Cardiovascular System - Blood Development
| Embryology - 26 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Initially blood develops within the core of "blood islands" along with blood vessels in mesoderm. Blood is considered as a form of "liquid conective tissue" consisting of a fluid and cellular component. Mesoderm both within the embryo (mesoderm) and outside the mesoderm (extra-embryonic mesoderm). Within the embryo the aorta in the aorta-gonad-mesonephros (AGM) region (para-aortic splanchnopleura), and the vitelline (yolk sac) and placental arteries are initial sites. There then follows a series of "relocations" of the stem cells to different organs (liver, spleen and thymus) within the embryo and fetus. In the adult, these stem cells are located in the bone marrow. At the time when blood first forms there are no bones, and it is only with bone development that we see bone marrow formation and relocation of blood stem cells.

|
In 1949 the embryologist George Streeter[1] used the replacement of cartilage within the humerus by bone marrow as an arbitrary definition of the embryo to fetus transition.
|
The clavicle is also one of the first fetal bone to contain marrow.[2] Granulocyte-colony stimulating factor (G-CSF) may initiate neutrophil production, with neutrophils first appearing in the clavicle marrow at 10 - 11 weeks.[3] The fetal white blood cells (neutrophils, monocytes, and macrophages) develop, though mononuclear phagocytes do not mature until after birth.
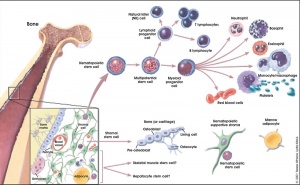
Stem cells that form blood cells (Hematopoietic Stem Cells, HSCs) change their location during development moving from tissue to tissue until their adult bone marrow location is formed and populated.
Angioblasts initially form small cell clusters (blood islands) within the embryonic and extraembryonic mesoderm. These blood islands extend and fuse together making a primordial vascular network. Within these islands 2 populations of cells exist: peripheral and core. The peripheral cells form endothelial cells while the core cells form blood cells (haemocytoblasts).
Blood formation occurs later (week 5) throughout embryoic mesenchyme, then liver, then spleen/thymus, bone marrow, lymph nodes.
Adult human red blood cells normally survive between 90 and 120 days, and are being continually replaced and their contents recycled.
- Historic Blood: 1941 Blood Formation
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Blood Development | Blood Embryology | Liver Haematopoiesis | Yolk Sac Haematopoiesis | Bone Marrow Haematopoiesis | Haematopoiesis |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Blood Stem Cells
A recent study in embryonic mouse development mapped the location of Hematopoietic stem cells (HSCs) during development. In the adult, blood cell formation is restricted to bone marrow, where a population of blood "stem cells" reside and differentiate into both red and white blood cells.
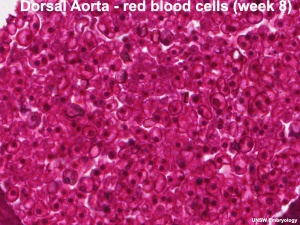
Hematopoietic stem cells (HSCs) origins have been the source of some recent controversy, as to yolk sac and dorsal aorta contributions.
[8] "It is thought that HSCs emerge from vascular endothelial cells through the formation of intra-arterial clusters and that Runx1 functions during the transition from 'haemogenic endothelium' to Haematopoietic stem cells (HSCs). ...Collectively these data show that Runx1 function is essential in endothelial cells for haematopoietic progenitor and HSC formation from the vasculature, but its requirement ends once or before Vav is expressed."
[10] "Hematopoietic system involves sequential transfers of hematopoietic stem cells (HSCs) generated in the yolk sac blood islands, to successive hematopoietic organs as these become active in the embryo (fetal liver, thymus, spleen and eventually bone marrow). 4.5 day gap between appearance of the yolk sac blood islands and the stage of a fully active fetal liver. Avian studies identified yolk sac produce only erythro-myeloid precursors that become extinct after emergence of a second wave of intra-embryonic HSCs from the region neighbouring the dorsal aorta." (text modified from paper abstract)
[11] "In the 1960s a series of ontogenetic studies in birds and subsequently in mice revealed that hematopoietic and lymphoid development involved migration streams of primitive cells that colonized developing primary lymphoid organs as well as spleen, marrow, and liver. The yolk sac was proposed as the ultimate origin of these lympho-hematopoietic precursors. Subsequent studies identified a region associated with the dorsal aorta as the primary site of "definitive" stem cells. These opposing views are currently achieving a compromise that recognizes that both sites contribute stem cells involved in seeding the developing tissues." (text from abstract)
Fetal Blood Facts
Fetal red blood cells (rbc) can also be identified by the presence of a nucleus that is absent in the adult red blood cell. Fetal red blood cells also contain a fetal haemoglobin which has different oxygen/carbon dioxide binding characteristics to adult red blood cell haemoglobin.
Maternal and fetal blood never mix, with exchange occuring across a number of membranes found in the placenta. (More? see placenta)
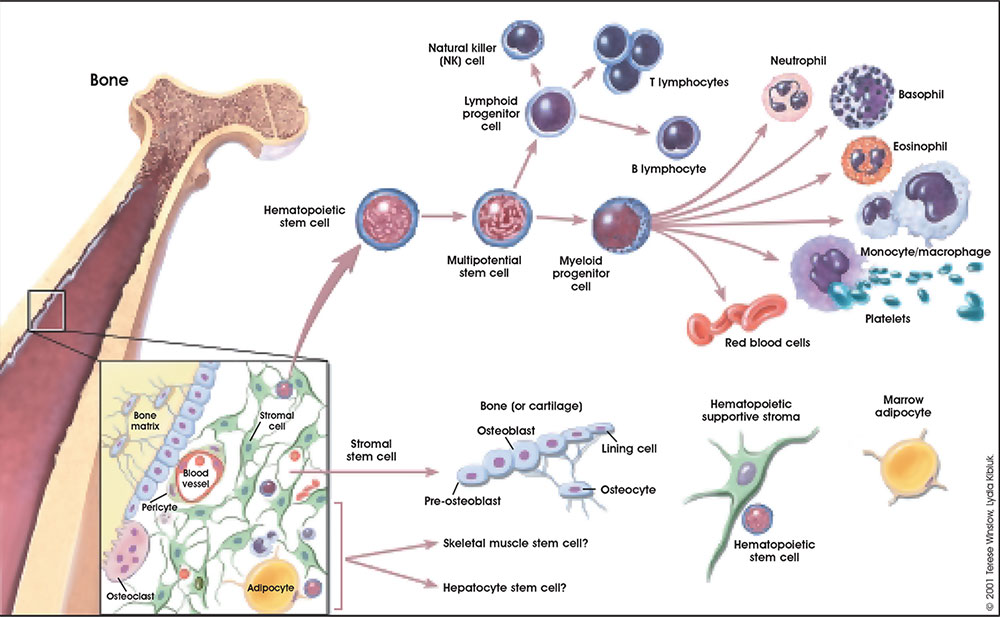
Adult Blood Cell Differentiation
- Links: bone
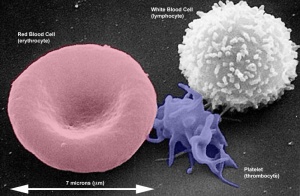
Red Blood Cells
Red blood cells (rbc) are the transporters of oxygen and carbon dixide in the blood.
When blood is centrifuged, the total % amount is known as the haemocrit. A low haemocrit or haemoglobin level leads to anemia. The lower oxygen tension at high altitudes leads to the body producing more rbc to compensate.
Adult red blood cells contain no nucleus and have a limited lifespan, of about 120 days[12], though apparently much lower (mean remaining life span) when used in transfusion.[13]
Most fetal red blood cells retain their nucleus, while adult red blood cells undergo enucleation as part of normal reticulocyte maturation within bone marrow before being released into circulation.
Adult reticulocyte maturation, as described in a recent article.[14]
- initially vesicles coalesce at the nuclear cytoplasmic junction.
- this creates a new limiting membrane.
- the sides are pinched inwards by the combined action of vesicle trafficking and microfilaments.
Organelles, such as mitochondria, are also eliminated by selective autophagy, by targeting to autophagosomes, and subsequently undergo degradation and exocytosis.
White Blood Cells
White blood cells are a family of many different cell types that mediate many different functions including: immune defence, clotting, bacteria and virus destruction and cell debris scavenging. These cells are not formed in the initial embryonic blood, mainly nucleated erythroid cells RBCs with small numbers of macrophages and megakaryocytes. White blood cells begin to develop in the early fetal period.[15] The fetal and neonatal neutraphils differ from adult neutrophils, based upon their maturation and environmental factors. These cells will form the majority of granulocyte precursors within the bone marrow.
Second trimester (GA 15-21) fetal blood study[16] showed significant changes in erythropoietic system though little change in myeloid series, with no significant increase or decrease in numbers. The only exception was eosinophils and basophils which increase significantly with gestational age while the platelet count remains constant.
Third trimester fetal cord blood study[17] showed no gender differences in counts of white blood cells, neutrophils, monocytes, eosinophils and lymphocytes that all increased. Platelets also increased from 30-35 weeks. The percentages of lymphocytes and monocytes decreased overall though, due to the large increase in the absolute neutrophil count.
Tissue Macrophages
Adult - liver (Kupffer cells), brain (microglia), epidermis (Langerhans cells) lung (alveolar macrophages)
Arise from erythro-myeloid progenitors (EMPs) in the yolk sac that are a separate population from haematopoietic stem cells (HSCs)[18]
Lymphocyte Cells
- Lymphocyte EM Images: T and B Lymphocytes 1 TEM | T and B Lymphocytes 2 TEM | T Lymphocyte SEM | B lymphocyte 1 TEM | B lymphocyte 2 TEM | B lymphocyte 3 TEM | Plasma Cell TEM | T2 Lymphocyte 1 TEM | T2 Lymphocyte 2 TEM | lymphocyte rosettes | T lymphocyte 1 | T lymphocyte 2 | T lymphocyte 3 | T lymphocyte 4 | T lymphocyte 5 | T lymphocyte 6 | B lymphocyte | B lymphocytes TEM | Immune System Development | Blood
B Cells
T Cells
Eutherian mammals prenatally initiate T cell development.
A recent study in Monodelphis domestica has shown that the marsupial opossum, like the eutherian mammals, prenatally initiate T cell development.[19]
- day 14 embryos - CD3ε+ cells were only found at the site of the early thymus.
- 48 h prior to parturition - CD3ε+ lymphocytes in late-stage embryos at the upper thoracic cavity (thymus).
- postnatal day 1 αβT cells were present and likely initiated development prenatally.
- Links: immune
Blood Progenitor Development
In the mouse, the yolk sac has an early important role in the provision of progenitor cells; before E8.0 all progenitors are found in the yolk sac, which remains enriched compared with the embryo from E9.5 to E10.5. (More? mouse)
4 to 8 somite stage (E8.25 - E8.5): small numbers of erythroblasts first enter the embryo (yolk sac-derived primitive erythroblasts)
26 to 30 somite stage (E10): 40% red cells steady state
Anemia
The cut-offs for haemaglobin and haemocrit which are used to define anemia in people living at sea level.
| Data from - World Health Organization | ||
Blood Cell Numbers
The adult ranges of cells / 1 litre (l), total blood volume is about 4.7 to 5 litres. Blood Development | Blood Histology
Red Blood Cells
- Male: 4.32 - 5.66 x 1012/l
- Female: 3.88 - 4.99 x 1012/l
Leukocytes (white blood cells)
- Male: 3.7 - 9.5 x 109/l
- Female: 3.9 - 11.1 x 109/l
Granulocytes
- 1.8 - 8.9 x 109/l
- Neutrophils: 1.5 - 7.4 x 109/l
- Eosinophils: 0.02 - 0.67 x 109/l
- Basophils: 0 - 0.13 x 109/l
Non-Granulocytes
- Monocytes 0.21 - 0.92 x 109/l
Lymphocytes
- 1.1 - 3.5 x 109/l
- B-cells: 0.06 - 0.66 x 109/l
- T-cells: 0.77 - 2.68 x 109/l
- CD4+: 0.53 - 1.76 x 109/l
- CD8+: 0.30 - 1.03 x 109/l
- NK cells: 0.20 - 0.40 x 109/l
Platelets
- 140 - 440 x 109/l
- not a cell, a cell fragment.
Altitude
The lower oxygen tension at high altitudes leads to the body producing more rbc to compensate. This means that people living at high altitudes have a higher haemocrit and/or haemoglobin level. This is also the reason why atheletes train at high altitude, to give them a higher gas carrying level when they return to sea level. This altitude effect on returning to sea level is gradually lost.
Alternately, this is also the basis of "altitude sickness" when people move rapidly from sea level to high altitude regions and their body has not yet been able to compensate.
- Links: Hypoxia | PMID 22724609
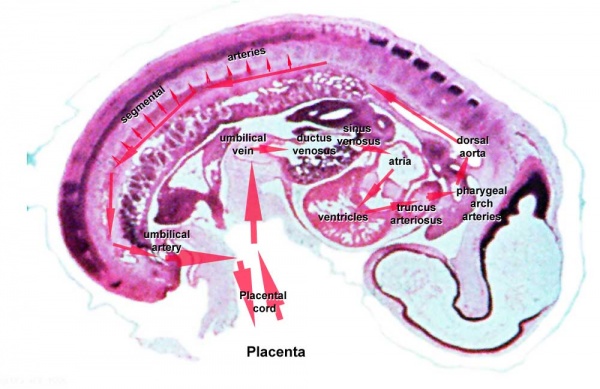
Circulation
Embryo
Maternal Blood | -> umbilical vein -> liver -> anastomosis -> sinus venosus -> atria ventricles-> truncus arteriosus -> aortic sac -> aortic arches-> dorsal aorta-> pair of umbilical arteries | Maternal Blood
Fetal
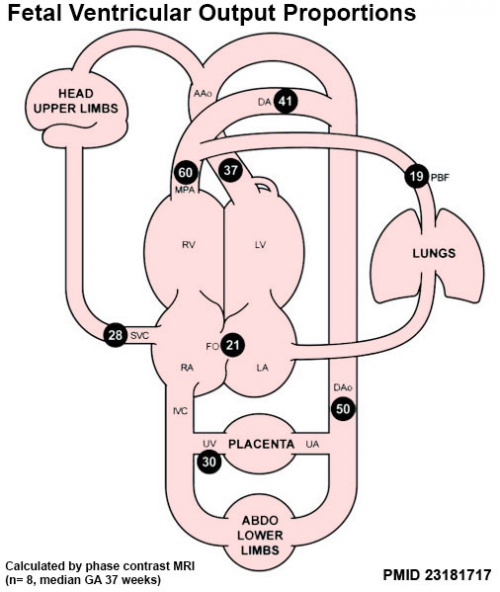
|
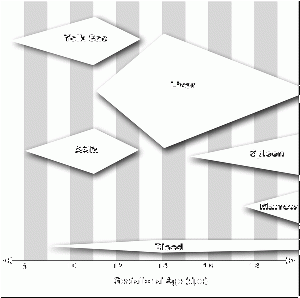
Proportions of the combined ventricular output in the major vessels of the human fetal circulation by phase contrast MRI. Mean flows (8 subjects) in the major vessels of the human fetal circulation by phase contrast MRI (median gestational age 37 weeks, age range of 30–39 weeks).[22]
|
Angiogenesis
- blood vessel formation
- vasculogenesis
- also occurs in adult and disease
- begins week 3 in extraembryonic mesoderm
- yolk sac
- connecting stalk
- chorion
- Growth Factors - Vascular endothelial growth factor (VEGF), PIGF
- angioblasts form clusters - blood islands
- blood islands extend and fuse together forms a network
- 2 populations of cells
- peripheral- form endothelial cells
- core- form blood cells (haemocytoblasts)
- all vessels (arteries and veins) appear initially the same
Blood formation
- blood formation occurs later (week 5)
- occurs throughout embryoic mesenchyme
- liver
- then spleen, bone marrow, lymph nodes
Maternal Blood
During pregnancy, maternal blood volume increases by about 50% and the uterine blood flow increases 10 to 12 fold. Uterine flow increase is due mainly to the trophoblast cell invasion of the spiral arteries opening them into blood-filled spaces of the placenta.
There are also changes in circulating glucose due to increases in insulin resistance during pregnancy.
- Links: Placenta Development
Molecular
- Sox17 - transcriptional regulator is specifically expressed in fetal and neonatal but not adult HSCs.[23]
- Steel factor (SF)
Histology
Adult Bone Marrow
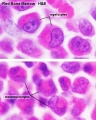
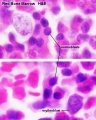
- Bone Marrow Histology: Blood Development | Marrow overview | Megakaryocyte | Megakaryocyte detail | Myelocyte | Normoblast | Reticulocyte | Blood Histology | Bone Development | Category:Blood
Adult Blood Cells
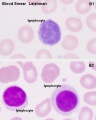
- Blood Histology: Blood Development | Blood Cell Number Table | Lymphocyte 1 | Lymphocyte 2 | Lymphocyte 3 | Lymphocyte 4 | Monocyte 1 | Monocyte 2 | Monocyte 3 | Monocyte 4 | Neutrophils 1 | Neutrophil 2 | Neutrophil 3 | Neutrophil 4 | Eosinophil 1 | Eosinophil 2 | labeled Neutrophil and Eosinophil | unlabeled - Neutrophil and Eosinophil | Basophil 1 | Basophil 2 | Basophil 3 | Platelet 1 | Platelet 2 | Reticulocyte | Megakaryocyte | Movie | Bone Marrow Histology | Category:Blood
| Blood Cells |
|---|
Adult human blood cell numbers shown in the table below is for reference purposes.
Blood Cell NumbersThe adult ranges of cells / 1 litre (l), total blood volume is about 4.7 to 5 litres. Blood Development | Blood Histology Red Blood Cells
Leukocytes (white blood cells)
Granulocytes
Non-Granulocytes
Lymphocytes
Platelets
|
Abnormalities
Haemolytic Disease of the Newborn
Haemolytic Disease of the Newborn (fetal erythroblastosis) is an immune problem arising from fetus Rh+ /maternal Rh-. Leakage of blood from fetus leads to maternal anti-Rh antibodies, which can then be dangerous for future pregnancies. This has in the past been identified by blood typing fetal blood by invasive prenatal diagnostic techniques or postnatally from the neonate. A recent study has shown that Non-Invasive Prenatal Testing (NIPT) can be used to identify presence or absence of the RhD type from circulating fetal DNA in the maternal blood after about 11 weeks gestation.[24]
Rhesus factor D (RhD)
|
Maternal immune system
|
- Coombs tests - anti-immunoglobulin antibodies used to detect the maternal RhD antibodies.
- Immunoprophylaxis - introduction of active immunization through vaccines or passive immunization through antisera.
- Links: Non-Invasive Prenatal Testing | Blood Groups and Red Cell Antigens | Immunologists' Toolbox | Image - Coombs test
Sickle Cell Anemia
| People who have this form of sickle cell disease inherit two sickle cell genes (“S”), one from each parent. This is commonly called “sickle cell anemia”, and is usually the most severe form of the disease. The name comes from the "sickle" shape of the RBC compared to the normal "donut" shape.
|
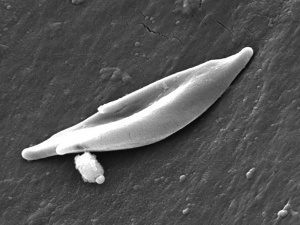
Sickle cell RBC (Image CDC) |
A recent study has shown that Sickle cell disease can induce resistance to cutaneous carcinogenesis.[25]
Thalassemia
Thalassemia is a group of inherited (genetic) blood disorders most frequently in people of Italian, Greek, Middle Eastern, Southern Asian and African Ancestry. The most severe form of alpha thalassemia, affecting mainly people of Southeast Asian, Chinese and Filipino ancestry, results in fetal or newborn death.
The two main types of thalassemia are called "alpha" and "beta," depending on which part of an oxygen-carrying protein in the red blood cells is lacking. Both types of thalassemia are inherited in the same manner. A child who inherits one mutated gene is a carrier, which is sometimes called "thalassemia trait." Most carriers lead completely normal, healthy lives.
References
- ↑ Streeter GL. Developmental horizons in human embryos (fourth issue). A review of the histogenesis of cartilage and bone. (1949) Carnegie Instn. Wash. Publ. 583, Contrib. Embryol., 33: 149-169. PMID: 18144445
- ↑ Enzan H, Hara H, Izumi T & Ohkita T. (1983). Morphologic and radiological observations on the earliest bone marrow formation in human embryos and fetuses. Acta Pathol. Jpn. , 33, 439-46. PMID: 6624441
- ↑ Slayton WB, Li Y, Calhoun DA, Juul SE, Iturraspe J, Braylan RC & Christensen RD. (1998). The first-appearance of neutrophils in the human fetal bone marrow cavity. Early Hum. Dev. , 53, 129-44. PMID: 10195706
- ↑ Popescu DM, Botting RA, Stephenson E, Green K, Webb S, Jardine L, Calderbank EF, Polanski K, Goh I, Efremova M, Acres M, Maunder D, Vegh P, Gitton Y, Park JE, Vento-Tormo R, Miao Z, Dixon D, Rowell R, McDonald D, Fletcher J, Poyner E, Reynolds G, Mather M, Moldovan C, Mamanova L, Greig F, Young MD, Meyer KB, Lisgo S, Bacardit J, Fuller A, Millar B, Innes B, Lindsay S, Stubbington MJT, Kowalczyk MS, Li B, Ashenberg O, Tabaka M, Dionne D, Tickle TL, Slyper M, Rozenblatt-Rosen O, Filby A, Carey P, Villani AC, Roy A, Regev A, Chédotal A, Roberts I, Göttgens B, Behjati S, Laurenti E, Teichmann SA & Haniffa M. (2019). Decoding human fetal liver haematopoiesis. Nature , 574, 365-371. PMID: 31597962 DOI.
- ↑ McMahon KE, Habeeb O, Bautista GM, Levin S, DeChristopher PJ, Glynn LA, Jeske W & Muraskas JK. (2018). The association between AB blood group and neonatal disease. J Neonatal Perinatal Med , , . PMID: 30347622 DOI.
- ↑ Nguyen PD, Hollway GE, Sonntag C, Miles LB, Hall TE, Berger S, Fernandez KJ, Gurevich DB, Cole NJ, Alaei S, Ramialison M, Sutherland RL, Polo JM, Lieschke GJ & Currie PD. (2014). Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature , 512, 314-8. PMID: 25119043 DOI.
- ↑ Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA & McCune JM. (2010). Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science , 330, 1695-9. PMID: 21164017 DOI.
- ↑ 8.0 8.1 Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E & Speck NA. (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature , 457, 887-91. PMID: 19129762 DOI.
- ↑ Furuta C, Ema H, Takayanagi S, Ogaeri T, Okamura D, Matsui Y & Nakauchi H. (2006). Discordant developmental waves of angioblasts and hemangioblasts in the early gastrulating mouse embryo. Development , 133, 2771-9. PMID: 16794034 DOI.
- ↑ Godin I & Cumano A. (2005). Of birds and mice: hematopoietic stem cell development. Int. J. Dev. Biol. , 49, 251-7. PMID: 15906239 DOI.
- ↑ Moore MA. (2004). Commentary: the role of cell migration in the ontogeny of the lymphoid system. Stem Cells Dev. , 13, 1-21. PMID: 15068689 DOI.
- ↑ SHEMIN D & RITTENBERG D. (1946). The life span of the human red blood cell. J. Biol. Chem. , 166, 627-36. PMID: 20276177
- ↑ Kuruvilla DJ, Nalbant D, Widness JA & Veng-Pedersen P. (2014). Mean remaining life span: a new clinically relevant parameter to assess the quality of transfused red blood cells. Transfusion , 54, 2724-9. PMID: 24611672 DOI.
- ↑ Ney PA. (2011). Normal and disordered reticulocyte maturation. Curr. Opin. Hematol. , 18, 152-7. PMID: 21423015 DOI.
- ↑ Lawrence SM, Corriden R & Nizet V. (2018). The Ontogeny of a Neutrophil: Mechanisms of Granulopoiesis and Homeostasis. Microbiol. Mol. Biol. Rev. , 82, . PMID: 29436479 DOI.
- ↑ Millar DS, Davis LR, Rodeck CH, Nicolaides KH & Mibashan RS. (1985). Normal blood cell values in the early mid-trimester fetus. Prenat. Diagn. , 5, 367-73. PMID: 4088972
- ↑ Glasser L, Sutton N, Schmeling M & Machan JT. (2015). A comprehensive study of umbilical cord blood cell developmental changes and reference ranges by gestation, gender and mode of delivery. J Perinatol , 35, 469-75. PMID: 25634517 DOI.
- ↑ Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F & Rodewald HR. (2015). Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature , 518, 547-51. PMID: 25470051 DOI.
- ↑ Hansen VL & Miller RD. (2017). On the prenatal initiation of T cell development in the opossum Monodelphis domestica. J. Anat. , 230, 596-600. PMID: 28052333 DOI.
- ↑ McGrath KE, Koniski AD, Malik J & Palis J. (2003). Circulation is established in a stepwise pattern in the mammalian embryo. Blood , 101, 1669-76. PMID: 12406884 DOI.
- ↑ Palis J, Robertson S, Kennedy M, Wall C & Keller G. (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development , 126, 5073-84. PMID: 10529424
- ↑ Seed M, van Amerom JF, Yoo SJ, Al Nafisi B, Grosse-Wortmann L, Jaeggi E, Jansz MS & Macgowan CK. (2012). Feasibility of quantification of the distribution of blood flow in the normal human fetal circulation using CMR: a cross-sectional study. J Cardiovasc Magn Reson , 14, 79. PMID: 23181717 DOI.
- ↑ Kim I, Saunders TL & Morrison SJ. (2007). Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell , 130, 470-83. PMID: 17655922 DOI.
- ↑ Chitty LS, Finning K, Wade A, Soothill P, Martin B, Oxenford K, Daniels G & Massey E. (2014). Diagnostic accuracy of routine antenatal determination of fetal RHD status across gestation: population based cohort study. BMJ , 349, g5243. PMID: 25190055
- ↑ Soutou B, Senet P, Lionnet F, Habibi A & Aractingi S. (2020). Sickle cell disease induces resistance to cutaneous carcinogenesis. Orphanet J Rare Dis , 15, 66. PMID: 32143660 DOI.
Reviews
Lawrence SM, Corriden R & Nizet V. (2018). The Ontogeny of a Neutrophil: Mechanisms of Granulopoiesis and Homeostasis. Microbiol. Mol. Biol. Rev. , 82, . PMID: 29436479 DOI.
Klaus A & Robin C. (2017). Embryonic hematopoiesis under microscopic observation. Dev. Biol. , 428, 318-327. PMID: 28728681 DOI.
Yumine A, Fraser ST & Sugiyama D. (2017). Regulation of the embryonic erythropoietic niche: a future perspective. Blood Res , 52, 10-17. PMID: 28401096 DOI.
Barminko J, Reinholt B & Baron MH. (2016). Development and differentiation of the erythroid lineage in mammals. Dev. Comp. Immunol. , 58, 18-29. PMID: 26709231 DOI.
Golub R & Cumano A. (2013). Embryonic hematopoiesis. Blood Cells Mol. Dis. , 51, 226-31. PMID: 24041595 DOI.
Magnon C & Frenette PS. (2008). Hematopoietic stem cell trafficking. , , . PMID: 20614595 DOI.
Jean D & Moore LG. (2012). Travel to high altitude during pregnancy: frequently asked questions and recommendations for clinicians. High Alt. Med. Biol. , 13, 73-81. PMID: 22724609 DOI.
Tavian M & Péault B. (2005). Embryonic development of the human hematopoietic system. Int. J. Dev. Biol. , 49, 243-50. PMID: 15906238 DOI.
Articles
Glasser L, Sutton N, Schmeling M & Machan JT. (2015). A comprehensive study of umbilical cord blood cell developmental changes and reference ranges by gestation, gender and mode of delivery. J Perinatol , 35, 469-75. PMID: 25634517 DOI.
Golub R & Cumano A. (2013). Embryonic hematopoiesis. Blood Cells Mol. Dis. , 51, 226-31. PMID: 24041595 DOI.
Davidson AJ & Zon LI. (2000). Turning mesoderm into blood: the formation of hematopoietic stem cells during embryogenesis. Curr. Top. Dev. Biol. , 50, 45-60. PMID: 10948449
Lensch MW & Daley GQ. (2004). Origins of mammalian hematopoiesis: in vivo paradigms and in vitro models. Curr. Top. Dev. Biol. , 60, 127-96. PMID: 15094298 DOI.
van der Schoot CE, Tax GH, Rijnders RJ, de Haas M & Christiaens GC. (2003). Prenatal typing of Rh and Kell blood group system antigens: the edge of a watershed. Transfus Med Rev , 17, 31-44. PMID: 12522770 DOI.
Avent ND. (2001). Molecular biology of the Rh blood group system. J. Pediatr. Hematol. Oncol. , 23, 394-402. PMID: 11563778
Urbaniak SJ & Greiss MA. (2000). RhD haemolytic disease of the fetus and the newborn. Blood Rev. , 14, 44-61. PMID: 10805260 DOI.
McGrath KE, Koniski AD, Malik J & Palis J. (2003). Circulation is established in a stepwise pattern in the mammalian embryo. Blood , 101, 1669-76. PMID: 12406884 DOI.
Palis J, Robertson S, Kennedy M, Wall C & Keller G. (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development , 126, 5073-84. PMID: 10529424
Forestier F, Daffos F, Galactéros F, Bardakjian J, Rainaut M & Beuzard Y. (1986). Hematological values of 163 normal fetuses between 18 and 30 weeks of gestation. Pediatr. Res. , 20, 342-6. PMID: 3703624 DOI.
Search PubMed
Search NCBI Bookshelf: Blood Development
Search PubMed: Blood Development | Maternal Blood Development
Cardiovascular Terms
| Cardiovascular Terms |
|---|
Cardiovascular System Development See also Heart terms, Immune terms and Blood terms.
|
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
- anastomose - a direct connection between arteries, veins or arteries and veins. The Circle of Willis is an arterial anastomosis.
- angioblasts - stem cells in blood islands generating endothelial cells
- angiogenesis - the formation of blood vessels also called vasculogenesis in the embryo
- anlage - (Ger. ) primordium, structure or cells which will form a future structure.
- atrial septal defects - (A.S.D.)
- blood islands - earliest sites of blood vessel and blood cell formation, seen mainly on yolk sac chorion
- branched villi - or terminal villi, grow from sides of stem villi, region of main exchange, surrounded by maternal blood in intervillous spaces
- cardinal veins - paired main systemic veins of early embryo, anterior, common, posterior.
- cardiogenic region - region above precordal plate in mesoderm where ceart tube initially forms.
- cord knotting- umbilical cord knotting occurs in 1%, prevents the passage of placental blood. pseudoknots also occur usually with no effect.
- cotyledons - on maternal face of placenta, form cobblestone appearance, originally placental septa formed grooves
- cytotrophoblast - extraembryonic cells of trophoblastic shell surrounding embryo, contribute to villi and placental membranes.
- decidua basalis-
- decidual reaction -
- ectoderm - the layer (of the 3 germ cell layers) which form the nervous system from the neural tube and neural crest and also generates the epithelia covering the embryo.
- endoderm - the layer (of the 3 germ cell layers) which form the epithelial lining of the gastrointestinal tract (GIT) and accessory organs of GIT in the embryo.
- endothelial cells - single layer of cells closest to lumen that line blood vessels
- extraembryonic mesoderm - mesoderm lying outside the trilaminar embryonic disc
- fetal erythroblastosis - see [#Haemolytic Disease Haemolytic Disease of the Newborn]
- haemocytoblasts - stem cells for embryonic blood cell formation
- Haemolytic Disease of the Newborn - fetal erythroblastosis, fetus Rh+ /maternal Rh-, fetus causes anti Rh antibodies, dangerous for 2nd child
- chorionic villi - the finger-like extensions which are the functional region of the placental barrier and maternal/fetal exchange. Develop from week 2 onward as: primary, secondary, tertiary villi.
- estrogens - support maternal endometrium
- fetal drug addiction - occurs when drugs used maternally cross the placental barrier and can establish addiction in the unborn fetus.
- growth factor - usually a protein or peptide that will bind a cell membrane receptor and then activates an intracellular signaling pathway. The function of the pathway will be to alter the cell directly or indirectly by changing gene expression. (eg VEGF, shh)
- hCG - [#hCG see Human chorionic gonadotrophin]
- Human chorionic gonadotrophin - (hCG) like leutenizing hormone, supports corpus luteum
- Human chorionic somatommotropin - (hCS) or placental lactogen stimulate mammary development
- Human chorionic thyrotropin - (hCT) placental derived hormone equivilant to thyroid
- Human chorionic corticotropin - (hCACTH) placental derived hormone equivilant to
- maternal antibodies - immune molecules capable of crossing placental barrier
- maternal decidua - region of uterine endometrium where blastocyst implants. undergoes modification following implantation, decidual reaction.
- maternal sinusoids - placental spaces around chorionic villi that are filled with maternal blood. Closest maternal/fetal exchange site.
- mesoderm - the middle layer of the 3 germ cell layers of the embryo. Mesoderm outside the embryo and covering the amnion, yolk and chorion sacs is extraembryonic mesoderm.
- neural crest - cell region at edge of neural plate, then atop the neural folds, that remains outside and initially dorsal to the neural tube when it forms. These paired dorsal lateral streaks of cells migrate throughout the embryo and can differentiate into many different cell types(=pluripotential). Neural crest cells also contribute to major cardiac outflow vessels.
- patent ductus arteriosus - (P.D.A.)
- pharyngeal arches - (=branchial arches, Gk. gill) form structures of the head. Six arches form but only 4 form any structures. Each arch has a pouch, membrane and groove.
- placenta - (Gk. plakuos= flat cake) refers to the discoid shape of the placenta, embryonic (villous chorion)/maternal organ (decidua basalis)
- placenta accreta - abnormal adherence of placenta, with absence of decidua basalis
- placental arteries - paired, carry deoxygenated blood (from dorsal aorta) and waste products to the placental villi
- placental lactogen - see [#hCS Human chorionic somatommotropin]
- placenta percreta - villi of placenta penetrate myometrium
- placenta previa - placenta overlies internal os of uterus, abnormal bleeding, cesarian delivery
- placental veins - paired initially then only left at end of embryonic period, carry oxygenated blood to the embryo (sinus venosus)
- primary villi - week 2, first stage of chorionic villi development, trophoblastic shell cells (syncitiotrophoblasts and cytotrophoblasts) form finger-like extensions into maternal decidua.
- protein hormone - usually a protein distributed in the blood that binds to membrane receptors on target cells in different tissues. Do not easliy cross placental barrier.
- relaxin - hormone
- secondary villi - week 3, second stage of chorionic villi development, extraembryonic mesoderm grows into villi, covers entire surface of chorionic sac
- sinus venosus - cavity into which all major embryonic paired veins supply (vitelline, placental, cardinal)
- splanchnic mesoderm - portion of lateral plate mesoderm closest to the endoderm when coelom forms.
- stem villi - or anchoring villi, cytotrophoblast cells attached to maternal tissue.
- steroid hormone - lipid soluble hormone that easily crosses membranes to bind receptors in cytoplasm or nucleus of target cells. Hormone+Receptor then binds DNA activating or suppressing gene transcription. Easliy cross placental barrier.
- syncitiotrophoblast - extraembryonic cells of trophoblastic shell surrounding embryo, outside the cytotrophoblast layer, involved with implantation of the blastocyst by eroding extracellular matrix surrounding maternal endometrial cells at site of implantation, also contribute to villi. (dark staining, multinucleated)
- tetralogy of Fallot- Named after Etienne-Louis Arthur Fallot (1888) who described it as "la maladie blue". The syndrome consists of a number of a number of cardiac defects possibly stemming from abnormal neural crest migration.
- tertiary villi - third stage of chorionic villi development, mesenchyme differentiates into blood vessels and cells, forms arteriocapillary network, fuse with placental vessels, developing in connecting stalk
- umbilical cord
- umbilical cord knotting
- vascular endothelial growth factor - (VEGF) protein growth factor family that stimulates blood vessel growth, a similar factor can be found in the placenta (PIGF).
- ventricular septal defects - (V.S.D.)
- virus - small infectious agent able to cross placental barrier. Can infect embryo and cause developmental abnormalities. (e.g. cytomegalovirus, rubella, measles)
- vitelline blood vessels - blood vessels associated with the yolk sac.
- waste products - products of cellular metabolism and cellular debris, e.g.- urea, uric acid, bilirubin
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 26) Embryology Cardiovascular System - Blood Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cardiovascular_System_-_Blood_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G