging changes our understanding of postnatal lung development. Histochem. Cell Biol. , 150, 677-691. PMID: 30390117 DOI.
- ↑ Yamamoto M, Wilting J, Abe H, Murakami G, Rodríguez-Vázquez JF & Abe SI. (2018). Development of the pulmonary pleura with special reference to the lung surface morphology: a study using human fetuses. Anat Cell Biol , 51, 150-157. PMID: 30310706 DOI.
- ↑ Peralta CF, Cavoretto P, Csapo B, Falcon O & Nicolaides KH. (2006). Lung and heart volumes by three-dimensional ultrasound in normal fetuses at 12-32 weeks' gestation. Ultrasound Obstet Gynecol , 27, 128-33. PMID: 16388511 DOI.
- ↑ Miserocchi G. (1997). Physiology and pathophysiology of pleural fluid turnover. Eur. Respir. J. , 10, 219-25. PMID: 9032518
- ↑ McGillick EV, Lock MC, Orgeig S & Morrison JL. (2017). Maternal obesity mediated predisposition to respiratory complications at birth and in later life: understanding the implications of the obesogenic intrauterine environment. Paediatr Respir Rev , 21, 11-18. PMID: 27818069 DOI.
- ↑ Azad MB, Moyce BL, Guillemette L, Pascoe CD, Wicklow B, McGavock JM, Halayko AJ & Dolinsky VW. (2017). Diabetes in pregnancy and lung health in offspring: developmental origins of respiratory disease. Paediatr Respir Rev , 21, 19-26. PMID: 27665512 DOI.
- ↑ McEvoy CT & Spindel ER. (2017). Pulmonary Effects of Maternal Smoking on the Fetus and Child: Effects on Lung Development, Respiratory Morbidities, and Life Long Lung Health. Paediatr Respir Rev , 21, 27-33. PMID: 27639458 DOI.
- ↑ McGillick EV, Orgeig S, Giussani DA & Morrison JL. (2017). Chronic hypoxaemia as a molecular regulator of fetal lung development: implications for risk of respiratory complications at birth. Paediatr Respir Rev , 21, 3-10. PMID: 27692868 DOI.
- ↑ Miller MD & Marty MA. (2010). Impact of environmental chemicals on lung development. Environ. Health Perspect. , 118, 1155-64. PMID: 20444669 DOI.
- ↑ Kleven GA & Ronca AE. (2009). Prenatal behavior of the C57BL/6J mouse: a promising model for human fetal movement during early to mid-gestation. Dev Psychobiol , 51, 84-94. PMID: 18980217 DOI.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 1) Embryology SH Lecture - Respiratory System Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/SH_Lecture_-_Respiratory_System_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G