Paper - The development of the cranial venous system in man, from the viewpoint of comparative anatomy
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Padget DH. The development of the cranial venous system in man, from the viewpoint of comparative anatomy. (1957) Carnegie Instn. Wash. Publ. 611, Contrib. Embryol., 36:
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Cranial Venous System in Man, from the Viewpoint of Comparative Anatomy
Contributions To Embryology, No. 247
Dorcas H. Padget
Department of Embryology, Carnegie Institution of Washington, Baltimore; Division of Neurologicall Surgery, University of MarylandSchool of Medicine, Baltimore
With six plates (two colored) and eighteen text figures
Introduction
The present communication is the logical outcome of the author’s companion study of the development of the cranial arteries in the human embryo (Padget, 1948, 1954). Following a pioneer report on the developing vasculature of the brain in man (Mall, 1904), there appeared the major contributions of Streeter (1915, 1918), which have been regarded as authoritative and are widely quoted. Streeter was chiefly concerned, however, with the dural sinuses as illustrative of fundamentals of the vascular apparatus dependent on changing factors in its environment (1918), and dealt only incidentally with the veins (and arteries) of the brain and extracranial parts. Although the present survey was begun to supplement Streeter's work, it became apparent that his interpretations regarding several important vessels could not be accepted. Near the close of the present author’s study, attention was directed to a monograph on the development of the dural sinuses and vessels of the human brain by Markowski (1922), which work, essentially completed but delayed in publication, had been summarized earlier (191r). His detailed contribution was buried as a supplement to a relatively inaccessible journal and suffers in contrast to Streeter’s exposition (I918) embellished with colored plates by James Didusch. Owing chiefly to the limitations of Markowski’s illustrations (meager diagrams, 1911, plus photographs of sections and of models in color, 1922), his reports may not be fully understood except by one familiar with comparable embryos. With such material at hand, however, the author had come, independently, to similar conclusions regarding the identity of certain vessels. Furthermore, the points of agreement with Marko\vski coincided with some of the points of disagreement with Streeter. The present extended observations have shown in general that the developmental pattern, both phylogenetic and ontogenetic, of the whole cranial vasculature, especially of the venous part, must be visualized in order to prevent misconceptions regarding its subdivisions in embryonic or mature specimens.
The fact that there has long been wanting a comprehensive account of the veins of the head region is readily explained. Many vessels, together with the common variations of venous patterns, can be comprehended only from the comparative standpoint of their evolution in other vertebrates. Moreover, there is no overstatemcnt in Mall’s picturesque comment that the “history of the arteries is relatively simple when compared with the gyrations the veins undergo.” In respect to the arteries, the author was forearmed, from the literature and from personal observation, with a reasonably wide knowledge_of the human adult configuration and its variations. For many reasons, similar knowledge of the venous system is less readily available. Routine removal of the brain from the skull tears the delicate veins that connect the pial and dural systems; careful removal to identify each connection is both diHicult and time consuming. A number of the large but thin-walled veins are buried under the arteries in the great cerebral fissures, so that a considerable resection of brain tissue is necessary for their exposure. The blood, or even certain types of injection material (e.g. ink), may not always fill or be retained in all parts of a continuous channel, owing to uneven compression of the more deeply situated veins by the brain, before or after fixation. In either infant or adult, though for different reasons, a complete dissection of the dural channels at the base of the skull is difficult; the obstructing bone is densely adherent to the dura around the nerves and certain related vessels (Walker, 1933). To supplement observations on adult heads, a number of infants, in which the delicacy of the skull bones somewhat offsets the disadvantage of minute vessels, were dissected with the original intention of establishing a norm for the adult pattern. Although the present evidence is limited, it is apparent that at birth certain important venous channels differ from the adult configuration as generally described. Added to this unexpected finding were the differences in certain details found in embryos and fetuses of the same age group.
- This work was primarily aided by grants made through Dr. George W. Corner by the Life Insurance Foundation, 19461950, and by a fellowship granted to the author by the Carnegie Institution of Washington, 1950-1953; it was completed in the Division of Neurological Surgery, University of Maryland School of Medicine, with aid from the I-Ioffberger Fund.
In spite of the confusion presented at the outset by all these factors, the constant comparison of embryonic, infant, and adult patterns, supplemented by what is known of other species, gradually clarified the picture, and what may be called, at least tentatively, a typical configuration in each case finally emerged. Attention is directed to a publication concerning obscure features of the adult pattern in man from the developmental point of view (Padget, 1956); this article includes a discussion of embryologic considerations regarding the origin of congenital arteriovenous aneurysms.
The final problem in the present study was the adequate pictorial presentation of the material within the limited number of figures. When preparing some of the illustrations, the author was faced with a choice between showing the complete or exact graphic reconstructions, which were too complicated to be readily understood, and risking errors of personal judgment by conversion to a semidiagrammatic picture. The resulting compromise makes no undue sacrifice to either clarity or accuracy.
Most of the material for this study was provided by the Carnegie Collection of Embryos, many being the same ones used in the earlier study of the arteries. From enlarged photographs of serial sections, graphic reconstructions were prepared in the three standard planes: lateral, horizontal, and coronal. Because of variations in details, it was necessary to compare the findings in more embryos of each stage than for the arteries, and more than 65 views of over 30 specimens were prepared. Dissections and sketches were made of 10 late fetal and newborn heads (3 were injected); a similar study of about 15 adult beads was possible through the courtesy of Dr. Allan L. Grafilin of the Iohns Hopkins School of Medicine. Much appreciation is expressed to Dr. E. Carl Sensenig of the University of Alabama for helpful sug gestions, and to Dr. George VV. Smith and Mr. Carl VV. Mueller, who facilitated study of their excellent corrosion preparations of the adult made in the department of Professor Eduard Uhlenhuth of the University of Maryland School of Medicine. (Unusually informative demonstrations of venous channels in several skulls were obtained by latex injections followed by digestion of the soft parts.) The essential comparative information, when not provided by the literature, was supplemented by study of several adult mammals, regarding which especial gratitude is expressed to the following: Dr. Karl R. Reinhard of the National Biological Institute, for the loan of many photographs of injected dog heads prior to his published report; Dr. David Bodian of the Department of Epidemiology, Iohns Hopkins University, for specially prepared heads of the rhesus monkey; Dr. and Mrs. Frederic A. Gibbs of the University of Illinois College of Medicine, who sent a collection of their venous injections of the cat head. The author is particularly indebted to Dr. George W. Corner, who obtained the special grants for this study, and to Dr. George \V. Bartelmez for his gracious help in review of the manuscript.
Embryonic Stages of Development
It was desirable to divide the complex process of venous differentiation in the head and neck into eight stages, including the postnatal pattern; the process can be stunmarized in eight illustrations to permit correlation with the arteries of this region.” Although arterial development falls naturally into this subdivision, based on essentially one specimen of a single age group, the prolonged emergence of definitive venous channels covers a considerably wider range of older embryos and must be more arbitrarily fitted into the classification. Actually, reduction to eight basic illustrations is feasible only for the lateral view (pl. 1); more figures are needed to show the most important changes in the vessels as viewed at the base of the brain and skull (pl. 2). The introductory text figures for each stage, showing its typical pattern in correlating lateral and basal views, in sotne instances are simplified composites of several embryos of the age group, necessitated by certain variations in detail. Furthermore, a designated venous stage sometimes covers two age groups, because the arteries resemble the adult conformation much earlier than do the veins, at about 40 and 80 mm., respectively; several important anastomoses typical of adult sinuses usually do not appear until after birth. Since the formation of venous channels depends 9It was not expedient in the present account to depict the exact venous pattern for vascular stage 1 (horizon xiii) as previously designated for the arteries. Its essentials, however, are present earlier, as described for horizon xii (fig. 1B). To avoid repetition, the reader is asked to assume henceforth that any untloctnnentetl reference to the arteries is to the author's 1948 monograph.
upon the more precocious arteries, certain major arteries are shown in most of the text figures. As each important sinus or vein becomes definitive, its name, whether that of a temporary embryonic or a permanent adult vessel, is emphasized by italics. The Roman numerals refer to the developmental horizons (age groups) determined by Streeter (1942-1951) for the Carnegie Collection of Embryos that are less than 40 mm. in crown—rump length.
Stage (1 and) 2 Embryos of Horizon XIV (5 to 8 mm)
The First stage fully described in the present series (fig. 2), and comparable to stage 2 of arterial development, consists of a relatively simple vascular plan. This plan, like that of earlier stages (fig. 1), is typical of vertebrate embryos: for instance, reptiles (Grosser and Brezina, 1895); birds, represented by the chick (Hughes, 1934, and others); and mammals like the bat (Grosser, 1901) and pig (Sabin, 1917). The head in stage 2 shows the three constant pharyngeal bars (“branchial" or “visceral arches”), namely the mandibular, hyoid, and glossapharyngeal, each with its contained cranial nerve. Subdivisions of the human neural tube at this stage indicate the five parts of the brain: the telencephalon and diencephalon (derived from the prosencephalon), the metencephalon or midbrain, the mctencephalon and myelencephalon (derived from the rhombencephalon). Stage 2 is characterized by the indentation of the ectodermal lens vesicle within the optic cup. The internal carotid artery now supplies the fore parts of the brain by way of its cranial (anterior and middle cerebral) and caudal (posterior communicating) divisions, and the basilar artery is emerging by consolidation of bilateral neural arteries. The veins are relatively much less mature.
Primary Head-Sinus Continuous with Anterior Cardinal Vein
The head is drained by the anterior cardinal vein (future internal jugular vein), which meets the posterior cardinal vein from the body to form the common cardinal vein (duct of Cuvier), entering the sinus venosus of the primitive heart. Caudal to the 10th and 12th nerve roots, in embryos younger than stage 2, the anterior cardinal is continuous with a channel that lies not only medial to all the cranial nerve roots but, more importantly, directly upon the neural tube (fig. IA). Although once considered part of the anterior cardinal vein. or called the “vena capitis medialis,” this channel antedates the time of true circulation in the head. It is transitory, and is fundamentally the proliferative endothelial material from which both pial arteries and veins are soon derived. For these reasons it was renamed the P}‘I.1}10J‘(iftIZ /1ind— brain c/Jamie! (“primitive rhombencephalic vessel” of Sabin, 1917) by Streeter (1918).
By stage 2 (fig. 2), the medial primordial channel has disappeared, but has given origin to a definitive venous channel in a more lateral position. This channel is still medial to the 5th and 10th nerves, but is outside the 7th, 8th, and 9th nerves and the otocyst. It constitutes the first true drainage channel of the craniocervical region. Formerly called the “vena capitis lateralis,” the new channel was renamed the “primitive head vein" by Evans (1912), and the “primary head-vein” by Sabin and by Streeter; in birds and mammals, it has also been called the “primary Stammvene” (van Gelderen, 1924; Hughes, 1934). These terms refer to the cranial part, as opposed to the future upper-cervical part, of the “anterior cardinal vein” designated by certain other writers.
The precise naming of embryonic vessels often presents a problem, not clearly resolved in this instance. The old designations, “medialis” and “lateralis," are often used for the most primitive head—veins of vertebrate embryos, but may be confusing unless it is realized that the later channel (“lateralis"), though lateral to the earlier channel (“meclialis"), is not necessarily lateral to the nerve roots; for instance, “medialis" has been applied to the part of the secondary channel, i.e. “lateralis,” which always lies medial to the 5th nerve root. Regarding the most recent terms noted above, which have the weight of distinguished authority, one must be careful to differentiate exactly in early embryos between the primordial hindbrain channel medial to all the nerve roots (horizon xi. fig. rA), and the later “primary bead-vein," which is medial to the 5th and Ioth (horizons xii to xiv, figs. IB, 2). Both have been observed simultaneously in the chick and pig injected in viva (Sabin). The primordial channel can be identified by the fact that it lies directly upon the neural tube and is usually represented after horizon xii only by veins (and arteries) of the pial layer. In contrast, it is soon seen that the “primary headvein." even when it remains medial to certain nerve roots, is dural in position. Since the present account emphasizes the distinction between vessels of the dural and pial layers, and since Streeter designated the dorsal tributaries of the “primary head-vein" as “dural” at this stage (see below), this vessel will henceforth be called the prinmry /zemidirzm‘ (figs. 1B, 2).
Tributaries of anterior cardinal System. Only the maior tributaries of the primary head—sinus are shown in the present illustrations. It must be understood that most of the neural tube is covered by a primitive capillary plexus (Padget, 19-36, figs. 24, 27), as is well shown in the colored plates of Sabin and Streeter. This plexus drains laterally, at the dorsolateral aspect of the neural tube, into a more superficial venous plexus, which in turn drains into the head—sinus through three well defined stems in the future dural layer. Having a relatively constant relation to the nerve roots, these stems were called the “anterior, middle, and posterior cerebral veins” by Mall (1904), terms commonly used for other vertebrate embryos. The designation “vein,” howevcr, is misleading, for several reasons. The so-called “middle cerebral vein,” for example, has nothing to do with the adult veins of the same name, which are derived later from the so-called “anterior cerebral vein” of this stage. In reference to the human embryo, Streeter grouped the superficial plexus drained by these “veins” of stages I and 2 into three parts, the mzterior, nziddlc’, and posterior‘ dural plexmcr. The first part drains the forebrain and midbrain; the middle part, the future cerebellar region; and the last part comes from the medullar region near the rotb nerve root. For simplicity, the terminal “vein” draining each plexus may be called the anterior, nziz/(I16, or poswrior dural stem, and figures IB and 2 show the levels relative to the nerve roots at which each joins the head—sinus. Since the three stems have important roles in the shaping of the adult dural sinuses and seem to be typical in the vertebrate line, their fate is of considerable interest phylogenetically.
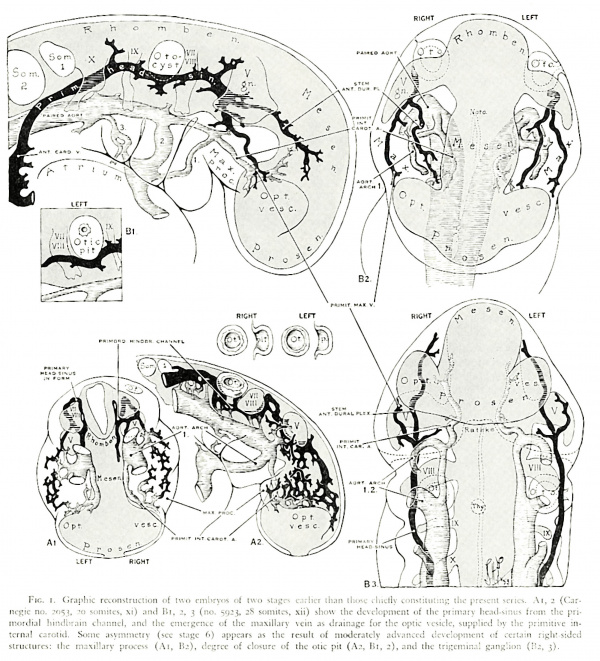
At stage 2, only one noteworthy ventral tributary of the primary head-sinus appears. Typically, this vein is as prominent in young vertebt'ate embryos as the betterknown dorsal tributaries just named, and ramifies extensively in the emerging maxillary process. It drains the ventrocaudal aspect of the optic vesicle at the lens pit through lateral tributaries, and the olfactory region, cranial and lateral to Rathke's pouch, through a medial vessel. The name primitive maxillary vein (cf. illustrations of chick and pig embryos in Sabin, I9l7) is used her than those chiefly constituting the prescnt :~‘.e|'ic5. .-\l, 2 (Carshow the development of the primary l1e:ul-sinus from the priitic \'c.~:iclc_. supplied hy the pt'imiti\'c in(levelopment of certain right—sid':d FIG. 1. Graphic reconstruction of two emhryos of two st:ige.< ea: and Ill, 2, 3 (no. 5923, :9 somites, xii) cncc of the maxillary vein as drainage for the 0; cars as the result of moderately advanced I clostlre of the otic pit (A2, B1, 2), and the trigeminal ganglion (B2, 3).
Carnegie no. 2053, so somites, xi) mordial hin(ll.ir:1in channel. and the cmcrg tcrnal carotid. Some asymmetry (sec stage 6) app structures: the maxillary process (A1, B2), degree
Fig. 2. Vascular stage 2 (which begins the present series) is typical of vertebrate embryos. The head-sinus, a lateral derivative of the primordial hindbrain channel (fig. 1), drains the hrain through three stems consistent in their relation to the nerve roots. The optic vesicle is drained by the maxillary vein (cf. fig. I), medial to the 5th nerve ganglion. A ventral pharyngeal vein primarily curves around the heart (fig. 1) to join the common cardinal, but soon migrates cranially upon the anterior cardinal vein (A). B shows the increased curvatures of the head-sinus and the definition of its initial pia-arachnoidal tributaries, coincident with expansion of the brain and otocyst in stage 3. (Crown-rump lengths in this and the other introductory illustrations of the typical pattern in each stage are approximate.)
here in preference to “infraorbital” or “ophthalmic” used by other writers, if they name it at all.
The primary significance of the maxillary vein is better understood by a glance at embryos younger than the stage under discussion. In embryo Carnegie no. 5923 of horizon xii (fig. IB),8 the primary head—sinus, recently derived from the primordial hindbrain channel, lies in its more primitive position medial to the 5th and 10th cranial nerve roots, and has just by—passed laterally the otic vesicle together with the acousticofacial and glossopharyngeal nerves, which border it. At this stage the head-sinus is formed by the junction of two vessels of about equal caliber: medially, the stem of the future anterior dural plexus; laterally, the maxillary vein, which originates from the top (superficial caudal margin) of the optic vesicle and courses through the bulging primitive maxillary process. The primary function of the maxillary vein, i.e. optic drainage (cf. injected chicks in Sabin’s pl. 6; Hughes, 1934, pl. 4), is clear in embryos still younger that show the plexiform components of the vein (horizon xi; fig. IA). In view of its conspicuous form in other vertebrate embryos illustrated in the literature, the maxillary vein is undoubtedly a morphological constant, the development of which, even before the three well known dorsal tributaries of the primary head—sinus, corresponds to the notably early formation of the optic vesicle. The history of the vein is of particular interest in man. Although its early role of draining the eye is soon supplemented by other vessels and is obscured by the addition of several larger tributaries from other regions, the stem of the maxillary vein finally becomes that of all the ophthalmic and orbital veins in the adult (stage 7).
The anterior cardinal vein, with which the primary head—sinus is directly continuous and which later constitutes the primitive internal jugular vein, is still medial to the 10th to 12th cranial nerves, instead of lateral as in the mature configuration. Furthermore, it must be emphasized that use of the adult designation does not imply the existence of an external jugular vein during early mammalian stages, or at any stage in many other vertebrates. Confusion has long existed regarding the developmental relation of the external and internal jugular systems, probably because the external system typically predominates over the internal in adult mammals except certain primates. Often called simply the “jugular" vein in reference to the adult of various species, the external jugular drains not only the face and extracranial parts, but also the intracranial sinuses through a temporal skull foramen (so—called “spurious jugular"; see stage 7a). In such cases the internal jugular vein, together with the “true” jugular foramen, is correspondingly small or essentially absent. Nevertheless, the internal jugular, representing the anterior cardinal, is the primary jugular vein of vertebrates. The external jugular vein is its secondary derivative, which does not become definitive in man until after horizon xx (ca. 20 mm.; stage 6); at the early human stage under consideration, it is represented only remotely by a ventral plmryngeal vein (fig. 2) draining the prominent mandibular and hyoid pharyngeal bars, from which the face and neck will he constituted. As indicated in figure 2, the pharyngeal vein primarily joins the common cardinal vein after coursing over the top of the heart, but its stem soon “migrates" " cranially to the anterior cardinal vein. At either position of exit, it is identified with the linguofacial vein (figs. 19-22, pl. 1), a “morphological constant" in vertebrates (F. T. Lewis, 1909). Although the vein is primarily a tributary of the internal jugular, it is eventually taken over, entirely or in part, by the exterior jugular vein at a much later stage; this secondary annexation occurs to the greatest extent in the many mammals in which the internal jugular dwindles in favor of the later external jugular (pl. 6). STAGE 3. E.\unn’os or HORIZONS XV, XVI (6 T0 12 MM.) Externally, the most striking advance of stage 3 is the expansion of the frontal and maxillary regions relative to the mandibular and hyoid bars, together with the elongation of the limbs and the development of s. definite hand plate. The optic stalk and cup, closed lens vesicle, and deep nasal pit characterize these embryos. The regularity in form of the pharyngeal bars has been changed by the marked growth of the maxillary process, the elevation of the auricular hillocks, and overgrowth of the third (glossopharyngeal) bar in the formation of the cervical sinus. Lateral evagination of the cerebral hemisphere has clearly differentiated the telencephalon from the diencephalon, and the growth of the cerebellar plate is beginning to produce the pontine flexure. Although most of the arteries of the brain are represented, including the recent basilar artery and the first stages in the formation of the vertebral artery, the adult venous pattern is not recognizable (fig. 3).
3 The head—vessels in this embryo as illustrated by Strceter (1942) are schematic for the purposes of his communication, and do not agree with the present figure.
Elaboration of Dural Channels Joining the Head-Sinus: Initial Pia—arachnoidal Tributaries
The anterior dural plexus is now considerably elaborated, in keeping with the differentiation of the more cranial parts of the brain. Its foremost component is the primitive margirml sinus (Markowski's “marginal vein,” see p. 104), which borders the craniodorsal margin of ‘Streeter (1918, p. 26) fully described the way in which vessels are shifted in the primordial plexuses, and the initiating factors of such “migration of veins,” “spontaneous" as opposed to “passive." A term he used for the former process, anaxtomotic progression, is particularly descriptive.
Fig. 3. Stage 3. With lhc development of thc dienccpliztlon, the anterior (lural plexus is clalmratctl; its component marginal sinus encircles the tclcnccpliztlon, expansion of which hrings a (liL‘l'lCL'pl111llC pia—araclmoi(la1 vein into View (cf. .-\I and B). A similar vein from the ITl}’L‘lL’I'lCC[)lI(ll0l'I cmcrgcs (cf. A1 and ll). as the licatl-sinus migrates lateral to the 10th ncrvc (A), thus defining the upper end of the primitive internal jugular (anterior cartlinal) win. From the choroid fissure :1 tributary (future central vein of retina) joins the prominent maxillary win; the stump of a primitive supraorbital vcin is elongated in stage 4 (B).
the emerging cerebral hemisphere from the mid—line region, and includes elements of the future superior sagittal and transverse sinuses. Another component of the anterior dural plexus borders the caudoventral margin of the hemisphere, and may be called a telencephalic vein, because it drains the primordial striatal (nuclear) region; being dural, however, its stem represents the future tentorial sinus (pl. 1). A medial tributary of the anterior dural plexus traverses the primitive arachnoid layer. It is the stem of an initial pial vein, the ventral dicnccplzalic, an augmented remnant of one of many vessels, short and laterally directed, that connected the pial and dural layers of earlier stages. The vein is now visible because the anterior dural plexus has become further removed from the neural tube owing to telencephalic expansion (see stage 4).
Although the middle dural plexus and its stern, draining the metencephalon, are larger and better defined, a more notable alteration in stage 3 concerns the stem of the posterior dural plexus at its junction with the headsinus and the primitive internal jugular (anterior cardinal) vein. The head-sinus has moved laterally to the 10th nerve, a development foreshadowed by the venous ring around the nerve in stage 2 (fig. 2); this secondary anastomosis takes place dorsocranially to the distal fibers of the nth nerve, leaving the toth nerve to enter the primordium of the cervical muscles. As a result, the nth nerve is deviated around the cranial border of the headsinus before proceeding caudolaterally, and lies just above the entrance of the sinus into the primitive internal jugular vein (fig. 3). The lateral migration of the head—sinus is accompanied by a caudal migration of the posterior dural stem (cf. figs. 19, 20, pl. 1), which thus becomes directly continuous with the primitive jugular vein and will constitute the caudal end of the future sigmoid sinus.
After the permanent lateral position of the head-sinus is established, the more medial and cranial part of the venous ring around the 10th nerve, noted above, does not disappear. Since this part was derived earlier from the primordial hindbrain channel (“vena capitis medialis”), lying directly upon the brain wall, it is in position to receive a definitive ventral myelcncc-p/mlic vein, more recently proliferated from the primordial channel (cf. fig. 3A1, B). The stem of this pia-araehnoidal vein, lying between the roots of the 9th and roth nerves, is significant because it much later gives origin to the inferior petrosal sinus. The identical position, relative to these nerves, of the caudal end of the adult sinus, which is unusual in lying outside the skull (below the jugular foramen), is explained by its derivation from this primitive vein extending from the pial to the dural layer (see stage 7).
Primitiz/e Internal Iugular Vein The anterior cardinal vein may now properly be called the primitive internal jugular vein, since the apparent descent of the common cardinal vein to the level of the 7th or 8th cervical nerve is beginning to define the neck region (cf. figs. I9, 20, pl. 1). During this descent, the cranial end of the primitive jugular vein migrates from the medial to the lateral side of the 10th to 12th nerves. Such alterations are not foreordained by the adult configuration but are determined by adjacent structures. As Streeter (1918) wrote, embryonic channels should not be thought of as busily engaged in building mature vessels, but as carrying on their functional activity in the best manner possible for the moment with regard to the available space and the amount of work to be done.
A good example of the principle underlying the developmental alterations of embryonic vessels is the lateral migration of the internal jugular vein and the head-sinus. Sections of embryos of stage 2 show that the head—sinus, although appearing large in diameter in profile reconstruction (fig. 2A), is actually flattened into a crescent (fig. 4, insert) by the prominent inferior (nodosal) ganglion of the vagus around which it is detoured. By stage 3 (fig. 3A), the primitive internal jugular also appears to be subjected to mechanical compression in the wedge between two converging nerve roots. The separate roots of the 12th nerve unite, as they pass lateral to the primitive jugular vein, into a trunk which is closely applied to the lateral margin of the 10th nerve (at the caudal border of the nodosal ganglion), where these nerves are surrounded by a common fibrous sheath in the adult. lust cranial to this juxtaposition, in embryos of this period, a marked compression 5 of the jugular is effected by the 10th nerve in front and the 12th behind (fig. 4, insert). In one embryo of age group xvi (Carnegie no. 792), which is certainly atypical, if not abnormal, with reference to the head—veins, the 12th nerve is applied, not only to the lateral, but also to the caudal, border of the Ioth nerve for a considerable distance; the primitive jugular vein, although conspicuous above and below, is not identified at all in this area. Subsequently, in stage 4, the confined position of the internal jugular vein will be overcome by its migration to the lateral side of the nth nerve (fig. 5). The initial juxtaposition of the 10th and 12th nerves also appears to block the upward migration of the linguofacial vein on the jugular (fig. 3). Figure 4 shows a detail of some of the lateral anastomotic channels by way of which the jugular vein and its 5Good sections of \vell preserved embryos show an amazing integrity of the vessel walls consisting of but a single entlothelial layer; even in channels empty of primitive red cells, their original contour seems to be smoothly maintained by the mesenchymal strands, including the loose mesh of the primitive piaarachnoid.
linguofacial tributary shift into a less restricted position outside these nerves.
In the preceding stage 2, the arm bud is drained by a plexiform tributary of the t/zomcoepigastric (lateral thoracic) z/c>:'n, which joins the posterior cardinal vein (fig. 2.) The arm in stage 3 is in a more cranial position, and the hand plate is drained by a vein along its superficial border, the so—called marginal vein, which joins the r l"l ya I e 1'1. ,___g ;\ ‘right side) CL =" ment is coincident with the formation of a definitive upper jaw by differentiation of the maxillary process, and includes growth of the lateral (nasal) rim of the nose, which places the primitive nostril (nasal pit) in a more medial position. Parts of the future external car can be recognized in the hillocks surrounding the hyomandibular groove. A distinctive advance is the appearance of finger rays of the hand plate.
Fig. 4. Stage 3. Details of embryo Carnegie no. 3335, 3.3 mm., xv. Note the secondary anastomoses between vessels lateral to the nerve roots, by means of which the parent primitive internal jugular vein, compressed between nerves (insert), “migrates"* to its adult position. Similarly, the linguofacial vein, accompanying the Lltll nerve, is migrating cranially on the jugular to become lateral to the nerve. The right otie vesicle is slightly more advanced than the left, which is still attached to the skin over a wider area; the right head-sinus is notably larger than the left.
pr.r'nz:'r:':2e ulnar vein (fig. 3). The common stem formed by the junction of the ulnar and lateral thoracic veins, having now moved cranially upon either the common or anterior cardinal (internal jugular) vein, may be called the prinzizive mbclauian at-irz.
Stage 4. Embryos of Horizons XVII, XVIII (10 to 16 mm)
At stage 4, called the “postbranchial phase" because the row of pharyngeal bars homologous to the branchial arches of fishes no longer exists, the hyoid bar is more prominent than before, and the mandibular bar persists in its entirety as the lower jaw (fig. 5). This develop The more caudal parts of the chondrocranium outlining the base of the future skull are represented in horizon xviii by the basioccipital plate surrounding the notochord, by a dense area in the position of the sella, and by the bilateral otic capsules. The prominent condensation of mesenehyme called the membranous skull radiates dorsally from these primarily separate cartilages. It clearly defines the contiguous dural condensation, which contains the prirnary head-sinus with its three dorsal tributaries draining the three dural plexuses, as defined for stage 2. Particularly ventrally, the dural layer has become widely separated from the pial layer on the brain wall, in which lie the emerging pial veins and the definitive arteries (fig. 6); the intervening space. a relatively \vide layer of loose mesenchyme, is the primitive arachnoid.
Fig. 5. Stage .;. I".x1»umim1 nf lhc futuru hk‘11liS1!hLTL‘5 of the iuruin clcurly wp:1;';1:cx' thc duml and pin] l;1y('1's of vcnuus ch;11111u1.~. C0l‘ISCqL1L'nl]}-‘. the mnncrnus :1n:1summ.<us Lr.':\'crsing Lhc primitive }vi:n-Ln’-.1chm:id lrcg.:in to Llccrvnsc, and can thus he idcmificd: Uflcn at lcast one such lr:1ns\'r:rsc vain for each division of the hmin is xccn in older cxnbryns of this stage (B: cf. fig. (2). Such pi;1-:1r:1cl1noidal veins hccnmz: cnnncctcd by lnn_s;i1udin:1I :1IT.15»tn111uSt‘H (H). surcccdcd by tr:1ns\'crsc ;u1;\.~‘I<'nno\‘cs lwt\\'ccn Llzcm (cf. fig. SB).
thus initiating f0r1n:U.ion of the pinl vcnous plexus and definative veins.
Except for the clear demarcation of the three meningeal layers, stage 4 is not notable for definitive changes The only prominent ventral tributary of the head-sinus of earlier stages, namely the primitive maxillary vein, borders the primitive cartilaginous sella laterally. In keeping with the differentiation of the upper jaw, its tributaries are voluminous. They include a vein from
Fig. 6. Stage 4. Embryo Carnegie no. 492 (t(i..‘~f mm.. late xviii, ink injection). The insert shows the largest extracranial veins and the major components of the primitive dural channels (cf. fig. 5), which are omitted in the tnain picture above to permit view of their tributaries. At least two laterally directed (transverse) veins of the pia-arachnoid, which are primary, leave each division of the primitive brain. Their most distal tributaries, in part joined by secondary pial anastomoses. pass subjacent to the definitive arteries (for iden tification of the arteries, cf. Padgel, 1948, Figs. 5a, 7.1).
in dttral channels (figs. 2o, 21. pl. 1). The head—sinus, however, is paralleled dorsally by plexiform elements of a channel that will soon connect the anterior, middle, and posterior dural plexuses dorsal to the 5th nerve root and the otocyst (fig. 5A); its significance will be clear in the next stage.
the choroid fissure in the caudoventral aspect of the elongated optic stalk; hence, the future central vein of the retina is probably already represented (sec stage 7). This vein, now subordinate, represents the optic drainage to which the primary maxillary vein of stages I and 2 was restricted, i.e. the exclusive drainage of the optic vesicle and, later, the lens region (figs. 1-3, 5). By stage 4, the maxillary vein receives a medial nasal tributary from the region of the olfactory pit and nerve, but it is the lateral tributaries that are most conspicuous; they ramify extensively in the regions lateral and caudal to the maxillary branch of the 5th nerve, i.e. the bulk of the upper jaw and the primordia of the pterygoid and temporal muscles (fig. 5).
Although drainage of the optic region before this stage has been exclusively through the voluminous primitive maxillary vein caudal and ventral to the eye, a smaller but well defined vein now comes from the superficial tissues cranial and dorsal to the eye. VVith the exception of its caudal end, this pririzitive .rupra0rbz'tal vein, coursing between the frontal nasociliary branches of the ophthalmic nerve, not only becomes the stem of the definitive vein, but will also constitute the major part of the adult so-called superior ophthalmic vein. The caudal end of the primitive vein entering the head-sinus or its anterior dural stem is temporary; it is dorsal and lateral to the junction of fibers between the 4th nerve and the Ophthalmic division of the 5th (fig. 5A). Although this relationship remains constant for several stages, it will finally be changed during the emergence of the adult common stem of all ophthalmic and orbital veins in stage 7.
During stage .-.j, the definitive irztcrmzl jugular vein completes its migration to the lateral side of the hypoglossal nerve trunk; embryos of horizons xvii and xviii show successive steps in this transposition, the nerve often passing through a hole in the vein (fig. 5). At the same time, the proximal (jugular) end of the linguofacial vein is freed from its previous confinement at the juxtaposition of the IOtl‘l and 12th nerves; by secondary anastomotic channels (fig. 4) this vein shifts to its permanent and less-fixed position, either lateral or just cranial to the 12th nerve, which swings medially to enter the tongue primordium. The descending branch of the hypoglossal nerve is now joined, as in the adult, by fibers of the 2d and 3d cervical nerves forming the cervical loop (ansa hypoglossi). These findings readily explain the adult variations in the relation of all these nerves to the jugular vein (see Grant, 1951, fig. 516), including reported occurrences of double internal jugular veins. The lateral migration of the jugular and its linguofacial tributary, the adult common facial vein, appears to be the result of its relative immobility in early stages, when it is compressed by the connections of the roth and 12th nerves and sometimes by the upper cervical nerves.
Principle: Governing the Formation of Vein: Draining the Neural Tube
Stage 4 of the present series affords the first opportunity to see how the pia-arachnoidal veins are formed. Ini * tially, the primitive capillary network on the surface of the neural tube is drained, by way of numerous and relatively short veins near its dorsolateral border, into the adjacent ramifications of the more or less continuous anterior, middle, and posterior dural plexuses. As the cerebral hemisphere and cerebellar primordium enlarge, and as the otocyst with its cartilaginous capsule expands, the head-sinus with its dorsal tributaries, the dural plexuses, are carried laterally, thus elongating their medial tributaries, the veins from the pial layer. Hence, a “cleavage of blood vessels” (Streeter, 1918) results in the sense that most of the numerous veins once connecting the primitive dural and pial layers are lost (Padget, 1956, figs. 1, 24, 27). The few elongated and augmented veins that remain, and are directed laterally (transversely) for some distance, can consequently be identified (fig. 7; see also p. 105). Definitive veins of the pial layer are formed by secondary anastomoses between the pial tributaries of the primary transverse veins coursing through the arachnoidal layer. The stems of two of the primary pia-arachnoidal veins were identifiable in stage 3, namely the ventral diencephalic and myelencephalic veins (fig. 3). The former is usually the more easily seen as it traverses the loose mesenchyme representing the arachnoid; it is well shown in a transverse head section of this stage by Markowski (1922, text fig. 2), who called it the “inferior” diencephalic vein. In some late embryos of stage 4, and more readily in stage 5, at least one pia-arachnoidal vein can usually be identified for each of the five regions of the primitive brain: the tclenccp/zalic, nzcxcnccp/zrzlic, and metenccp/zalic veins, in addition to the veins from the diencephalon and myelencephalon, identified in stage 3 (figs. 3, 5, 6). Since these definitive veins emerge as augmented remnants in the reduction of many short pial-dural connections, variation in their position and number is inevitable.
An excellent embryo of horizon xviii (Carnegie no. 492, 16.8 mm.), which is injected with ink, shows fundamental details in the formation of the veins of the piaarachnoid, as seen in figures 6, 7B, and 8B, lateral, coronal, and basal views, respectively. Together with an embryo somewhat younger (fig. 8A), this embryo reveals pertinent details most clearly in the basal view, including the basilar artery (fig. 8B2), description of which follows.
The actual or approaching anastomoses between the pial tributaries of the separate primary groups of piaarachnoiclal veins, named above (shown diagrammatically in fig. 513), are seen particularly well in the reconstructions of embryo Carnegie no. 492 (figs. 6, SB). Essentially, the secondary anastomoses are directed longitudinally and in line with each other, and thus parallel the basilar artery bilaterally. Secondary formation of such pial anastomoses furthers the reduction of the primary anastomoses, once multiple, between the pial and dural venous layers, a reduction that brings the regional pia—arachnoidal veins (telen- to myelen-cephalic) into view, subsequent to their elongation. In the present embryo, the transverse pia—arachnoidal veins have been reduced in most instances to two veins that are identifiable for each region of the brain.
Steps in the further reduction of the pia—arachnoidal veins, i.e. to essentially one regional vein in each instance, are particularly indicated in this embryo by the metencephalic group of veins, including their bilateral asymmetry (fig. 8B2). On each side, this group constitutes essentially two pia—arachnoidal veins, one in front of and one behind the 5th nerve root; their stems join chiefly the stem of the middle dural plexus on each side. On the left side of the embryo, the more cranial metencephalic vein is the favored of the two, being the larger; by way of the secondary longitudinal pial anastomosis noted above, it has annexed the pial tributary of the more caudal vein, the stem of which is about to disappear, leaving one ventral metencephalic vein, a significant vessel (see stage 7a). On the right side of the embryo, in contrast, the secondary longitudinal veins of the metencephalic pial layer drain more into the primary metencephalic vein caudal to the 5th nerve than into its cranial counterpart; they also join the head—sinus medial to the nerve. Such asymmetry shows how variations occur in the reduction of the pial-dural anastomoses, primarily numerous.
In certain areas of the hindbrain wall, further secondary pial anastomoses that are transverse, i.e. pass across the mid-line beneath the basilar artery (see below), have begun to connect the secondary longitudinal pial anastomoses formed earlier (fig. 8132). In this way the venous net and the main pial veins of the cord, medulla, and pons are formed. The steps just outlined are similar for all the cerebral veins, including, in particular, the important basal cerebral vein. Components of this vein, which is not recognizable until stage 7a, involve secondary pial anastomoses cranially that are similar to those just described for the hindbrain region.
Another basal view of this embryo (fig. 8B1) includes the forebrain, internal carotid artery, and internal jugular vein. Pial tributaries of the mesencephalic veins are anastomosecl with those of the diencephalic veins, two of which persist on each side (cf. figs. 6, 8). The ventral diencephalic vein, much better developed than it was in stage 3, lies on the primitive hypothalamus; it is dorsolateral and subjacent to the posterior communicating artery, in the position of the later basal cerebral vein, of which it is a component; it still empties, as earlier, into the head-sinus. Pial tributaries of the diencephalic veins are anastomosed with those of the lateral tclencep/zalfc (adult superficial middle cerebral) vein—the first stages in the formation of the medial tclenccp/zalic (deep middle cerebral) vein, which connects the adult basal and middle cerebral veins. On the right side of the embryo, the ventral diencephalic vein empties chiefly into the stem of the anterior dural plexus, a typical arrangement before stage 6 (figs. 21, 22, pl. 1).
Relation of pial vein: to the arteries.
The present study, based on many embryos of the previous report on the cerebral arteries, facilitated observations on the relation of veins and arteries to each other, a subject that apparently has received little if any comment in the literature, in reference to either embryo or adult. As for the adult brain, it is well known that the largest veins on its external surface are superficial to the arteries, and that arteries and veins in general do not accompany each other. Such large veins obviously are those immediately derived from the primitive dural plexus, specifically from channels in the inner dural layer that are the proximal ends of the primary veins traversing the arachnoidal layer (see below). A publication by Scharrer (1940) brought out an important principle regarding the extracerebral tributaries of the largest cerebral (terminal) veins just noted. His work includes beautiful photographs of the surface of the cerebral convolutions of the adult opossum and rhesus monkey, in which the arteries and veins were injected separately with different colors demonstrable in half-tone photography. These pictures show the pial arteries and veins to comprise two separate precapillary networks, one lying over the other (see also Padget, 1956, fig. 24). It can be seen (as Scharrer notes in the legend to his fig. 2) that the network of veins, including some veins of relatively large size, is not superficial to the arterial branches, as might be supposed, but rather lies between the arterial network and the brain wall.
The fact that pial veins typically lie medial to the pia—arachnoidal arteries is probably contrary to the general impression, as was exemplified indirectly by Scharrer's discussion. His injections (starch grains, which do not go beyond the capillaries) proved that arteries had been mistaken for veins, and vice versa, by Pfeifer (I928, I930, detailed studies on the adult angioarchitccture of human and cat brains; see also Solnitzky’s report on the monkey, I940); Scharrer’s finding confirmed Campbell (1938), who had made the same observations in regard to Pfeiffer’s reports. The author examined the cerebral convolutions of several human brains (newborn) with an excellent natural injection, which showed repeatedly the same relation of arteries to veins that was demonstrated by Scharrer for other mammals. Such evidence was supported by observations in all the embryos of the present series, beginning with the stage under discussion.
In reference to Scharrer’s report, it is also interesting that, even before there is any difference in thickness of
Fig 7. ;. Stages 3, 4, 6. , reconstructed from coronal sections (same magnification): .-\x, 2. Carnegie no. 1|2r. 11.9 mm., xvi, stage 3; I5, no. 49:. 16.3 mm., xviii, stage .3; C, no. 966, :3 mm., xx, stage 6. The anterior duml plexus, from which the sngittnl zmtl straight sinuses urn: derived, drains to the right sigh; predominantly‘, in all. In I! and C the tr:1ns\'er5:: pi.1—:tr.1chm)it\:11 veins 1eu\':: the brain wall sul)i;tceI1t to the arteries and cross them at a right angle. The greater depth of the mesenchyxnc on the right in A and B (see p. 105) is apparently not an artifact.
Fig. 8. Stage 4. Basal views of two embryos (A middle, B late stage 4) reconstructed from transverse sections (same magnification). In A2 (Carnegie no. 940, 14 mm., xvii) and B2 (no. 492, 16.8 mm., xviii), the fore part of the brain and certain venous channels are eliminated to sho\v fundamentals of pial venous development. Note: the secontlary anastomoses forming the pial venous net and veins, also their pattern and position in relation to the definitive arteries; the way in which the multiple stems of primary pia-arachnoidal (transverse) veins, here reduced to about two from each region of the brain (telen- to myelen—cephalon), can be further re duced to essentially one outlet from each region. This reduction is possible subsequent to the secondary pial anastomoses, longi— tudinal and later transverse, between the initial pial tributaries.
cerebral arterial and venous walls, certain morphologic dififerences between pial arteries and veins can, nevertheless, sometimes be seen in the human embryo of about horizon xx. As Scharrer noted in detail regarding adult intracerebral vessels, there are more arteries than veins, but veins have more tributaries than arteries have, and anastomose with each other less (Padget, 1956, fig. 24).
To return to stage 4, the embryo of figure 8B2 (xvii, 16.8 mm.), showing the base of the hindbrain, is to be compared with figure 8A2, which shows the same region in a younger specimen (xvii, Carnegie no. 940, 14 mm.). In both, the basilar artery is paralleled by the bilateral longitudinal veins that have just developed from pial anastomoses between the transverse myelencephalic, metencephalic, and mesencephalic pia-arachnoidal veins as described above. In embryos preceding stage 6, particularly those with a notable congestion of the vessels (e.g. Carnegie no. 940, which is atypical; fig. 8A; cf. Padget, 1948), it may be difiicult to distinguish parts of certain cerebral arteries from veins: their walls as yet do not differ in thickness and consist of a single layer of endothelial cells; furthermore, such vessels are often in contact when crossing each other. In early stages, therefore, arteries and veins can be identified with certainty only by tracing each from beginning to end; in the present study both were reconstructed in every case. Knowledge of the principles of their interrelation facilitates the task of distinguishing between arteries and veins and should help to clarify the interpretation of vascular findings in other vertebrates (e.g. the chick, Feeney and Watterson, 1946).
One rule about primary vessels is that a vein and an artery cross each other approximately at right angles (figs. 6, 8B2; Padget, I956, fig. 1). In this manner the bilateral longitudinal pial veins are crossed by the transverse branches of the basilar artery. Subsequently (horizon xviii and later), the secondary pial venules that cross the mid-line are perpendicular to the basilar artery (fig. 8132). Another rule is that the pial veins lie between the arteries and the brain wall. Such basic relations of arteries to veins are also shown in a model by Markowski (1922, fig. 12, pl. 6), in which the bilateral longitudinal (“basal metencephalic") veins are overlain by the transverse branches of the basilar artery.
In other regions of the brain, during this and later stages, the same pattern of primitive veins, including their crossing the arteries at right angles, is observed. For instance, the pial tributaries of the diencephalic veins cross beneath the two choroid arteries, anterior and posterior; those of the mesencephalic veins cross beneath the mesencephalic arteries (figs. 6, S, tr; Padget, 1956, figs. 24, 26). The only veins that are superficial to the arteries, as is shown by Markowski and the accompanying figures, are the terminal pia-arachnoidal veins (telen to myelen-cephalic), which, now or earlier, pass laterally away from the neural tube. In most parts of the primitive brain, by late stage 4, these veins traverse a relatively wide layer of loose mesenchyme, the arachnoid, to enter the primitive sinuses in a layer of condensed mesenchyme, the dura. (These layers at the 40-mm. stage are not clearly demarcated by the arachnoid membrane and subdural space.) Hence, the largest veins on the surface of the adult brain belong more to the dural than to the pial layer, and are superficial to the arteries.
In consideration of the primary relations of vessels, confusion must be avoided regarding the relative position of the primary telencephalic veins and arteries as they appear in most of the reconstructions (figs. 6, 8, II; pls. 4A2, 5C1). Though not so readily apparent, the situation is the same. The telencephalic vein drains the primordial striatal region in stages 3 and 4 (figs. 3, 6), by way of inconspicuous short remnants of primary pialdural anastomoses. It never has the obviously transverse course of other pia-arachnoidal veins, e.g. the diencephalic, because of the lesser depth of the primitive arachnoidal mesenchyme over the cerebral hemisphere, the most superficial part of the brain. Furthermore, owing to early cerebral expansion in all directions, the elongating stem, or stems, of telencephalic veins are always in intimate contact with the dura. These veins are directly continuous with the tentorial sinus to be described below, and become the superficial group of middle cerebral veins. In contrast, the deep group of middle cerebral veins, to be enclosed within the late fetal Sylvian fissure, constitutes secondary pial anastomoses not clearly identifiable until stage 6 or later. They are the pial middle cerebral veins, which ramify beneath the proximal ends of the middle cerebral arteries and the brain in the anterior perforated area.
The largest cerebral veins on the surface of the postnatal brain, including not only the superficial middle cerebral veins but also the immediate tributaries of the superior sagittal and transverse sinuses, are well known to be superficial to the cerebral arteries. For the reasons noted above, these veins are often more or less intimately attached to the inner dural surface for some distance before they enter the sinus (Bailey, 1948). Only their more distal tributaries that form the smaller veins and the precapillary net on the surface of the convolutions lie beneath the arteries and their network of branches, as is shown for other adult mammals by Scharrer (1940; see fig. 24 in Padget, I956). It is presumed that the relation of the pial arteries and veins just outlined is the same in all mammals, and probably in all vertebrates. The only direct evidence known to the author, however, is incidentally shown by Shindo (195) for a mouse embryo; here the “collateral cerebral vein” (comparable to the ventral diencephalic or basal cerebral vein) lies next to the brain wall and is medial to two branches of the “cerebral carotid artery.” It is interesting that the dural meningeal arteries and veins also have the same relation to each other in respect to the structure vascularized, the membrane bones (pp. 127, 35).
Speculation on the reasons for the primary relation between arteries and pia-arachnoidal veins is inevitable. The primitive pial veins undoubtedly represent endothelial material not utilized by the arteries, which are developed earlier than the veins from the primordial capillary net covering the neural tube (stage 2 and earlier; p. 83), inasmuch as the arteries are elevated from the primordial net as they become definitive. This sequence of development seems to explain the pattern of veins crossing beneath arteries at right angles, as is illustrated diagrammatically in another publication (Padget, 1956, fig. 27).
While the many primary pial-dural anastomoses are being reduced in number to result in definitive veins traversing the arachnoid layer, they appear to be “stretched," owing to the fact that the dural layer is continually being shifted laterally by the expanding brain and related structures. Since the main arteries of the cranial region are centrally located from the beginning, their branches are not subject to such “pull” between origin and destination. Although both arteries and veins of this region are little more than endothelial tubes before stage 7 (see below), the arteries are much better defined than the comparable veins; figures 6 and 8 show that many definitive branches of the internal carotid and basilar arteries have formed by stage 4, in contrast to the rudimentary (plexiform) venous configuration. The decrease in the number of primary transverse veins from pia to dura characteristic of venous development (Streeter, I918; Padget, 1956, figs. I, 26) initiates the formation of well defined pial anastomoses between the tributaries of those that remain. During the increasing separation of pial and dural layers, this secondary formation of pial veins might be facilitated because the primordial pial network is held in place on the neural tube, particularly before its penetration by blood vessels (under way in stage 2), by the relatively precocious arteries supplying an elevated, and thus overlying, arterial network.
It is impossible to consider the interrelation of the pial veins and arteries without speculation on the formation of congenital arteriovenous aneurysms of the brain (see discussion in Padget, 1956). In view of the actual close contact of certain primitive arteries and veins, with walls of endothelium only—not until stage 7 are the walls of cerebral arteries definitely thicker—such anomalies might be expected to occur more often. Is it possible, for instance, that the typical crossing of arteries and veins at a right angle is a factor in preventing anomalous connections as long as certain aspects of the blood flow in both are normal and the integrity of the thin walls is maintained? In the injected embryo of horizon xviii (Carnegie no. 492, fig. SBI) described above, the right internal carotid artery is crossed by a component of the ventral diencephalic vein that is about to lose its connection with the head-sinus. This vein crosses the artery at a right angle in two directions—in fact, is closely wrapped around it.
Stage 5. Embryos of Horizon XIX (16 to 21 mm)
At stage 5, development of the eye, nose, jaws, and ear has reached the point at which the human face is first recognizable (fig. 9). The cerebral hemisphere and cerebellar plate have expanded notably, and this growth, together with the enlargement and differentiation of the otic capsule, is responsible for conspicuous changes in the veins of the dural layer. The venous system, however, is far less advanced than the arteries of the brain, many of which are already definitive (cf. fig. 6).
The emerging pial veins with their arachnoidal stems, described for late embryos of stage 4, may be dismissed briefly since no fundamental changes are apparent. Some of them can more readily be identified, however, because their stems that pass laterally through the primitive arachnoid mesh toward the sinuses have elongated (fig. 9A1), owing to expansion of the cerebral hemisphere and, to a lesser extent, of the cerebellar plate (pl. 2). Such expansion increases the distance between the neural tube and the dural layer, particularly at the diencephalon and mesencephalon. At least five of these veins, one (or more) for each subdivision of the embryo brain, telencephalon to myelencephalon, can sometimes be identified at this time (fig. 9A), and are illustrated in an injected embryo of late stage 4 (fig. 6).
Involution of Primary Head-Sinus, Paralleling Formation of the Sigmoid Sinus Elements of a new longitudinal channel, which connected the stems of the anterior, middle, and posterior dural plexuses dorsal to the nerve roots and otic capsule at stage 4 (fig. 5), are now definitely consolidated (figs. 22, 23, pl. 1). The conspicuous and continuous sinus thus formed is parallel and dorsal to the primary heatisinus, which is dwindling below the otic capsule and appears, in fact, to be compressed between it and the adjacent distal part of the 7th nerve. The new dorsal sinus is obviously being substituted for the more ventral head-sinus in the role of conveying blood from the brain into the internal jugular vein. The part of the secondary anastomotic channel between the middle and posterior dural plexuses, together with the main stem of the posterior dural plexus which it joins, remains virtually unchanged in later stages and constitutes the definitive sary of mammalian development. Apparently, there is no definitive external jugular vein in either reptiles or birds, in contrast to the situation in mammals generally, wherein it becomes predominant (see below).
Primary Extracranial Veins A noteworthy advance of stage 5 concerns the emergence of definitive extracranial veins. The region around the primitive orbit, now demarcated by the developing chondrocranium, is drained by two orbital veins, which have become almost equally prominent (fig. 9A): the most recent one is craniodorsal to the eye, the other caudoventral, and both now include ophthalmic tributaries. Earlier and later, however, these veins differ fundamentally. Naming them presents a problem, because of lack of precise terminology for the adult orbito— ophthalmic veins and because of their complicated development (see stage 7 and pl. 1).
In stage 3, the vein traversing the dorsal orbital region emerges dorsal to the primordial ophthalmic division of the 5th nerve, and is well developed by stage 4 (figs. 20, 21, pl. 1). By the present stage 5 (or 4; fig. 12), the distal end of the vein is associated with the supraorbital (and, soon, the frontal) branch of the ophthalmic nerve in the region supplied by the supraorbital division of the stapedial artery. An ophthalmic tributary, probably representing a primitive dorsal (superior) vorticose vein, joins the dorsal orbital vein. Except for its most proximal end (at the head-sinus), which is temporary, the present dorsal orbital vein constitutes the stern of the adult supraorbital vein from the earliest stages; in addition, however, its intraorbital part constitutes the most con— spicuous adult vein among the superior ophthalmic group. In consideration of all the factors (see below), and granted that no simple name is adequate, the term prinzitivc uzprrzorbital vein is preferable to “ophthalmic" (e.g. Streeter, 19x8) for this embryonic vein.
More appropriately than in the case of the supraorbital vein, the stem of the vein traversing the ventral orbital region, namely the primitive maxillary, has also been called the “ophtbalmic" by various writers, including l\'I:1rl>{0\VSl{l (1922) and Thyng (1914), in reference to an embryo of stage 5. The maxillary vein of the maxillary process has emerged before stage 2, in the role of exclusive ophthalmic drainage, i.e. of the optic cup (or vesicle) and stalk (see stage 2). ln succeeding stages, however, its primary ophthalmic and later orbital tributaries become more and more disguised among the other voluminous and plexiform maxillary tributaries, particularly the components of the adult pterygoid plexus (see below and fig. 12); among them in stage 5 are included the primitive vcntral (inferior) vorticose and ciliary veins, and also the future central vein of the retina, represented as early as stage 2. Another primitive ophthalmic remnant, which is anomalous postnatally (p. 116), often persists in embryonic stages; it extends from the cranial cavity in company with the optic nerve, by way of the optic foramen (fig. 14133).
The primitive maxillary (infraorbital) vein reaches the height of its development in stage 5. Although it is the primary ophthalmic vein, as was noted above, it now shares drainage of the orbit and, to a lesser extent, of the eye with the augmented supraorbital vein. Its major territory has come to include the emerging nose and all of the expanded upper jaw (fig. 9). Figure 12 shows that its most medial tributaries are associated with the nascciliary branch of the ophthalmic nerve; the prominent lateral tributaries come from the primordia of the pterygoid and temporal muscles in close association with motor branches of the mandibular nerve. The maxillary vein, therefore, which was once confined to the maxillary process with the maxillary division of the 5th nerve, has begun to invade, cranially and caudally, the territory of the other two divisions of the 5th nerve (figs. 19-23, pl. 1). This development initiates the formation of the future anterior facial vein in stage 6. The lower jaw is still drained by the linguofacial vein, consisting of two main divisions. The medial tributary, as before, accompanies the nth nerve to the base of the tongue, and the larger lateral tributary now drains structures associated with the mandibular division of the 5th nerve. Figure 9 shows that a plexiform anastomosis between lateral tributaries of the linguofacial and maxillary veins has already formed around the outer labial margin. Among the many plexiform and more medial tributaries of the maxillary vein, the adult pterygoid plexus is already represented.
Modifications in tributaries of the internal jugular vein are seen in figure 9. In the preceding stages, the only vein from the arm was caudal or ulnar in position; this primitive ulnar vein joined the lateral t/zoracic (thoracoepigastric) vein to form the primitive subelavian trunk. An inconspicuous cranial tributary of this trunk in stage 3 (fig. 5) has developed into a prinzitiue cephalic z/c-in following the radial border of the arm; this vessel is continuous with the ulnar vein by way of the marginal vein outlining the hand plate. The proximal end of the new cephalic vein is not that of the adult, however, but curves cranially around the superficial aspect of the clavicular primordium (fig. 9). This arched part of the vessel, which is the temporary proximal end of the cephalic vein, has been called the jugulocep/zalic vein (e.g. F. T. Lewis, 1909), a name appropriate for three reasons: it emphasizes the temporary cervical detour of the primitive cephalic vein, identifies the segment which usually disappears after the permanent infraclavicular relocation of the cephalic trunk, and suggests the imminent external jugular vein which will adopt the discarded postclavicular part of the cephalic vein as its terminal trunk.
The plexiform vertebral vein has now been formed comparably to its companion artery (Padget, 1954)? It is seen (figs. 19-22, pl. x) that each cervical nerve is primarily accompanied by a cervical intersegmental vein (and artery), the most caudal veins joining the posterior cardinal vein. As elongation of the neck region separates the heart from the base of the cranium, the lower cervical nerve roots appear to rise to the level of the common cardinal vein. Consequently, the proximal ends of the lower cervical intersegmental veins, like those of the arteries, become grouped closely together in migrating cranially to and upon the anterior cardinal vein (fig. 9). Now a secondary longitudinal anastomosis between the primary transverse intersegmental veins has produced a primitive vertebral vein, and most of the jugular ends of the intersegmentals have dropped out. The vertebral vein remains plexiform around its companion artery and empties into the internal jugular or the primitive subclavian vein, before the formation of the left innominate vein in stage 6.
In the adult the caudal end of the vertebral vein passes through the transverse foramen of either the 6th or the 7th cervical vertebra, or it may be bifid, traversing both. Its cranial end, together with components of the deep cervical vein, joins one or more of the veins around the foramen magnum: specifically, the primitive comlylar (condyloid) en2i.c.rar_v, a tributary of the sigmoid sinus; or, particularly if the condylar is absent, the prirnz'tirJe hypoglossal enzissary, which is a tributary of the primitive myelencephalic vein (future stem of the inferior petrosal sinus). These emissary veins. before the emergence of the external jugular system (stage 6), drain extracranial structures medially through their respective well defined foramina in the chondrocranium. Together with the primitive mastoid c-mt'5.rary 1/ez'n—its foramen, sometimes formed at this stage, is characteristic of stage 6—these emissaries will finally constitute a source of voluminous collateral circulation between the sigmoid sinus and the external jugular system of the adult.
Stage 6. Embryos or Horizon XX, XXI (18 to 26 mm)
At this significant stage in vertebrate embryology, the head has begun to lift off the chest, the expansion of cerebral hemispheres and cerebellar plate has brought the brain llexures to the height of their development, and the facial region is well defined (fig. 10). In the human embryo the fingers have appeared, and the arm is flexed 3The earlier study (I948) was supplemented because the modern substitution of the preferable term :’nter.rrgn:cr:tal for “segmental," in reference to the arteries and veins passing between the embryonic somites, had resulted in much uncertainty about the identification of the vessels involved in the formation of the vertebral and subclavian arteries.
at the wrist and elbow. Arterial development is particularly marked by the major components of the external carotid system, which emerged in stage 5. This system has annexed the three branches of the temporary stapedial artery (from the internal carotid), which are associated with the three divisions of the 5th nerve; in many mammals (e.g. the cat, Davis and Story, 1943), the external carotid takes over additional branches of the internal carotid, e.g. the ophthalmic, and finally becomes predominant to the primary carotid.
Similarly, the basic component of the external jugular system has now appeared—a system that will later annex most of the tributaries of the internal jugular vein, over which it is conspicuously predominant in many mammals. In man, although the tributaries of the primary head—sinus and, more variably, the primitive linguofacial (common facial) tributary of the internal jugular become anastomosed with the external jugular, they are only partly taken over by it. Apparently this stage is a crossroad in the development of craniocervical vessels, particularly the veins. From here on, in different species the growth of the brain relative to that of the face and neck determines which carotid and jugular systems, internal or external, will predominate in the adult; this concept is illustrated in plate 6.
Pro-otic and Tentorial Sinme: Two major primary sinuses, prominent throughout fetal life, become clearly defined at this stage. Their history explains the fate of the typical primitive channels they represent, namely the stems of both the anterior and middle dural plexuses (stage 2). As was described in stage 5, the stem of the posterior dural plexus is stable in contrast to the two stems just named; it is merely augmented to form the caudal end of the sigmoid sinus.
The head-sinus, which the three dural stems originally joined, has essentially disappeared in stage 5 with the exception of its short segment (lateral wing of the future cavernous sinus) medial to the 5th nerve. A small remnant, however, accompanying the 7th nerve extracranially and immediately ventral to the otic capsule (fig. 10A), must be emphasized for these reasons: it is the only remains of the primary drainage vessel of the head region, typical of vertebrates, which is permanently retained in this capacity in the pattern of reptiles (see stage 5); it must not be identified, as has been done for stage 5 and later (p. 124), with the primitive temporal emissary vein of the foramen jugulare spurium, the augmented counterpart of which becomes the permanent major exit for intracranial blood in many mammals (pl. 6). Study of the developing vasculature of the ear region showed that the caudal remnant of the head—sinus in stage 5 is incorporated into the veins accompanying the superficial petrosal and the stylomastoid arteries, which anastomose with each other in accompanying the intraosseous course of the 7th nerve (Padget, 1956).
Fig. 10. Stage 6. The superficial middle cerebral veins, particularly on the right (A1), now drain dorsally through the tentorial sinus into a component of the future transverse sinus; as a result, the anterior dural stem is dwindling. \Vith the disappearance of the caudal part of the head-sinus, the veins from the eye region drain dorsolaterally through the middle dural stem, now called the prootic sinus. Note (At) that the primitive sagittal and straight sinuses both drain more to the right side. The new anterior facial vein (cf. A and left side AI) is annexing lateral tributaries of the maxillary vein, and is about to anastomose with a cranial (external jugular) tributary of the jIIgulocephalic vein.
In the augmented stem of the middle dural plexus, the blood flow has presumably undergone a reversal, from the former head-sinus, which was ventral, to the new sigmoid sinus, dorsally situated. The stem now constitutes the pro-otic rirmr. Markowski (I911, 1922) called this prominent and constant dural channel the pro-otic “vein,” but Mall (I904) and Streeter (I918) thought it to be the superior petrosal sinus. Granting that the present position of this channel bordering the craniodorsal aspect of the superior semicircular canal (fig. 10) does suggest the latter inference, such an error is explained by a prevalent lack of information regarding the two channels thus confused: the prominent remnant of the pro-otic sinus as usually found in the late fetus or infant, and the medial end of the adult superior petrosal sinus (the part connecting it to the cavernous sinus), which is both secondary and inconstant. Although the most caudodorsal (lateral) end of the pro-otic sinus is in the position of the adult superior petrosal sinus, which it later constitutes, its greater part has a different course. This course is more cranial than dorsal to the crest of the otic capsule, and is extracranial in reference to the chondrocranium. Furthermore, the pro-otic sinus leaves the cavernous sinus ventral and medial to the trigeminal ganglion at the origin of its mandibular division (pls. I, 2). In contrast, the secondary medial end of the adult superior petrosal sinus, never in contact with the mandibular nerve, is usually formed dorsal and lateral to the 5th nerve root; the communication, moreover, between the superior petrosal and cavernous sinuses is a late secondary development (see stage 7a), which often does not occur until after birth, if at all, and is absent in mammals generally. The significance of the pro-otic sinus will be clear in later stages when it gives origin to the middle meningeal sinuses (dural veins), elements of which can already be seen lateral to the trigeminal ganglion (fig. to). The name pro-otic, as used hereafter, will include for simplicity the last remaining short segment of the primary head-sinus (“vena capita lateralis”) of stages I and 2. From this segment, which has always been media] to the trigeminal ganglion, the cavernous sinus is derived during stage 7.
Comparison of figures 22 and 23 (pl. 1) shows that the pro-otic sinus, unlike the former middle dural stern it represents, does not chiefly concern drainage of the brain. Instead, it is the direct outlet for two extracranial veins, the supraorbital (superior ophthalmic) and the maxillary. The reason for this situation is that the once voluminous stem of the anterior dural plexus has dwindled to an insignificant channel or has disappeared. The blood from the fore parts of the head and brain, which hereto fore drained ventrally through this stem into the headsinus, now goes dorsally into the lateral segment of the primitive transverse sinus.
Concomitant with the definition of the pro-otic sinus, another important embryonic channel, to be called here the tentorial .rirzu.r,° becomes prominent. This sinus, represented earlier in the anterior dural plexus by a channel that drains telencephalic pial—dural tributaries, has become longer and more conspicuous (pl. 1). It borders the caudoventral aspect of the cerebral hemisphere, and receives not only the superficial and deep telencephalic (middle cerebral) veins but also the ventral diencephalic vein. The tentorial sinus of stage 6 (fig. 10) was called the “tentorial vein” by Markowski (1911, 1922) and the “inferior cerebral vein” by Streeter (1918). It must be emphasized, however, that the channel is dural, not pial, in position. Lying just superficially to the 3d and 4th nerves, it accompanies them for some distance through the primitive tentorium, which is now represented by a relatively wide wedge of condensed mesenchyme separating the cerebral hemisphere from the cerebellar plate. The phylogenetic significance of the tentorial sinus will be discussed in stage 7a, after its subsequent elongation coincident with the expansion of the caudal part of the brain (cf. figs. 22, 23, pl. 1). For :1 long time, sometimes permanently, the sinus is the only drainage for the largest veins of the cerebrum; before the formation of the basal cerebral vein (stage 7a), it also drains the diencephalon and the corpus striatal region.
The venous pattern of stage 6 (fig. 10) indicates the fate of the three prominent stems of the primitive dural plC.\'tI5CS, leading to the head-sinus, which were formed in stages I and 2 (before 8 mm.). The posterior stem (sigmoid sinus) is permanent, and the middle one (prootic sinus) often persists throughout fetal life, leaving definitive postnatal remnants. In contrast, the stem of the anterior dural plexus is temporary and is usually dwindling or absent at stage 6. Streeter thought, however, that the anterior dural stem represents the adult communication, as commonly described, between the middle cerebral (tclencephalic) veins and the cavernous sinus (Streeter, I918, fig. 27, pl. 5; p. 29). His interpretation again reflects a prevalent failure to appreciate certain details of human and comparative anatomy: the middle cerebral veins of many adult mammals drain exclusively to the junction of the sigmoid and transverse sinuses, or their counterparts, through a channel that represents the present tentorial sinus; a homologous rctention of this arrangement is regularly found in the late human fetus or newborn and often in the adult (see stage 7a).
9 This must be distinguished from the postnatal tentorial sinus of Gibbs and Gibbs (1934), which drains cerebellar veins (see stage 7a, p. 133).
Only in general may it be said that the “middle cerebral” veins of the early human embryo empty by way of the anterior dural stem into the “cavernous sinus,” since this sinus will be derived in stage 7 from the primary head—sinus (pl. 2). This anterior stem later becomes anchored cranially by the sheath surrounding the juxtaposition of the 4th and 5th (ophthalmic) nerves (figs. 21-24, pl. 1). As such, it cannot represent the inconstant and secondary adult communication between the cavernous sinus and middle cerebral veins, which is lateral to the 4th nerve and borders the lesser sphenoid wing (fig. 39, pl. 2). Furthermore, at the present stage 6 the anterior dural stem usually has either dwindled considerably or has disappeared. The result is that the middle cerebral veins now drain dorsally, instead of ventrally, into the new primitive transverse sinus by way of the tentorial sinus.
The tentorial sinus must not be confused with the tentorial plexus, an appropriate name (Streeter) for the combined anterior and middle dural plexuses of preceding stages; from the former, the tentorial sinus was earlier derived (cf. figs. 20-23, pl. 1). Whereas the tentorial plexus is gradually reduced during caudal cerebral expansion and the elaboration of the major adult sinuses (pl. 1), and is finally represented by the adult torcular (confluence of sinuses), the tentorial sinus remains prominent throughout fetal life, and not infrequently later. Coincidently with the loss of the stem of the anterior dural plexus and the compensatory development of the tentorial sinus emptying into the primitive transverse sinus, a second forebrain vessel belonging to the tentorial plexus has come into conspicuous view, namely the marginal sinus. This channel, bordering the dorsocaudal margin of the emerging cerebral hemisphere, was identified in stage 3 (cf. figs. 20-23, pl. 1), but now appears to be the direct continuation of the primitive transverse sinus to the mid-line (fig. 10). Although called for simplicity the medial component of the transverse sinus, the marginal sinus is not a stable vessel, as was fully discussed by Streeter (1918): for some time, as the hemisphere expands, it will be replaced by succeeding anastomoses with more caudal loops in the tentorial plexus. By stage 6 the marginal sinus (“anterior marginal vein” of Markowski, 1911, I922) meets that of the other side in a plexiform anastomosis, named the sagittal plexus by Streeter, which lies between the cerebral hemispheres and constitutes the elements of the future superior sagittal sinus and its derivative, the inferior sagittal sinus. From now on, it will be simpler to refer to the more precocious and stable lateral part of the primitive transverse sinus and the shifting marginal sinus as one, namely the priniitive trans:/err: 5/7111:.
Asymmetry of Venous C/tunnels, Notably in the Tentorial Plexus (Torcular) Consecutive stages (figs. 20-23, pl. 1) show that the anterior and middle dural plexuses become consolidated in the condensed mesenchyme representing the tentorium, and together constitute a tentorial plants at stage 6. Typically, the tentorial plexus, which includes the sagittal plexus just noted, does not drain equally to the right and to the left sides. Instead, asymmetrical drainage is indicated by the relative size of the two transverse sinuses, specifically, of the marginal sinuses constituting their proximal (medial) ends: that on the left is often still notably plexiform, whereas that on the right is better defined and is the larger, because it receives most of the drainage of the primitive superior sagittal sinus (plexus)—a common adult pattern. A deep component of the sagittotentorial plexus is the future straight sinus, a continuation of the primitive internal cerebral vein, which is an irregular channel lying on the membranous roof of the diencephalon. This mid-line channel (fig. 10) drains the choroid plexus of both lateral ventricles at the interventricular foramina (of Monro) and, it should be noted particularly, also empties into the larger primitive transverse (marginal) sinus on the right. The configuration, in other words, is the opposite of that in a typical adult, in which predominant drainage of the straight sinus is to the left side. Streeter (1918) stated that the prinritiue sagittal sinus, derived from the sagittal plexus, drained more to the right side in 16 of 18 cmbryos from 17 to 80 mm. long. This configuration was noted by the author in most of these and in 9 other specimens within this range; furthermore, Streeter’s observation of left-sided drainage could not be confirmed in the 2 specimens so designated. He noted that no explanation had been advanced for this interesting asymmetry.
According to the present study, it appears that the tendency toward venous asymmetry at the torcular, which is evident even earlier (stages 3, 4, fig. 7), depends upon the developmental alterations of the great veins leading to the heart, as summarized in plate 3. Initially, these veins are almost bilaterally symmetrical (pl. 3A). By stage 2 (5 to 8 mm.), although the right common cardinal vein still empties directly into the right atrial section of the heart, the left reaches it somewhat less directly by way of a channel caudal to the heart tube. At first, this embryonic sinus venosus (represented by the adult coronary sinus) is relatively wide and short. Owing to the differentiation of the heart, it is soon forced to elongate; while also becoming relatively reduced in size, it takes a marked curve from the front toward the back of the chest before entering the right atrium (pl. 313). During and after stage 6 (horizons xx, xxi), the considerable subcardiac detour of the blood from the left side of the head can be counteracted to a variable extent by a secondary anastomosis—namely the formation of the left imzomimzte ac-in, which connects the two internal jugulars (anterior cardinals) cranial to the heart (pl. 3E). Initial stages of this new channel are seen earlier, at stage 5 (horizon xix), as a plexus of small veins in the mid-line just cranial to the arch of the aorta (pl. 3D). These t/zymicotfiyroid veins, right and left, which drain the corresponding primordia, empty into the internal jugulars near the level of the subclavian veins. As was noted by Anikew (1909) and by Thyng (1914) in embryos of stage 6, one of these vessels enlarges to become the transverse left innominate vein, which receives the inferior thyroid veins in the adult.
The secondary left innominate may eventually permit almost as direct access to the heart for blood from the left side of the head as that from the right; this, however, is not typical of man. The ultimate difference, anatomically, of drainage on the two sides conceivably depends upon the variable length and direction of the secondary left innominate vein in the adult (see pl. 6; Padget, 1956, fig. 23).
It was not possible within the limits of the present study to explore the occurrence of venous asymmetry and possibly related factors in any orderly or conclusive way, and the observations below are merely for the record. The tendency of the veins, particularly near the top of the head, to drain more to the right than to the left side was most obvious and consistent at stage 6 in the present series. \Vhenevcr a difference was seen in either younger or older specimens, drainage to the right invariably predominated (figs. 7, 10B). Furthermore, it was noted that venous development tends to be a little more advanced on the right in embryos before horizon xxi, as shown in figures 1, 4, 7, II, and 12.
Such observations raised the question whether there are any other dissimilarities on the two sides, especially in those embryos that definitely show the asymmetry in drainage. Essentially no disparity was detectcd in most cases, but, in a few, certain structures on the right side appeared to be somewhat more advanced than those on the left. Only particularly well preserved embryos, sectioned symmetrically and enlarged sufliciently in photographs for reconstruction, can demonstrate minor disparities, such as were detected in several younger embryos of this series. Here, the following factors provide a gross index to growth: (1) reduction of the opening of the otic pit to form the vesicle; (2) approximation of the optic vesicle to the skin stimulating the lens formation; (3) the relative size of these vesicles, of the nerve roots, and of the emerging maxillary process; and (4) the depth of the mesenchyme in general. In reference to such indices, the disparities found invariably suggested a certain lag of development on the left side, as shown in figures I, 4, and 7.
‘Whether or not structural asymmetry is related to that of the veins noted above, including patterns which permit the anterior dural plexus to drain predominantly toward the right side (fig. 7), is problematical. No comparable discrepancy in the arteries happened to be observed in any em bryo, although the arterial pattern frequently differs in detail on the two sides.
Modification and Reduction of Pia-arachanlal Vein: The intracranial venous system of stage 6 shows relatively minor advances in the cerebral veins, of which the conspicuous and constant ventral diencephalic vein is the most noteworthy. Expansion of the brain and elaboration of the sinuses described above have brought about not only an elongation and a change in direction of this transverse pia-arachnoidal vein, but also an apparent change in its dural exit, now the tentorial sinus (Figs. 10, 11). Fundamentally, however, the tentorial sinus is a part of the anterior dural plexus in which the diencephalic vein has terminated from its beginning in stage 2. As seen in the basal view of stage 6, the recent pattern has occurred only on the right side of the embryo, which is the more advanced from the venous standpoint (fig.10AI). On the left, although the telencephalic veins now empty into the tentorial sinus, the ventral diencephalic vein empties, as more primitively, into the stem of the anterior dural plexus, which is still large compared with that on the right. On either side, the ventral diencephalic vein now drains 11ot only the more ventral (liencephalon but also the voluminous choroid plexus of the lateral ventricle. In fact, this ventral choroid drainage is primarily more marked than the dorsal route through the primitive internal cerebral vein (fig. 10), as discussed for stage 7.
The ventral diencephalic vein is often augmented at stage 6 by a dorsal diencephalic vein (“lateral diencephalic vein,” Markowski, 1911, I922) from the more caudal, dorsal, and lateral aspects of the primitive thalamus (fig. 11). Other Findings include elaboration of the superficial lateral and deep medial telencephalic veins; there are often two (dorsal and ventral) mesencephalic and metencephalic veins. The stem of the mesencephalic vein (or veins), at its proximal junction with the tentorial plexus, usually disappears, as does that of the dorsal diencephalic at the transverse sinus after its tributaries are annexed by the ventral diencephalic vein, or vice versa. Similarly, the ventral metencephalic vein often annexes tributaries of the dorsal metencephalic, when present, to form one conspicuous stem (essentially the superior petrosal sinus of stage 7a), which empties into the prootic sinus at its junction with the sigmoid. The ventral diencephalic vein may remain prominent for some time, but finally (ca. 80 mm.) its stem of exit in the tentorial sinus is lost in most cases (pl. 5C).
Fig. 11. Stage 6. Embryo Carnegie no. 966, 23 mm., xx, showing the typical relation of the primitive pial veins to the definitive branches of the internal carotid and basilar arteries. Two (or more) pirt-arachnoitlal veins from each region of the primitive brain are often identifiable. Their number becomes reduced as one of :1 pair annexes another, resulting in :1 single stem (cf. Fig. 8), eg. the ventral diencephulic vein, which joins the tentoriztl sinus. Note the cliflerent patterns on the two sides (reversed for comparison with other figures): the stem of the left common facial vein, as primarily, joins the internal jugular (insert); on the right, the stem has been annexed by the juguloccphalic vein, a component of the later external jugular vein.
As was discussed in stage 4, and by Streeter (1918), cranial venous development is characterized by a diminishing number of channels draining the pial venous system into the dural system (see Padget, 1956, figs. 1, 26). The reader must not be confused, therefore, by the fact that more of these transverse connecting veins are often readily visible at stage 6 (18 to 26 mm.) than previously. Such duplication of the regional veins (telen- to myelencephalic), even though not always identifiable in earlier stages, is not a secondary development in a strict sense, but rather represents the persistence of primary vessels, which are secondarily visible, but are eventually lost in most instances. Many pial-dural veins, relatively short and small, exist originally near the dorsomedial margins of the dural plexuses. These veins are reduced as the dural plexuses become smaller in elaboration of the sinuses, and as the dural layer becomes more and more separated from the pial layer. Those veins that remain are rendered more conspicuous as they become larger and longer in traversing the arachnoidal layer. Thus, although their number has been diminishing, it may appear to be maximum at the present stage. Hereafter, the stems of most of these veins will also drop out after secondary pial anastomoses between them have formed the definitive pial veins—a process coincident with the gradual reduction of the primitive tentorial and posterior dural plexuses, which constitute the adult torcular and the marginal sinus of the foramen magnum, respectively (figs. 23-26, pl. 1).
Emergence of External Jugular System; Facial Vein: By horizon xx the chondrocranium is prominent at the base of the brain (VV. H. Lewis, 1920) and surrounds most of the cranial nerve roots; it is most advanced in the basioccipital and temporo-occipital regions, including the large otic capsule. Thus, the components of the intracranial venous system just described and the extracranial veins, which present notable changes in the developing face and neck, are readily distinguishable. Figures 21 to 23 (pl. 1) show that the new anterior facial vein has formed as follows: a prominent lateral tributary of the linguofacial vein has anastomosed at the outer labial angle with a posterolateral tributary of the maxillary vein, the stem of which is now considerably reduced in size. Caudal to the mandibular division of the 5th nerve, the anterior facial vein receives from the superficial temporal region a strong tributary representing the adult vein of the same name or, as it is sometimes called, the posterior facial vein. Hence, the embryonic linguofacial tributary of the internal jugular vein may now be called the definitive common facial vein (fig. 11). After anastomosis with the anterior facial vein, the lateral tributary of the dwindling maxillary vein constitutes the definitive deep facial vein (fig. 10). The cranial end of the anterior facial vein, which develops near the bridge of the nose in association with the frontal and nasociliary branches of the ophthalmic nerve (fig. toAI), also anastomoses with the primitive supraorbital (superior ophthalmic) vein at the inner angle of the eye during this stage or the next (fig. 13). This emissary connection through the orbit between intracranial and extracranial veins is particularly significant in the adult configuration.
Figure 12 shows that the stem of the primitive maxillary vein, the lateral tributaries of which are being annexed by the new anterior facial vein, undergoes a marked reduction before stage 6 (figs. 20-23, pl. 1); its medial tributaries drain the optic nerve, nasal cavity, and pharyngeal roof. A remnant of one of its very primitive optic tributaries of stages I and 2 sometimes persists (cf. figs. 14B2, B3). In one injected embryo (Carnegie no. 460, xx, 21 mm.), this vessel ramifies intracranially around the cerebral end of the optic nerve before accompanying it through the optic foramen. It should be noted that the maxillary vein does not accompany the maxillary nerve through the foramen rotundum, now definitive in the primordial temporal wing of the Chondrocranium H. Lewis, 1920); instead, the vein is substantially medial to this cartilage, and borders the margin of the sella turcica at the site of the medial end of the future infraorbital fissure (cf. pl. 4A1).
As is generally known, and is well illustrated by F. T. Lewis (1909) and the Grant Atlas (1951), some, or most, of the adult tributaries of the common facial vein may empty into the external jugular vein, or sometimes into its anterior jugular tributary. The common facial (linguofacial) vein of the embryo, however, primarily joins the internal jugular (stages 2 to 6). Not until stage 6 (averaging 22 mm.) does the external jugular vein begin to emerge. Clearly, therefore, communications between the common facial and external jugular veins are secondary; they are present in stage 7, although variable (fig. 13).
Comparison of figures 22 and 23 (pl. 1) shows how the external jugular vein is formed. A small cranial tributary of the primitive cephalic vein in the arm at stage 6 (fig. 9) has grown larger in the differentiating tissues of the neck, an(l joins the juguloeephalic vein craniodorsal to the cartilaginous clavicle, which is now surrounded by a venous ring (fig. 10). The caudoclavicular part of this ring is a new anastomosis whereby the definitive cephalic vein overcomes its jugulocephalie detour and becomes directly continuous with the subclavian vein, now definitive. The old proximal end of the primitive cephalic vein, which is the craniodorsal part of the clavicular venous ring, may be recognized as the trunk of the adult external jugular vein. The part of the venous ring ventral and superficial to the clavicle, i.e. the jugulacephalie segment of the primitive cephalic vein (p. too), often dwindles at stage 6 (fig. 10) and is usually lost.
Fig. 1:. Two embryos of late stage .1 and >11.-igc (2 (same mrtgnilicutiun): A and 151, fruntnl \'lC\\'S nf cnrumtl rccnnstructions of Cztrncgic no. 491, 16.3 mm., xviii, and no. 966. 23 mm., xx, respectively: B: is :1 correlating basal \'lC\\’ of no. 966. Notc the voluminous m:1_\'ill;tr_v vcin (A), which includes ittfrzlurliitul trihumrics. Sy hori'/.0n .\.\' (lit). the primitive supr:t0rhit:tl vcin has hccomc dctourccl dorsal to the iuxtrtposition of the 4th ncrvc with the ophthalmic ncrvc. L‘S[t1l)llSl1L'Ll c:u'licr (A): this intlircct Ct)llrSC prcccdcs :1 secondary amtstomosis whereby the stem of the Innxillztry will will l)L‘C()t1)C that of all the adult nrhitnl '\'L'il1S (cf. fig. 14). Owing to secondary anncxzttion of its lateral trihutzlrics lay thc new anterior facial vein, the stctn of thc I1T.lXlll;1l'y vcin clwimllcs (B), :1 dc\'cl<':pmcnt more .'td\';tnccLl on the right Sltlti of this embryo.
Cases of atypical retention of the jugulocephalic vein in the adult are illustrated by F. T. Lewis (1909; see also fig. 1068 of the Toldt Atlas, 1941). Similarly, the adult cephalic vein may join either the external jugular vein or its anterior iugular tributary (sometimes definitive as small vessel at the present stage) cranial to the clavicle. emnants of the embryonic venous ring around the avicle may also persist (F. T. Lewis), so that the adult ibclavian is either double or passes between the clavicle id subclavius muscle. Plate 3E (coronal view of age 6) shows bilateral asymmetry: on the left of the nbryo, which is typically somewhat less advanced from te venous standpoint than the right side (see above), te stem of the external jugular, joining the supraclavicur (jugulocephalic) arch of the primitive cephalic vein, ill defined and has no anastomosis with the common .cial tributary of the internal jugular; on the right side, re external jugular stem not only is well developed but 1s annexed essentially all the tributaries of the common .cial vein, the origin of which is represented by a small vig of the internal jugular (fig. IIAI). The secondary tastomosis between the external jugular and common .cial veins, which is sometimes seen at this time and is -gularly found in stage 7 (fig. 13), is commonly repre-nted in the adult by a prominent channel; often called re posterior facial vein, the anastomosis is also desig1ECCl as part of either the superficial temporal or the tternal jugular vein.
The considerable adult variety in detail of these veins, ell illustrated in diagrams by F. T. Lewis (1909), can aw be understood. Lewis emphasized that the linguocial, later common facial, tributary of the internal tgular is a primary constant in mammals (and other trtebrates). In species except certain primates, it gives 3 most of its tributaries to the secondary external jugur coincident with a secondary retrogression of the iternal jugular vein (pl. 6). In adult man, however, 3 derivatives, notably the anterior facial, lingual, super:ial temporal, and internal maxillary veins, are comionly drained by both jugular systems, the external and at€I‘l'13l. l
STAGE 7. Threshold or Fetal Period (CA. 21/; .\IOS. AND 40 MM.)
The change in designation from embryo to fetus, altough somewhat arbitrary, is useful in denoting the tquisition of essentially all the characteristics recogZzable in later life. By the 4o—mm. stage, the cranial arteries that radiate from the completed circle of VVillis ‘e readily comparable to those of the adult. Whereas re pattern of these arteries, arising near the mid-line, ill not be essentially changed by the great expansion 3 the cerebral and cerebellar hemispheres yet to come, tat of the venous channels, except in the face and neck, .vaits many major alterations before recognizable ma trity (fig. 13). rtperior Sagittal Sinus and Primitiz/e Galenic System In previous stages, only one component of the adult nuses has become definitive—the sigmoid. Although re whole length of the primitive transverse sinus is out lined in the tentorial plexus, its course is opposite to that of the adult (figs. 22-24, pl. 1). Its lateral end joining the sigmoid sinus is well defined, but its lessadvanced medial segment, the marginal sinus, is still being transferred caudally; this process, called anastomotic progression (spontaneous migration),* is possible in the meshes of the tentorial dural plexus, or, more specifically, its cranial component near the mid-line, the sagittal plexus. This process initiates the formation of the superior sagittal sinus, described by Strccter (1918). As the cerebral hemispheres grow caudally and become approximated at the mid-line over a greater area, the marginal sinuses, which follow their dorsocaudal borders, join in a mid-line anastomosis. Like several other midline vessels derived from bilateral channels, notably the basilar artery, the superior sagittal sinus emerges by a combination of two processes: the unequal or alternate enlargement of the channel of one side with a dwindling of the opposing vessel; and, to a lesser extent perhaps (Streeter), a coalescence of the bilateral channels. As was discussed above, the sagittal sinus drains more into the right transverse sinus than into the left in all the embryos of this series (fig. 7), as it frequently does in the adult. Since its dural sheath is still histologically immature, the typical triangular shape of the adult sinus in cross section is not seen until much later. The sinus receives a number of ill-defined tributaries, remnants of the anterior dural plexus, on the dorsocaudal convexity of the hemisphere. Sometimes identifiable is the anastomosis between one or more of these primitive superior cerebral veins and the superficial middle cerebral (telencephalic) veins that represents the great anastomotic vein, or veins (of Trolard, 1868), commonly described for the adult. In contrast to their secondary pial tributaries, the stems of these major veins, immediately derived from the anterior dural plexus, are superficial to the arteries of the cerebral convexity (see stage 4).
Another differentiation of the mid-line sagittal plexus includes the first stages in the establishment of the Galenic system of intracerebral drainage. The primitive straiglzt sinus, continuous with the primitive great cerebral vein (of Galen), leaves the membranous (liencephalic roof near the pineal primordium (fig. r3). Like the primitive sagittal sinus, a plexus that drains predominantly to the right from its earliest stages, the channel representing the straight sinus also drains chiefly to the right side in the embryos of the present series. Although this configuration is compatible with the history of all the related channels until this time, the adult straight sinus is usually described as draining more to the left, i.e. to the side opposite that of maximum drainage of the superior sagittal sinus, as described in stage 6. An intermediate stage in the secondary transference of more drainage to the left side, which presumably occurs in many cases, is shown in Streeter's illustrations (I918, figs. 7-9) of the formation of the superior sagittal sinus; in the last stage pictured (a 54-mm. fetus), the primitive straight sinus has developed a relatively equal connection with each transverse sinus. It is here postulated (p. 104) that the early tendency of the mid-line venous channels which belong to the sagittal and tentorial plexuses to drain more toward the right side depends upon the temporary ventrocardiac detour, through the sinus venosus, of the blood in the left internal jugular vein in reaching the right cardiac atrium (pl. 3). The left innominate vein, formed in stage 6, reduces this detour to a variable extent in man and certain other mammals; it is a late development phylogenetically (McClure and Silvester, I909).
Fig. 13. Stage 7 (when the arteries resemble the adult configuration; cf. pl. 4.-\2). Although tI1e ventral diencephalic vein continues to drain the lateral choroid plexus (A1, B), its dorsal drainage is esmblishetl by way of the superior choroid vein, the only primary tributary of the primitive internal cerebral vein. The new cavernous and inferior pctrosnl sinuses are medial extensions of the pro-otic sinus and iiiyelenceplmlic vein, respectively. The stem of the m:1xiIlary vein has become that of all the definitive orbito-ophthalmic veins. Anrtstomoses have ccurretl between components of the new external jugular vein and the primitive cmiss;1r_\' veins. Note the elongation of the tenlorial sinus (cf. A1 with B of stage 7a) coincident with expansion of the hemisphere.
The degree of persistent asymmetry of the sinuses at the torcular in the adult conceivably depends on that of the two innominate veins, since these develop differently: the vein on the left is a secondary anastomosis in its entirety; at first it is in transverse position and is relatively long, in contrast to the vein on the right, which is merely a segment of the primary anterior cardinal (internal jugular) vein (pl. 3), and hence is vertical. The relative length and direction of the two innominates, therefore, can vary considerably in the adult of different individuals and species; they are essentially symmetrical in mammalian types (McClure and Silvester). On evidence based in part on cases of persistent left superior vena cava (e.g. Halpert and Coman, 1930; Halpert, 1942), it is assumed that, the more nearly symmetrical the innominates become, the greater is the tendency toward equalization of drainage of the veins leading to the torcular (Padget, I956, fig. 23). Since the developing straight sinus empties into the midline remnants of the tentorial plexus, i.e. the future torcular, it is in better position than the longer transverse sinuses, in the dwindling lateral parts of the tentorial plexus, to compensate for the primary inequality. Accordingly, a typical adult torcular in man appears to be one in which the superior sagittal sinus passes somewhat more to the right, and the straight sinus similarly to the left, with accessory channels, i.e. remnants of the tentorial plexus, on the contralateral sides.
At stage 7, the channel representing the combined primitive straight sinus and great cerebral vein of the adult is formed by the junction of a bilateral vein, the prz‘1m'tz'z/e interim! cerebral. It must be understood that this vein, so named for convenience, is not definitive in the present series (under 80 mm.) in respect to either its adult position or its tributaries. Essentially, it is the continuation of a prominent superior‘ c‘/ioroid vein in the voluminous choroid plexus now almost filling the lateral ventricle, over which the hemisphere is a thin shell. Conversely, in the adult, the superior choroid vein is an inconspicuous tributary of the internal cerebral vein, formed by the junction of the septal vein (of the septum pellucidum) with the terminal (so-called thalamostriate) vein, which includes superior striate tributaries. Not until the formation of the corpus callosum and changes in the hippocampal complex at a stage beyond the present series is the internal cerebral vein enclosed within the brain, nor does it receive, with one exception, any intracerehral tributaries; they emerge later in response to the relatively great increase in the substance of the cerebral hemisphere.
The present drainage of the choroid plexus of the lateral (and third) ventricle into a superior choroid vein is secondary. At first, the choroid plexus, originating near the interventricular foramen (of Monro), is drained ventrally by way of an inferior c/zoroid vein, a tributary of the prominent ventral diencephalie vein (figs. 9, 10). These veins will be incorporated into the later basal cerebral vein and its well defined inferior ventricular tributary (stage 7a). Apparently, the earlier inferior choroid vein is more or less annexed by the later superior choroid vein, while the internal cerebral tributaries are becoming predominant during cerebral expansion; anustomosis between the internal cerebral and basal cerebral veins by way of their choroid tributaries is frequent in the postnatal configuration.
Primitive patterns showing the Galenic system of intracranial drainage in stage 7 are well exemplified by an excellent embryo of 39 mm. with an unusually good natural injection (Carnegie no. 6203; photographs of the sections, X25, facilitated graphic reconstructions, which unfortunately are among those that could not be included here). The corpus striatum, protruding into the ventrolateral aspect of the ventricular cavity, is drained ventrally, as primarily, by inferior xtriate tributaries of the anterior cerebral and deep middle cerebral veins, which are secondary pial derivatives of the primary superficial middle cerebral veins, shown in figures 33 and 34 (pl. 2). The thickened diencephalie wall, protruding into the narrowed third ventricle, is drained bilaterally by about six well defined tributaries of the dorsal diencephalie vein, sometimes annexed at stage 6 or 7 by the ventral diencephalie vein, definitive earlier (cf. figs. 10, II).
The only exception in this embryo to the primary drainage of the basal nuclear masses ventrally into components of the future basal cerebral vein (stage 7:1) is in the region of the interventricular foramen. It must be noted that the primitive internal cerebral vein is still extracerebral. Lying in dorsal contact with the epithelial diencephalie roof (future choroid membrane of the third ventricle), it is in contact with brain tissue, namely the diencephalon, only at the interventricular foramen. Here the vein receives one intracerehral tributary from the region of the anterior thalamic nucleus. This tributary, probably comparable to the adult “deep vein of the thalamus” (Browning, 1884) or to the “epithalamic vein” of Schlesinger (1939), but not of Bedford (1934a, b, in reference to the dog and rhesus monkey), may be called the primitive deep t/mlamic vein.
The single intracerebral (thalamic) tributary of the primitive internal cerebral vein, as it is found in the 39-mm. embryo identified above, is a clue to the way in which the conspicuous adult terminal vein is probably “’ formed at a later stage. At the interventricular foramen, this vein (unlabeled in fig. 13A), between its exit from the anterior thalamic region and its junction with the primitive internal cerebral (superior choroid) vein, lies for an appreciable distance in the pia—arachnoid still separating the primitive thalamus and corpus striatum. These two structures, primarily parts of the diencephalon and tclencephalon, respectively, will become united by the increase of the internal capsule fibers which spread over the lateral surface of the thalamus as the lentiform nucleus extends caudally. Although it is well known that the adult internal cerebral vein, once extraventricular, is secondarily enclosed within the brain in a double fold of pia—arachnoid, an cxtraventricular derivation of the terminal vein is much less obvious in the adult, because it is covered by ventricular ependyma and its main stem also by the terminal stria (Spalteholz Atlas, 1937, fig. 720), which outlines the thalamostriate groove. In the 39—mm. embryo, a large venous plexus, bordering the internal cerebral veins on the epithelial diencephalic roof, lies adjacent to the hippocampus, which then extends caudally from the region of the interventricular foramcn. This plexus anastomoscs cranially with the stem of the thalamic vein, and caudally with the primitive internal cerebral. How it is possible for a derivative of this plexus to become enclosed in the floor of the lateral ventricle may be visualized in connection with embryonic stages in the secondary juxtaposition of the primitive thalamus and caudate nucleus, as illustrated by Hamilton, Boyd, and Mossman “ (1952, fig. 326).
“‘:‘t definitive conclusion could not be reached, because the meninges concerned had been distorted by technical procedures in the few early fetuses available for this study.
1‘ Regarding their description. however, it was noted by Dr. George W. liartelme-2 (personal communication) that what appears to be :1 “fusion" of the hemisphere and dicncephalon is a thickening of the ventricular wall, at the dien—telencephalic sulcus. as the internal capsular fibers begin to pass through it. Hence, any enclosure of the terminal vein is not likely to occur within this sulcus. More probably. an extraventrieular vein is enclosed within the choroid membrane of the third ventricle, in association with the development of the rhinencephalic complex’, of which the terminal stria is a part. There is one other possibility. lf it should be found that the veins of the ventricular \\'alls (which include the transverse and longitudinal caudate veins of Schlesinger: see Paclgct. to-‘,6, p. 315) result from secondary anastomoses between the primary veins stemming from the embryonic ependymal layer, the terminal vein itself conceivably may be formed in the same way. Furthermore, it may be that most of the proximal tributaries of the terminal vein (as 1 I . 1
Sometime after the 8o mm. stage, the secondary terminal vein (not identified in the present series) receives the superior striate veins, which conceivably represent an annexation of certain inferior striate tributaries of the ventral diencephalic vein, well established at stage 7. Postnatally, there is some evidence (Padget, 1956) of a secondary anastomosis between the superior and inferior striate veins. In the 39-mm. embryo described above, one of several well defined ventral (inferior) striate veins is particularly long and prominent; it is also notable for its symmetrical configuration bilaterally. Its distal end coursing longitudinally through the primordial caudate just beneath its craniodorsal (intraventricular) surface is in the position, therefore, of the subependymal caudate tributaries of the adult terminal vein that receive the superior striate and other intraventricular veins. Such a vein could readily be involved in a later secondary anastomosis with the Galenic internal cerebral system, which is completed relatively late in cerebral venous development, paralleling cerebral dominance.
Inferior Petrosal and Cavernous Sinuses The advanced growth of the chondrocranium and the beginning development of the frontal, parietal, and occipital components of the membranous skull at this stage (Macklin, 1921) lead to the emergence of the permanent derivatives of the pro-otic sinus. Its most lateral tributarics, associated with branches of the new middle meningeal artery, drain the primitive dura and adjacent bones, and are components, therefore, of the middle meningeal sinuses, to be discussed for stage 7:: (figs. 13. 15; pl. 4). Of particular importance now are the new medial derivatives of the pro«otic sinus, the definitive inferior petrosal and cavernous sinuses, which appear simultaneously.
Although no well defined venous channel in the position of the inferior pctrosal sinus is seen before the present stage 7, it is interesting that the adult trunk of the sinus, typically lying between the roots of the 9th and toth nerves, appeared much earlier in stage 3. This trunk, which in all stages joins the internal jugular vein extracranially, represents the segment of the primary head-sinus that originally passed from the lateral aspect of the otocyst and the 9th nerve to the medial side of the toth nerve during the transitory stage when the sinus lay between the mth to 12th nerves and the brain (figs. 19, 20, pl. 1; figs. 27-30, pl. 2). After the headsinus and the primitive internal jugular vein, continuous with it, are shifted outside the 10th ttervc by way of secondary anastomoses (fig. 4), the former trunk of the sinus is retained as the stem of a transverse pia-arach—
precisely defined, only that part of the vein related to the terminal stria) that are prominent grossly in the ventricular walls result from -.1 subependymal elongation of the primary embryonic veins as the cerebral hemisphere expands.
rfoidal vein from the medulla. Relatively early, this ventral myelencephalic vein, which drains the neural tube between the 6th and 10th nerves, also receives components of other adult veins, the hypoglossal emissary and the vein of the cochlear aqueduct. just distal to these tributaries between 20 and 40 mm., the myelencephalic vein receives a dural plexiform collateral that extends ventrocranially in the angle of secondary junction between the well developed otic and basioccipital cartilages. Thus is clarified the trabecular conformation of the inferior petrosal sinus, now in definitive position, as shown in plate 4.
At the level of the 6th nerve root, which it surrounds, the emerging inferior petrosal sinus is continuous with another new plexiform channel medial to the trigeminal ganglion. Derived from the medial side of the pro-otic sinus, this plexus surrounds the carotid artery at the level of the hypophysis (fig. 15; pl. 4). For the first time, therefore, one may properly speak of a primitive cavernous sinus, although the part of the pro-otic sinus that is always medial to the 5th nerve ganglion, and is the sole remaining segment of the primary head—sinus of initial stages, has often been so designated (Streeter, 1918; and others). This conspicuous primary sinus is always notably lateral to the carotid artery and is smoothly outlined, however, and thus cannot represent the trabecular cavernous sinus through which the carotid and nerve roots pass in the adult—a singular anatomical configuration which can now be explained. The new cavernous and inferior petrosal sinuses, located medially and ventrally, will provide a more direct caudal exit (to the internal jugular vein) for the orbital venous blood than does the lateral, and soon devious, pro-otic sinus (cf. figs. 31-37, pl. 2). The irztercauernozu sinuses (forming the circular sinus) and the basilar sinus (plexus) of the adult are merely the mid-line plexiform extensions already beginning to anastomose the bilateral cavernous and inferior petrosal sinuses, respectively (pls. 3, 4).
Modification: of Inter- and Extra-cranial Channel
The alterations in the pia—arachnoidal veins resulting from cerebral growth, especially their trunks entering the dural sinuses, are notable in stage 7 (figs. 23, 24, pl. 1). The superficial middle cerebral veins, belonging more to the dural than to the pial layer, are particularly prominent. Their stem is joined in front of the optic nerves by a pial tributary from the mid-line, which is the primary component of the anterior cerebral and deep middle cerebral veins, and receives the inferior striate veins referred to above (fig. i3A1). All these cerebral veins, including the prominent ventral diencephalic vein with its strong inferior choroid tributary, are directly continuous with the tentorial sinus, which has been considerably elongated. Curved around the ventral margin of the future temporal lobe in following the course of the 3d and 4th nerves, the sinus is now unmistakably enclosed within the primitive tentorium. Through it the dorsal diencephalic and mesencephalic veins pass laterally to join components of the still immature medial end of the transverse sinus, after forming in each case a characteristic dorsal arch (figs. Ii, 13). Their stems are often smaller at their entrance into the primitive dura surrounding the sinus, thus presaging their ultimate disappearance. Secondary anastomoses between some of the pial ramifications of these separate veins (see stage 4) foreshadow the emergence of the basal cerebral vein during stage 7a. It must be emphasized that, as before, all the veins of the brain empty directly into the sigmoid sinus. The cavernous sinus, just formed, plays no part in cerebral drainage; it is merely a secondary caudal outlet for the veins of the orbit, by way of the new inferior petrosal sinus.
The primitive venous communication between the tentorial sinus (draining the middle cerebral veins) and the pro-otic sinus (component of the cavernous sinus at this stage) is by way of the primary stem of the anterior dural plexus. This stem dwindled during stage 6 and is usually not found later (figs. 19-23, pl. 1). Its exceptional late retention, however, has led to errors of identification of important related vessels, as will be seen.
One 43-mm. embryo (Carnegie no. 886) of stage 7 is atypical in that the anterior dural stern has persisted as a small channel on the right side (pl. 4A2, unlabeled vessel connecting pro—otic and tentorial sinuses). Moreover, a large channel representing the same primary channel on the left side of this embryo still drains the middle cerebral veins, asioriginally, into the pro-otic sinus (see also Streeter, 1913, fig. 27, pl. 5); in keeping with this pattern, the tentorial sinus, which usually at this time connects these veins dorsally with the transverse sinus, is relatively small. The atypical configuration of this specimen, particularly on the left, is probably responsible in part for Streeter’s (1918) impression that the termination of the middle cerebral veins in the cavernous sinus, although inconstant even in the adult, is an early primary connection. In his illustration of the same embryo, the prominent channel on the left labeled “cavernous sinus" is not medial but lateral to the trigeminal ganglion (not illustrated by Streeter, but see pl. 4A2). As such, it cannot represent the adult sinus. It does represent plexi— form anastomoses sometimes occurring between the prootic sinus (middle dural stem) and the anterior dural stem, which lie upon the lateral surface of the ganglion.
Anastomoses similar to those just noted in stage 7 may occur in earlier stages, as shown by Mall (1904, fig. 13, pl. 3, in an embryo of horizon xviii), who erroneously thought” that they represented first stages in 12 The material of the Carnegie Collection begun by Dr. Mall was itself in an embryonic stage in 1904 and thus was barely adequate for his brief but often-quoted report.
the formation of the primitive transverse sinus. He noted the fact that the cranial nerves, from the 5th to the 12th, very early “wander through” the “anterior cardinal vein" (head-sinus; see p. 83) during its transposition from their medial to lateral aspects, as described for the present stages 2 to 4. The 5th nerve ganglion alone is not extensively involved in this general migration, probably because it is a relatively large mass superficially situated, and because the head—sinus is anchored medially by its voluminous and constant maxillary tributary (pl. 1). At this level the head-sinus may share, however, in the tendency toward lateral migration, as witnessed by the small anastomoses just noted. The atypical dilatation of one of these vessels on the left side of the 43-mm. embryo is accompanied not only by a corresponding dwindling of the segment of the pro-otic sinus medial to the trigeminal ganglion, but also by the retention of the anterior dural stem; the 4th nerve, usually outside the stem, passes through it in this instance. This pattern results in drainage of the middle cerebral veins into the pro-otic sinus, and thus directly into the sigmoid, not the cavernous, sinus. Since the atypical channel is lateral to the trigeminal ganglion, drainage is essentially the same as in the typical pattern of middle cerebral veins that terminate in the tentorial sinus; both routes, leading into the transverse sinus, are lateral to the ganglion (pl. 4B).
In conclusion, the embryo described above is no exception to the typical history of the outlet of the superficial middle cerebral veins (pls. 1, 2): They first empty into the primary head-sinus (later pro-otic sinus), which is the lateral component of the future cavernous sinus. By the time the definitive cavernous sinus emerges, the primary communication, i.e. the anterior dural stem, has been lost. A similar communication, but in a notably different position, is finally regained in an indeterminate number of cases by a late secondary anastomosis, usually postnatal (fig. 18C, D).
The external jugular system, which emerged during stage 6, already resembles the adult conformation, far in advance of the intracranial venous channels (fig. 13), as can best be summarized by reference to the consecutive stages shown in plate I. To be noted are the subclavian and external jugular veins with their major tributaries, including secondary anastomoses with ophthalmic and emissary veins. VVith growth of the lower jaw and the concomitant elevation of the external ear, the new external jugular has anastomosed with the definitive superficial temporal vein, continuous with the posterior facial tributary of the anterior facial vein. By way of this secondary anastomosis, the external jugular often becomes the chief drainage of the superficial vein and may even annex the primary common facial stem from the internal jugular (pl. 313, F).
Vein: of Orbit A notable advance in the extracranial veins is the belated formation of the permanent stem of all the orbital veins, including the ophthalmic, of which the adult superior op/it/mimic (so-called, BNA) is the major orbital component. In the outline below, it can be seen that these veins, briefly noted in stage 5, have a complicated development recalling that of the ophthalmic artery (fig. 14). Understanding of this development is further complicated by inexact terminology regarding the adult vessels.
Most of the dorsal orbital part of the primitive supraorbital vein, which emerged in stage 4 (fig. 5) and later also constitutes the chief superior ophthalmic (orbital) vein (pls. I, 2), is permanent; its stem, however, is temporary. In stage 5 (or late stage 4) the course of this vein is still direct, but its proximal end at the head-sinus has become wedged against the dorsocaudal aspect of the tissue surrounding the juxtaposed 4th and 5th (ophthalmic) nerves, as shown in figures 9 and 12A. By stage 6 (figs. to, 14A), shifting and unequal growth including the orbital structures have resulted in a circuitous course for the same vein. The course, best seen in a coronal view (fig. I2BI), is obviously the result of the anchoring of the vein by the approximation of the two nerves. Furthermore, a consideration of adult anatomy in this region shows that the primitive course, which is dorsal to all the orbital nerve roots and caudal to the juxtaposition of the 4th and 5th nerves within the region of the cavernous sinus, would be even more devious if it persisted after formation of the anterior clinoid process and lesser sphenoidal wing.
At the present 4o—mm. stage, the devious primary trunk of the primitive supraorbital vein of earlier stages is replaced. Medial to the 4th and 5th (ophthalmic) nerves, a secondary anastomosis joins the supraorbital vein with the stern of the primary maxillary vein (fig. 14A, BI), which, by stage 6. has lost most of its tributaries to the anterior (and deep) facial vein (fig. 12). Since two vessels, previously continuous, i.e. the stems of the primitive supraorbital vein and of the anterior dural plexus (figs. 23, 24, pl. 1), have both disappeared, the stem of the old maxillary (infraorbital) vein has become the outlet of all the adult veins of the orbital region into the cavernous sinus. Some of the medial maxillary tributaries, which have always drained the caudoventral parts of the eye and orbit, are incorporated as the socalled in /crior op/zt/zalmic veins of the adult (see below).
FIG. 14. Reconstruction of details around the right eye in three embryos of two stages (same magnification). A, lateral view of Carnegie no. 966, 23 mm., xx, stage 6: B1, 2, 3, no. 886, 40 mm., stage 7, viewed from the right, in front, and below, respectively; C, no. 6203, 39 mm., stage 7, viewed from below. At horizon xx (A) the orbital region is drained by two veins, one dorsal orbital and supraorbital, the other ventral orbital and infraorbital or maxillary. By 40 mm. (Br, 2, 3), the most proximal end of the primitive supraorbital vein, dctoured by the junction of the 4th and ophthalmic nerves (A), has disappeared (B1); :1 new anastomosis joins it to the stem of the maxillary vein, which thereby becomes that of all the adult orbital and ophthalmic veins. The secondary anastomosis between the orbital and anterior facial veins is among the earliest of the emissary connections.
With the definition of the permanent stem of all the orbital veins, it can be understood why the adult superior ophthalmic vein—since it is also the early primary stem of the supraorbital vein, it is the best developed and most constant vein of the orbit—typically does not join the cavernous sinus in the upper lateral extremity of the superior orbital fissure, although its course is dorsal and then lateral to the orbital structures, including the common tendinous ring (of Zion). Instead, the end of the vein, a secondary anastomosis noted above, follows the outer edge of the orbital fissure to its lowest medial extremity, where it joins the cavernous sinus ventral to all orbital structures. Development shows that the adult outlet of the superior ophthalmic vein could more precisely be called the common orbital (orbito-ophthalmic) vein, comprising two main divisions, superior and inferior orbital, with respective Ophthalmic tributaries. This common trunk is medial to the foramen rotundum, i.e. to the maxillary division of the 5th nerve, a structure that, even before stage 2, has marked the level of the maxillary vein, which the trunk represents (pls. r, 2). The true inferior ophthalmic veins, contained within the cone of muscles, are, of course, augmented remnants of the maxillary vein. Outside the muscle cone, the inferior orbital veins, usually included in the adult group designated inferior “ophthalmic,” are also primary maxillary remnants; they may include infraorbital, facial, ethmoid, and nasal tributaries. A very primitive ophthalmic tributary may persist (figs. I4B2, B3) as a vestige of the early stages when the maxillary vein was the only drainage of the optic stalk, vesicle, and cup (figs. 19-21, pl. 1) : the dilated, tortuous remnant of this vein, emerging from the intracranial cavity by way of the optic foramen under the nerve (fig. 1.jB3), was seen by the author in an abnormal juvenile brain (congenital arteriovenous aneurysm; cited in Padget, 1956).
According to Mann (1950), no central retinal vein accompanying the hyaloid artery (later central artery of the retina) is seen until the third month. Its stem, however, appears to be represented very early (stages 1, 2) among the tributaries of the primitive maxillary vein that first ramify at the base of the optic vesicle (fig. 1) and later receive a vessel from the choroid fissure in the ventral part of the optic stalk (figs. 2, 3, 5). The adult retinal vein is said to join the cavernous sinus, and to have a communication with the superior ophthalmic vein (Wolff, 1954), or to be a tributary of either; such variation accords with the developments just described, but the vein is a primary tributary of the common orbital, i.e. the maxillary, vein. Similarly, reports that the adult stem of the inferior ophthalmic veins enters the cavernous sinus either alone or together with the superior ophthalmic vein are readily explained by the variable size and form of the adult cavernous sinus, which forms secondarily from a plexus, and by the position of the secondary anastomosis of stage 7 that joins the supraorbital (superior ophthalmic) vein to the stem of the primitive maxillary (infraorbital) vein. Since the resulting common orbital (ophthalmic) vein may be very short, the adult superior and inferior ophthalmic veins may appear to enter the sinus separately.
The intrinsic veins of the primitive embryonic eye, including the annular vessel of the pupil and the choriocapillary net on the optic cup (Mann), all drain primarily into the primitive maxillary (infraorbital) vein of stages 1 and 2 (under 8 mm.), which is before any supraorbital vein is present (figs. 1, 2). Voluminous orbital anastomoses often obscure specific vessels (fig. 14), but prirrzitive uorticore veins could be identified in two embryos of late horizon xviii and xx (stages 4, 6, fig. 12B2): the craniodorsal tributary joins the primitive superior ophthalmic, and the caudoventral one joins the primitive maxillary vein (“inferior ophthalmic vein" of Mann, who pictures the latter veins diagrammatically between 13 and 18 mm.).
Emissary Veins The emergence of the definitive emissary veins can be seen during stage 7. A frontal tributary of the primitive supraorbital vein anastomoses with the anterior facial vein at the inner angle of the eye, thus constituting the relatively constant angular (nasofrontal) vein (fig. 14); dorsal extensions in the scalp plexus (see below) of the superficial tributaries of the primitive supraorbital vein (figs. 22-24, pl. 1) form the definitive frontal and supraorbital veins. In various mammals below the primate level (Dennstedt, 1904; and others), such emissary communications incorporating the orbital veins develop to the extent that these veins drain more laterally into the external jugular system, by way of the facial vein, than medially into the atrophic internal jugular, by way of the cavernous sinus. In the human embryo of stage 6, an anastomosis between the primitive maxillary vein and the emerging anterior facial vein formed the deep facial vein (fig. 10). Since the stem of the old maxillary vein of stage 7 is now the permanent stem of all the orbital veins, as just described, the deep facial vein has become its tributary at the caudolateral margin of the orbit, and will pass through the adult inferior orbital fissure. Communicating with the pterygoid plexus, derived from the medial components of the voluminous maxillary vein of the early stages, the deep facial vein is the most proximal tributary of the adult stem of the common orbital vein (so-called superior ophthalmic stem) before its junction with the cavernous sinus.
With the formation of all essential elements of the external jugular system, of the dural sinuses, and of veins in the chondrocranial foramina in the present 4o-mm. stage, the other emissary veins become definitive. It must be understood that the primitive emissaries of earlier stages (figs. 21-23, pl. 1), so called for convenience, drain cxtracranial structures medially into the primitive sinuses; the chondrocranium develops around their stems, thus forming the emissary foramina. Now that secondary anastomoses with the new tributaries of the external jugular vein are being formed, flow in the emissaries can be reversed peripherally.
As was noted above in the order of development of their foramina, the primitive hypoglossal emissary was the first to appear in stage 4 (fig. 5). It drains veins around the upper spinal cord medially into the ventral myelencephalie vein. This also constitutes a definitive emissary, namely the stem of the future inferior petrosal sinus, as soon as the jugular foramen is completed (late stage 4); it joins the internal jugular vein below its foramen. In stage 5 (fig. 9), the primitive condylar (condyloid) emissary drains the more superficial vessels around the upper spinal cord, which include components of the deep cervical and vertebral veins, into the sigmoid sinus at the level of the posterior dural plexus (adult marginal sinus of the foramen magnum). By stage 6 (fig. to), the primitive mastoid emissary from the primordia of the occipital muscles drains into the sigmoid sinus just cranial to the conspicuous stem of the posterior dural plexus. These emissaries will constitute an important collateral between intracranial venous channels and veins around the vertebral column, by way of the external jugular system (Batson, 1940, and others). Whereas the recent anastomosis of the cavernous sinus with the anterior and deep facial veins, by way of the common stem of all orbital veins, represents an adult emissary, neither the orbital fissures, superior and inferior, nor the middle fossa are clearly demarcated in the fetal chondrocranium, or completed in bone until much later.
Formation of the cavernous sinus at stage 7 clarifies the origin of the remaining emissary commonly found at the base of the adult skull, the sp/zenoid emissary (of the foramen ovale). As early as stage 3 or 4 (figs. 3, 5), the dorsal pharyngeal vein, a tributary of the primary headsinus, accompanies the great superficial petrosal nerve. When the head-sinus disappears ventrally to the labyrinth, the pharyngeal vein becomes a tributary of the prootic sinus (fig. 15A). By stage 7 it drains medially into the cavernous sinus, a medial derivative of the pro-otic sinus (fig. 158, C). The vein becomes truly emissary when its lateral tributaries, already ramifying around the carotid artery as in the adult, anastomose with the deep facial tributaries of the primitive maxillary vein that comprise the adult pterygoid plexus.
Successive stages of the embryonic dorsal pharyngeal vein also clarify the origin of the inconstant and accessory splzenoid emissary (of the foramen of Vesalius; fig. 15). Though usually minute, this vessel is of interest because it explains the occurrence of its not-uncommon adult foramen, which lies just cranial and medial to the foramen ovale (Cunningham, 1951; Morris, 1953). Since neither of these sphenoid foramina occurs in embryonic cartilage—very little of the middle fossa is laid down in the fetal chondrocranium—nor is usually well defined by bone until after birth, it cannot be said which opening contains the embryonic pharyngeal vein. Obviously, however, the Vesalian emissary, when it occurs, is merely accessory to the relatively constant and more voluminous emissary vein (or venous plexus) of the foramen ovale. Both veins drain what is here designated the lateral wing of the cavernous sinus, a remnant of the prootic sinus; in the adult they also drain the ventral end of the middle meningeal sinus (fig. 18C, D).
By the present 40 mm. stage, the last opening has been formed in the caudal half of the fetal chondrocraniumthe conspicuous spurious jugular (“capsuloparietal”) foramen, of phylogenetic interest (see stage 7a). This foramen is unusual in the embryo in that it contains a vein relatively much smaller than the foraminal diameter (fig. 13A1). Plexiform anastomoses between this primitive temporal emissary (stem of petrosquamosal sinus; p. 123) and a deep tributary of the superficial temporal vein may be found at this time. The spurious jugular foramen must not be confused with the near-by mastoid (“capsulo-occipital”) foramen, which earlier (stage 6) is formed around the relatively large and constant mastoid emissary vein. Although the primitive petrosqzm— mosal sinus on the lateral aspect of the chondrocranium is not definitive until stage 7a (fig. 16), the anastomosis that it constitutes, between the primitive temporal emissary and a lateral tributary of the pro-otic sinus, is sometimes identified at stage 7 (fig. 13A; figs. 33, 36, pl. 2).
Meningeal Drainage of Primary Membrane Bones,‘ Ina’epena’ent Scalp Plexus A prominent tributary of the pro-otic sinus in stage 7 is the stem of irregular plexiform vessels representing the adult middle meningeal sinuses in the outer part of the dural layer. These extrachondrocranial vessels accompany the two primary branches of the adult dural artery of the same name (fig. 15C), which are best understood by reference to the developing skull, as fully described by Macklin (192r); his models (adapted for fig. 279 in Hamilton, Boyd, and Mossman, 1952) were made from the same 43-mm. embryo shown here (figs. 13, 15B; pl. 4A).
In two embryos of stage 7 (Carnegie no. 886, just noted, and no. 6203), the frontal meningeal (dural) artery supplies the frontal ossification center, which is the most advanced of the primitive membrane bones and overlaps about half of the cerebral hemisphere. The region of the primordial parietal and squamous temporal membrane bones (fig. r3Ar) is supplied by a parietal meningeal (dural) artery, which already comprises two branches. These two primary dural arteries, frontal and parietal, of the present stage must not be confused with the two major branches of the parietal artery in the adult, i.e. the so-called “anterior” and “posterior" branches of
Fig. I5. Coronal reconstruction of three embryos of two stages: A, Carnegie no. 492, 16.8 mm., xviii, stage 4, viewed from the front; ll, no. 886, 43 mm., and C, no. 6203, 39 mm., both of stage 7 and viewed from behind. All show the dorsal pharyngeal vein, which accompanies the great superficial petrosal nerve. It is represented by the sizable emissary veins of the adult foramen ovale, which drain the cavernous and middle meningeal sinuses, and also by the emissary vein of the sphenoid forztmen (of Vesalius), when present. Note the relation of the middle meningeal arteries to the accompanying venous channels, derived from the pro-otie sinus: the veins, after crossing the arteries, lie between them and the membranous hones they vasculztrize. Tributaries of the ventral metencephalic vein (C), the stem of which later becomes the superior petrosal sinus, lie between the branches of the basilzir artery and the pons (dotted line). [The meningeal vessels in C are in the outer dural condensation at (1 level in front of the section through lirain stem and otic capsule]
the middle meningeal artery vascularizing most of the adult cranial vault. As the embryonic growth of the parietal membrane bone overtakes that of the frontal bone, the frontal artery becomes subsidiary, and finally appears to be a branch either of the anteroparietal (“anterior”) branch of the middle meningeal, or of its main stem.
The primary frontal dural artery, supplying the more lateral (greater) aspect of the frontal bone, has no specific name ” in the adult; indeed, its proximal end in the middle fossa is often obscured in passing through a canal formation in the osseous thickening at the pterion. A medial branch of the frontal dural artery, namely the laerimal or ophthalmomeningeal artery of potential importance, frequently passes through the superior orbital fissure, or :1 separate foramen, to anastomose with the lacrimal branch of the ophthalmic artery. Varying in size, this anastomosis represents the supraorbital branch of the temporary embryonic stapedial artery, which becomes the stem of the adult middle meningeal artery. Any venous channel accompanying the arterial anastomosis (fig. 18B—D) constitutes an op/it/ialmomeningeal rimtr (“vein"; the origin of the name in the anatomical literature is not clear, but evidently it often refers erroneously to a remnant of the tentorial sinus; p. 122). The ophthalmomeningeal sinus may be continuous with the ventral end of the adult rp/Jerzoparietal rimts, a channel sometimes both meningeal and diploic, i.e. the sinus of Breschet (fig. 18D); it may, in general, be conHuent with the stem of the anteroparietal menirzgeal ‘3 rirmr, from which it is derived postnatally.
Veins in the dorsal scalp region are first clearly identified in the present 40-mm. stage. It must be emphasized that the membrane bones are primarily vascularized by the dural meningeal vessels just noted, not by scalp vessels (see below). Furthermore, apparently only one significant anastomosis develops between these intra- and extra-cranial systems of the skull vault, and it is often small and not constant. The fact that the parietal emissary (arterial or venous) was not identified except in the late fetal stages available for the present study does not mean that it never is formed between 40 and 80 mm., a stage that seems more likely. In any event, the parietal obviously is the last of the emissaries to be formed and, unlike the others near the skull base, apparently does not comprise a typical primary vessel.
In the gross specimen between 30 and 50 mm., the
'3 The term po:tr-ro- (or /rvlz.-ro-) frontal would distinguish it from another adult meningeal artery-a branch of the ophthalmic artery, i.c. of its anterior ethmoid braneh—which supplies the antero- (metlio—) frontal dura and bone. Of the two major branches of the middle meningeal artery, primarily m1tcr0— and po.r!cro—{7m‘ic-ta/, the latter gives off the anastomotic branch passing through the parietal foramen (p. no); an artery, albeit small, typically accompanies all emissary veins through their foramina.
main stems of the superficial veins can often be seen through the transparent scalp region, namely the frontal, superficial temporal, posterior auricular, and occipital veins,‘ the corresponding, but smaller, scalp arteries with their thicker walls can be identified in sections. This superficial cranial group, together with the dural meningeal arteries and veins, was graphically reconstructed in an excellent embryo (Carnegie no. 6203, 39 mm.; see p. 120) : the occipital artery supplying the occipitocervical musculature is particularly conspicuous in length and gives off the so—called mastoid branch (latero—occipital meningeal artery,” the largest dural artery in the adult posterior fossa); as this artery enters the mastoid foramen, it lies upon the thin wall of the mastoid emissary vein, much larger in diameter (fig. I 3), after bordering it outside the chondrocranium. Such configuration is notably in contrast with the pattern of arteries and veins of the endothelial tube stage, which do not accompany each other, but cross at a right angle (see stage 4). The parallel contiguity of certain vessels of stage 7 is significant in that the walls of even the smaller cranial arteries, at least, outside the brain, have become definitely thicker at 40 mm. than the walls of cranial venous channels, the diameter of which has become relatively much larger. Not until now do arteries and veins in certain areas accompany each other contiguously for the first time.
The superficial cranial vessels just named have developed in the meshes of a scalp plexus, the history of which has been considerably misinterpreted (see Padget, 1956). Erroneously, it is thought that the venous components of the scalp are primarily connected with those of the dura, and that the two layers are subsequently separated by the intervention of the membranous skull, leaving a number of emissary veins as remnants. Although Streeter’s (I918) remarks about vascular “cleavage" have frequently been so misinterpreted, such secondary separation of vascular layers applies only to the dural and pial venous channels, as the result of expansion of the cerebral and cerebellar hemispheres and of the otic capsule (see stage 4). The arteries of the pia, dura, and integument are always separate systems.
Before stage 6, the thin scalp region covering the membranous skull is avascular. Between 20 and 40 mm., a scalp plexus, derived from the most superficial dorso— cranial components of the emerging external carotid and jugular systems, specifically from the scalp vessels named above, advances dorsally from all sides up over the vault of the developing skull toward the region of the future parietal foramen. Initially, it seems, the scalp plexus does not vaseularize the primitive membrane bones. In two embryos of about 40 mm. (identified below), the plexus is everywhere bounded medially by a conspicuous, though narrow, mesenchymal condensation, the primitive galea (aponeurotica), which, furthermore, is separated from the membranous skull by a considerable interval of loose avascular mesenchyme. As was described in detail by Finley (1922) and noted by Streeter (1918, 195i), the advancing margin of this superficial plexus is peculiarly well defined and is often conspicuous in the gross specimen. By 40 mm. it outlines a closing avascular area at the cranial vertex toward which all the meningeal and the superficial scalp vessels converge (graphically reconstructed in embryos Carnegie nos. 886, 6203). Presumably, later extension of the scalp plexus normally closes the primary vascular hiatus.
The significance of the area of the terminal hiatus in the closing scalp plexus is genetically vestigial. It marks the area of the late fetal or postnatal parietal (so-called “_sagittal”) fontanel and its remnant, the parietal foramen, together with the relatively late and inconstant secondary anastomoses, namely arterial and venous emissaries that traverse these skull-hiatuses. This area in normal and abnormal cases, briefly discussed elsewhere (Padget, 1956), calls for study of more specimens, particularly between 40 mm. and term.
Stage 7A. Fetus at the Three Month (60 to 80 mm.)
Whereas the external appearance of the fetus, doubled in length between stage 7 and the end of stage 7a (fig. 16), the last of the present series, is not conspicuously altered, the expanding cerebral hemispheres grow caudally over the midbrain and the emerging cerebellar hemispheres (figs. 24, 25, pl. 1). This expansion, continuing throughout fetal life, together with the late ossification of the skull, necessarily delays the advent of certain channels. Nevertheless, the adult pattern of most venous sinuses and cerebral veins can be recognized by the end of this period. Notable changes, particularly seen in the basal views of the brain and skull (figs. 3339, pl. 2), involve extension or modification of two channels that are prominent and primary, but are not always persistent, namely the tentorial and pro-otic sinuses, and their derivatives. For an understanding of these developments, frequent reference to other mammals is essential.
The transverse sinus, as distinguished from the sigmoid sinus, definitive in stage 5, is pushed into definitive position by cerebral expansion at about 80 mm. (pl. 5). Consequently, the tentorial plexus, which marks the junction of the transverse sinus with the superior sagittal and straight sinuses, is reduced in size to be recognizable as the adult torcular (confluence of sinuses). Comparison of figures 24 and 25 (pl. 1) shows that the marginal sinus, constituting the medial end of the primitive transverse sinus, has been shifted far caudally since the 40-mm. stage by the caudal growth of the cerebral hemisphere, the migration being accomplished by the successive use and discard of loops in the dwindling" tentorial plexus (anastomotic progression)." The transverse sinus, having been swung caudally on the sigmoid sinus as on a hinge, has attained the approximate adult direction, which is opposite to that of earlier stages. This caudal swing is reminiscent of that of the posterior communicating artery between 18 and 40 mm., and it occurs for the same reason.
Significance and Modification: of the Tentorial Sinus Another result of the notable expansion of the cerebral hemisphere between .10 and 80 mm. is a considerable elongation, under its temporo-occipital margin, of the tentorial sinus, which is still the sole drainage for the only definitive vessels now present on the surface of the hemisphere, the conspicuous middle cerebral group of arteries and veins.
It is now obvious that the tentorial sinus is the human counterpart of the mammalian “posterior rhinencephalic vein” described by Hofmann (1901), as indicated in plates 5 and 6. In many adult mammals (e.g. rabbit, pig, cat, and dog), this prominent vein follows the rhinal fissure; it collects veins from the region of the olfactory bulb, drains the middle cerebral veins, and empties into the transverse sinus near its junction with an inconspicuous channel comparable to the sigmoid sinus. In the horse, the rhinencephalic vein is represented by a dural channel for the greater part of its length (Hofmann’s fig. 27), thus resembling the tentorial sinus during fetal stages in man. A comparable vessel, part vein and part sinus, follows the rhinal fissure of the rhesus monkey, according to the author’s observations. After receiving the middle cerebral veins, which, it must be noted, have no primary connection with the cavernous sinus, the vessel passes caudolaterally under the temporal lobe into the lateral junction of sinuses, namely the transverse, sigmoid, and superior petrosal; the last-named sinus in man becomes definitive at this stage, as described below.
Between approximately 20 and 40 mm. in human development (figs. 23, 24, pl. 1), the tentorial sinus empties into the marginal sinus, i.e. the medial segment of the primitive transverse sinus, a route both short and direct. By 60 to 80 mm. (fig. 3; cf. AI, B), growth of the hemisphere, responsible for the caudal swing of the transverse sinus just noted, has also necessitated the marked elongation of the tentorial sinus emptying into it. Its caudal end, now attenuated and plexiform (pl. 4B). has begun a compensatory migration, by anastomotic progressionf caudally along the transverse sinus toward its junction with the sigmoid. Thus, the tentorial sinus attains the shortest route available through existing channels for drainage of the middle cerebral veins into the internal jugular vein (pl. 5); the configuration in stage 7a resembles that of the adult in other mammals (pl. 6).
The cranial end of the elongated tentorial sinus, which directly receives the superficial cerebral veins, is larger than its caudal end and is now curved ventrally, following the contour of the new middle cranial fossa (cf.
FIG. 16. Stage 7:7, in l:ttcr:I| (A) and basal views of the brain (AI) and chondrocranium (A2), when adult patterns become recognizable. The elongated tcntoriul sinus, draining the superficial cerebral veins, begins to migrate to the junction of the sigmoid sinus with the tmnsverse sinus, which hzis been swung into definitive position. The superior pctrosnl sinus becomes definitive as the durnl end of the metcnccphnlic Vein, which it represents, surmounts the expanding optic capsule. Tributaries of the prootic sinus drain the membrane bones. Note the spurious jugular fornmcn and its vein. The basal ccrcbral vein is formed by pial anastomoses between the primary transverse veins of the pia-arachnoid (AI).
i pl. 5A, B). Specifically, the sinus often borders the edge of the cartilaginous lesser sphenoid wing (pl. 413) in the position of the adult so-called sphenoparietal sinus (a term of dubious identity, which has obviously been applied in some instances to this part of the persistent fetal sinus, p. 119).
Considerable variation in the relations and position of the tentorial sinus has become apparent since its elongation. In this study, venous channels in all layers of the dural condensation of mesenchyme are called sinuses. Whereas the pro-otic sinus and its tributaries, eg. the middle meningeal “veins” (usually so called; see below), belong to the outer dural layer, the tentorial sinus, directly continuous with the stem of superficial middle cerebral veins (pl. 1), belongs to its inner layer. The relatively late expansion of the temporal lobe and its fossa cranioventrally, while the eerebrum is expanding dorsally in all directions, not only elongates the tentorial channel; it also pulls the channel and the continuous arachnoidal stem of the veins diagonally for an increasing distance through the innermost and outermost layers, respectively, of the dense dural and loose arachnoidal mesenchyme. (No subdural space intervenes initially.) Hence, the transition between arachnoid-al vein and dural sinus is a very gradual one, and varies considerably. All or part of the postnatal tentorial channel, therefore, may be either more or less deeply situated in the dura, or merely attached to it, in different individuals and mammalian species (Hofmann, Igor). VVhen it is part “sinus" and part “vein,” which is to say lying either in, on, or near the inner dural layer of the middle fossa, the tentorial channel resembles the dural end of other superficial cerebral veins as they approach the superior sagittal (and transverse) sinus (Bailey, 1948); their conformation is similarly explained.
Why the position of the tentorial sinus is not constant is now clear. It may be relatively lateral in the floor of the fetal‘ middle fossa as seen in a 60-min. specimen (fig. ISA), or medial near the edge of the tentorium as in one of 80 mm. (fig. ISB); either position occurs in newborn and adult heads (fig. ISC, D). The remnant of the sinus in postnatal stages has been designated in many ways, for example: erroneously it is called the “ophthalmomeningeal” or the “sphenoparietal” sinus (p. 119); a definitive term is sp/zerzotenzporal rinm' (fig. 1189, Cunningham, Textbook of Anatomy, 1951). By way of the Sylvian vein, the channel obviously is the frequent adult termination into the transverse sinus, or secondarily into the superior petrosal, of the great anastomotic cerebral vein (of Trolard, 1868) according to this author's description (see also Bailey). Details of the pial tributaries of the tentorial sinus at stage 7:: will be de scribed below in connection with the formation of the basal cerebral vein.
Significance and Modification: of the Pro-otic Sum: and It: Tributaries The modifications of the primary embryonic pro-otic sinus between 40 and 80 mm. depend more on the growth of the skull than on that of the brain (fig. r8A—D). A recent derivative, the inferior petrosal sinus, has become the caudal and intrachondrocranial outlet for the orbiteophthalmie veins by way of the cavernous sinus, similarly derived in stage 7. This drainage, however, is still shared about equally with the extracbondrocranial pro-otic sinus, which leaves the cavernous sinus at the tip of the otic capsule and ventral to the trigeminal ganglion, as before, but has become deviated cranially and laterally by the expanded capsule, and borders its cranial aspect caudally to the junction of the transverse and sigmoid sinuses. The pro-otic sinus still receives the small remnant of the primary head-sinus, namely the definitive superficial petrosal vein, which accompanies the nerve of the same name by way of the facial hiatus of the otic capsule (pl. 4B). At its caudal end the pro-otic sinus has two conspicuous tributaries, which emerged at 40 mm., but are now recognizable as permanent adult channels: the medial tributary is the prominent old stem of the ventral metencephalic vein from the primitive cerebellum and pons, a stem that constitutes the new superior petrosal sinus (see below) ; the plexiform lateral tributaries, closely associated with branches of the middle meningeal artery (fig. 15B, C), are components of the primitive meningeal sinuses. In response to the emergence of the meningeal bones, particularly the frontal and parietal, the middle meningeal vessels develop extensive ramifications between 40 and 80 mm. (p. 126). The parietal bone superficially overlaps the parietal plate of the chondrocranium at the end of this period (pl. 4B; Hamilton, Boyd, and Mossman, 1952, fig. 380). The primary drainage of these bones in fetal stages is lateral into the pro-otic sinus, rather than ventral (through the foramen ovale) and dorsal (to the sagittal sinus lacunae) as in the adult.
Superior petrosal sinus. The dural channel that is augmented by stage 7a to constitute the superior petrosal sinus, the last of the major adult sinuses to become definitive in human development, has been present since stage 4. It is the dural end of the primary ventral metencephalic vein, typical of vertebrates (see below). Changes in the primitive brain and skull that bring the sinus into view can best be understood by referring to successive stages, particularly in the basal view (pls. t, 2).
Between stages 5 and 7, the proximal end of the ventral metencephalic vein traverses the primitive tentorium, which is not shown in the accompanying illustrations. The embryonic origin of the tentorium, it must be understood, is the site of the future middle fossa: a Condensation of mesenchyme, arising ventrally from the chondrocranial anterior clinoid process and temporal wing (ala temporalis, surrounding the foramen rotundum; pl. 4B), extends dorsally to the membranous skull vault, where it contains the tentorial plexus. As the result of its primary ventrotlorsal direction, before the caudal expansion of the cerebral hemisphere, the primitive tentorium at first borders the cranioventral, rather than the dorsal, aspect of the otic capsule. Not until the tentorium has been swung caudally by cerebral expansion into more nearly transverse position at 80 mm., as was described above in reference to the tentorial sinus it contains, does the part of the tentorium containing the meteneephalic channel come in contact with the dorsal crest of the expanded otic capsule.
During the time that cerebral expansion between 40 and 80 mm. carries the tentorium and its channels caudally (figs. 24, 25, pl. 1), otic expansion carries the pro-otic sinus cranially, thus separating the sinus from its tentorial tributary, the stern of the metencephalic vein (figs. 36, 37, pl. 2; fig. 18). The separation has been completed because the caudal end of the pro-otic sinus, as the result of a secondary anastomosis, now empties into the sigmoid by way of the petrosquamosal sinus, definitive in stage 7a (see below). ‘While being pushed forward by the otic capsule, the middle part of the pro-otic sinus dwindles; meanwhile the new cavernous and inferior petrosal sinuses, emerging in stage 7, annex the orbito-ophthalmic veins, of which the pro-otic sinus was formerly the exclusive drainage. Consequently, the tentorial end of the metencephalic vein, now the superior petrosal sinus, empties into the junction of the transverse and sigmoid sinuses. This secondary pattern of stage 7a, typical of the adult, obscures the fundamental pattern of the metencephalic channel of stage 7 and earlier, i.e. its derivation from the pro-otic sinus (fig. 18A, B). Expansion of the otic capsule dorsally tends to bring it into contact with the primary metencephalic channel. Thus, the superior petrosal sinus is pushed into definitive position on the dorsal margin of the enlarging otic capsule (later petrosal bone) by two advances: otic expansion cranially and from below, and cerebral expansion caudally and from above, the latter being reflected in the caudal swing of the tentorium.
Although definitive in position by 80 mm., the fetal superior petrosal sinus, it must be noted, is still not that of the classic textbook picture: it has no well defined communication eranially with the cavernous sinus. The primary role of the sinus in all stages is drainage of the cerebellar and pontile regions, as is notably true of its counterpart in other mammals. The inference that the definitive position of the sinus is the result of the relatively great enlargement of the cerebral hemisphere, plus chondrocranial otic expansion, explains the absence of a definitive channel in mammals generally.
Apparently the significance of the superior petrosal sinus, belated in mammalian development, has not been under stood. The channel has long been confused with the pro-otic sinus (e.g. Shindo, I915; Streeter, 1918; van Gelderen, 1933), which is a prominent primary channel, i.e. the stem of the middle dural plexus typical of early vertebrate embryos. Essentially, the superior petrosal is its tributary. inexact knowledge of the morphology and variations of the superior petrosal sinus in adult man—it is rarely as large as the inferior petrosal sinus, and its communication with the cavernous sinus is both secondary and inco11stant—is probably chiefly responsible for many errors in its identification in other mammals.
The superior petrosal sinus has also been confused with the tentorial sinus: for instance, the channel so identified in the adult horse, cow, dog (Dennstedt, 1904, pls. 1-3), and rat (Greene, 1935, figs. 218, 219) does lie in the tentorium but is not contiguous to the petrosal bone. Since the channel originates in the anterior, not the posterior, cranial fossa, there draining the anterior and middle cerebral veins, it is the tentorial sinus, i.e. the dural end of the “posterior rhinencephalic vein” described above; its form and connections in these adult mammals resemble those in the human embryo at 60 to 80 mm. (fig. I6). Confusion regarding these vessels is a fundamental error, because the tentorial sinus drains the largest veins cranial to the tentorium, whereas the superior petrosal sinus drains the chief vein of the posterior fossa.
Not only does the definitive superior petrosal sinus appear late in human development; it is a new formation phylogenetically. The following examples of its counterpart in adult mammals recapitulate stages of its development in man: (I) in the bat (Grosser, I901, fig. 36, pl. 20), the sinus is represented only by the dural end of the major metencephalic vein, i.e. the constant trigeminal vein (of I-lofmann, I901) characteristic of vertebrates; (2) in the horse and dog (Sisson, 1933), a homologous sinus, which, however, does not touch the petrosal crest, is the dural (tentorial) end of the major cerebellar vein, and enters the yentral (caudal) end of the transverse sinus, a pattern comparable to the 40-mm. stage in man; and (3) in the rhesus monkey, according to the author's studies, a definitive superior petrosal sinus is present, as the continuation of the great cerebellar vein, but it is relatively short, for the reason that it touches only the lateral part of the petrosal crest.
Fundamentally, in all species, the superior petrosal sinus is the major metencephalic vein of the posterior lossa, the proximal end of which becomes surrounded to a variable extent by dura, namely the tentorium, in so far as this is present. Application of the channel to the petrous crest apparently depends on the otic and cerebral expansion in certain primates. Furthermore, in contrast to the inferior petrosal, the superior petrosal sinus has no primary connection with the cavernous sinus. Any communication with a dural channel in the anterior fossa is a late development, perhaps belonging exclusively to man (the situation in most primates is unknown to the author). The primary cerebellar vein involved in the formation of the superior petrosal sinus, however, eommonly does anastomose with a pial vein of the anterior fossa, as will be discussed below in reference to the basal cerebral vein.
Petro_cqt1zm2o.ml _:irm.¢, an cnzismry of the c/zom1rocranirzl spurious jugrtlar foramen. Although the fore going discussion shows why it can be said in general that the superior petrosal sinus is an elaboration of a metencephalic tributary of the pro-otic sinus (Marl;owski, 1911, 1922), close inspection of consecutive stages (pls. 1, 2) shows it to be the result of a rather complicated rearrangement of these channels. This complex includes the petrosquaniosal sinus, which now constitutes, secondarily, the caudal end of the pro-otie sinus, leading into the sigmoid sinus. These alterations, dependent upon the developing skull, involve the interesting spurious jugular (“capsuloparietal") foramen of the chondrocranium, an opening definitive in stage 7.
Between 40 and 60 mm., a lateral (temporal emissary) tributary of the sigmoid sinus passes through the spurious jugular foramen and anastomoses, external to the temporoparietal lamina of cartilage, with a lateral (primitive dural meningeal) tributary of the pro-otic sinus (figs. 13A, 18A). This anastomosis is the primitive petrosquamosal sinus (stage 7). By 80 mm. (fig. 18B) the sinus is augmented to become the new caudal (sigmoid) end of the pro-otic sinus, the change being coincident with the utilization by the stem of the metencephalic vein of the old caudal (sigmoid) end of the pro-otic sinus in the formation of the definitive superior petrosal sinus. The petrosquamosal sinus, on the outer aspect of the chondrocranium, is now definitive in position, because it is being overlapped laterally by the emerging membranous skull (fig. ISB); thus it already suggests the subsequent intracranial and later diploic situation of the adult channel. A photomicrograph of a 69-mm. fetus (van Gelderen, x924, fig. 65) shows well the fact that the “emissary of the capsuloparietal foramen" is medial to the bone after becoming lateral to the cartilage.
The junction of the transverse sinus with the sigmoid sinus, or with any less well developed channel comparable to it, a junction that includes the stern of the petrosquamosal (pro-otic) sinus, is a region of considerable significance in mammalian development. VVe have seen that, ever since its formation in stage 5, the cranial (dorsal) end of the sigmoid sinus has received, more or less directly, all the drainage from the brain and also that from the orbit and dura (figs. 22-25, pl. 1). By 80 mm., coincidentally with the migration of the tentorial sinus described above, the sigmoid receives cerebral drainage even more directly; it drains the posterior (cerebellar) fossa by way of the superior petrosal sinus.
In spite of the fact that ventral drainage into the primary internal jugular vein is predominant at all stages of human development, evidence exists, between .jo and 80 mm., of potential drainage laterally into the external jugular system: sometimes a deep tributary of the external jugular anastomoses with a lateral tributary of the petrosqtiamosal sinus, which is the embryonic temporal emissary; this route, only potential in man. is augmented in a typical mammal (see below and pl. 6). Although the petrosquamosal sinus has become continuous with the pro-otic sinus, it is still emissary in that it passes through the spurious jugular foramen (cf. fig. ISA, B). This lateral opening in the chondrocranium is at least as large as the true jugular foramen, i.e. the internal, and is much larger, therefore, than the vessel it usually contains in man. The opening is adjacent to the junction of the major fetal sinuses, the transverse and the sigmoid.
The relatively constant spurious jugular foramen in cartilage (see stage 7) must not be confused with the later temporal foramen in bone, rare in man, which nevertheless is related to it, and has often been called by the same name. That both foramina, particularly the osseous in the human adult, have elicited the interest of comparative anatomists for a hundred years is reflected by many names, e.g. the foramen of Verga, Rathke, Fischer, Krause, Luschka, and Flower. The spurious jugular foramen transmits, pre- and postnatally, the caudomedial end of the petrosquamosal sinus, the embryonic temporal emissary. The temporal foramen, when present in man—it is typically large in other adult mammals—represents late ossification around an inconstant lateral tributary of the craniolateral end of the petrosquamosal sinus.
The significance of the embryonic spurious jugular and the postnatal temporal foramina and the vessels concerned has often been misinterpreted. Because of its predominance in many mammals, often to the extent of virtual absence of the internal jugular vein (pl. 6), the external jugular was assumed to be the primary jugular of vertebrates (see stages 1, 2). On this erroneous premise, it was believed that a lateral route of intracranial drainage, by way of a petrosquamosal emissary of the temporal region, characterizes early fetal life in man. Furthermore, both foramina and their vessels have been confused, as by Streeter (1918), with the extrachondrocranial remnant of the primary head-sinus (“vena capitis lateralis”); this remnant is just superficial and ventral to the otic capsule during stages 5 to 7. In one 80-mm. embryo of stage 7a, plexiform remnants of the head-sinus still accompany the 7th nerve (pl. 5C1) on the lateral aspect of the otic capsule. Although persistence in adult man of a large remnant of the head-sinus traversing the petrous bone is probably rare (except in cases of abnormal venous dilatation), a small remnant is normally represented by the plexiform superficial petrosal and stylomastoid veins; they accompany arteries of the same name throughout the intraosseous course of the 7th nerve. Any remnant in association with this nerve would, of course, be caudal to the external acoustic mcatus. In contrast, a position that varies but is cranial to the meatus, and sometimes also dorsal, is the rule for the osseous temporal foramen. The mammalian temporal foramcn, dependent not only on the volume of drainage it carries but also on the skull development of the region, varies considerably in size and exact location, examples of which follow. The conspicuous foramcn typical of mammals except certain primates lies ventrolaterally between the external acoustic meatus and the posterior glenoid process; it is usually called the “postgler1oid" foramcn (Greene, 1935; Miller, 1948; Sisson, 1953). In the rhesus monkey, an inconspicuous foramcn is more medially situated in the depths of the glenoid fossa; in accordance with the dural drainage conveyed, it was called the “posterior meningeal foramcn" (Hartman and Straus, 1933). A small foramcn that, nevertheless, is conspicuous, owing to its more dorsal position on the lateral wall of the skull at or near the root of the zygoma, has been seen in fetal monkeys and adult apes (Schultz, 1921, 1950, respectively), and is not uncommon in the human newborn (Limson, 1932). Anomalously, at any time after birth, a large foramcn may be found in the human squamous temporal bone (Streit, 1903). In any of these positions in man, a small foramcn (diameter about I mm.) for accessory drainage of tympanic or meningeal emissary veins may occur normally (p. 126); it is related developmentally to the lateral end of the so—called petrotympanic fissure (Glaserian; for precise naming of this fissure see later editions of the Cunningham Textbook, I951).
The significance of the emissary temporal vein of the temporal (“spurious jugular”) foramcn in mammals was appreciated by Shindo (1915). He called it the cranial tributary (“vordere Ast") of the transverse sinus, as distinguished from the sigmoid sinus, established much earlier, which is its caudal tributary (“hintere Ast"). Shindo describes the predominance of one or the other route of intracranial drainage in a series of adult mammals, on the basis of several reports, particularly Dennstedtls (r904): for example, the anterior route into the external jugular system by way of the temporal foramcn is well developed in the opossum, hedgehog, mole, bat, weasel, mouse, rabbit, cat, dog, cow, and horse. Although there was said to be no sigmoid sinus or internal jugular vein (small?) in one species of monkey (/1crol7:1.-ms pygmacm‘), the primary drainage through these channels does persist in the rhesus monkey (author's studies), to a variable degree. In the cat and pig, the anterior route was reported by Shindo to be undeveloped; Mivart (1895), however, found a posterior glenoid foramcn "occasionally" in the adult cat skull. Among the excellent illustrations of rat anatomy by Greene (1935), the transverse sinus is shown leaving the cranium through the postglenoid foramcn to join the internal maxillary vein (Greene, fig. 223).
Regarding comparative embryonic stages, the author has little information. The situation in the fetal pig, however, according to Mead (1909), resembles a comparable human stage: the spurious jugular (“capsuloparietal”) foramcn is notably larger than the vessel it contains, which represents the primitive petrosquamosal sinus of the human embryo at 40 mm. (fig. 16). The assumption is that a chondrocranial temporal emissary, which may or may not develop a sizable communication with the external jugular vein, is the rule in mammalian development. In any event, the basic pattern of representative adult mammals (cf. many illustrations of jugular veins by McClure and Silvester, 1909) is as follows: The temporal emissary (“vordere Ast”) is considerably augmented to become the vein called “dorsal cerebral" (Sisson, 1953) or “superior cerebral” (Dennstedt, 1904); the vein is the direct continuation of the transverse sinus and empties into the largest vein of the neck, the “jugular.” On the other hand, underdevelopment of a channel homologous to the human sigmoid sinus (“hintere Ast") of man is represented, respectively, by a small “ventral” or “inferior" cerebral vein that passes through the foramen lacerum in its part homologous to the internal jugular foramcn. Whether or not this vein, together with the primary internal jugular, becomes notably atrophic, it may also enter, secondarily, the “jugular” vein, i.e. the secondary external jugular, which becomes dominant during the embryonic development of a typical mammal (pl. 6A).
The relatively constant spurious jugular foramcn of the human chondrocranium, as first seen at 40 mm., was called the “capsuloparietal fissure” by Macklin (1914, 1921) because it occurs between two parts of the Chondrocranium, the otic capsule and the parietal lamina. This name is in distinction to the equally constant “capsulo-occipital" (mastoid) foramcn, similarly named and identified earlier in stage 6 (about 20 mm.; W. H. Lewis, 1920), which contains the primitive mastoid emissary vein. Lewis’ figures show that the future spurious jugular foramcn is indicated during horizon xx by a notch at the cranial end of the partly conjoined parietal lamina and otic capsule (cf. dotted lines, figs. 10A, r3A). By 80 mm., when the chondrocranium reaches its maximum development, both foramina are conspicuous: in reconstructions of 40- and 8o—mm. skulls by Macklin and by Hertwig (1907), respectively, according to adaptations of their illustrations elsewhere (Hamilton, Boyd, and Mossman, 1952; Gray's Anatomy, 1954), the spurious jugular is the more dorsocranial of the two openings (unlabeled) dorsal to the otic capsule; the more ventro caudal foramcn is the mastoid (cf. fig. 16A, dotted lines; fig. 18A).
Both emissary openings also occur regularly in the chendrocranium of other mammals as illustrated by de Beer (1937; the spurious jugular and mastoid foramina are labeled “superior” and “inferior occipito-capsular fissures,” respectively). The fact that the spurious jugular foramcn is relatively large and irregular in outline in many species, including man, and is thus notably out of proportion to the size of its contained vein, as compared, for instance, with the mastoid emissary vein and foramcn, is probably incidental but significant for the following reasons: the cartilaginous lamina that forms the craniodorsal border of the foramcn varies considerably in size and shape in clifierent species (and probably individuals); this lamina is consolidated much later than the otic capsule, to which it becomes partly joined by the parietocapsular commissure (so called in the human fetus)—a development which brings the irregular foramen (or fissure, often the more descriptive term) into existence. The spurious jugular is the most dorsally situated foramen, and the last to form, in the mammalian chondrocranium, which does not extend much above the base of the future adult skull, and varies most in its dorsal parts.
The early membranous bones of the skull in certain mammals are also shown by de Beer, including those adjacent to the spurious jugular foramen, namely the parietal and squamous temporal bones. Although none of these fetal skulls was sufficiently advanced to show ossification completely surrounding the foramen laterally, photographs of coronal sections of mammalian embryo-heads (in studies quoted by de Beer) show that conditions around the foramen resemble those in the human fetus, and subsequent development can readily be postulated. The temporal canal of mammals represents ossification around a channel comparable to the petrosquamosal sinus. Essentially, in all species this channel passes, at its medial end, through the spurious jugular foramen, typical in the cartilage, and, at its lateral end, through the temporal foramen, highly variable in the bone (cf. fig. 18B, C).
At the 80 mm. stage (fig. 18B) of human development, the definitive petrosquamosal sinus, i.e. the secondary channel that now receives the dorsal (caudal) end of the pro-otic sinus from which it was derived, lies between the chondrocranium and the primitive parietal and temporal bones. As ossification extends in later stages, the parietal lamina of cartilage in man is absorbed, to some extent, at least (Macklin, 1921). Consequently, the petrosquamosal sinus, in spite of lying on the outer aspect of the cartilage, comes to lie within the skull, since it is always medial to the membrane bones. Its position in all stages, cartilaginous and later osseous, is within a groove at the site of the adult petrosquamosal suture.
The fetal petrosquamosal sinus (fig. 18C) is homologous to the prominent “superior" or “dorsal cerebral vein" in the temporal groove or canal of typical mammals, as was noted above; by way of this sinus, the external jugular, which may become the only jugular vein, annexes most intracranial drainage from the primary internal jugular (pl. 6). The boundaries of the sinus vary in different species, but, to a greater or lesser extent, it is ultimately bridged over by bone. Such variable ossification also characterizes the occasional presence of the sinus in adult man. (See the Toldt Atlas, 1941, fig. 13,2; the osseous channel is part groove and part canal.) The petrosquamosal sinus frequently persists in the newborn, according to the author’s studies: it may be partly covered internally by paper-thin cartilage, obviously representing the fetal capsuloparietal commissure (cartilage) noted above, outside of which the channel always lies: its caudal end at the sigmoid sinus sometimes traverses a cartilaginous foramen. the fetal spurious ‘ jugular; in the young adult an osseous foramcn here may be the only remaining superficial evidence of the embryonic sinus.
In the typical adult, the petrosquamosal sinus, which is the relatively constant temporal emissary vein of the fetal chondrocranium, becomes part of the diploic system. The remnant of the sinus receives the posterior temporal diploic vein and also superior tympanic veins. The secondary and inconstant anastomosis between the fetal temporal emissary and the external jugular is represented by any adult anastomosis between a superior tympanic vein and an inferior tympanic tributary of the deep temporal vein; the inferior tympanic passes through the Glaserian fissure (p. 125) or a separate foramen lateral to it (identified in several skulls by the author). Any such anastomosis between inconspicuous tympanic veins is homologous to the major route of intracranial drainage into the external jugular, typical of many mammals (pl. 6).
Relation of dural meningeal sinuses to the arteries; other venous channels fundanzcntally extrac/zondrocranial. The ultimate diploic character of the pro-otic sinus in man is seen not only in its adult petrosquamosal derivative, just described, which may groove the inner table of the skull, but also in the conformation of its other important embryonic tributary, the stem of the rnirlclle meningeal sinuses accompanying the artery of the same name. Developing between 40 and 30 mm. (figs. 24, 25, pl. 1), these venous channels in the dura are the primary drainage of the membrane bones, and their primary relation to the middle meningeal arteries is of particular interest.
The primitive meningeal channels are also clarified by the situation in other mammals. For the adult bat, Grosser (1901, figs. 36, 37, pl. 20) described the counterpart of the petrosquamosal sinus as “diploic,” a situation comparable to the temporal canal of other species. The transverse sinus terminates in two channels: a vessel representing the sigmoid sinus (“hintere Ast" of Shindo, 1915; see above); and a “diploic transverse sinus” (his “vordere Ast”), which passes cranially over the petrous bone, enters the conspicuous temporal foramen, and joins the external jugular, a vein notably larger than the internal jugular, as is typical of mammals in general. In two adult rhesus monkeys examined by the author, the petrosquamosal sinus is not enveloped medially by ossification, but occupies an intracranial sulcus. the “posterior meningeal groove" (p. 125; Hartman and Straus, 1933), which is directly continuous with the meningeal groove in the anterior part of the middle cranial fossa. I-lcre it anastomoses with the external jugular system by way of a vein in a small temporal foramen.
The anatomy of the adult bat (Grosser) also illustrates the relation of the meningeal venous channels to the artery they accompany, a configuration comparable to that of the 40-mm. human embryo. The greater part of the embryonic stapedial artery constitutes the major meningeal (dural) artery, as in man, but even its stem from the internal carotid is permanent in the bat. Ventrally, this stem borders the lateral margin of the “transverse” (pro-otic) sinus. More dorsally, however, the relative position of artery to vein is reversed, so that the “meningeal ramus of the stapedial artery” is medial to the “diploic transverse sinus” (noted above), and partly enveloped by it.
The adult mammalian pattern just noted, which resembles that of the 40- to 80-mm. stages of human development (fig. 15C; cf. Grosser’s text fig. 18), exemplifies the typical relation of primitive arteries and veins to each other, discussed for the pial vessels in stage 4 (see figs. 6, 7, 8, I1; pls. 4A2, 5C2). In reference to the structure 1/ascz:lat1'izer1—-in the present instance, the membranous bone—arteries are superficial to veins, even though they are media] to the same veins in reference to the whole head. Their development explains the unusual conformation of the larger meningeal (dural) vessels in adult man. As the skull matures, i.e. as ossification occurs laterally around the dural vessels that vascularize it, a relatively large venous channel, consolidated from a previous plexus, often appears to be compressed lengthwise between the smaller artery it accompanies and the skull. The resulting partial obliteration of the long axis of the venous lumen, together with secondary venous anastomoses, gives the appearance of two veins bordering the meningeal artery, well illustrated for late fetal and adult stages by (Wood) Jones (1912). It should be noted that most arterial walls, external to the brain, at least, become definitely thicker than those of the veins by the 40-mm. stage, before which time arteries and veins do not accompany each other (cf. p. 119 and discussion in stage 4).
By stage 7:: it is clear that the pro-otie sinus, which has long been erroneously identified with either the superior petrosal or the tentorial sinus in several species including man (see above), is concerned with drainage not of the brain, but of its dural and osseous coverings. Although the primordial cartilage is not shown in the illustrations of earlier stages, it must be noted that the pro-otic sinus, which includes the stem of the middle dural plexus and a short segment of the primary headsinus (so-called “vena capita lateralis”; see stages 1, 2), typically lies on the outer aspect of the chondrocranium. In illustrations of a mouse embryo by Shindo (1915; pls. 28, 29; text fig. 4), comparable to stage 5 (fig. 9), the counterpart of the pro-otic sinus (labeled “anastomosis between transverse sinus and vena capita lateralis”) and its tributaries, the maxillary (infraorbital) and ophthalmic (supraorbital) veins, are also extracranial. The territory traversed by the pro-otic sinus, which is rostral to the otic capsule (later petrous bone), thus representing the middle cranial fossa and site of the adult foramina lacerum, ovale, and spinosum, remains permanently membranous in various mammals.
In man, ossification here is late, often postnatal, and is superficial to the entire course of the pro-otic sinus, including its petrosquamosal component. The remnant of the sinus, therefore, in late fetal life is intracranial and finally becomes diploic in the adult (fig. 18).
There is evidence that the inferior petrosal sinus, together with the cavernous sinus, both derivatives of the pro-otic sinus (stage 7), is also an extracranial channel from the phylogenetic standpoint, and is secondarily enclosed within the skull of primates exemplified by man. We have already seen that both ends of the inferior petrosal in the human embryo are derived from extracranial channels: cranially, from the pro-otic sinus, and caudally, from the dural end of the ventral myelencephalic vein (of stage 2), which joins the internal jugular below its foramen (figs. 20-25, pl. 1). Although the main part of the inferior petrosal sinus develops intraeranially, in reference to the cartilage, a similar anastomosis superficial to the bone constitutes the plexiform adult petro-occipital win,’ this lies just outside the skull and borders the fissure of the same name, as was well demonstrated in two adult corrosion specimens (acknowledged in the Introduction). As opposed to the main part of the pro-otic sinus, its cranial end, which gives rise to the cavernous sinus, must be considered intracranial in human development, since it is dorsal (medial) to the narrow root of the sphenoid cartilage (ala temporalis, fig. 18A, B) attached to the sella; even this end of the primary sinus is undoubtedly extrachondroeranial in other mammals.
In the horse and cow, the region of the pro-otic sinus is neither ossified nor cartilaginous in the adult (Sisson, 1953). Dennstedt’s plates I and 2 (1904) show a conspicuous “emissary vein,” the counterpart of the pro-otic sinus, in a position just superficial to the skull; this vein anastomoses both the ophthalmic veins and the “veins of the subtemporal fossa” (maxillary vein) with the “superior cerebral vein" (comparable to the petrosquamosal sinus of man), which traverses the temporal (postglenoid) foramen. Moreover, the inferior petrosal sinus in the adult horse is also extracranial (Dennstedt’s pl. I and text fig. 2). Whether this sinus, a secondary anastomosis, develops in the embryo horse on the inner or on the outer side of the chondrocranium is uncertain (see below). It unites channels, however, which are extracranial in all stages of man and are probably comparable, namely the pro-otic sinus (cranially) and the primary myelencephalic tributary of the internal jugular vein (caudally). Furthermore, the otic and basioccipital eartilages have fused by the 40-mm. stage (de Beer, 1937), so that the site of the sinus (also the site of the osseous petro-occipital fissure in man) in the equine ehondrocranium resembles that in the human embryo of the same length (Macklin, 1914, 1921). Although de Beer's specimen may be atypical, apparently more fetal absorption of the secondary cartilage at the otic-basioccipital junction must occur in the horse than in man. In any event, this region is notably unossified in the adult equine skull, according to Sisson (1953, fig. 53), thus constituting a large “foramen lacerum" around the whole medial half of the petrous bone. As a result, the inferior petrosal sinus lies in dural membrane at the floor of the skull.
The apparent absorption of embryonic cartilage just noted is comparable to that presumed to take place elsewhere in the human fetus, for instance, in the chondrocranial capsuloparietal commissure medial to the petrosquamosal sinus (see above); here some variable absorption must occur coincidently with the ossification of the membrane bones (fig. 18) in order to result in the intracranial position of this sinus (sometimes in man and in certain primates, e.g. the rhesus monkey).
In summary, it is interesting that the embryonic prootic sinus—the primary stem of the middle dural plexus, not very aptly called the “middle cerebral vein” in vertebrate embryology—togetber with its tributaries seems to be transitional from the standpoint of both ontogeny and phylogeny: The sinus first drains the brain (meteocephalon, stages 2 to 5), later the orbit (stages 6, 7), and finally the dura and bone (stage 7:1,‘ see pls. I, 2). Its medial and cranial end, relatively short, is intrachondrocranial, i.e. the part of the primary head—sinus between the narrow cartilaginous temporal wing, when present, and the trigeminal ganglion; its greater length is extrachondrocranial and extends laterally in bordering the otic capsule (fig. 2B). The sinus may ultimately be represented in mammals by venous channels lying on either the inner or the outer side of the mature skull, or both, or by those within its substance (diploic).
Formation of the Basal Cerebral Vein
It has been seen that the growth of the brain and primitive skull between stages 7 and 7a has initiated significant alterations in the dttral sinuses. These alterations, continued until birth and after, require adjustments of the cerebral and cerebellar veins and are responsible for a completely new channel, which has not been well understood, the important [marl (cc’r'c'[>m[)” vein (of Rosenthal) (figs. 16, 17, 18). Although this vein and many of its tributaries are noted briefly in most anatomical descriptions, one of the very few current illustrations is a diagram in Bailey (1948). In the adult the basal cerebral vein is often as large as the internal cerebral vein, which it joins (bilaterally) to form the great cerebral vein (of Galen). Unlike that of the primary Galenic veins, however, the formation of the basal vein is complicated, and certain aspects of the development of the primitive brain up to this time must be reviewed to understand why it comes into existence.
1" The modifier ¢‘rr':*[mz/ (agreeing with Hofmann, 1901) is used here for emphasis. The term /Jami (RNA) is preferable to “basilar," because the vein is not comparable to the basilar artery and has no primary connection with the basilar dural plexus ventral to it.
In spite of the conspicuous expansion of the primordial cerebral hemisphere by 80 mm., its bulk still consists chiefly of the lateral ventricle almost filled by the relatively huge choroid plexus (fig. 16). The dorsolateral brain wall is relatively little thicker than that of the 40-mm. embryo, and still comprises the three primary layers, ependymal, mantle, and marginal, of which the ependymal, where the cells of the future cortex are arising, appears to be the most active. Well injected specimens show the many notably straight veins (and arteries) that pass directly laterally from the ependymal layer to the external surface; these vessels are collected by the pial tributaries of the superficial middle cerebral veins and, to a considerably lesser extent, those draining into the primitive superior sagittal and transverse sinuses.
It is important to recall (see stage 7) that, in contrast with the adult brain, little if any part of the primitive brain of stage 7a, including the corpus striatum protruding into the ventricle, is drained as yet into the conspicuous, but primitive, internal cerebral vein. The explanation is that this vein is primarily the continuation of the superior choroid vein and courses over the part of the diencephalon that is membranous (tela choroidca of the third ventricle). Accordingly, the internal cerebral vein touches brain tissue only at the interventricular foramen (of Monro). Here it receives one primitive intracerebral vein from the region of the anterior thalamus, as was noted for stage 7. The thickened thalamic wall, which has reduced the third ventricle to a narrow slit, is drained by tributaries of the primary ventral and dorsal diencephalic veins. The hypothalamic region is also drained by the ventral diencephalic vein, reinforced cranially by tributaries of the deep tclencephalic vein (secondary pial derivative of the superficial middle cerebral veins), which is still the exclusive drainage of the corpus striatum. Until stage 7:1, all the veins draining the forebrain are still tributaries of the tentorial sinus emptying into the transverse sinus near its junction with the sigmoid sinus. This drainage route, however, is now remote, owing to cerebral expansion and the resulting caudal swing of the definitive transverse sinus, described above. A shorter route is about to be substituted, therefore, into the great cerebral vein by way of a new basal cerebral vein.
It is interesting that the internal cerebral tributary of the great cerebral vein—essentially a superior choroid vein, the first component of the adult Galenic system to appear, and the most constant and uncomplicated in form at all stages—does not fulfill its final role of draining parts of the internal brain until sometime after the separate components of the basal cerebral vein, a secondary Galenic tributary that develops in a complex way, have assumed this function.
To understand the emergence of the basal vein. it is useful to review fundamental principles of the formation of all pial veins discussed in stage 4, as supplemented by basic adult patterns in a series of vertebrates (well illustrated by Hofmann, Igor). The simplest pattern is seen on the spinal cord, where an intervertebral (intersegmental) collecting vein primarily accompanies each nerve root. Later, pial tributaries of these transverse veins are joined by secondary longitudinal anastomoses, and these, in turn, are irregularly joined in similar fashion across the mid-line; as a result, some of the transverse collecting veins accompanying the nerve roots from pia to dura drop out (see also Herren and Alexander, 1939). The anterior and posterior spinal veins, however variable in detail in different species, are formed in this way. At the medulla and pons, where the nerve roots are relatively closer to each other, the primary transverse piaarachnoidal veins extending from the pia to the dura (originally numerous along the dorsolateral aspect of the neural tube) are typically reduced to three in the vertebrate series: a “hypoglossal,” a “vagal," and a “trigeminal" (Hofmann). It was seen in stage 4 (fig. 8) that the ventral myelencephalic (“vagal”) vein of the human embryo, anastomosed caudally with a spinal vein, makes another longitudinal anastomosis cranially on the medulla and pons with a pial tributary of the ventral metencephalic (“trigeminal”) vein. The specialization of the fore parts of the neural tube obscures the basic pattern somewhat, but the veins at its base are formed in the same way. According to a succinct remark of Dr. Frederic A. Gibbs (personal communication) about his venous injections of the cat, “the cerebral venous drainage may be interpreted as a bulge in the cord drainage.” As illustrated by Hofmann, the “basal cerebral vein" of adult mammals is the cranial counterpart of the “basal vein of the medulla oblongata” on the hindbrain, with which it is usually more or less continuous. The caudal end of the basal cerebral vein is an anastomosis between a “lateral mesencephalic” tributary of the “trigeminal vein”—this is the largest and most constant of the three transverse piaarachnoidal veins of the hindbrain—and the "superior mesencephalic” tributary of the great cerebral vein. The cranial end of the basal vein lies between the hypothalamic and temporal-lobe regions, where it receives telencephalic (anterior and deep middle cerebral) and diencephalic tributaries; it is usually not symmetrical in the mammals illustrated, and is not always continuous, being sometimes represented by more than one vein along its course.
Hofmann shows that the most primitive exit for the basal cerebral vein (his text figs. 1-6) is lateral, i.e. by way of the “trigeminal" vein, the stem of which is the mammalian counterpart of the human superior petrosal sinus draining the cerebellar region, as was described above. This finding again exemplifies the fact that, typically, in adult mammals, as in the human embryo, virtually all the veins of the brain are laterally drained into the junction of vessels representing the sigmoid and transverse sinuses (pl. 6). Medial drainage of the basal cerebral vein into the Galenic system, by way of a channel lying between the midbrain and thalamus, is secondary from the phylogenetic standpoint; this route is often predominant in certain primates typified by adult man (see below). According to I-Iofmann, the basal cerebral vein, as exemplified in the chick, rabbit, guinea pig, cat, dog, and horse, empties more laterally into the counterpart of the transverse sinus by way of the “trigeminal vein” than medially into the Galenic vein. In the rabbit, pig, cat (Hofmann), and dog (Bedford, 1934(1), it has both lateral and medial communications with the transverse and straight sinuses, respectively, by way of the veins just mentioned. Bedford’s studies (193412) show the lateral route to be also frequent in the rhesus monkey. In a specimen dissected by the author, the basal cerebral vein on one side drained more laterally, that on the other side more medially—a “combination or transitional type,” according to Bedford.
From the appearance of adult mammalian veins at the base of the brain as compared with those of the present fetal stage 7:1, it is clear that the basal cerebral vein of all species emerges in essentially the same way. In the 60 to 80 mm. embryo, the course of the vein is well outlined by segments of a new longitudinal anastomosis connecting the pial ends of three or four of the primitive transverse (pia-arachnoidal) cerebral veins, as follows: (1) the telencephalic vein, near the junction of its deep middle cerebral and anterior cerebral tributaries; (2) the ventral diencephalic vein, draining the choroid plexus and the region of the descending horn of the lateral ventricle through a well defined tributary; (3) the dorsal diencephalic vein, which may be represented by a dorsal tributary of the ventral diencephalic vein; and (4) the dorsal diencephalic vein, anastomosing with one of several tributaries of the great cerebral vein in the region of the pineal primordium (fig. 16; cf. figs. 34, 35, pl. 2).
The primitive great cerebral vein is seen earlier in stage 7 (40 mm.) on the membranous roof of the third ventricle near the dorsolateral aspect of the thalamus (fig. 13); it drains a plexus extending cranially to the interventricular foramen, which communicates with the internal cerebral vein at its junction with the voluminous superior choroid vein and a small, deep thalamic tributary. As was noted above (stage 7), a component of this plexus undoubtedly represents the future terminal vein, the major tributary of the adult internal cerebral vein, which either supplements or annexes internal cerebral drainage from the primary pia-arachnoidal veins of the ventral surface of the brain; the vein ultimately becomes enclosed in the ventricular wall during the secondary embryonic juxtaposition of the thalamic and caudate nuclear regions, owing to development of the internal capsule.
The formation of the basal cerebral vein, like that of the terminal tributaries of the internal cerebral vein, is a result of the expansion of the cerebral hemisphere, particularly caudally. The vein compensates for the elongation not only of the tentorial sinus emptying into the transverse sinus but also of the primitive transverse diencephalic and mesencephalic veins, which are now stretched over the expanded temporo-occipital lobe in reaching these sinuses; these veins become attenuated near their entrance into the dura, where they will usually sun, mr.
Adult
Adult patterns (in an infant head). Note particularly the following: (A) the conformation of the basal and internal ll vein; remnants of the tentorial, pro-otic (clural meningeal), and (bilaterally) to form the great cerebr: petrosquamosztl sinuses, l exit of the inferior petrosal sinus; the emissary veins; three primary veins draining the otie region (unlabeled); (13) the remnant of the tentorial sinus, sometimes constituting a superficial vein; the great ns. The tributaries of the basal vein, itself second anterior cerebellar vein: the tentorial lacuna that receives the posterior cerebellar vei ith the great anterior cerebellar vein constitutes the lateral mesence ary and variable, are significant primary veins; its anastomosis w pltalic vein, potential drainage for both the basal and internal cerebral veins.
130 A1. INFANT on Fm. 17.
cerebral veins, meeting - the deep facial vein; the extracrania Fm. 18. Four stages in the vessels at the base of th D to be correlated with fig. I pro-otic sinus tl\\'in(llCS and la generally); the tlural end of the metencephnlic (anterior cerebellar) the tentorial sinus varies in pre— and post-n:1t;tl life (:\-D). \V arily connect the cavernous sinus cranially (:trro\\'s, D).
C. INFANT "7""-'9"-" D. Aouu 131 I-:m:Bt-:L.v._ e chondrocranium (A, B) and the skull of the infant (C) and adult (D; C and 7). Expansion of the one capsule between 60 and 80 mm. modifies several prominent channels: the L-comes continuous with the petrosqurtmosnl sinus (comparable to the temporal emissary of mcunmals. vein (A) becomes the superior petrosal sinus The position of hen it is situated metliatlly (B, C), remnants of the sinus may secondwith the superficial mitltlle cerebral veins, and crtuclnlly with the superior petrosztl sinus be obliterated. When fully developed, the adult basal cerebral vein is often as large as the internal cerebral vein and may drain as much (if not more) territory. These veins, together with those of the opposite side, meet to form the great cerebral (Galenic) vein (fig. 17). The complicated formation of the basal vein from elements of widely separated embryonic veins explains its frequent existence in somewhat fragmentary form, as is well illustrated for other mammals by Hofmann (I901).
Variable lateral outlet of basal vein. The significance of an important adult modification affecting the caudal terminus of the basal cerebral vein in man, apparently unknown in general (see Padget, 1956), should be emphasized. According to the usual description, the vein empties medially into the internal (or great) cerebral vein. It may empty laterally, however, either in part or entirely, into the sigmoid sinus by way of the superior petrosal sinus, which represents the primary metencephalic vein.
The alternative or supplementary routes by which the basal vein empties either caudomedially or caudolaterally are better understood by reference to the development of comparable channels in other mammals, referred to above. Apparently lateral drainage of the basal vein is the more primitive type; it is cerebellar (infratentorial) with respect to the vessels involved, in contrast to medial drainage, which is cerebral (supratentorial). Drainage through the posterior fossa, frequently predominant in vertebrates, is by way of the “trigeminal vein,” which is obviously a biological constant; the vein is homologous to the great anterior cerebellar vein (see below), including its outlet, the superior petrosal sinus of man, which is derived from the pro-otie sinus (stem of the middle dural plexus, typical of vertebrate embryos). The stem of the embryonic metencephalic (later trigeminal or anterior cerebellar) vein lies at the level of the 5th nerve root. A secondary anastomosis between one of its tributaries and the developing basal cerebral vein on the diencephalon must pass across the midbrain and thus incorporate part of the embryonic mesencephalic vein. The first stages in the longitudinal basal anastomoses between the primitive telencephalic, diencephalic, mesencephalic, and metencephalic veins were seen in several well injected embryos of the present series (figs. 6, 8, 11, 16; pl. 5C1).
A postnatal vein, often well defined in or near the lateral mesencephalic sulcus of the brain stem (fig. 17A1), results from the embryonic anastornoses forming the basal vein, and hence may be called the lateral mesencephalic (anastomotic) vein. Constituting an anastomosis between the great anterior cerebellar vein and the basal vein, near its junction with the internal cerebral vein, the mesencephalic vein was conspicuous in several newborn and adult brains dissected for the present study, and in one of two corrosion specimens (see Introduction). The only adequate illustration of an anatomical specimen known to the author, however, is by Hédon (1888, pl. 2). I-Iocbstetter (1938) reported in detail on a human adult in whom the left basal cerebral vein emptied into the superior petrosal sinus, thus obviously representing a persistent and well developed lateral mesencephalic vein; on the right side, the basal cerebral joined the great cerebral vein, as is commonly described for adults—an example of Bedford's “combination type" (in the monkey), referred to above. Such exclusive or accessory lateral drainage of the basal cerebral vein, potentially important and including, of course, that of the internal cerebral vein, is undoubtedly more frequent than is generally realized.
Cerebellar Veins
Thus far, drainage of the cerebellum has been mentioned only incidentally. With regard to the veins of the cerebrum, it has been seen why those near its base are the first to develop. The often conspicuous, but highly variable, anastomoses in the adult between the superficial middle cerebral veins and the tributaries of the superior sagittal and transverse sinuses, namely the great anastomotic veins of Trolard and L’Abbe', are secondary; they are not identified until sometime after 80 mm., when the expanded dorsolateral cerebral wall, as contrasted to the more precocious medial parts comprising the basal nuclei, has begun to differentiate (between 3 and 6 months, according to Hédon; see his pl. 5). Similarly, very few of the larger adult cerebellar veins may be identified at the 60- to 80-mm. stage, because the adult hemispheres are still represented by the cerebellar plates only; these primordia have become fused cranially in the mid-line, but have not yet grown caudally to cover the membranes of the fourth ventricle (fig. 16A). The primary and most conspicuous vein of the cerebellum, therefore, is located at its base in the fore part of the posterior fossa. This is the ventral metencephalic vein, which emerged in stage 4 (fig. SB) and has constantly provided drainage of the primitive pons and the cerebellar plate (pls. 1, 2). Although its proximal end becomes enclosed in the dura above the petrosal crest, to constitute the essential part of the definitive superior petrosal sinus, as was discussed above, the ventral metencephalic vein is clearly homologous to the relatively constant “trigeminal vein” of vertebrates (Hofmann, 1901); it has a comparable position and drainage.
The important primary cerebellar vein just noted has been given no specific anatomical name in the human adult. lts conspicuous trunk, entering the superior petrosal sinus, however (shown unlabeled in several textbook illustrations of actual specimens), is well known to neurosurgeons, and has been called the “petrosal" or “petrous” vein (Bailey, 1948); Hédon (I888, fig. I, pl. 4) designated it the “vein of the pneumogastric lobule,” in reference to an old name for the Hocculus, near which its tributaries are consolidated into one or more main stems before they enter the superior petrosal sinus. The vein drains parts supplied by all three cerebellar arteries, particularly the anterior inferior, and also the territory of the internal auditory artery. As a primary vein constituting the predominant drainage of the metencephalon, it is comparable in the adult to the great cerebral vein, which secondarily develops intra- and extra-cerebral tributaries. The superior, inferior, lateral, and medial cerebellar tributaries of the metencephalic vein in the adult include drainage of the pons and medulla, and also the dentate nucleus (fig. 17B), as was noted by Bailey. In view of its importance, the name great anterior cerebellar vein is appropriate (fig. 16AI).
In the embryo of stage 4, the conspicuous ventral metencephalic (“trigeminal”) vein anastomoses over the pons and medulla with the smaller ventral myelencephalic (“vagal”) vein, from which the inferior petrosal sinus is derived (stage 7). Hence, the myelencephalic vein may become an inferior nzea"ullm-y tributary of this sinus; it is often annexed, however, by the predominant great cerebellar vein, which drains, therefore, all brain tissue of the posterior fossa except the most dorsal and caudal parts of the cerebellum, the last to develop. VVhen present, the mezliarz superior cerebellar vein of the adult (designated and pictured by I-Iédon; also called the “superior vermian,” Morris’ Textboo/( of Anatomy, 1953) leaves the summit of the vermis to enter the great cerebral vein; it may ultimately be represented among the many tributaries of the great anterior cerebellar vein. At stage 7a, its stem is sometimes seen among the plexiform channels in the region of the tentorial plexus, the future torcular (fig. IGA).
A relatively inconspicuous vein of the embryo is the dorsal metencephalic vein of stage 7a (exaggerated in fig. 16A), first identified in late stage 4. This vessel is ultimately represented, however, by a prominent group of adult veins, (including those exposed by the surgical suboccipital approach), which must he classified; to them, again, no precise anatomical name has been assigned. From the developmental standpoint, the cerebellar veins are essentially comprised of only two main groups, commonly joined by anastomoses. The more primitive group, developmentally and phylogenetically, constitutes the anterior cerebellar veins, which often terminate in the one conspicuous primary trunk just described, the essential component of the superior petrosal sinus (see above). In many cases, these veins drain all except the most caudal aspects of the cerebellar lobe. The posterior cerebellar group, which develops parallel with later cerebellar expansion, includes prominent superior and inferior tributaries, which were well illus trated for the adult by Hédon (1888, pl. 3). The interesting way in which they often enter the transverse sinus is clarified in the present study.
Primarily, the posterior cerebellar group of veins do not enter the transverse sinus as commonly portrayed, i.e. at various intervals between the superior sagittal and sigmoid sinuses. Instead, posterior cerebellar tributaries, superior, inferior, and lateral, meet in loops, either within the tentorium or densely attached to it, and join a lacunalike intratentorial sinus; this in turn joins the transverse sinus through a more or less constricted (necklike) stem near the torcular (fig. 18C, D). The stem of the lacuna represents the tentorial end of the dorsal metencephalic vein of the present fetal stage (fig. 18B). In other words, the posterior cerebellar group of veins, like the anterior cerebellar, commonly enters the sinus by way of a single trunk, a primary vessel. This pattern, which must be frequent, has been well demonstrated bilaterally in adult specimens, including two corrosion preparations acknowledged in the Introduction. It is interesting that Gibbs and Gibbs (1934), in a dynamic study of the torcular region, incidentally pictured this tentorial lacuna: as a diverticulum of the transverse sinus, it was noted in 19 of their 25 corrosion preparations, bilateral in 17. The significance of the diverticulum was not relevant to their study, but they designated it the “tentorial sinus”; from the developmental point of view, it may more precisely be called the cerebellar tentorial sinus in distinction to the cerebral tentorial sinus,” described above, which is the primary drainage of the superficial middle cerebral veins. Although the caudal ends of these separate dural channels, various remnants of which are often found at birth and even later, may become essentially identical, one is primarily related to the cranial or cerebral layer of the tentorium, the other to its caudal or cerebellar side.
Whereas the cerebellar tentorial sinus superficially resembles a lateral lacuna of the superior sagittal sinus, the tributaries of each are primarily different: the cerebellar tentorial sinus receives essentially all the posterior cerebellar veins, but the cerebral dural lacunae are dilatations in adult life of the dorsal ends of the middle meningeal sinuses (“veins”; well illustrated in the Grant Atlas, 1951, fig. 479). Primarily, the parasagittal lacunae do not receive the superior cerebral veins, as was emphasized for the adult by Bailey (1948). Nevertheless, since both groups of dural channels are derived from the anterior dural plexus (see stages 1, 2), specifically, from its components in the outer and inner dural layers, respectively, communications between them may persist, particularly in pathologic vascular conditions.
1"‘ Sp/ieltotempoml sinus is often definitive for its postnatal remnant (p. 122). It should be noted that the term “tentorial sinus” was formerly applied to the straight sinus (rcctus).
Summary
Full understanding of the complex pattern of veins in the region of the human head has been delayed because of inadequate knowledge of the basic plan. Particularly if complicated by variations, including arteriovenous anomalies, the plan is clear only when the stages of venous development in man are illuminated by comparison with embryonic and adult patterns in other vertebrates. Definitive venous channels emerge from the primitive vascular net later than do the arteries, which influence their development; furthermore, the complicated venous anastomoses are essential to facilitate a greater adjustment to the changing needs of their environment over a considerably longer period. The following outline of stages, which necessarily overlap, can be followed by reference to plates I and 2; these are comparable to plates similarly numbered in the arterial study (Padget, 1948), and are also accompanied by legends that summarize the sequence of events in man. (Vascular stage I is not represented in this pictorial summary of the veins; see p. 82.) Stages 1 and 2 (fig. 19, pl. 1; fig. 27, pl. 2) of vascular development are similar in vertebrates. Characteristic features of the head are the pharyngeal bars, which are homologous to the gill-bearing arches of fishes and larval amphibians. In amniotes, the aortic arches in the first three bars contribute to the formation of the common and internal carotid arteries. Their dorsal branches supply a capillary plexus in the future pial layer, which drains through many short vessels, dorsolateral to the neural tube, into a superficial venous plexus of the future dural layer. By way of three relatively constant dural stems, anterior, middle, and posterior, this plexus drains ventrally into'a primary head-sinus (“head-vein"), continuous with the anterior cardinal vein, which constitutes the primitive (internal) jugular vein. As the first two aortic arches are succeeded by a ventral pharyngeal artery (forerunner of the external carotid), the future facial region is drained by a corresponding vein, which becomes the typical linguofacial (common facial) tributary of the internal jugular vein. The optic vesicle is vascularized by prominent vessels in the emerging maxillary process, the spinal cord by intersegmental vessels, which are precursors of the vertebral system of arteries and veins (figs. 19-21, pl. 1).
During stages 3 and 4, the dural venous channels come to lie more laterally as the cerebral hemisphere and rhombic lip expand and the otic vesicle enlarges (figs. 2;—_2,o, pl. 2). As a result, the head-sinus and the primitive internal jugular—this vein, not the external jugular, is the primary neck vein of vertebrates——migrate laterally to certain cranial nerve roots except the trigeminal ganglion, medial to which the head-sinus appears to be anchored by its voluminous maxillary tributary (figs. 20, 21, pl. 1). VVith continuing separation of the dural and pial layers of venous channels, most of the numerous primary anastomoses between them drop out. The few remaining anastomoses enlarge and thus constitute the stems of the primary veins, which pass transversely through the pia-arachnoid (fig. 30, pl. 2). Sometimes, by late stage 4, at least one such vein for each of the five divisions of the brain can be identified (fig. 6). The pial veins develop as secondary anastomoses between the primary pia-arachnoidal veins; they probably represent an elaboration of channels in the parts of the primitive capillary mesh not used by the preceding arteries, which, in becoming definitive, are somewhat elevated from the neural tube. The intrinsic pial veins consequently pass under and at a right angle to the arteries; this fundamental pattern is particularly clear around the basilar artery and its branches (fig. 8).
At the end of stage 4 (fig. 21, pl. 1; figs. 29, 30, pl. 2), drainage of the mammalian head is comparable to that of adult reptiles. The brain is still drained through the primary head-sinus, which leaves the primitive skull between the medial side of the 5th nerve and the otic complex. In birds, the caudal end of the head-sinus, after becoming crowded dorsally by the hyoid pharyngeal pouch, is replaced by a secondary anastomosis between'two vessels: one is comparable to the linguofacial vein; the other, to the ventral end of the middle dural stem, which constitutes the extrachondrocranial pro-otic sinus. In all stages of reptiles and birds, the head is drained by the primary internal jugular system representing the anterior cardinal vein. An external jugular vein apparently is not a typical development.
By stage 5 (fig. 22, pl. 1), which represents a fundamental pattern in mammals, the caudal end of the head-sinus, continuous with the internal jugular vein, has become crowded by otic development as in birds, but more laterally than dorsally. The head-sinus, therefore, is replaced by a secondary anastomosis that is intrachondrocranial, namely the sigmoid sinus, dorsal to the otic capsule. A similar anastomosis formed cranially is the very primitive transverse sinus, at first recognizable only through its connections. The facial region is drained cranially by the prominent ophthalmic and maxillary tributaries of the head-sinus. and caudally by the linguo— facial tributary of the internal jugular.
Stage 6 (fig. 25. pl. 1) is particularly significant in mammalian development. It marks the emergence of the external jugular system, which succeeds the external carotid. Thereafter, the essential difference between the venous pattern of most macrosmatic mammals and of primates resembling man is that the secondary external jugular system annexes most components of the primary internal system in those species in which the brain becomes relatively less developed than the face and neck (pl. 6). At stage 6, however, all the brain except the medulla is drained into the junction of the sigmoid sinus (or its counterpart, underdeveloped in many mammals) with the primitive transverse sinus. Here, the cerebral veins empty by way of a conspicuous tentorial sinus (rhinencephalic vein typical of mammals), bordering the ventrocaudal aspect of the hemisphere. The primitive supraorbital and infraorbital (maxillary) veins also drain to this significant junction of channels by way of the extrachondrocranial pro-otic sinus.
By stage 6 (figs. 31, 32, pl. 2), venous asymmetry of mid—line channels has become conspicuous in human development, for which compensation is possible after the appearance of a new channel. VVhen, as a result of cardiac development, the sinus venosus constitutes a substantial detour for flow from the left side of the head, both the primitive superior sagittal and straight sinuses in the embryo drain more to the right. In certain mammals, including man, an excessive detour to the heart is either more or less counteracted by the formation of the left innominate vein—a transverse anastomosis between the bilateral anterior cardinals (internal jugulars). If the secondary innominate later becomes essentially symmetrical with the primary right innominate vein (segment of the anterior cardinal), as does occur in many mammals (pl. 6A), a relative symmetry of the torcular pattern can result in the meshes of the tentorial plexus. Persistence of asymmetry of the innominate veins in anthropoid primates typified by man, however, is often reflected in persistent asymmetry of the sinuses at the torcular (pl. 6B).
In stage 7 (fig. 24, pl. 1), the secondary external jugular vein, just formed, begins to annex many tributaries of the primary internal jugular system, including the anterior facial (external maxillary) vein; this facial vein was formed in stage 6 by anastomosis of a tributary of the linguofacial vein with that of the maxillary vein, the stem of which has now become the outlet of all the veins in the orbit. The primitive emissary veins, which arose earlier to drain extracranial structures medially into the sinuses, become definitive by anastomosis with external jugular tributaries. The mastoid and temporal emissaries are particularly significant, and traverse foramina relatively constant in the mammalian chondro— cranium (fig. 33, pl. 2). The mastoid vein, developed in response to the cervical musculature, thus may compensate for the small sigmoid sinus and internal jugular vein typical of adult mammals (pl. 6A). The embryonic temporal emissary (of the spurious jugular foramen in the chondrocranium) at first drains the region of the temporoparietal ossification centers medially into the sinuses; its stem later constitutes the fetal petrosquamosal sinus, which finally becomes diploic (tympanic) in man (fig. 24, pl. 1). The human pattern, however, is the exception in mammals. Generally, the petrosquamosal channel enlarges to become the chief drainage of the brain into the external jugular system, a secondary anastomotic development that is accompanied by secondary dwindling of the internal jugular vein. These contrasting patterns are shown diagrammatically (pl. 6).
The primary stems of the pia-arachnoidal veins draining the brain (stage 4) increase in length as the cerebral hemisphere expands before stage 7a. Meanwhile the Galenic system of intracerebral drainage emerges as the result of accelerated growth of the basal nuclear masses and the choroid plexus almost filling the lateral ventricle, of which the bulk of the primitive cerebral hemisphere is constituted. The straight sinus, continuous with the great cerebral and internal cerebral veins, is primarily, however, the outlet of the superior choroid vein only (fig. 24, pl. 1); the terminal vein, and its intracerebral tributaries, are developed later, parallel with pallial expansion. In this and earlier stages, the choroid plexus, corpus striatum, and diencephalon are drained by primary pia-arachnoidal (telencephalic and diencephalic) veins, tributaries of the tentorial sinus that are incorporated into the later basal cerebral vein.
The extrachondrocranial pro-otic sinus (primary stem of the middle dural plexus) is the caudal outlet of the supraorbital and infraorbital veins until stage 7, when development of its secondary medial derivatives, the cavernous and inferior petrosal sinuses, substitutes an intracranial route in man. In other mammals, orbital drainage is chiefly annexed by the external jugular vein. V‘Vhile being pushed craniolatcrally by the expanding otic capsule, the middle part of the pro-otic sinus dwindles in the human fetus (figs. 36, 37, pl. 2). Meanwhile, its lateral tributaries emerge to drain the frontal and subsequent parietal ossification centers; these centers are supplied by the middle meningeal artery, which annexes the stapedial branch of the internal carotid. The stem of the meningeal artery is just lateral to the pro-otic sinus, but the branches of the artery and tributaries of the sinus reverse this position: the venous channels, in other words, lie between the arteries and the primitive bones. As is clear in stage 4, the pia-arachnoidal veins, in approaching the sinuses, likewise change their position relative to the arteries. VVhile they are both endothelial tubes, pial veins cross at right angles under the arteries, and consequently they do not parallel one another. VVhen the arterial walls have notably thickened, the dural meningeal vessels, for instance, accompany each other. Similarly, the intimate relations in the cavernous sinus are not established until stage 7 (40 mm. in man).
The primitive membrane bones of stages 7 and 7a (figs. 33-37, pl. 2) are not vascularized by the vessels of the scalp—a region initially avascular. Furthermore, the scalp plexus when it appears is not the result of a vascular cleavage, which occurs, it must be noted, only between layers of venous channels, specifically those of the dura and pia. Instead, the scalp plexus is separately derived from the external jugular and carotid systems during stage 6. Marked externally by a conspicuous margin, the plexus advances up over the vault on all sides, separated from the bones by a considerable interval of avascular tissue. The hiatus in the plexus closes sometime after the so-mm. stage in the region of the future parietal foramen. Among the emissaries, which typically comprise primary vessels at the skull base, those of the parietal (and associated, but very rare, occipital) foramen appear to be unique. The entire complex, arterial and venous, seems to be a secondary anastomosis not incorporating primary vessels, and suggests genetically vestigial remnants of vascularity in the region of an ancestral pineal eye. This interesting region of the cranial vault, including the parietal (“sagittal") fontanel that ossifies to form the parietal foramen either before or after birth, calls for further study in reference to normal and abnormal morphology (Padget, x956).
Stage 7: (fig. 25, pl. 1) is not represented in the previous account of the arteries (Padget, 1948), for they have already attained the essential adult configuration by 40 mm. (stage 7). Not until approximately 80 mm., when the venous pattern in the human embryo is comparable to that of the adult in other mammals, does the transverse sinus begin to assume its definitive position; its caudal swing on the sigmoid as the result of cerebral growth (figs. 22-25, pl. 1) resembles that of the posterior communicating artery. The expansion of the cerebral hemisphere and otic capsule by the 80—mm. stage is responsible for the definition of the last of the major adult sinuses to appear, the superior petrosal (figs. 36, 37, pl. 2). The direct contiguity of the sinus with the petrosal crest is peculiar to certain primates typified by man. Nevertheless, the stem of the great anterior cerebellar vein, of which the sinus constitutes essentially a dural envelopment, is the primary metencephalic (trigeminal) vein characteristic of representative vertebrates.
The most important vein at the base of the brain is not clearly defined until stage 7: (60 to 80 mm.) in man. The basal cerebral vein represents a channel, or fragments thereof, typical of adult mammals, which is derived from primary veins typical of vertebrates. These vessels include the primary drainage of the basal nuclear masses, in spite of the earlier appearance of the internal cerebral vein, which emerges as the outlet of the superior choroid vein only. The basal vein develops as a longitudinal pial anastomosis between primary pia-arachnoidal veins, i.e. the transverse telen-, dien-, mesen-, meten(trigeminal), and myelen—cephalic (vagal) veins of stages 4 and 5. These primitive veins, particularly the first three, become the adult tributaries of the basal vein as shown in figure 35 (pl. 2). One of these tributaries, the lateral mesencephalic vein, is especially important. It may constitute part, or all, of the outlet of the basal cerebral (and/or internal cerebral) vein into the superior petrosal sinus by way of the great anterior cerebellar (metencephalic) vein. Such lateral drainage of the basal vein occurs often in many mammals (including certain primates), in which essentially all intracranial drainage converges to the junction of the transverse and sigmoid sinuses (pl. 6). This pattern typifies stages 6 and 7 in human development, and frequently persists, although the medial route into the great cerebral vein may usually be predominant in adult man.
Subsequent venous changes in human development depend chiefly upon the expansion of the cerebral and cerebellar hemispheres and the relatively late ossification of the skull. Since certain significant anastomoses in the dura often occur after birth, the typical configuration in the newborn differs from that in the adult 1“ (figs. 38, 39, pl. 2). These contrasting patterns, together with an outline of other obscure features of the postnatal configuration, and including comments on the embryologic background of congenital arteriovenous anomalies, have been published elsewhere (Padget, 1956).
Addendum
Since this paper was written, there has come to hand a monograph by Dr. Richard Lindenberg [1956: Die Gefiissversorgung und ihre Bedeutung fiir Art und Ort von kreislaufbedingten Gewebsschiiden und Gefl1'sspro— zessen, in Lubarsch, Henke, and Rossle, Handbuch der speziellen pathologischen Anatomic und Histologie, vol. 13 (ed. W. Scholz), pt. IB, chap. 2, pp. 1071-1164]. The section on anatomy is well illustrated by photographs of dissected specimens. Of these, his figure 28, a rare illustration of the basal cerebral vein, is directly comparable to the present figure I7Ar; it includes the important potential anastomosis, the lateral mesencephalic vein (1). sulci Iateralis; it is large in his fig. 18), together with two conspicuous tributaries draining the inferior “This evidence, though strong developmentally, is based on a small number of infant and adult heads (ca. 25; see Introduction). Owing to certain limitations of dissection, more corrosion specimens are particularly called for, especially of later fetal stages, although obtaining complete venous injections without extravasation is technically very tlillicult in postmortem material. Similarly, until more material has been collected, undue weight must not be given the author's observation (Padget, 1948, and earlier) that, typically, there is less relative difference in the size of the several arteries comprising the circle of Willis in infants than in adults; as stated in I948, no essential dillerence in arterial patterns has been demonstrated, contrary to the situation regarding a number of dural venous channels.
ventricular horn and thalamus. By way of this vein, the more anterior group of cerebellar veins may sometimes drain the region of the dentate nucleus into the basal or great internal cerebral vein, as he notes; primary drainage of this region, however, is by way of the superior petrosal sinus. (Essentially, his Vv. floccularer, together with the V:/. carebellarcr rortraler, rnedialer, and 1ate’ra1e.r, constitute the great anterior cerebellar system of the present account.) Other observations of interest here (supplemented by personal communication) include the following.
1. The frontal emissary vein that traverses the region of the adult foramen cecum (Padget, 1956, p. 328) is identifiable with Zuckerkandl's vein of infancy (Lindenberg, pp. 1078, 1114), as it is called on the continent. This vein, apparently secondary in human development, passes through the skull at least prior to completed ossification. Its incidence, especially in juveniles, and whether its large size is abnormal or a normal variant are not apparent.
2. This ventral or orbital frontal emissary must be distinguished from a more dorsal c-n1ir.rarz'zmz frontal: (Lindenberg’s diagram after Clara, fig. 29), seen by Lindenberg in several specimens. Occasionally bilateral, but apparently not common, its foramen has been found just in front of the coronal suture near the mid—line. This perforating skull foramen, like the parietal (and rare occipital), is undoubtedly the remnant of one of the normal fontanels (Padget, 1956, p. 328); the vein is assumed to be a secondary anastomosis that forms sometime during fetal as opposed to embryonic life (pp. 119, 136).
3. The deep thalamic vein (Padget, 1956, p. 314), which Lindenberg refers to as the “Zentralvene der oralen Thalamushiilfte” (his p. 1110; also fig. 64), is prominent and significant; in the very early fetal stages that conclude the present series, it is the only thalamic drainage into the internal cerebral vein.
4. A rich network of sinusoids is frequent in the tentorium, especially in juveniles, and represents remnants of the primary drainage of the embryonic cerebral and cerebellar hemispheres.
5. Lindenberg believes that there is strong evidence supporting, though not necessarily proving, the existence of the postnatal intracerebral anastomotic veins of Schlesinger (see Padget, 1956, p. 315). Certainly, most of the length of such veins would represent a persistence of embryonic conditions, in which all drainage of the ependymal region is directed laterally to the periphery. But how the veins concerned become anastomosed with the tributaries of the terminal vein, indeed, just how the stem of this vein beneath the stria terminalis, and possibly at first extracerebral, becomes enclosed within the ventricular wall (see footnote 11, p. 112), are interesting features of stages beyond the scope of this study that await clarification. Various details near the superior sagittal sinus and its lateral lacunae, which involve seemingly few and minor emissaries under normal conditions, also require exploration. It is to be hoped that future studies on the development (fetal and postnatal?) of the subarachnoid and subdural spaces, especially of the arachnoid granulations—and current observations (unpublished) show that macroscopic arachnoid villi are by no means limited to the region of the cranial vault——will answer these questions regarding venous development in later fetal stages.
Literature Cited
NXKEW, A. 1909. Zur I-‘rage iiber die Entwickelung der Venn anonyma sinistra. Anat. Anz., vol. 34, pp. 24-29.
Bailey, P. 1948. Intracranial tumors. 2d ed. 478 pp. Baltimore.
Ba'rsoN, O. V. 1940. The function of the vertebral veins and their role in the spread of metastases. Ann. Surg., vol. 112, pp. 138-149.
Bsnronn, T. H. B. 193442. The great vein of Galen and the syndrome of increased intracranial pressure. Brain, vol. 57, pp. 1-24.
1934]). The venous system of the velum interpositum of the rhesus monkey and the effect of experimental occlusion of the great vein of Galen. Brain, vol. 57, pp. 255-265.
BROWNING, W. 1884. The veins of the brain and its envelopes. Their anatomy and bearing on the intracranial circulation. 79 pp. Brooklyn.
CAMPBELL, A. C. P. 1938. The vascular architecture of the Cat's brain. A study by vital injection. In The circulation of the brain and spinal cord. A symposium on blood supply. Assoc. for Research in Nervous and Mental Disease Pub., chap. 3, vol. 18, pp. 69-93.
3ux.\'1N<;HA.\1, D. J. 1951. (Ed. I. C. Brash.) Textbook of anatomy. 9th ed. 1604 pp. London.
Davis, D. D., and H. E. Sronv. 1943. The carotid circulation in the domestic cat. Field Museum Nat. Hist., Zool. Series, no. 1, vol. 28, pp. 1-47.
121-: BEER, G. R. 1937. The development of the vertebrate skull. 552 pp. London.
DENNSTEDT, A. 1904. Die Sinus durae matris der Haussiiugeticre. Anat. I-Iefte, vol. 25, pp. 1-96.
III. The Development of the Vascular System Evans. HM. Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
FEENEY, I. F., ]R., and R. L. Waterson. 1946. The development of the vascular pattern within the walls of the central nervous system of the chick embryo. Jour. Morphol., vol. 78, pp. 231-303.
Finley EB. The development of the subcutaneous vascular plexus in the head of the human embryo. (1922) Pub. 277 Contrib. Embryol., Carnegie Inst. Wash. 14:155 - 161.
Gsrneaew, C. vars. 1924. Die Morphologie der Sinus durae matris. Zweiter Teil. Die vergleichende Ontogenie der neurokraniellen Venen der Vogel und Siiugctiere. Ztschr. f. Anat. u. Entwicl-:lungsgesch., vol. 74, pp. 432-508.
1933. Venensystem, mit einem Anhang fiber den Dotterund Plazentarkreislauf. In Bolk, Goppert, Kallius, and Lubosch, Handbuch der vergleichenden Anatomic der Wirbeltiere, vol. 6, chap. 6, pp. 685-744.
Gums, E. L., and F. A. Gibbs. 1934. The cross section areas of the vessels that form the torcular and the manner in which flow is distributed to the right and to the left lateral sinus. Anat. Rec., vol. 59, pp. 419-425.
Gttaxr, I. C. I}. 1951. An atlas of anatomy by regions. 3d ed. 503 pp. Baltimore.
Gan, H. 1954. (Ed. C. 1\l. Goss.) Anatomy of the human body. 26tl1 ed. 1480 pp. Philadelphia.
Gar-:1=..\:1:, E. C. 1935. Anatomy of the rat. 370 pp. Philadelphia.
Gsossrza, O. 1901. Zur Anatomie und Entwickelungsgcschiehte des Gefassystemes der Chiropteren. Anat. Hefte, vol. 17, 129- 203-424
and E. BREZINA. 1895. Ueber die Entwicklung der Venen des Kopfes und Halses bei Reptilien. Morphol. )ahrb., vol. 23, pp. 289-325.
H.1L1=e1t'r, B. 1942. Situs inversus of the superior vena cava. Arch. Pathol., vol. 33, pp. 75-77.
and F. D. Conan. 1930. Complete situs inversus of the vena cava superior. Amer. Iour. Pathol., vol. 6, pp. 191-198.
Hamilton, W. I., I. D. Bow), and H. W. Mosstxr.-tx. 1952. Human embryology. 2d ed. 432 pp. Baltimore.
I"L\RT.\1:\N, C. G., and W. L. Sraaus, Ia. (eds.). 1933. The anatomy of the rhesus monkey. 383 pp. Baltimore.
Hénox, C. E. 1888. Etude anatomique sur la circulation veineuse de l’ence'phale. Thesis. 96 pp. Bordeaux.
Heiutex, R. Y., and L. 1\1.F.XA-.\'DER. 1939. Sulcal and intrinsic blood vessels of the human spinal cord. Arch. Neurol. and Psychiat., vol. 41, pp. 678-687.
Hertwig, O. 1907. In Kollmann, Handatlas der F.nt\vicklungsgeschichte des Menschen, vol. 1, figs. 262-264. Iena.
Hoc11s1'1=:rT1-:11, F. 1933. Ueber cine Varietiit der Vena cerebralis basialis des Mcnschen nebst Bemerkungen tiber die Entwicklung bestimmter I-lirnvencn. Ztschr. f. Anat. u. Ent\vicklungsgesch., vol. 108, pp. 311-336.
I‘l0X-‘.\1.—\!\'N, M. 1901. Zur vergleichenden Anatomic der Gchirnund Riieken-marksvenen der Vertebraten. Ztschr. f. Morphol. u. Anthr0pol., vol. 3, pp. 239-299.
I-lL'<;111-ts, A. F. W. 1934. On the development of the blood vessels in the head of the chick. Phil. Trans. Roy. Soc. London, B, vol. 224, pp. 75-130.
Ioxes, F. Woon. 1912. On the grooves upon the ossa parietalia commonly said to be caused by the arteria meningea media. Iour. Anat. and Pl1ysiol., vol. 46, pp. 228-238.
Lewis FT. On the cervical veins and lymphatics in four human embryos, with an interpretation of anomalies on the subclavian and jugular veins in the adult. (1909)
LF.\vts, Vt’. H. 1920. The cartilaginous skull of a human embryo 21 millimeters in length. Carnegie Inst. Wash. Pub. 272, Contrib. to Embryol., vol. 9, pp. 299-324.
LIMSON, M. 1932. Observations on the bones of the skull in white and negro fetuses and infants. Carnegie Inst. Wash. Pub. 433, Contrib. to Eml)ryol., vol. 23, pp. 205-222.
Macklin CC. The skull of a human fetus of 40 mm 1. (1914) Amer. J Anat. 16(3): 317-386.
Macklin CC. The skull of a human fetus of 40 mm 2. (1914) Amer. J Anat. 16(3): 387-426.
Macklin CC. the skull of a human fetus of 43 millimeters greatest length. (1921) Contrib. Embryol., Carnegie Inst. Wash. Publ., 48, 10:59-102.
— 1921. The skull of a human fetus of 43 millimeters greatest length. Carnegie Inst.Wash. Pub. 273, Contrib. to Embryol., vol. 10, pp. 59-103.
Mall FP. On the development of the blood-vessels of the brain in the human embryo. (1905) Amer. J Anat. 4(1): 1–18.
M.\x.\', I. 1950. The development of the human eye. 2d ed. 312 pp. New York.
lVl.-\Rl-i0\\-‘SKI, I. 1911. Ueber die Enttvicklung der Sinus durae matris u11d der Hirnvenen bei menschlichen Embryoncn von 15.5-49 mm. Scheitcl-Steissliinge. Bull. dc l‘acad. sci. de Cracovie, B, sci. nat., pp. 590-611.
1922. Entwicl-dung der Sinus durae matris und der I-Iirnvenen des Menschen. Bull. internat. de l'acad. polonaise sci. et lettres, B, sci. nat., Cracovie, pp. 1-269.
MCCLURE, C. F. W., and C. F. S11.vr.s'r1~:a. 1909. A comparative study of the lymphatico-venous communications in adult mammals. Anat. Rec., vol. 3, pp. 534-552.
Mr..tt>, C. S. 1909. The chondrocranium of an embryo pig, Su: scro/a,’ a contribution to the morphology of the mammalian skull. Amer. Iour. Anat., vol. 9, pp. 167-209.
M11.1.£11, M. E. 1948. Guide to the dissection of the dog. 2d ed. 427 pp. Ithaca.
Mtvaar, S1‘. G. 1895. The cat. An introduction to the study of backboncd animals, especially mammals. 557 pp. New York.
MORRIS, HENRY. 1953. (Ed. I. P. Schaeficr.) Human anatomy. 11th ed. 1718 pp. New York.
Padget DH. The development of the cranial arteries in the human embryo. (1948) Carnegie Inst. Wash. Pub. 575, Contrib. to Embryol. 32: 205-261.
Padget DH. Designation of the embryonic intersegmental arteries in reference to the vertebral artery and subclavian stem. (1954) Anat. Rec. 119: 349-356. PMID 13197795
Padget DH. The cranial venous system in man in reference to development, adult configuration, and relation to the arteries. (1956) Amer. J. Anat. 98: 307-356. PMID 13362118
P1=e11=1-:11, R. A. 1928. Die Angioarchitektonik der Grossl1irnrinde. 157 pp. Berlin.
1930. Grundlegende Untersuchungen fiir die Angioarchitektonik des menschlichen Gehirns. 220 pp. Berlin.
S.t111.\*, F. R. 1917. Origin and development of the primitive vessels of the chick and of the pig. Carnegie Inst. Wash. Pub. 226, Contrib. to Embryol., vol. 6, pp. 61-124.
SCH.-\RRF.R, E. 1940. Arteries and veins in the marnmalian brain. Anat. Rec., vol. 78, pp. 173-196.
SCHLESINCER, B. 1939. The venous drainage of the brain, with special reference to the Galcnic system. Brain, vol. 62, pp. 274-291.
SCHULTL, A. H. 1921. Fetuses of the Guiana howling monkey. Zoologica (New York Zool. Soc.), vol. 3, pp. 243-262.
— 1950. In VV. K. GREGORY (ed.), The anatomy of the gorilla. Morphological observations on gorillas. The studies of Henry Cushier Raven, Part 5, pp. 227-251. New York.
SHINDO, T. 1915. Ueber die Bedeutung des Sinus cavernosus der Siiuger mit vergleichend-anatomischer Beriieksiclitigung andercr Kopfvenen. Anat. Hefte, vol. 52, pp. 319-495.
S1ssoN, S. 1953. The anatomy of the domestic animals. 4th ed., rev. by ]. D. Grossman. 972 pp. Philadelphia.
SOLNITZKY, O. 19.10. The angioarchitecture of the cerebral cortex of 1\Iacacm‘ r/zc-.m.r. Anat. Rec., vol. 76, Suppl. 2, pp. 51-53 Sr=.\t.T1-:Ho1.z, W’. 1943. I-land-atlas of human anatomy. 7th ed. i11 English (figures clearer in earlier editions). 902 pp. Philadelphia.
Streeter GL. The development of the venous sinuses of the dura mater in the human embryo. (1915) Amer. J Anat.18: 145-178.
Streeter GL. The developmental alterations in the vascular system of the brain of the human embryo. (1918) Carnegie Inst. Wash. Pub. 271, Contrib. to Embryol., 8: 5-38.
Streeter GL. Developmental horizons in human embryos. Description of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites. (1942) Contrib. Embryol., Carnegie Inst. Wash. Publ. 541, 30: 211-245. For subsequent articles on the Horizon series. see ibid., 557 (1945), 575 (1948), 583 (1949). and 59: (1951); the whole series was reproduced in Embryology Reprint Volume Il, 1951.
Smaxr, H. 1903. Ueber otologisch wichtige Anomalien der Hirnsinus, iiber accessorische Sinus und bedcutcndere Venenvcrbindungen. Arch. f. Ohrenheilk., vol. 58, pp. 161167.
Thyng FW. The anatomy of a 17.8 mm human embryo. (1914) Amer. J Anat. 17: 31-112.
Toldt, C. 1941. An atlas of human anatomy for students and physicians. 2 vols. 2d ed. 957 pp. New York.
Tnoumn, P. 1868. Recherches sur l'anatomie du systéme vcineux de l'encéphale et (in crime. Thesis. 3: pp. Paris.
WA1.xEI<, A. E. 1933. The attachments of the dura mater over the base of the skull. Anat. Rec., vol. 55, pp. 291-295.
Wolff, E. 1954. The anatomy of the eye and orbit, including the central connections, development, and comparative anatomy of the visual apparatus. 4th ed. 491 pp. New York.
List of Abbreviations in Figures
(2., am, artery, arteries mm.rt., anastomosis, anastomotic rm:., anterior rzort., aorta, aortic m'acI':., arachnoid rm:1:'t., auditory am'ic., mm, auricular rzx., axillary 6., bone 12:15., basal, basilar brr.tioc‘c., basioccipital [)r., 1')r.r., branch, branches; brachial br.-cap/1., brachioccphalic bmc/:., brachial bram/2., branchial Brcrc/2., Breschet mp:., capsule mrz1., cardinal mrot., carotid m:-ot.—lm_r., caroticobasilar mm, cartilage cam’rrz., car/., cavernous c‘c'C., cccum cc.-2r., central ccplml., rep/2., cephalic ccrclz, c‘c'r., cerebral c'c'rc[2:'/., cerebellar cc-rt/., cervical c/i., channel c/iamlranz, chondrocranium ci1ar., choroid c/ror(I., chnrda (tympani) c/zw., clavicle c1in., clinoid com, common rommm1., communicating comIy1., condylar or condyloid carom, coronary Cm/., Cuvier dcfir2it., definitive dent, dentate rlimccp/a.,
dien., diencephalon,
diencephalic d.-‘pl, diploic a'or:., dorsal duct. r/£11., ductus vcnosus dmz, dural c'm., emissary rt/m:., ethmoid e.rt., external fan, facial
for-., foramen form., formation frzmt., frontal ga.r:., gum-r., Gasserian (trigeminal) gn_, ganglion gr., great /:cm1'.s°., hemisphere /2.-c. c/1., hepatocardiac channel (of Strectcr) /n'mI'br., hindbrain /2yp., hypophysis /1ypoglo:., /zypog1., hypoglossal in/., inferior 1'rmom., innominate int., interval int.~:/:nt., interventricular intercom, intcrcostal int:-r/Jca'., interpcduncular intcr.rcgn1., intersegmental iugn1., jug., jugular jug.-cc-p/1., iugulocephalic j:mct., junction L., left 1a!2., labial lat., lateral l1'g., ligament Iing., lingual ling.-fac., linguofacial nmg., nmgn., magnum 1mm2., mammilary margim, marg., marginal (sinus; vein of hand plate) ma5t., mastoid max, maxillary nu-d., medial mcm'ng., mcnin., nu-n., meningeal me:c'nc‘r'[1/1., mc:c'n., mcsenccphalon, mesenccphalic mctenccp/1., mc-tc'n., ma-1., metencephalon, metencephalic mid., middle rrzyclcrzccp/1., myclc-n., myelencephalon, myelencephalic 11., nerve rm.c., nasal 1m.rocil., nasociliary 11010., notochord 1mcl., nucleus obl., oblique occrm, occasional o¢‘cip., 066., occipital 01]., olfactory Of!/It/1., op/1., ophthalmic op/1.-mem'ng., ophthalmomeningeal apt., optic orbit, orl7., orbital, orbitalis ot., 020., otic (pit), otocyst or/171., ovale parict., parietal patron, pz'tr., petrosal f1ctro:(]mm1., pctro:q., petrosquamosal p/mryng., pharyngeal pIex., pl., plexus p1exif., plcxiform pas-it., position pa:t., posterior - pouglg-:z,, posrglenoid :ubc1atr., 5111251., subclavian prim, f7l'., primary .m[2mcnt., submcntal primiz., primitive 5:1/1., superior primord., primordial sup], superficial pro:-., process :uprzIorb., supraorbital proot, prootic (sinus) tclcnccp/1., tcIcn., telcncephalon, tclenccphalic prorcncc-p/:., pron-n., proscnccphalon, prosenccphalic lcmp., temporal, temporalis p:eryg., pterygoid tcntor., !cnt., tenlorial R., right tcrm., terminal 7'c'ct., rcctus (muscle) t/1or., thoracic re-mm, remnant t/zor.-cpig., thoracoepigastric r/zinelzccpln, rhinenccphalic t/1ym.—t/zyr., thymicothyroid 2'/loin/1071., rhombencephalon t}'zyr., thyroid (primordium) ro:zmd., mtundum tarc., torcular (I-Icrophili; confluence sinuses) 5., .rin., sinus transza, tramz, transverse (lateral) sagita, mg., sagittal tril2., tributary scrip, scapular trigmz., trigcminal .rccom1., secondary tymp., tympanic, tympani sect, section uIn., ulnar -WP!-. Scptill umb., umbilical signh. sigmoid u., m»., vein, veins St-H. !«"€’fl05., Sll'lllS VCDOSUS 1/_ 5" Von; cava mm-, somitc um-., variable spit.-pal” sphcnopalatinc ucno:., vcnosus :pl1c'nopm'ict., sphenoparictal z/cnt., ventral spun, spurious t't’7IlI'., ventricle, ventricular :£., stem uz*rt., vertebral .m2p., :i‘apct1., stapcdial z/05., zIcsc., vesicle 5tr., straight VcsaI., Vcsalius
Plates
Plate 1 The development of cranial venous channels as related to the growth of their environment. The numbering of the vascular stages” facilitates correlation with the arteries (Padget, 1948, pls. r, 2); Roman numerals denote the horizons (age groups) of Streeter (1942-1951); crown-rump lengths are approximate.
Fig. 19. Stage (1 and) 2, xiv, 5 to 8 mm. The walls of the neural tube, covered by a continuous plexus (not shown), drain dorsolaterally into a superficial (primitive dural) plexus, which empties through three (dural) stems into :1 head-sinus continuous with the anterior cardinal vein. The mandibular and hyoid pharyngeal bars are drained by a ventral pharyngeal vein; the emerging maxillary process, by a maxillary (infraorbital) vein.
Fig. 20. Stage 3, xvi, 8 to 11 mm. The head-sinus remains medial to the trigeminal ganglion, but is migrating lateral to cranial nerves 7 to 10. A remnant of the medial channel is part of a myelencephalic vein; this and a diencephalic vein are the first pia-arachnoidal veins. The ventral pharyngeal vein enters the primitive internal jugular at the junction of the 10th and 12th nerves. The maxillary vein drains both optic and olfactory regions. A marginal vein of the hand plate empties into the primitive subclavian vein.
Fig. 21. Stage 4, xvii, II to 14 mm. Having completed its migration lateral to the lath nerve, in which the ventral pharyngeal vein sharcs, the anterior cardinal vein has the position of the adult internal jugular vein. The cervical intersegmcntal veins are uniting to form the vertebral vein. Each subdivision of the brain is drained laterally into the dural plexuses by at least one primitive pia-arachnoidal vein, as designated. Note the primitive supraorbital vein and its relation to the junction of the 4th and 5th nerves.
Fig. 22. Stage 5, xix, 17 to 20 mm. As the pharyngeal bars difiercntiate and their nerves develop, a channel between the middle and posterior dural plexuses forms the sigmoid sinus; accordingly, the head-sinus dwindles ventral to the labyrinth. Lateral tributaries of the maxillary and linguofacial veins drain the maxilla and mandible, respectively. Components of the anterior dural plexus, the marginal and tentorial sinuses, border the expanding hemisphere caudally. A jugulocephalic vein, derived from the primitive subclavian vein, arches over the primordial clavicle (x) and receives a vein from the radial border of the arm.
Fig. 23. Stage 6, xxi, 22 to 24 mm. The supraorbital vein no longer drains into the anterior dural stem, which has d\vindlecl', it is continuous, by way of the head-sinus, with the pro-otie sinus (middle dural stern; note the reversal of its flow), which empties into the ne\v sigmoid sinus (incorporating the posterior dural stem). After anastomosis of its lateral tributaries with the new anterior facial vein (lateral tributary of linguofacial vein), the stem of the maxillary vein is attenuated. A secondary left innominate vein unites the internal jugulars. An anastomosis involving the jugulocephalic vein forms the definitive subclavian vein, from which the external jugular vein arises. Cerebral growth has elongated the tentorial sinus draining the superficial middle cerebral veins. The shifting marginal sinus constitutes the medial end of the primitive transverse sinus. Drainage of the choroid plexus of the lateral ventricle is primarily by the ventral diencephalic vein, supplemented later by the primitive internal cerebral vein. Note the consolidation of the anterior and middle dural plexuses into a tentorial plexus (future torcular).
Fig. 24. Stage 7, 40 mm. Plcxiform elements of the newly developing cavernous sinus are medial extensions of the pro-otic sinus. They are continuous with the new inferior petrosal sinus, a similar extension of the ventral myelencephalic vein, which joins the internal jugular below its foramen. The primary maxillary vein is no longer recognizable, because its trunk, after anastomosis with the supraorbital vein, has become the stem of all the veins of the orbit. A lateral tributary of the pro-otie sinus, which primarily receives the middle meningeal sinuses, anastomoses (through the spurious jugular foramen) with a primitive temporal emissary tributary of the sigmoid sinus to form the petrosquamosal sinus. An anastomosis between the external jugular and common facial veins constitutes the posterior facial vein. The primitive emissary veins near the skull base primarily drain medially into the sinuses; later they anastomose with the external jugular system.
Fig. 25. Stage 71:, 60 to 80 mm. Cerebral growth and expansion of the otic capsule are responsible for a new superior petrosal sinus, which is the dural (tentorial) end of the ventral metencephalic (great anterior cerebellar) vein. The primitive transverse sinus has been swung backward on the sigmoid sinus, and receives the elongated tentorial sinus continuous with the superficial middle cerebral veins. Being primarily the drainage of the superior choroid vein, the internal cerebral vein is still primitive; it receives only one intracercbral (anterior thalamic) tributary. The basal cerebral vein is formed by longitudinal pial anastomoses between several primitive veins: the telencephalic (deep middle or anterior cerebral), the ventral and dorsal diencephalic, the mesencephalic, and ventral metencephalic veins, and a caudal diencephalic tributary of the primitive internal cerebral vein; anastomosing the new basal vein with the ventral metencephalic (great anterior cerebellar) vein, the lateral mesenccphalic vein is a potential lateral outlet of the basal (and internal) cerebral vein by way of the superior petrosal sinus. The pro-otic sinus is continuous with the definitive petrosquamosal sinus.
Fig. 26. Adult pattern (shown in an infant head). Between the fetus of the 3d month and term, secondary intracerebral tributaries of the internal cerebral vein supplement similar primary components of the basal cerebral vein, completed earlier: included are superior striate tributaries of the terminal vein, formed by extraventricular vessels that are secondarily enclosed in the lloor of the ventricle. In the development of the superficial cerebral groups of veins, the superior and posteroinferior emerge considerably later than does the primary middle cerebral group. The petrosquamosal sinus and the tributaries of the prootic sinus primarily drain the dura and bone. They become diploic in adult life, when the middle meningeal sintlscs drain secondarily through the foramen ovale and into the superior sagittal sinus. Remnants of the tentorial sinus, frequently persistent, may be involved in two late anastomoses (arrows) that unite the cavernous sinus cranially with the middle cerebral veins, and caudally with the superior petrosal sinus. l’t..\ 1' ti These figures. to he C(ll‘I'L‘l:ll(.'(l \\ith those of plate I, also shmv intertnetliate stages. Iiigttres :7 to 33 are venous channels at the ltase of the hrain as vietvetl from helotv.
Fig. 27. Stage (1 anti) :_. xiv. 5 to X tntn. The pritnary headsinus ts medial to the 5th and toth nerves, hut has‘ migrated laterttllv to the 7th to 9th tterves '.ll1(l the otie vesicle. Its tlorsal trihutaries are the three l.ltll':t.l stems of the sttperlicial plexttses tlraittittg the tteural tuhe (lUt'5t)l:llCt'ttll‘_\‘. \'entral|_v. :t ntaxillatv trihutary tlrains the optic antl olfaetorv regions. .-\ ventral pharyngeal veitt joins tlte attterior eartlinal vein.
Fig. 28. Stage 3, xvi, N to it mm. With tlitierentiation of tlte teleneephalon and tlieneephalon. t\vo veins (lt‘;tll1il1_L' these regions etnerge: they ioin the anterior tlural plexus. in the fore part of \vhieh is the primitive marginal sinus hortlering the cereht';tl |temi.s'phere. The Iteatl-sinus has hegun to migrate laterally to the toth nerve.
Fig. 29. Stage 4. xvii. it to 14 tntn. .-‘tlthough the heatl—sinus is now essentially lateral to the toth nerve, its former metlial position is representetl h_\' the stem of a ventral tnyeleneephalie vein. \'nlt1tninous trihutaries of the maxillary vein inclutle the pritnitive central vein of the retina. Note the primitive supraorhital vein.
Fig. 30. Stage .1. xviii. 1.; to 1!} min. At least one vein identiIietl from each .s'tthtlivi.sion of tlte pritnitive ht'aitt tt'averse.s the pritnitive araehnoitl to join eotnpottettts of the (llll':tl plexuses. Longitutlinztl (antl later trttnsverse) anastotnoses hettveen the distal ends of tlte.se veins form the pial venotts ttet and veins. svhieh are .suhiaeettt to the - ‘teries. .-\ tlorsal phar_vn;:eal vein accompanies the great .s'uperhtial petrosal nerve.
Fig. 31. it (in tlevelopmental sequence). Stage i. xix. I7 to 10 mtn. Note the vettotts as}'mmett'v. ()n the etnhrvo'.s left (;\). the seeontlary ana.stotno.sts (hettveen the lltl(lt.llL‘ and posterior tlural .stent.si) that forms the sigmoid sintts is less atlvaneetl tlt:ttt tltat on the right sitle (l3): thtts. the left heatl-sinus. \\‘ltlClt the ne\v eh-.tnnel sttpersetles. has (l\\'ll](llL'tl less tltau the t‘lt:ltl. l)rainage from the mitl-line. lit the region of the superior sagittal antl transverse sinuses. pa.sse.s pretlominatelv to the right (1!) hv \va_v of the marginal sinus. The primitive straight sinus also passes to the right. Tlte vetttral plt:tt‘ytt;_:ettl vein (s'ho\vn on the left onlyi is now a (lL‘lil\ill\'C litttzttofaeial vein: its lateral trihutaries \vill ana.s'totno.se \vitlt those of the maxillary vein.
Fig. 32. .-\. l’; (in (le\'elop:ttet‘.t'.tl seqttenee‘). Stttge ft, xxi. 2: to 24 mitt, .-\svtntnetry of tlrainage front tnitl—line channels is again evitlent. so tltat tlte lateral ehoroitl plexus tlrains tnostlv to the right tlt1‘ttlI_t1lt the pritnitive straight sinus (H). The ntitltlle tlural stem. notv the pro-otie sinus. heeomes the tlireet continuatiott of the primitive sttpraorhital vein. The anterior tlural stein has thvintlletl. more on the ritzht. resulting in tlorsal tlt';titt:tge of the mitltlle cerehral veitts into the primitive trattsverse sinus. .-\na.stotno.sis hettveen the litt;.:nofaei.'t| antl maxillary veins (shotvn on the left only) fot'ttts the anterior facial vein. \\'ltit.‘lt annexes" lateral trihut:tries ol' the tlsvintlling tn-axillary vein.
Fig. 33. Stage 7. to into. l’lexil'orm extensions of tlte pro-otie s'itttI.s aml of the tlural entl of the mveleneephalie vein. respeetively. are the cavernous and inferior petrosal sinuses. The pritnarv eontlvloitl antl mastoitl etnissarv teins drain tnetliallv ittlo the sigmoid sinus ltefore their :lt'I:t.\[()tttt).s'i.\ Will! the external ittgttlar s_vsteni. The pro-otie sinus has t\vo tt'ihtttat‘ie.s': the \'Cl\tral tneteneephalie vein. to constitute the superior petrosal sinus: and the primitite mitltlle tneningeal sintts. tlrainittg the metnhrane hones. ltteltttlitn: anterior and tleep cerehral (pi-.tlJ trihtt tat'ie.s. the superficial Iltl(l(llC cerehral veins are still tlireetlv eon. tittuous \\'ill't the tentorial sinus. svhieh is t‘lt)l‘l}1;tlL‘(l he the espantlittg hetttisphere. antl ioins the primitive tt'tttts\'t:t'.s‘e sinu.s.
Fig. 34. Stage jtt, (to nun. .-\ longitudinal ttttastontosis hettveen the tleep telett—. tlien-. antl mesen-eephalie veins eonstitutes the tttaior part of the ltasal eerehral vein. .\ similar att:tstomo.sis' heL\\'t‘L‘tt the tlieneephalie antl tneteneephalie veins heeomes a sig s nilieant lateral tneseneepltalie vein. The stem of the \et‘ttt';tl meteneephalie (anterior eerehellar) vein heeomes the superior petrosal sinus. ’tt-coming plexifortn antl tltvintlling. the eautlal entl of the tentorial .sintt.s is sltifted l()\\':|l'Ll the signtoitl sinus.
Fig. 35. The postnatal pattern. Parts of the left temporal lohe are ettt atvay to sltoxv the basal cerebral vein. Note these tributaries: the variable anterior cerehral vein: the inferior .striate veins: the interpetlttneular vein from the posterior perforatetl area: the inferior ventricular vein with trihtltaries from the vetttrieular walls. inelutling an inferior L'llt)t‘t)ltl vein: the lateral meseneephalie vein—-att anastomosis with the great attterior eerehellar vein. The superficial ntitltlle cerehral veins tlrain throttgh the remttant of the tentorial sinus (sometitnes a superlieial vein) or. .secontlaril_v. into the eavcrttous sinus. .\'ote the internal occipital vein frotn the visual cortex, and the posterior eerehellar veins. emptying ittto a tentorial lacuna helore reaelting the tt'ansver.se sinus.
Fig. 36.. Stage 74/, (it! into. The orhito-ophthaltnie veins tlrain laterallv tltrottgh the printary pro-otie sinus. and metlial|_t' through the eavernous and inferior petrosal sittttses. hoth seeotttlarv. The tentorial sinus. constituting the only tlt‘aitta;.:e of the primitive tnitltlle cerehral veins. ntav follow the etlge of the lesser sphenoitl wing. Trihtttaries of the primitive superior petrosal sinus (end of the meteneephalie vein) tna_v annex those of the mveleneephalie vein, \vhose stem is the eantlal entl of the inferior petrosal sitttts. l’»et\veen the primitive temporal etniss-.n'v vein (of the spurious iugttlar foratnett) and the tlnral tneningeal trihutary of the pro-otie .sinus is the ana.stomo,s'is constituting the petro.squatno.s‘al sinus.
Fig. 37. Stage ';tt. No min. The tlelinitive sttperior petrosal sitttts sttrtnottnts the enlarged otie capsule. Lying hettveen tltis eat'tilat.:e and the parietal (and temporal) hotte. the petrosqtttttttttsttl sinus heeotnes continuous \\'iIlt the pro-otie sinus. \vhiclt is tl\vintllin;.',. since it is no lon,t.v,er the exelusise outlet of the orltittt-ophthalmic veins. .\lthoug.:lt eraniallv anastotnosetl tvith the anterior faeial tein. the orhital veins tlraitt eautlallv ittto the ne\v eaverttotts sinus continuous tvith the inferior petrosal sinus.
Fig. 38. Typical infant at birth, The eavernons antl interior petrosal sinuses. the new eautlal outlet of the orhital ve':n~. tlo not eonttnunieate \vith the ltti(l(llC cerehral vi. '. \vhieh tlt".tl1t. insteatl. throu;_v_lt the tentorial sinus. \\hieh has a \.'tt‘i.tltle position. Veins of the posterior lossa. only. are tlrainetl hv the superior petrosal .s'inus: it is not eonneetetl with the cavernous sintts. Since the tuitltlle tttettittgeal sittttses are sttperlieial to the .trter_\'. thL‘)' look like two “veins" hortlerittsl it. Tl‘tL' 1‘s‘r.sis'tent l‘l‘tro— squantosal sinus ma_v he eonneetetl with tleep temporal \eiIts' hv tvay of a temporal Clttis\:lr}‘ vein. The marginal sintts of the loramen lnttgltllltt is the retnnanl of the posterior (llII'.ll plexus.
Fig. 39. Typical adult. Note the seeontlarv attttstotnoses (arrotvs) ltet\\'eett the cavernous sinus :ll'ttl (1) the tentorial sinus. tvhieh may lie in the position ol the spltetioparietal sinus. ztntl tlrains the superlieial ntitltlle cerehral veitts‘. anti (2) the superior pelntsttl sitttts. the tlural eotttittttatiott of the },:t‘e.tt atttet'ior eereltellar vein: \\hen the tentorial sitttts is loeatetl more l;tterall_v. stteh anastomoses are less‘ likely to oeeur. l-'.xtentliu_t; helotv the tttantliltttl:n' tterte root. the tvpieal lateral tsing oi‘ the cavernous sitttts is a retnnant of the pro-otie sinus. continuous tvith the emissary \eins oi the lot';ttttett ovale. The opltth-.tltttotttettittgeal sinus accompanies the artery of the satne name. a primary .tn-astotnosis (variahle) hettveen the oplttltahnit‘ antl tnitl (lle lttt.'ltiH}{L‘:tl arteries. Ilaving heeome tlip|oi' the petrosquamosal sinus reeeix es superior tvmpanie veins. The he.ttl—sinus is representetl ltv the sttpertieial petrosttl vein. sthieh aeeotnp.uties' the nerve of the satne name.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The development of the cranial venous system in man, from the viewpoint of comparative anatomy. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_development_of_the_cranial_venous_system_in_man,_from_the_viewpoint_of_comparative_anatomy
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G