Introduction
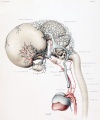
Human Embryo vascular development (week 8, stage 20 Carnegie Embryo No.
460)
This page relates specifically to vascular development associated with the central nervous system (CNS). There is an additional page that looks at vascular development and remodelling. The CNS is extensively vascularised as neurons have a mainly oxygen requiring (aerobic) metabolism. At the cellular level the finer blood vessel endothelium is separated from direct contact by glial cells, that form the so-called "blood-brain barrier".[1]
There is also an extensive vascular network throughout the surrounding meninges, the skull bone, and the scalp. During the embryonic period blood vessels are also modified to form the extensive choroid plexus that lies within the ventricular space and is a source of the cerebrospinal fluid (CSF).
- Links: Ventricular System | Meninges | artery | vein
| Neural System Parts
|
|
|
Neural Tube Development
| Neural Tube
|
Primary Vesicles
|
Secondary Vesicles
|
Adult Structures
|
| week 3
|
week 4
|
week 5
|
adult
|
neural plate
neural groove
neural tube
Brain
|
prosencephalon (forebrain)
|
telencephalon
|
Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles
|
| diencephalon
|
epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle
|
| mesencephalon (midbrain)
|
mesencephalon
|
tectum, Cerebral peduncle, cerebral aqueduct, pons
|
| rhombencephalon (hindbrain)
|
metencephalon
|
cerebellum
|
| myelencephalon
|
medulla oblongata, isthmus
|
| spinal cord, pyramidal decussation, central canal
|
|
See also these historic articles on human vascular development by:
Mall FP. On the development of the blood-vessels of the brain in the human embryo. (1905) Amer. J Anat. 4(1): 1–18.
Streeter GL. The developmental alterations in the vascular system of the brain of the human embryo. (1921) Contrib. Embryol., Carnegie Inst. Wash. 8:7-38.
Human embryo 50 mm long (Carnegie Collection, No.
96.
Some Recent Findings
- Variations of the CNS Venous System Mimicking Pathology - Spectrum of Imaging Findings[2] "Variations in the venous drainage of the central nervous system can have imaging and clinical findings that mimic pathology, presenting a challenge for neuroimagers and clinicians. Patients with these variants may undergo unnecessary testing, and patients with pathology may receive delayed diagnoses because of overlap with benign findings. Consequently, the accurate identification of venous variations on cross-sectional imaging and angiography and their potential causes are critical for differentiating benign imaging variants from potential pathologic processes requiring further evaluation. For example, in the epidural space, benign dilation of the epidural venous plexus may be mistaken for evidence of a fistula, abscess, or metastasis. Hypoplasia of a dural venous sinus or an arachnoid granulation may mimic venous sinus thrombosis. The superior ophthalmic vein may demonstrate benign dilation in intubated patients, mimicking thrombosis, increased intracranial pressure, orbital varix, inflammatory pseudotumor, or other conditions. Furthermore, certain venous variations, such as the occipital sinus or emissary veins, may complicate surgery or herald pathology and should be reported. In addition, some supposedly benign variations, such as the developmental venous anomaly, can be complicated by pathology. The objective of this review article is to provide a descriptive and pictorial review of common anatomic and physiologic variations in the venous drainage system of the brain, spine, and orbits that can mimic pathology. Neuroimaging findings of related pathologies and differences in clinical presentations will also be discussed to assist in the approach to differential diagnosis."
- A review of extraaxial developmental venous anomalies of the brain involving dural venous flow or sinuses[3] "This paper is a narrative review of extraaxial developmental venous anomalies (eDVAs) of the brain involving dural venous flow or sinuses: persistent embryonic sinuses, sinus pericranii, enlarged emissary veins, and venous varices or aneurysmal malformations. The article highlights the natural history, anatomy, embryology, imaging, clinical implications, and neurosurgical significance of these lesions, which the authors believe represent a continuum, with different entities characterized by distinct embryopathologic features. The indications and surgical management options are discussed for these individual intracranial pathologies with relevant illustrations, and a novel classification is proposed for persistent falcine sinus (PFS). The role of neurointervention and/or microsurgery in specific cases such as sinus pericranii and enlarged emissary veins of the skull is highlighted. A better understanding of the pathophysiology and developmental anatomy of these lesions can reduce treatment morbidity and mortality. Some patients, including those with vein of Galen malformations (VOGMs), can present with the added systemic morbidity of a high-output cardiac failure. Although VOGM is the most studied and classified of the above-mentioned eDVAs, the authors believe that grouping the former with the other venous anomalies/abnormalities listed above would enable the clinician to convey the exact morphophysiological configuration of these lesions, predict their natural history with respect to evolving venous hypertension or stroke, and extrapolate invaluable insights from VOGM treatment to the treatment of other eDVAs."
- Variations of the Circle of Willis at the End of the Human Embryonic Period[4] "Variations of the circle of Willis (CW) influence blood supply to the brain and adjacent structures in adults. We examined the formation of the CW in 20 human embryo samples at the end of the embryonic period using 3-D reconstructions of serial histological sections. The CW was closed in all samples, and did not form in a single plane, but was composed of multiple stair-like planes. The artery acutely curved at the caudal part of the CW, namely, at the inlet of the basilar artery and bifurcation of the P1 segment of the posterior cerebral artery (PCA), reflecting flexure of the mesencephalon and diencephalon at this stage. Variations were observed in 17 of 20 samples-only anterior parts (anterior communicating artery [Acom] and anterior cerebral artery [ACA]) in 10 samples, only posterior parts (posterior communicating artery [Pcom]) in one sample, and both anterior and posterior parts in six samples. Variations included the Acom formed as partially duplicated in three samples, duplicated in four, plexiform in three, and no channel as a result of a single azygos ACA in one. The ACA formed as duplicated in two, median ACA in two, and right hypoplasia in one. The Pcom formed in hypoplasia of either side in six samples. Variations observed in this study are similar to those observed in fetuses, neonates, and adults. The P1 segment of PCA was very large in all samples. The present observations indicate that variations in the CW are present from the initiation of CW formation."
- Formation of the circle of Willis during human embryonic development[5] "The circle of Willis (CW) is a circulatory anastomosis that supplies blood to the brain and adjacent structures. We examined the timing of formation of CW in 20 Japanese human embryo samples by using 3-dimensional reconstruction of serial histological sections. The CW was closed in 1 (n = 6), 2 (n = 8), 2 (n = 3) and 2 (n = 3) samples at Carnegie stages 20, 21, 22, and 23, respectively. The CW was unclosed in 13 samples (unclosed at ACOM alone, 6 samples; ACOM and bilateral P1, 4; left PCOM and right P1, 1; right PCOM and right P1, 1; ACOM and left PCOM, 1). It was difficult to predict whether the circle would close during further development, as such variations frequently exist in adults."
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Neural Vascular System Development | Neural Vascular Development
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- Vascular pattern of the dentate gyrus is regulated by neural progenitors[6] "Neurogenesis is a vital process that begins during early embryonic development and continues until adulthood, though in the latter case, it is restricted to the subventricular zone and the subgranular zone of the dentate gyrus (DG). In particular, the DG's neurogenic properties are structurally and functionally unique, which may be related to its singular vascular pattern. Neurogenesis and angiogenesis share molecular signals and act synergistically, supporting the concept of a neurogenic niche as a functional unit between neural precursors cells and their environment, in which the blood vessels play an important role. Whereas it is well known that vascular development controls neural proliferation in the embryonary and in the adult brain, by releasing neurotrophic factors; the potential influence of neural cells on vascular components during angiogenesis is largely unknown. We have demonstrated that the reduction of neural progenitors leads to a significant impairment of vascular development. Since VEGF is a potential regulator in the neurogenesis-angiogenesis crosstalk, we were interested in assessing the possible role of this molecule in the hippocampal neurovascular development. Our results showed that VEGF is the molecule involved in the regulation of vascular development by neural progenitor cells in the DG."
- Foxc1 is required for early stage telencephalic vascular development[7] "The brain vascular system arises from the perineural vascular plexus (PNVP) which sprouts radially into the neuroepithelium and subsequently branches off laterally to form a secondary plexus in the subventricular zone (SVZ), the subventricular vascular plexus (SVP). The process of SVP formation remains to be fully elucidated. We investigated the role of Foxc1 in early stage vascular formation in the ventral telencephalon. Results: The Foxc1 loss of function mutant mouse, Foxc1ch/ch , showed enlarged telencephalon and hemorrhaging in the ventral telencephalon by E11.0. The mutant demonstrated blood vessel dilation and aggregation of endothelial cells in the SVZ after the invasion of endothelial cells through the radial path, which lead to failure of SVP formation. During this early stage of vascular development, Foxc1 was expressed in endothelial cells and pericytes, as well as in cranial mesenchyme surrounding the neural tube."
- Review - The human brain intracerebral microvascular system: development and structure.[8] "The capillary from the meningeal inner pial lamella play a crucial role in the development and structural organization of the cerebral cortex extrinsic and intrinsic microvascular compartments. Only pial capillaries are capable of perforating through the cortex external glial limiting membrane (EGLM) to enter into the nervous tissue, although incapable of perforating the membrane to exit the brain. Circulatory dynamics and functional demands determine which capillaries become arterial and which capillaries become venous."
|
Cerebral Blood Supply Development
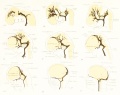
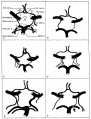
Figures in table below from the 1921 study by Streeter.[9]
Brain Vascular System Development[9]
|
|
|
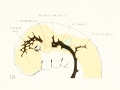
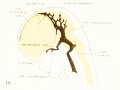
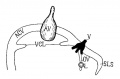
| Fig 13. 4 mm Embryo 588
|
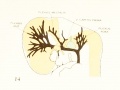
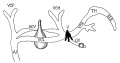
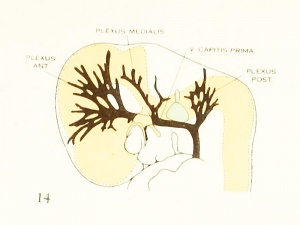
Fig. 14. 14 mm Embryo 940
|
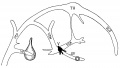
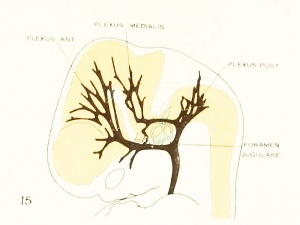
Fig. 15. 18 mm Embryo 144
|
|
|
|
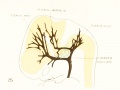
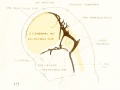
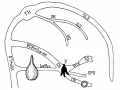
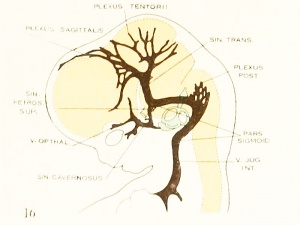
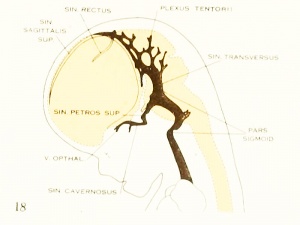
| Fig. 16. 21 mm Embryo 460
|
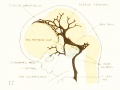
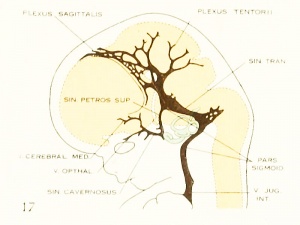
Fig. 17. 24 mm Embryo 632
|
Fig. 18. 35 mm Embryo 199
|
|
|
|
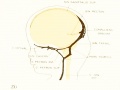
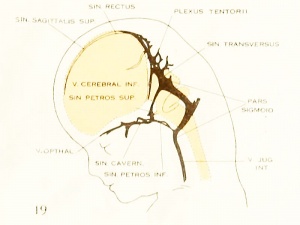
| Fig. 19. 50 mm Embryo 96
|
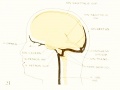
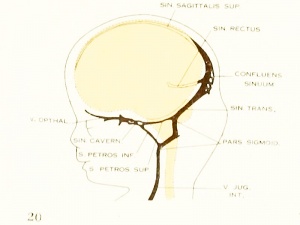
Fig. 20. 80 mm Embryo 234a
|
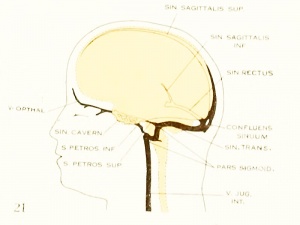
Fig. 21. Adult
|
Fig 13. 4 mm Embryo No. 588
Fig 14. 14 mm Embryo No. 940
Fig 15. 18 mm Embryo No. 144
Fig 16. 21 mm Embryo No. 460
Fig 17. 24 mm Embryo No. 632
Fig 18. 35 mm Embryo No. 199
Fig 19. 50 mm Embryo No. 96
Fig 20. 80 mm Embryo No. 234a
Cerebral Blood Supply Development[10]
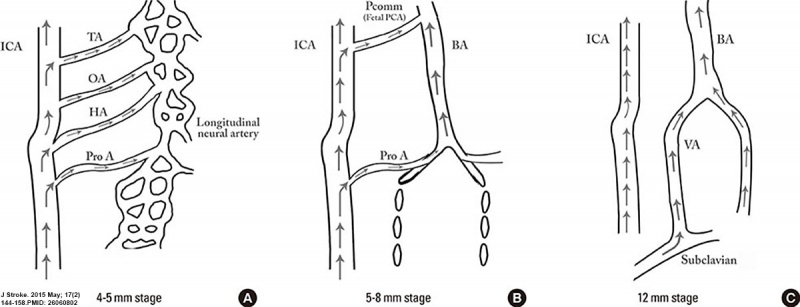
Cerebral Arterial Timeline
Cerebral Arterial Timeline
| Carnegie Stage
|
CRL (mm)
|
Event
|
| 13
|
4 - 5
|
hindbrain (future posterior fossa) is supplied by two parallel neural arteries (or channels). These arteries obtain their blood supply from carotid-vertebrobasilar anastomoses given by the trigeminal artery (TA), the otic artery (OA), hypoglossal artery (HA), and the proatlantal artery (ProA)
|
| 14
|
5 - 8
|
basilar artery (BA) forms from the consolidation of the neural arteries.
|
| 15
|
7 - 12
|
vertebral arteries (VA) forms from transverse anastomoses between cervical intersegmental arteries, beginning with the ProA and proceeding downward to the 6th intersegmental artery,
|
| 16
|
11 - 12
|
(35 days) development of the middle cerebral artery (MCA) is first identified as small buds originating proximal to the anterior cerebral artery (ACA) on the anterior division of the primitive internal carotid artery (ICA).
|
| 19
|
16 - 18
|
middle cerebral artery (MCA) becomes more prominent, the plexi fuse into a single artery and further branches pierce the cerebral hemisphere.
|
| 20
|
18
|
stem of the ACA gives rise to the olfactory artery.
|
| 21
|
21-24
|
formation of the anterior communicating artery (ACOMM).
|
| Early development - the posterior circulation relies almost entirely from blood supply coming from the anterior circulation through carotid-vertebrobasilar anastomoses.
|
| Later development - as the posterior fossa structures and the occipital lobe grow, the posterior circulation becomes progressively independent from the anterior circulation with obliteration of the anterior-posterior anastomoses from caudal to rostral maintaining in the majority of adult only one connection between the distal basilar arteries with the carotid artery via the posterior communicating artery.
|
| Data source[10]) Links: neural vascular
|
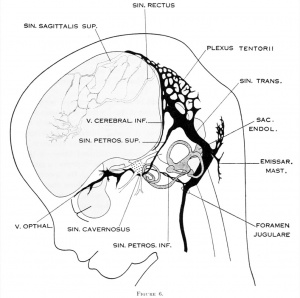
Cerebral Veins
- cavernous sinus - one of the dural venous sinuses forming a network of veins that sit in a cavity. The carotid siphon of the internal carotid artery, and cranial nerves III, IV, V (branches V1 and V2) and VI all pass through this blood filled space.
- superficial middle cerebral vein - (SMCV) drains venous blood from most of the superolateral surface of the brain into the cavernous sinus.
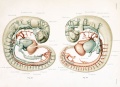
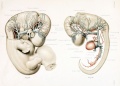
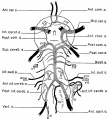
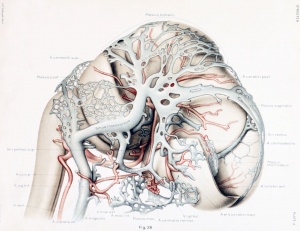
Figures from the 1905 study by Mall.[11]
Fig 14. Veins of the head of an embryo four weeks old.
Fig 15. Veins of the head during the fifth week.
Fig 16. Veins of the head beginning of third month.
Fig 17. Veins of the brain of an older foetus.
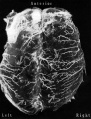
Blood-Brain Barrier
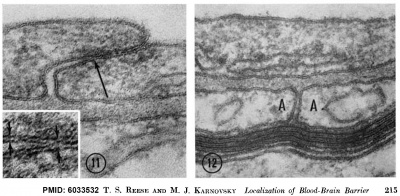
Mouse Blood-brain barrier (EM)[1]
Endothelial tight junctions nbsp; nbsp; Astrocytic end feet
|
Fig 11. Endothelial tight junctions Regions of overlap between neighboring endothelial cells, illustrating range of variation in structure of the intercellular cleft. In Fig. 11, the cleft is obliterated by a tight junction (arrow) extending throughout most of its length. The region of the intercellular cleft indicated by the arrow in Fig. 11 is shown in inset at higher magnification to illustrate that the total width of the tight junc- tion (between arrows at right) is less than twice the width of the adjacent plasma membrane (between arrows at left). Normal mouse; uranyl acetate block stain. Fig. 11, X 170,000. Fig. 11 inset, X 420,000.
Fig 12. Astrocytic end feet (A) lying between a myelinated axon and the basement lamina of the vascular endothelium. A cleft between the astrocytic end feet extends from the basement lamina to a perivascular myelinated axon and appears open except near the blood vessel where it is invaded by some basement lamina material. Normal mouse; uranyl acetate block stain. X 150,000.
|
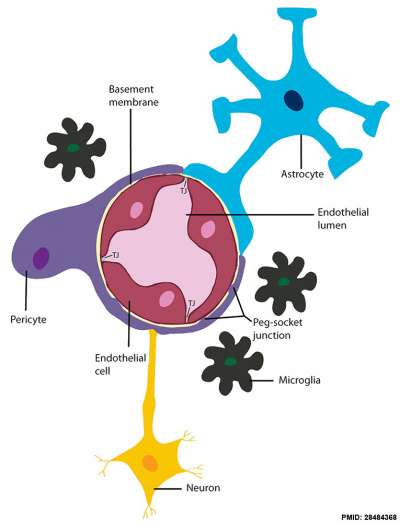
Blood-brain barrier cartoon[12]
|
A layer of brain endothelial cells connected by tight junctions forms the blood-brain barrier (BBB). The intimate contact of these specialized endothelial cells with different cell types constitutes the neurovascular unit (NVU).
- Basement membrane embeds the brain endothelial cells, the pericytes, and astrocytes.
- Brain endothelial cells and pericytes connect through peg-socket junctions in areas where the basement membrane is absent.
- Glial astrocytes extend end-feet and establish a close interaction with endothelial cells through transmembrane proteins, such as aquaporins.
- Astrocytes also connect with pericytes and neurons and together regulate BBB maintenance and function.
- Links: Glial Development
Terms
- aquaporins - transmembrane proteins that form channels for water and small solutes transfer across the membrane.
- astrocytes - glial cells named by their "star-like" branching appearance, are the most abundant cells in the brain.
- basement membrane - specialised extracellular matrix that underlies all epithelia.
- end-feet - specialised cellular club-shaped endings, often associated with neural synapses, but also found in other cell junctions.
- endothelial cells - squamous epithelial cells that line all blood vessels.
- pericytes - (Rouget cells) cells located at the abluminal surface of microvessels close to endothelial cells, mainly found associated with CNS vessels and involved in vessel formation, remodeling and stabilization.
|
Molecular
Foxc1
- required for early stage telencephalic vascular development[7]
Abnormalities
Developmental Venous Anomaly
Developmental venous anomaly (DVA) is a common, usually benign, congenital vascular malformation of the brain.[13]
References
- ↑ 1.0 1.1 Reese TS & Karnovsky MJ. (1967). Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. , 34, 207-17. PMID: 6033532
- ↑ Freeman CW, Lazor JW, Loevner LA & Nabavizadeh SA. (2019). Variations of the CNS Venous System Mimicking Pathology: Spectrum of Imaging Findings. J Neuroimaging , 29, 673-688. PMID: 31529762 DOI.
- ↑ Manjila S, Bazil T, Thomas M, Mani S, Kay M & Udayasankar U. (2018). A review of extraaxial developmental venous anomalies of the brain involving dural venous flow or sinuses: persistent embryonic sinuses, sinus pericranii, venous varices or aneurysmal malformations, and enlarged emissary veins. Neurosurg Focus , 45, E9. PMID: 29961384 DOI.
- ↑ Furuichi K, Ishikawa A, Uwabe C, Makishima H, Yamada S & Takakuwa T. (2018). Variations of the Circle of Willis at the End of the Human Embryonic Period. Anat Rec (Hoboken) , 301, 1312-1319. PMID: 29457875 DOI.
- ↑ Takakuwa T, Koike T, Muranaka T, Uwabe C & Yamada S. (2016). Formation of the circle of Willis during human embryonic development. Congenit Anom (Kyoto) , 56, 233-6. PMID: 27037515 DOI.
- ↑ Pombero A, Garcia-Lopez R, Estirado A & Martinez S. (2018). Vascular pattern of the dentate gyrus is regulated by neural progenitors. Brain Struct Funct , 223, 1971-1987. PMID: 29306978 DOI.
- ↑ 7.0 7.1 Prasitsak T, Nandar M, Okuhara S, Ichinose S, Ota MS & Iseki S. (2015). Foxc1 is required for early stage telencephalic vascular development. Dev. Dyn. , 244, 703-11. PMID: 25733312 DOI.
- ↑ Marín-Padilla M. (2012). The human brain intracerebral microvascular system: development and structure. Front Neuroanat , 6, 38. PMID: 22993505 DOI.
- ↑ 9.0 9.1 Streeter GL. The developmental alterations in the vascular system of the brain of the human embryo. (1921) Contrib. Embryol., Carnegie Inst. Wash. 8:7-38.
- ↑ 10.0 10.1 Menshawi K, Mohr JP & Gutierrez J. (2015). A Functional Perspective on the Embryology and Anatomy of the Cerebral Blood Supply. J Stroke , 17, 144-58. PMID: 26060802 DOI.
- ↑ Mall FP. On the development of the blood-vessels of the brain in the human embryo. (1905) Amer. J Anat. 4(1): 1–18.
- ↑ Haddad-Tóvolli R, Dragano NRV, Ramalho AFS & Velloso LA. (2017). Development and Function of the Blood-Brain Barrier in the Context of Metabolic Control. Front Neurosci , 11, 224. PMID: 28484368 DOI.
- ↑ Mullaguri N, Battineni A, Krishnaiah B, Migdady I & Newey CR. (2019). Developmental Venous Anomaly Presenting with Spontaneous Intracerebral Hemorrhage, Acute Ischemic Stroke, and Seizure. Cureus , 11, e5412. PMID: 31632865 DOI.
Online Textbooks
Reviews
Marín-Padilla M. (2012). The human brain intracerebral microvascular system: development and structure. Front Neuroanat , 6, 38. PMID: 22993505 DOI.
Raybaud C. (2010). Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg. Clin. N. Am. , 21, 399-426. PMID: 20561492 DOI.
Articles
Sumalatha S, Kotian SR & Shetty A. (2019). Persistent fetal superficial middle cerebral vein: an anatomical study. Anat Cell Biol , 52, 250-254. PMID: 31598353 DOI.
Chung JI & Weon YC. (2005). Anatomic variations of the superficial middle cerebral vein: embryologic aspects of the regressed embryonic tentorial sinus. Interv Neuroradiol , 11, 115-22. PMID: 20584490 DOI.
Allsopp G & Gamble HJ. (1979). Light and electron microscopic observations on the development of the blood vascular system of the human brain. J. Anat. , 128, 461-77. PMID: 468705
Search PubMed
Search Pubmed: neural vascular system development |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Additional Images
Historic
| Historic Disclaimer - information about historic embryology pages
|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers)
|
The Developmental Alterations in the Vascular System of the Brain of the Human Embryo (1921)
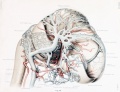
Gillilan, LA. Significant superficial anastomoses in the arterial blood supply to the human brain. J Comp Neurol. 1959 Jun;112:55-74. PMID 13850118
Fig. 1. Different patterns of the Circle of Willis
Fig. 2. Complex of arteries on the Ventral Surface of the Brain
Fig. 3. Fetal Brain (6 month) Large Superficial Arteries
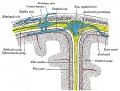
Gray, Henry. Anatomy of the Human Body Philadelphia: Lea & Febiger, 1918.
Fig. 769. Diagrammatic representation of a section across the top of the skull
Terms
| Neural Terms
|
Neural Development
- 3DMRI - Three-dimensional magnetic resonance imaging. A new technique that allows 3D analysis of embryonic structures. (More? Magnetic Resonance Imaging)
- 3rd ventricle - a fluid-filled space formed from neural tube lumen, located within the diencephalon (from the primary vesicle prosencephalon, forebrain).
- 4th ventricle - a fluid-filled space formed from neural tube lumen, located within the rhombencephalon (from the primary vesicle, hindbrain).
- adenohypophysis - (anterior pituitary) = 3 parts pars distalis, pars intermedia, pars tuberalis.
- afferent - refers to the direction of conduction from the periphery toward the central nervous system. Efferent is in the opposite direction.
- alar plate - embryonic dorsolateral region of the neural tube forming at spinal cord level dorsal horns (afferent) and brain level different structures.
- anlage - (German = primordium) structure or cells that will form a future adult structure.
- arachnoid mater - (G.) spider web-like used in reference to the middle layer of the brain meninges.
- astrocytes - cells named by their "star-like" branching appearance, are the most abundant glial cells in the brain, important for the blood-brain barrier.
- basal ganglia - (basal nuclei) neural structure derived from the secondary vesicle telencephalon (endbrain) structure from the earlier primary vesicle prosencephalon (forebrain).
- basal plate - embryonic ventrolateral region of the neural tube forming at spinal cord level ventral horns (efferent) and brain level different structures.
- brachial plexus - mixed spinal nerves innervating the upper limb form a complex meshwork (crossing).
- brain - general term for the central nervous system formed from 3 primary vesicles.
- buccopharyngeal membrane - (oral membrane) at cranial (mouth) end of gastrointestinal tract (GIT) where surface ectoderm and GIT endoderm meet. (see also cloacal membrane).
- cauda equina - (horse's tail) caudal extension of the mature spinal cord.
- central canal - lumen, cavity of neural tube within the spinal cord. Space is continuous with ventricular system of the brain.
- central cerebral sulcus - (central fissure, fissure of Rolando, Rolandic fissure) fold in the cerebral cortex associated with the sensorimotor cortex.
- cerebral aqueduct - ventricular cavity within the mesencephalon.
- cervical flexure - most caudal brain flexure (of 3) between spinal cord and rhompencephalon.
- choroid plexus - specialized vascular plexus responsible for secreting ventricular fluid that with further additions becomes cerebrospinal fluid (CSF).
- cloacal membrane - at caudal (anal) end of gastrointestinal tract (GIT) where surface ectoderm and GIT endoderm meet forms the openings for GIT, urinary, reproductive tracts. (see also buccopharyngeal membrane).
- connectome - term describing the detailed map of neural connections in the central nervous system.
- cortex - - CNS structure derived from the secondary vesicle telencephalon (endbrain) from the earlier primary vesicle prosencephalon (forebrain).
- cortical plate - outer neural tube region which post-mitotic neuroblasts migrate too along radial glia to form adult cortical layers.
- cranial flexure - (=midbrain flexure) most cranial brain flexure (of 3) between mesencephalon and prosencephalon.
- diencephalon - the caudal portion of forebrain after it divides into 2 parts in the 5 secondary vesicle brain (week 5). (cavity- 3rd ventricle) Forms the thalmus and other nuclei in the adult brain. (sc-My-Met-Mes-Di-Tel)
- dorsal root ganglia - (spinal ganglia) sensory ganglia derived from the neural crest lying laterally paired and dorsally to the spinal cord (in the embryo found ventral to the spinal cord). Connects centrally with the dorsal horn of the spinal cord.
- dura mater- "tough" (Latin, mater = mother) used in reference to the tough outer layer of the brain meninges.
- efferent - refers to the direction of conduction from the central nervous system toward the periphery. Afferent is in the opposite direction.
- ependyma - epithelia of remnant cells after neurons and glia have been generated and left the ventricular zone.
- floorplate - early forming thin region of neural tube closest to the notochord.
- ganglia - (pl. of ganglion) specialized neural cluster within either the CNS or PNS.
- glia - supporting, non-neuronal cells of the nervous system. Generated from the same neuroepithelial stem cells that form neurons in ventricular zone of neural tube. Form astrocytes, oligodendrocytes.
- grey matter - neural regions containing cell bodies (somas) of neurons. In the brain it is the outer layer, in the spinal cord it is inner layer. (see white matter white matter).
- growth factor - usually a protein or peptide that will bind a cell membrane receptor and then activates an intracellular signaling pathway. The function of the pathway will be to alter the cell directly or indirectly by changing gene expression. (eg SHH).
- HOX - (homeobox) family of transcription factors that bind DNA and activate gene expression. Expression of different Hox genes along neural tube defines rostral-caudal axis and segmental levels.
- hydrocephalus - abnormality as the result of an imbalance between the rate at which the CSF is being formed and the rate at which the CSF is passing through the arachnoidal villi back into the blood (hydrocephalus rate is a function of the degree of imbalance in these two). Very small imbalance exhibit subtle, if any, symptoms. Large imbalances will have rapidly evolving symptoms of unmistakable import.
- isthmus- (G. narrow passage).
- lamina terminalis - anterior region of brain where cranial neuropore closes.
- lumbar plexus - mixed spinal nerves innervating the lower limb form a complex meshwork (crossing).
- mantle layer - layer of cells generated by first neuroblasts migrating from the ventricular zone of the neural tube. Layers are rearranged during development of the brain and spinal cord. (Ven-Man-Mar-CP)
- marginal zone - layer of processes from neuroblasts in mantle layer. (Ven-Man-Mar-CP)
- mater - (Latin, mater = mother) used in relation to the 3 layers of the meninges.
- meninges - mesenchyme surrounding neural tube forms 3 layer (Dura-, pia-, arachnoid- mater) connective tissue sheath of nervous system. (D-P-A-cns)
- mesencephalon - (midbrain), the middle portion of the 3 primary vesicle brain (week 4). (sc-R-M-P)
- metencephalon - the cranial portion of hindbrain after it divides into 2 parts in the 5 secondary vesicle brain (week 5). Forms the pons and cerebellum in the adult brain. (sc-My-Met-Mes-Di-Tel)
- microglia - CNS innate immune cells that have a macrophage function, derive from yolk sac progenitor cells migrating into the CNS. microglia
- myelencephalon - the caudal portion of hindbrain after it divides into 2 parts in the 5 secondary vesicle brain (week 5). Forms the medulla in the adult brain. (sc-My-Met-Mes-Di-Tel)
- neural tube - neural plate region of ectoderm pinched off to form hollow ectodermal tube above notochord in mesoderm.
- neural tube defect - (NTD) any developmental abnormality that affects neural tube development. Commonly failure of neural tube closure.
- neuroblast - undifferentiated neuron found in ventricular layer of neural tube.
- neurohypophysis - (posterior pituitary; pas nervosa)
- neuromere - (prosomere) the model units for segmental brain development regions based upon a series of neural tube transverse subunits.
- neuron - The cellur "unit" of the nervous system, transmitting signals between neurons and other cells. The post-mitotic cells generated from neuroepithelial stem cells (neuroblasts) in ventricular zone of neural tube.
- neuropore - opening at either end of neural tube cranial (rostral, anterior) neuropore closes (day 25) about 2 days before caudal (posterior) that closes at somite level 32 to 34. Neural Tube Defects (NTDs) can be due to failure of these two neuropores to close.
- notochord - rod of cells lying in mesoderm layer ventral to the neural tube, induces neural tube and secretes sonic hedgehog which "ventralizes" the neural tube.
- olfactory bulb - (cranial nerve I, CN I) bipolar neurons from nasal epithelium project axons through cribiform palate into olfactory bulb of the brain associated with smell.
- optic nerve - (cranial nerve II, CN II) retinal ganglion neurons project from the retina as a tract into the brain (at the level of the diencephalon) associated with vision.
- optic vesicle - diencephalon region of neural tube outgrowth that forms the primordia of the retina associated with vision.
- opercularization - during fetal development of the sensorimotor cortex, the insula (located deep within the lateral sulcus) begins to invaginate from the surface of the immature cerebrum, until at term, the opercula completely cover the insula.
- otocyst - (otic vesicle) sensory placode that sinks into mesoderm to form spherical vesicle (stage 13/14 embryo) that will form components of the inner ear associated with hearing.
- pharyngeal arch - (branchial arch, Gk. gill) form the main structures of the head and neck. Humans have 5 arches appearing in week 4 that form 4 external swellings, each arch has a pouch, membrane and cleft.
- pharynx - uppermost end of GIT, beginning at the buccopharyngeal membrane and at the level of the pharyngeal arches.
- pia mater - (G.) (L. pius = soft, faithful + mater = mother) delicate vascular membrane which adheres to surface of brain and spinal cord, faithfully following their contours, the inner layer of the brain meninges.
- placode - specialized regions of ectoderm which form components of the sensory apparatus.
- pontine flexure - middle brain flexure (of 3) between cervical and cranial flexure in opposite direction, also generates thin roof of rhombencephalon and divides it into myelencephalon and metencephalon. ( sc-^V^ )
- posterior insula - during sensorimotor cortex development this region is composed of the anterior and posterior long insular gyri and the postcentral insular sulcus, which separates them.
- prosencephalon - (forebrain), the most cranial portion of the 3 primary vesicle brain (week 4). (sc-R-M-P)
- prosomere - (neuromere) a model for segmental brain development based upon a series of neural tube transverse subunits. PMID 12948657
- Rathke's pouch - a portion of the roof of the pharynx pushes upward towards the floor of the brain forming the anterior pituitary (adenohypophysis, pars distalis, pars tuberalis pars intermedia). Where it meets a portion of the brain pushing downward forming the posterior pituitary (neurohypophysis, pars nervosa). Rathke's pouch eventually looses its connection with the pharynx.
- rhombencephalon - (hindbrain), the most caudal portion of the 3 primary vesicle brain (week 4). (sc-R-M-P)
- rhombic lip - metencephalon posterior part extending from the roof of the fourth ventricle to dorsal neuroepithelial cells that contributes to the cerebellum.
- roofplate - early forming thin region of neural tube closest to the overlying ectoderm.
- spinal cord - caudal end of neural tube that does not contribute to brain. Note: the process of secondary neuralation contributes the caudal end of the spinal cord.
- spinal ganglia - (dorsal root ganglia, drg) sensory ganglia derived from the neural crest lying laterally paired and dorsally to the spinal cord (in the embryo found ventral to the spinal cord). Connects centrally with the dorsal horn of the spinal cord.
- spinal nerve - mixed nerve (motor and sensory) arising as latera pairs at each vertebral segmental level.
- sonic hedgehog - (shh) secreted growth factor that binds patched (ptc) receptor on cell membrane. SHH function is different for different tissues in the embryo. In the nervous system, it is secreted by the notochord, ventralizes the neural tube, inducing the floor plate and motor neurons.
- sulcus - (L. furrow) groove.
- sulcus limitans - longitudinal lateral groove in neural tube approx. midway between roofplate and floorplate. Groove divides alar (dorsal) and basal (ventral) plate regions.
- telencephalon - the cranial portion of forebrain after it divides into 2 parts in the 5 secondary vesicle brain (week 5). (cavity- lateral ventricles and some of 3rd ventricle) Forms the cerebral hemispheres in the adult brain. (sc-My-Met-Mes-Di-Tel)
- thalamus - (G. thalamos= bedchamber) cns nucleus, lateral to 3rd ventricle, paired (pl thalami).
- thyroid hormone - hormone required for brain development. T3 (3,5,3′-triiodothyronine) binding to nuclear receptors then act as a transcription factor in both neurons and glial cells. iodine deficiency
- transcription factor - a factor (protein or protein with steroid) that binds to DNA to alter gene expression, usually to activate. (eg steroid hormone+receptor, Retinoic acid+Receptor, Hox, Pax, Lim, Nkx-2.2)
- trigeminal ganglion - (cranial nerve V, CN V) first arch ganglion, very large and has 3 portions.
- vagal ganglion - (cranial nerve X, CN X) fourth and sixth arch ganglion, innervates the viscera and heart.
- ventricles - the fluid-filled interconnected cavity system with the brain. Fluid (cerebrospinal fluid, CSF) is generated by the specialized vascular network, the choroid plexus. The ventricles are directly connected to the spinal canal (within the spinal cord).
- ventricular zone - Neuroepithelial cell layer of neural tube closest to lumen. Neuroepithelial cells generate neurons, glia and ependymal cells. (Ven-Man-Mar-CP)
- vestibulocochlear nerve - (cranial nerve VIII, CN VIII, also called statoacoustic)
- white matter - - neural regions containing processes (axons) of neurons. In the brain it is the inner layer, in the spinal cord it is outer layer. (see grey matter).
|
| Cardiovascular Terms
|
Cardiovascular System Development See also Heart terms, Immune terms and Blood terms.
- angioblast - the stem cells in blood islands generating endothelial cells which will form the walls of both arteries and veins. (More? Blood Vessel)
- angiogenesis - the formation of new blood vessels from pre-existing vessels following from vasculogenesis in the embryo. (More? Blood Vessel)
- anlage (German, anlage = primordium) structure or cells which will form a future more developed or differentiated adult structure.
- blood islands - earliest sites of blood vessel and blood cell formation, seen mainly on yolk sac chorion.
- cardinal veins - paired main systemic veins of early embryo, anterior, common, posterior.
- cardiogenic region - region above prechordal plate in mesoderm where heart tube initially forms.
- ectoderm - the layer (of the 3 germ cell layers) which form the nervous system from the neural tube and neural crest and also generates the epithelia covering the embryo.
- endoderm - the layer (of the 3 germ cell layers) which form the epithelial lining of the gastrointestinal tract (GIT) and accessory organs of GIT in the embryo.
- endocardium - lines the heart. Epithelial tissue lining the inner surface of heart chambers and valves.
- endothelial cells - single layer of cells closest to lumen that line blood vessels.
- extraembryonic mesoderm - mesoderm lying outside the trilaminar embryonic disc covering the yolk sac, lining the chorionic sac and forming the connecting stalk. Contributes to placental villi development.
- haemocytoblasts - stem cells for embryonic blood cell formation.
- anastomose - to connect or join by a connection (anastomosis) between tubular structures.
- chorionic villi - the finger-like extensions which are the functional region of the placental barrier and maternal/fetal exchange. Develop from week 2 onward as: primary, secondary, tertiary villi.
- estrogens - support the maternal endometrium.
- growth factor - usually a protein or peptide that will bind a cell membrane receptor and then activates an intracellular signaling pathway. The function of the pathway will be to alter the cell directly or indirectly by changing gene expression. (eg VEGF, shh)
- intra-aortic hematopoietic cluster - (IAHC) blood stem cells associated with the endothelial layer of aorta and large arteries.
- maternal decidua - region of uterine endometrium where blastocyst implants. undergoes modification following implantation, decidual reaction.
- maternal sinusoids - placental spaces around chorionic villi that are filled with maternal blood. Closest maternal/fetal exchange site.
- Megakaryocytopoiesis - the process of bone marrow progenitor cells developMENT into mature megakaryocytes.
- mesoderm - the middle layer of the 3 germ cell layers of the embryo. Mesoderm outside the embryo and covering the amnion, yolk and chorion sacs is extraembryonic mesoderm.
- myocardium - muscular wall of the heart. Thickest layer formed by spirally arranged cardiac muscle cells.
- pericardium - covers the heart. Formed by 3 layers consisting of a fibrous pericardium and a double layered serous pericardium (parietal layer and visceral epicardium layer).
- pericytes - (Rouget cells) cells located at the abluminal surface of microvessels close to endothelial cells, mainly found associated with CNS vessels and involved in vessel formation, remodeling and stabilization.
- pharyngeal arches (=branchial arches, Gk. gill) series of cranial folds that form most structures of the head and neck. Six arches form but only 4 form any structures. Each arch has a pouch, membrane and groove.
- placenta - (Greek, plakuos = flat cake) refers to the discoid shape of the placenta, embryonic (villous chorion)/maternal organ (decidua basalis)
- placental veins - paired initially then only left at end of embryonic period, carry oxygenated blood to the embryo (sinus venosus).
- protein hormone - usually a protein distributed in the blood that binds to membrane receptors on target cells in different tissues. Do not easliy cross placental barrier.
- sinus venosus - cavity into which all major embryonic paired veins supply (vitelline, placental, cardinal).
- splanchnic mesoderm - portion of lateral plate mesoderm closest to the endoderm when coelom forms.
- steroid hormone - lipid soluble hormone that easily crosses membranes to bind receptors in cytoplasm or nucleus of target cells. Hormone+Receptor then binds DNA activating or suppressing gene transcription. Easliy cross placental barrier.
- syncitiotrophoblast extraembryonic cells of trophoblastic shell surrounding embryo, outside the cytotrophoblast layer, involved with implantation of the blastocyst by eroding extracellular matrix surrounding maternal endometrial cells at site of implantation, also contribute to villi. (dark staining, multinucleated).
- truncus arteriosus - an embryological heart outflow structure, that forms in early cardiac development and will later divides into the pulmonary artery and aorta. Term is also used clinically to describe the malformation where only one artery arises from the heart and forms the aorta and pulmonary artery.
- vascular endothelial growth factor - (VEGF) A secreted protein growth factor family, which stimulates the proliferation of vasular endotheial cells and therefore blood vessel growth. VEGF's have several roles in embryonic development. The VEGF family has 7 members (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF) that have a common VEGF homology domain. PIGF is the placental growth factor. They act through 3 VEGF tyrosine kinase membrane receptors (VEGFR-1 to 3) with seven immunoglobulin-like domains in the extracellular domain, a single transmembrane region, and an intracellular tyrosine kinase sequence.
- vasculogenesis - the formation of new blood vessels from mesoderm forming the endothelium. Compared to angiogenesis that is the process of blood vessel formation from pre-existing vessels.
- vitelline blood vessels - blood vessels associated with the yolk sac.
- waste products - products of cellular metabolism and cellular debris, e.g.- urea, uric acid, bilirubin.
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Neural - Vascular Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Vascular_Development
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G