Neural Crest Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
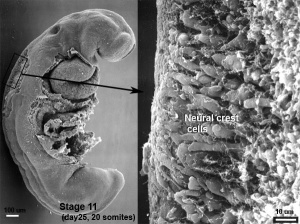
The neural crest are bilaterally paired strips of cells arising in the ectoderm at the margins of the neural tube. These cells migrate to many different locations and differentiate into many cell types within the embryo. This means that many different systems (neural, skin, teeth, head, face, heart, adrenal glands, gastrointestinal tract) will also have a contribution fron the neural crest cells. An in vitro study[1] has shown neural crest cell migration occurs at different rates along the embryo axis between Carnegie stage 11 to 13 in week 4.
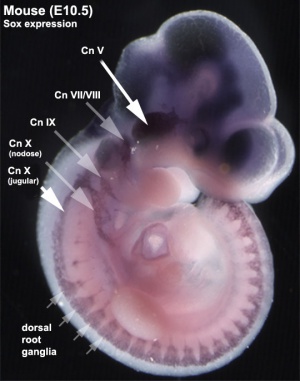
In the body region, neural crest cells also contribute the peripheral nervous system (both neurons and glia) consisting of sensory ganglia (dorsal root ganglia), sympathetic and parasympathetic ganglia and neural plexuses within specific tissues/organs.
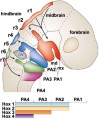
In the head region, neural crest cells migrate into the pharyngeal arches (as shown in movie below) forming ectomesenchyme contributing tissues which in the body region are typically derived from mesoderm (cartilage, bone, and connective tissue). General neural development is also covered in neural Notes.
Nicole Le Douarin has had a long research career, mainly on the development of neural crest cells using originally a Chicken-Quail chimera model she had developed[2], see also her recent review paper on the "beginnings" of the neural crest.[3]
| Historic Embryology |
|
Arthur Milnes Marshall (1852–1893) at Cambridge in 1879 historically first described this embryonic region. In his study of dogfish and chicken brain development, and identified it as "neural crest".[4] See neural crest history and the original 1879 article. Wilhelm His (1831-1904) in 1868 also described in the chick embryo the early neural structure that would form neural crest. |
Use the links listed below to study development of specific neural crest populations.
| Neural Crest Links: neural crest | Lecture - Early Neural | Lecture - Neural Crest Development | Lecture Movie | Schwann cell | adrenal | melanocyte | peripheral nervous system | enteric nervous system | cornea | cranial nerve neural crest | head | skull | cardiac neural crest | Nicole Le Douarin | Neural Crest Movies | neural crest abnormalities | Category:Neural Crest | |||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Neural Crest Embryology |Neural Crest Development | Neural Crest |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
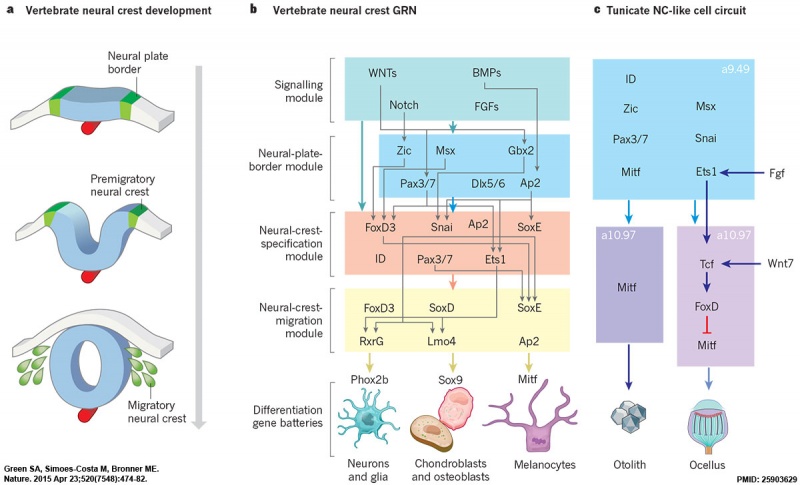
Neural crest formation stages and gene regulatory networks.[19]
| System | Cell Type |
|---|---|
| Peripheral Nervous System (PNS) | Neurons - sensory ganglia, sympathetic and parasympathetic ganglia, enteric nervous system, and plexuses
Glia (neuroglial cells) - Schwann cells[20], satellite cells, olfactory ensheathing cells[21] |
| endocrine | Adrenal medulla Calcitonin-secreting cells Carotid body type I cells |
| integumentary | Epidermal pigment cells melanocyte |
| Facial cartilage and bone | Facial and anterior ventral skull cartilage and bones |
| Sensory | inner ear, cornea endothelium and stroma |
| Connective tissue | tooth odontoblast
smooth muscle, and adipose tissue of skin in head and neck Connective tissue of meninges, salivary, lachrymal, thymus, thyroid, and pituitary glands Connective tissue and smooth muscle in arteries of aortic arch origin |
| Links: neural crest | Category:Neural Crest | Neural Crest collapsible table | |
Neural Crest Migration
Neural crest cell migration involves an initial epithelial mesenchymal transition to delaminate from the ectoderm layer. Then these neural crest cells, depending upon the rostrocaudal level within the embryo, migrate throughout the embryo with distinct morphological patterns:
- cephalic region - sheet-like mass migration
- trunk - chain migration
See also this recent review.[22]
Human
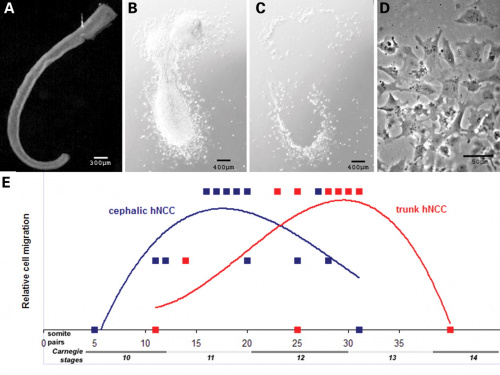
|
Human neural crest cell migration (in vitro)[1]
|
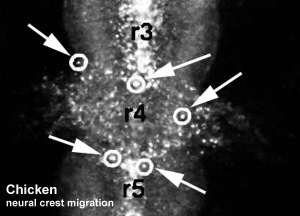
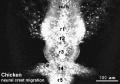
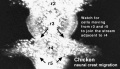
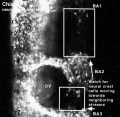
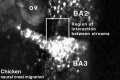
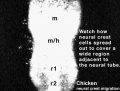
Chicken
Movie Source: Original Neural Crest movies kindly provided by Paul Kulesa.[23]
| Neural crest migration Chicken Head (movies overview) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
| |||||||||||||||||||||
- Neural Crest Movies: Migration 01 | Migration 02 | Migration 03 | Migration 04 | Migration 05 | Migration 06 | Migration 07
Textbooks

|
Hill, M.A. (2020). UNSW Embryology (20th ed.) Retrieved April 27, 2024, from https://embryology.med.unsw.edu.au
| |||||

|
Moore, K.L. & Persuad, T.V.N. (2008). The Developing Human: clinically oriented embryology (8th ed.). Philadelphia: Saunders. (chapter links only work with a UNSW connection). | |||||

|
Schoenwolf, G.C., Bleyl, S.B., Brauer, P.R. and Francis-West, P.H. (2009). Larsen’s Human Embryology (4th ed.). New York; Edinburgh: Churchill Livingstone. The following chapter links only work with a UNSW Library subscription
| |||||
Additional Resources
| ||||||
Objectives
- Understand the structures derived from ectoderm.
- Understand the formation of neural folds.
- Identify the initial location of neural crest cells in the trilaminar embryo.
- Identify pathways of neural crest migration throughout the embryo.
- To know the major tissues to which neural crest cells contribute.
- To know how abnormalities in development that result from abnormal neural crest cell migration.
- Understand how neural crest cells contribute to the pharyngeal arches and the head structures they form.
Neural Crest Derivatives
A key feature of neural crest is the migration into other embryonic tissues to form specific neural and non-neural populations and structures.
| Neural Crest Structures | ||||
|---|---|---|---|---|
|
Cranial neural crest
- migration - dorsolaterally and into pharyngeal arches
- craniofacial mesenchyme - cartilage, bone, cranial neurons, glia, and connective tissues of the face
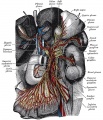
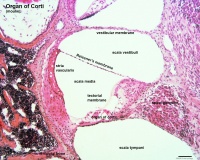
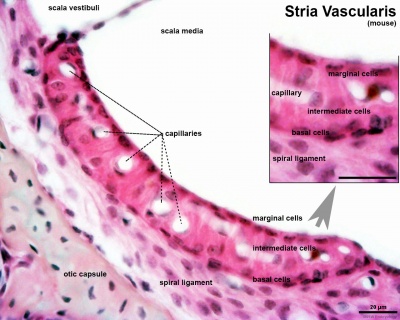
- pharyngeal arches and pouches - thymic cells, tooth odontoblasts, middle ear bones (ossicles), stria vascularis cells, and jaw (mandible)
| Cochlea - Stria Vascularis | |
|---|---|
|
|
| Inner ear cochlea, showing the stria vascularis intermediate cells that are derived from neural crest. | |
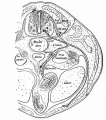
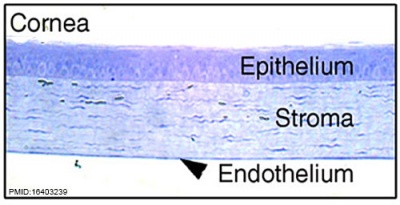
| Eye - Cornea | |
|---|---|
|
|
| Human embryonic cornea detail (Week 8, Carnegie stage 22)]] | Mouse cornea layers |
| The adult eye cornea has three layers: an outer epithelium layer (ectoderm), a middle stromal layer of collagen-rich extracellular matrix between stromal keratocytes (neural crest) and an inner layer of endothelial cells (neural crest) | |
Carotid body are chemoreceptors in the wall of the common carotid (3rd pharyngeal arch) [24][25]
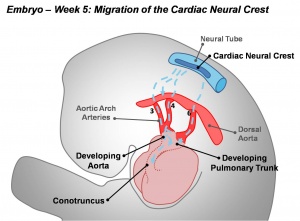
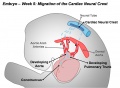
Cardiac neural crest
Trunk neural crest
- migration - two major pathways over somites (dorsolaterally) and between somite and neural tube (ventrolaterally)
- dorsolateral - skin melanocytes
- ventrolaterally - dorsal root ganglia, sympathetic ganglia, adrenal medulla, aortic nerve clusters
para-aortic body
(organ of Zuckerkandl, OZ) A neural crest derived chromaffin body, anatomically located at the bifurcation of the aorta or at the origin of the inferior mesenteric artery. Thought to act as a fetal regulator of blood pressure, secreting catecholamines into the fetal circulation.[26] In human, reaches its maximal size at 3 years of age and then regresses either by death, dispersion or differentiation.[27]
Named in 1901 by Emil Zuckerkandl (1849-1910) a Hungarian-Austrian anatomist at the University of Vienna.
Development Overview
The following cranial and trunk data is based upon 185 serially sectioned staged (Carnegie) human embryos.[28]
Cranial Neural Crest
| Carnegie Stage | Event |
|---|---|
| 9 | an indication of mesencephalic neural crest |
| 10 | trigeminal, facial, and postotic components |
| 11 | crest-free zones are soon observable in rhombomere 1, 3, and 5 |
| 12 | rhombomeres 6 and 7 neural crest migrate to pharyngeal arch 3 and then rostrad to the truncus arteriosus |
| 13 | nasal crest and the terminalis-vomeronasal complex are last of the cranial crest to appear |
| 9 to 14 | otic vesicle primordium descends |
| Week: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Carnegie stage: | 1 2 3 4 | 5 6 | 7 8 9 | 10 11 12 13 | 14 15 | 16 17 | 18 19 | 20 21 22 23 |
Data from a study of 185 serially sectioned staged (Carnegie) human embryos.[28] Links: vision | neural crest | timeline | Category:Timeline
Trunk Neural Crest
Spinal ganglia increase in number over time and are in phase with the somites, though not their centre. There are 3 migratory pathways: ventrolateral between dermatomyotome and sclerotome, ventromedial between neural tube and sclerotomes, and lateral between surface ectoderm and dermatomyotome.
Vagal Neural Crest
- Vagal segment arise at the level of somites 1–7
- leave neural crest at week 4 and also populate the pharyngeal arches.
- enter posterior wall of anterior gut, then surround and continue their rostrocaudal migration route
- migrate into external gut wall just beneath the serosa
- form an uninterrupted chain of cells that continues migration in the caudal direction
- mitotically divide during migration
- differentiate into neurons and glial cells of the myenteric plexus
- migration - ventrally into surrounding splanchnic mesenchyme of gastrointestinal tract
- splanchnic mesenchyme - parasympathetic (enteric) ganglia of the gut
Recent research suggests that the vagal neural crest cells are a transitional population that has evolved between the head and the trunk, taking separate pathways to the both the heart and to the gut.[29][30]
Sacral Neural Crest
- Sacral segment arises caudally to somite 28
- contributes to the enteric nervous system along the postumbilical gut
Neck and Shoulder
A mouse study using individually labelled cells of postotic neural crest followed the development of the shoulder girdle (clavicle and scapula) that connects the upper limb to the axial skeleton.[31]
- Clavicle is a neural crest-mesodermal structure, posterior dermal clavicle mesoderm.
- Cryptic cell boundaries traverse apparently homogeneous skeleton of the neck and shoulders.
- Bones and muscles code of connectivity that mesenchymal stem cells of both neural crest and mesodermal origin obey
- Neural crest anchors the head onto the anterior lining of the shoulder girdle
- Hox-gene-controlled mesoderm links trunk muscles to the posterior neck and shoulder skeleton.
- Skeleton identified as neural crest-derived is affected in human Klippel-Feil syndrome, Sprengel's deformity and Arnold-Chiari I/II malformation.
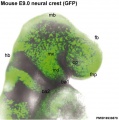
Skin Melanocytes
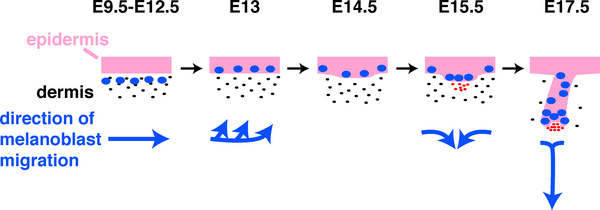
|
|
| Mouse melanocyte migration[32] | Movie Mouse Skin - Melanoblast Migration E14.5[33] |
Neural Crest Migration
A key event in neural crest development is migration from the original site that neural crest cells are generated (edge of the neural plate) to the different anatomical regions within the embryo.
Stimulators
- complement component C3a - (C3a) acts as an autocrine diffusible chemotactic agent attracting NCC toward the self-secreted source.
Inhibitors
- versican - (VCAN, Chondroitin Sulfate Proteoglycan 2; Cspg2) an extracellular matrix proteoglycan that acts as both an inhibitor of NCC migration and as a guiding cue by forming exclusionary boundaries.[34]
- Links: OMIM 118661
Historic
The paper by Marshall, Morphology of the Vertebrate Olfactory Organ (1879)[4], was historically the first time the term "neural crest" was used. In his own earlier papers he had referred to this as a "neural ridge" in describing development of the chicken embryo neural tube.
See paper text and his referenced comment:
- "I take this opportunity to make a slight alteration in the nomenclature adopted in my former paper. I have there suggested the term neural ridge for the longitudinal ridge of cells which grows out from the reentering angle between the external epiblast and the neural canal, and from which the nerves, whether cranial or spinal, arise. Since this ridge appears before closure of the neural canal is effected, there are manifestly two neural ridges, one on either side ; but I have also applied the same term, neural ridge, to the single outgrowth formed by the fusion of the neural ridges of the two sides after complete closure of the neural canal is effected, and after the external epiblast has become completely separated from the neural canal. I propose in future to speak of this single median outgrowth as the neural crest, limiting the term neural ridge to the former acceptation. Thus, while there are two neural ridges, there is only one neural crest, a distinction that will be at once evident on reference to my former figures."
- Links: Embryology History
References
- ↑ 1.0 1.1 Thomas S, Thomas M, Wincker P, Babarit C, Xu P, Speer MC, Munnich A, Lyonnet S, Vekemans M & Etchevers HC. (2008). Human neural crest cells display molecular and phenotypic hallmarks of stem cells. Hum. Mol. Genet. , 17, 3411-25. PMID: 18689800 DOI.
- ↑ Le Douarin NM. (2018). A life in Science with the avian embryo. Int. J. Dev. Biol. , 62, 19-33. PMID: 29616728 DOI.
- ↑ Le Douarin NM & Dupin E. (2018). The "beginnings" of the neural crest. Dev. Biol. , 444 Suppl 1, S3-S13. PMID: 30048640 DOI.
- ↑ 4.0 4.1 Marshall AM. The morphology of the vertebrate olfactory organ. (1879) Quarterly Journal of Microscopic Science. 19: 300–340.
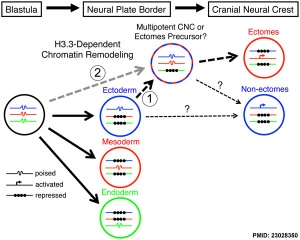
- ↑ 5.0 5.1 Cox SG, Kim H, Garnett AT, Medeiros DM, An W & Crump JG. (2012). An essential role of variant histone H3.3 for ectomesenchyme potential of the cranial neural crest. PLoS Genet. , 8, e1002938. PMID: 23028350 DOI.
- ↑ Coutiño BC & Mayor R. (2021). The mechanosensitive channel Piezo1 cooperates with Semaphorin to control neural crest migration. Development , , . PMID: 34822717 DOI.
- ↑ DiStasio A, Paulding D, Chaturvedi P & Stottmann RW. (2019). Nubp2 is required for cranial neural crest survival in the mouse. Dev. Biol. , , . PMID: 31733190 DOI.
- ↑ Martik ML, Gandhi S, Uy BR, Gillis JA, Green SA, Simoes-Costa M & Bronner ME. (2019). Evolution of the new head by gradual acquisition of neural crest regulatory circuits. Nature , , . PMID: 31645763 DOI.
- ↑ Hockman D, Chong-Morrison V, Green SA, Gavriouchkina D, Candido-Ferreira I, Ling ITC, Williams RM, Amemiya CT, Smith JJ, Bronner ME & Sauka-Spengler T. (2019). A genome-wide assessment of the ancestral neural crest gene regulatory network. Nat Commun , 10, 4689. PMID: 31619682 DOI.
- ↑ Odelin G, Faure E, Coulpier F, Di Bonito M, Bajolle F, Studer M, Avierinos JF, Charnay P, Topilko P & Zaffran S. (2018). Krox20 defines a subpopulation of cardiac neural crest cells contributing to arterial valves and bicuspid aortic valve. Development , 145, . PMID: 29158447 DOI.
- ↑ Pla P & Monsoro-Burq AH. (2018). The neural border: Induction, specification and maturation of the territory that generates neural crest cells. Dev. Biol. , , . PMID: 29852131 DOI.
- ↑ Radenkovic G, Radenkovic D & Velickov A. (2018). Development of interstitial cells of Cajal in the human digestive tract as the result of reciprocal induction of mesenchymal and neural crest cells. J. Cell. Mol. Med. , 22, 778-785. PMID: 29193736 DOI.
- ↑ Uribe RA, Hong SS & Bronner ME. (2018). Retinoic acid temporally orchestrates colonization of the gut by vagal neural crest cells. Dev. Biol. , 433, 17-32. PMID: 29108781 DOI.
- ↑ Mimura S, Suga M, Okada K, Kinehara M, Nikawa H & Furue MK. (2016). Bone morphogenetic protein 4 promotes craniofacial neural crest induction from human pluripotent stem cells. Int. J. Dev. Biol. , 60, 21-8. PMID: 26934293 DOI.
- ↑ Causeret F, Ensini M, Teissier A, Kessaris N, Richardson WD, Lucas de Couville T & Pierani A. (2011). Dbx1-expressing cells are necessary for the survival of the mammalian anterior neural and craniofacial structures. PLoS ONE , 6, e19367. PMID: 21552538 DOI.
- ↑ Betters E, Liu Y, Kjaeldgaard A, Sundström E & García-Castro MI. (2010). Analysis of early human neural crest development. Dev. Biol. , 344, 578-92. PMID: 20478300 DOI.
- ↑ Kulesa PM, Bailey CM, Kasemeier-Kulesa JC & McLennan R. (2010). Cranial neural crest migration: new rules for an old road. Dev. Biol. , 344, 543-54. PMID: 20399765 DOI.
- ↑ Lee G, Chambers SM, Tomishima MJ & Studer L. (2010). Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc , 5, 688-701. PMID: 20360764 DOI.
- ↑ Green SA, Simoes-Costa M & Bronner ME. (2015). Evolution of vertebrates as viewed from the crest. Nature , 520, 474-482. PMID: 25903629 DOI.
- ↑ Woodhoo A & Sommer L. (2008). Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia , 56, 1481-90. PMID: 18803317 DOI.
- ↑ Barraud P, Seferiadis AA, Tyson LD, Zwart MF, Szabo-Rogers HL, Ruhrberg C, Liu KJ & Baker CV. (2010). Neural crest origin of olfactory ensheathing glia. Proc. Natl. Acad. Sci. U.S.A. , 107, 21040-5. PMID: 21078992 DOI.
- ↑ Szabó A & Mayor R. (2018). Mechanisms of Neural Crest Migration. Annu. Rev. Genet. , 52, 43-63. PMID: 30476447 DOI.
- ↑ Kulesa PM & Fraser SE. (2000). In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development , 127, 1161-72. PMID: 10683170
- ↑ Smith P, Scraggs M & Heath D. (1993). The development of the nerve network in the fetal human carotid body and its subsequent function in cardiac disease. Cardioscience , 4, 143-9. PMID: 8400021
- ↑ Hempleman SC & Warburton SJ. (2013). Comparative embryology of the carotid body. Respir Physiol Neurobiol , 185, 3-8. PMID: 22902512 DOI.
- ↑ WEST GB, SHEPHERD DM, HUNTER RB & MACGREGOR AR. (1953). The function of the organs of Zuckerkandl. Clin Sci , 12, 317-25. PMID: 13107111
- ↑ Schober A, Parlato R, Huber K, Kinscherf R, Hartleben B, Huber TB, Schütz G & Unsicker K. (2013). Cell loss and autophagy in the extra-adrenal chromaffin organ of Zuckerkandl are regulated by glucocorticoid signalling. J. Neuroendocrinol. , 25, 34-47. PMID: 23078542 DOI.
- ↑ 28.0 28.1 O'Rahilly R & Müller F. (2007). The development of the neural crest in the human. J. Anat. , 211, 335-51. PMID: 17848161 DOI.
- ↑ Kuo BR & Erickson CA. (2010). Regional differences in neural crest morphogenesis. Cell Adh Migr , 4, 567-85. PMID: 20962585
- ↑ Kuo BR & Erickson CA. (2011). Vagal neural crest cell migratory behavior: a transition between the cranial and trunk crest. Dev. Dyn. , 240, 2084-100. PMID: 22016183 DOI.
- ↑ Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP & Koentges G. (2005). Neural crest origins of the neck and shoulder. Nature , 436, 347-55. PMID: 16034409 DOI.
- ↑ Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP & Koentges G. (2005). Neural crest origins of the neck and shoulder. Nature , 436, 347-55. PMID: 16034409 DOI.
- ↑ Mort RL, Hay L & Jackson IJ. (2010). Ex vivo live imaging of melanoblast migration in embryonic mouse skin. Pigment Cell Melanoma Res , 23, 299-301. PMID: 20067551 DOI.
- ↑ Szabó A, Melchionda M, Nastasi G, Woods ML, Campo S, Perris R & Mayor R. (2016). In vivo confinement promotes collective migration of neural crest cells. J. Cell Biol. , 213, 543-55. PMID: 27241911 DOI.
Books
Trainor, P. (ed) Neural crest cells: evolution, development and disease. ISBN: 978-0-12-401730-6 ScienceDirect Nelms BL, Labosky PA. Transcriptional Control of Neural Crest Development. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. PMID 21452438
Reviews
Erickson AG, Kameneva P & Adameyko I. (2022). The transcriptional portraits of the neural crest at the individual cell level. Semin Cell Dev Biol , , . PMID: 35260294 DOI.
Roth DM, Bayona F, Baddam P & Graf D. (2021). Craniofacial Development: Neural Crest in Molecular Embryology. Head Neck Pathol , 15, 1-15. PMID: 33723764 DOI.
Bhattacharya D, Khan B & Simoes-Costa M. (2021). Neural crest metabolism: At the crossroads of development and disease. Dev Biol , 475, 245-255. PMID: 33548210 DOI.
Leonard CE & Taneyhill LA. (2019). The road best traveled: Neural crest migration upon the extracellular matrix. Semin. Cell Dev. Biol. , , . PMID: 31727473 DOI.
Etchevers HC, Dupin E & Le Douarin NM. (2019). The diverse neural crest: from embryology to human pathology. Development , 146, . PMID: 30858200 DOI.
Szabó A & Mayor R. (2018). Mechanisms of Neural Crest Migration. Annu. Rev. Genet. , 52, 43-63. PMID: 30476447 DOI.
Le Douarin NM & Dupin E. (2018). The "beginnings" of the neural crest. Dev. Biol. , 444 Suppl 1, S3-S13. PMID: 30048640 DOI.
Martik ML & Bronner ME. (2017). Regulatory Logic Underlying Diversification of the Neural Crest. Trends Genet. , 33, 715-727. PMID: 28851604 DOI.
Bronner ME & Simões-Costa M. (2016). The Neural Crest Migrating into the Twenty-First Century. Curr. Top. Dev. Biol. , 116, 115-34. PMID: 26970616 DOI.
Green SA, Simoes-Costa M & Bronner ME. (2015). Evolution of vertebrates as viewed from the crest. Nature , 520, 474-482. PMID: 25903629 DOI.
Lee YH & Saint-Jeannet JP. (2011). Sox9 function in craniofacial development and disease. Genesis , 49, 200-8. PMID: 21309066 DOI.
Kish PE, Bohnsack BL, Gallina D, Kasprick DS & Kahana A. (2011). The eye as an organizer of craniofacial development. Genesis , 49, 222-30. PMID: 21309065 DOI.
Jiang M, Stanke J & Lahti JM. (2011). The connections between neural crest development and neuroblastoma. Curr. Top. Dev. Biol. , 94, 77-127. PMID: 21295685 DOI.
PubmedParser error: The PubmedParser extension received invalid XML data. ()
Articles
O'Rahilly R & Müller F. (2007). The development of the neural crest in the human. J. Anat. , 211, 335-51. PMID: 17848161 DOI.
Search PubMed
Search Pubmed: Neural Crest Development
NCBI - Policies and Guidelines | PubMed | Help:Reference Tutorial
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Neural Crest Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_Crest_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G