Talk:BGDA Practical 3 - Quiz
Introduction
--Mark Hill 06:58, 8 April 2011 (EST) This is a draft version of the all pages used in this practical class. Note that new updates and links may not appear in this early version.
- Links: 2011 Printable Version - Practical 3 Pages (48 pages)
We will study human development over a series of 3 practical classes spanning the overall human prenatal developmental timecourse. There will be an additional class covering the extramebryonic tissues formed from the conceptus (the embryonic membranes and placenta).
| Practical 3 - Fertilization to Implantation | Practical 6 - Implantation to 8 Weeks | Practical 12 - Fetal Period |
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Practical - Fertilization to Implantation
Aim
This laboratory is an introduction to the earliest event in development, from fertilization of the ovum (egg) by sperm through to implantation.
Key Concepts
Gonad, gametogenesis, ovary, testis, menstral cycle, oocyte development (oogenesis), spermatozoa (sperm) development (spermatogenesis), sperm morphology/motility, meiosis/mitosis, follicle development, ovulation, zona pellucida, polar bodies, hormonal changes, mechanism of fertilization, post-fertilization changes, corpus luteum, zygote, morula, blastocyst, inner cell mass (embryoblast), trophoblast, implantation, ectopic implantation, abnormalities.
Key Reading
- Human Embryology, WJ. Larsen Chapter 1, 2, 3
- The Developing Human: Clinically Oriented Embryology. Moore & Persaud Chapter 1, 2
Online Practical Help
- Online Practical Pages contain movies which work better using the Firefox browser on your desktop (Internet Explorer may crash)
- Bookmark this current page (so you don't get lost)
- Work through the series of linked online resources with the demonstrator (listed in order after the green BGD icon on each page)
- Online Page Organisation is the same on each page with a lefthand menu and righthand content.
- Page Content has a series of images, text and movies down the page in sequence, a list of Terms and a link to a Glossary.
- Highlighted words within the page text link to the Glossary brief description, all additional pages and resources are always shown as "Links:"
- External Links there are links on some pages which may take you to other places outside the current Practical (navigate carefully, remember point 2!)
- Finished when we have reached and discussed Week 3 Overview (additional pages are not covered in the Practical but for your own additional study)
Textbooks

|
Moore, K.L. & Persuad, T.V.N. (2008). The Developing Human: clinically oriented embryology (8th ed.). Philadelphia: Saunders.
The following chapter links only work with a UNSW connection and can also be accessed through this UNSW Library connection. |

|
Schoenwolf, G.C., Bleyl, S.B., Brauer, P.R. and Francis-West, P.H. (2009). Larsen’s Human Embryology (4th ed.). New York; Edinburgh: Churchill Livingstone.
The following chapter links only work with a UNSW connection and can also be accessed through this UNSW Library connection. |

|
Hill, M.A. (2011) UNSW Embryology (11th ed.). Sydney:UNSW. |
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
This page covers gametogenesis within the ovary. With the help of the tutors and other students you will work your way through identifying features described in the text.
Begin by looking at the ovary and the formation of the follicle containing the egg which matures and is released upon ovulation. The images are arranged in series so that progressive stages of the maturing follicle can be seen. The final image on this current page is a link to a movie showing follicle development and ovulation. Use the series of images of the cat ovary below to identify the key features described in the associated text.
Note: This should be a revision of the Ovary Histology Practical you have already completed. If you have trouble with the terms, there is a glossary at the bottom of each page.
Oogenesis
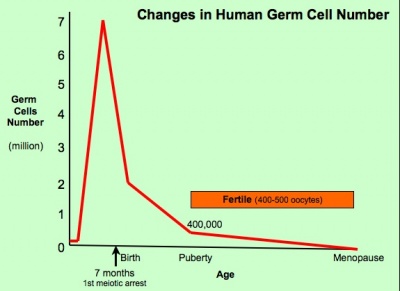
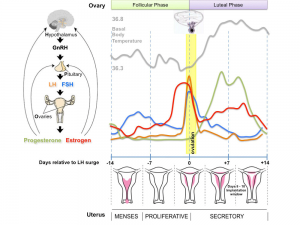
The graph below shows the changes in human germ cell numbers in the ovary with age, peaking at about 7 million (occuring in early fetal development) and then decreasing by apopotic cell death. At puberty there remain only about 400,000 and only about 10% of these will be released through reproductive life. (More? Menstrual Cycle)
(Based on data from: Hassold, etal., Environ Mol Mutagen 1996. 28: 167-175)
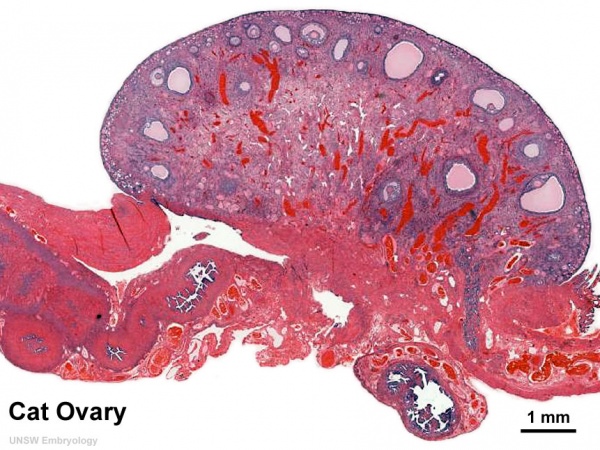
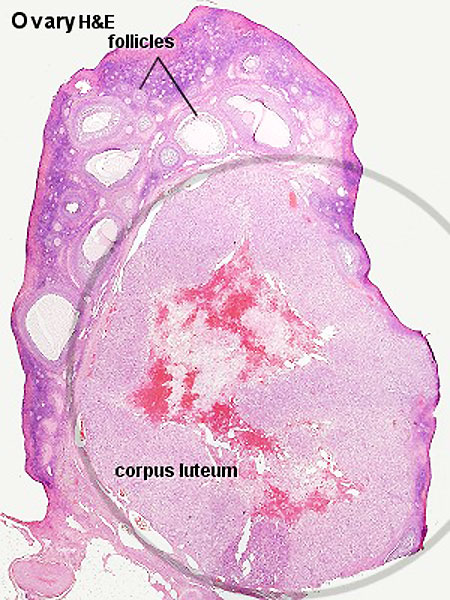
Whole Ovary
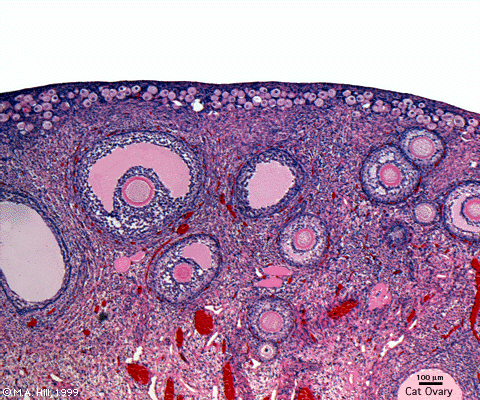
Ovary (cat, cross-section) showing histology and maturation of follicle.
Image (low magnification) showing cortical primordial follicles with primary (preantral) and secondary (antral) follicles lying deeper. Mesovarium at lower right and blood vessels in medullary region.
At this magnification, the overall organization of the ovary can be observed, cortex/medulla organization and arrangement of the maternal blood vessels, but few specific follicle details can be seen.
The next image is of the ovarian cortical region.
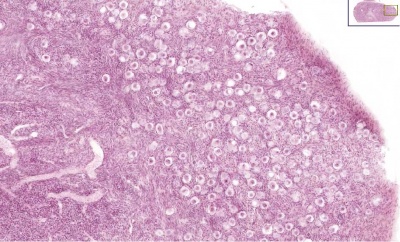
Ovary Cortex (low power)
Ovary cortex showing primordial follicles.
At the top of the image, is the outside of the ovary.
The thick connective tissue outer layer is the tunica albuginea. Over which a single layer of cells called the germinal epithelium (not visible) cover the surface of the ovary.
The next layer contains the earliest primordial follicles, single cells with pale cytoplasm and darkly stained nuclei.
The next layer contains many growing follicles at various stages of maturity and development. There is also evidence of degeneration as atretic follicles.
At the bottom of the image, is the medullary region of the ovary. Note the large number of maternal blood vessels which are the circulatory conduits for the estrogens and progesterones produced by the theca surrounding the ovarian follicles.
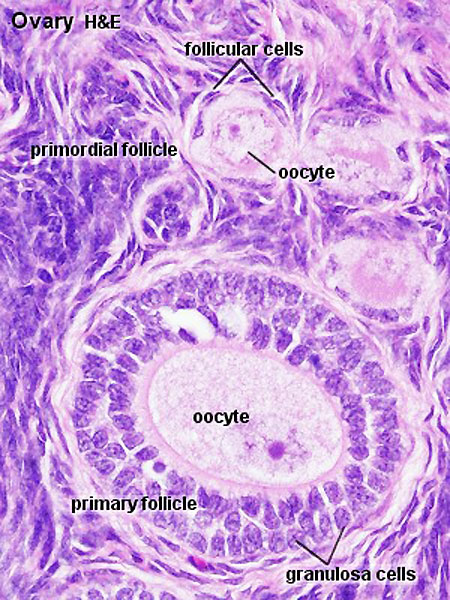
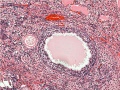
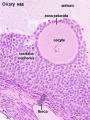
Note: [[G#germinal epithelium|germinal epithelium], tunica albuginea, primordial and atretic follicles. Note larger preantral follicle with (from the centre out) nucleus of maturing oocyte, oocyte cytoplasm, zona pellucida (pink ring), follicle cells, stromal cells.
Ovary Cortex Primordial and Primary Follicles
View of cortical ovary region showing primordial follicles and a single preantral follicle, with atretic follicle to its left. Bottom of picture shows outer cells of antral follicle.
High power view of ovary cortical region showing primordial follicles and a single preantral follicle.
Features: germinal epithelium, tunica albuginea, preantral follicle, nucleus of oocyte, oocyte cytoplasm, zona pellucida, Call-Exner body, stratum granulosa, basement membrane, theca, blood vessels surrounding follicle in theca layer.
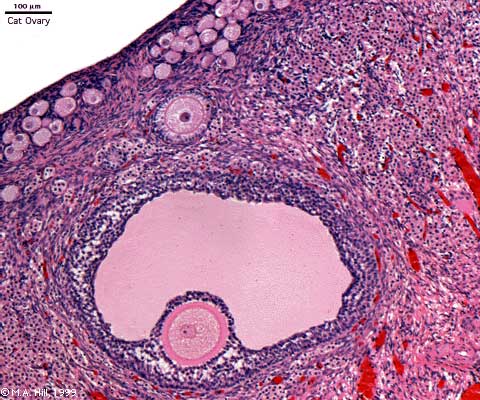
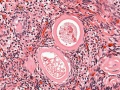
Ovary Cortex and Medulla
Low power view of ovary cortex and medullary region. Note 3 stages of follocle development (primordial, preantral and antral).
Features:
- cortical primordial follicles
- oocyte
- follicular cells
- stromal cells
- preantral follicle- zona pellucida, stratum granulosa, theca
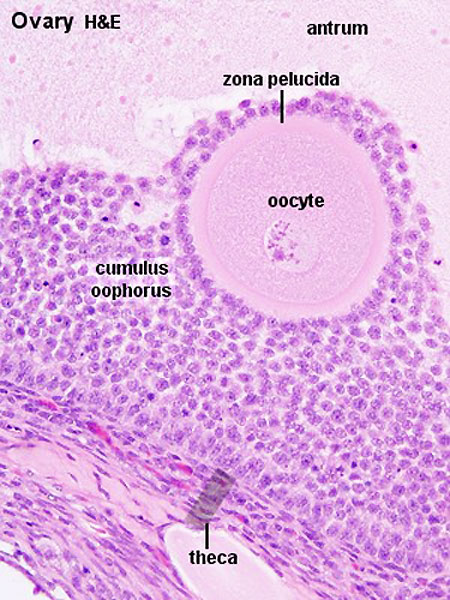
- early preovulatory follicle (Graafian) - oocyte, zona pellucida, corona radiata, cumulus oophorus, liquor folliculi, stratum granulosa, theca interna, theca externa, blood vessels surrounding follicle
Follicle Development
The development of a primordial follicle to a preovulatory follicle takes in excess of 120 days. After it has become a primary follicle of about 0.2 mm diameter it takes about 65 days to develop into a preovulatory follicle. Cohorts of follicles continually develop but only one is most sensitive to hormonal stimulation and is "selected", becoming the dominant follicle. All others in this cohort will undergo atresia.
Fertility Treatments
Superovulation therapy is a fertility drug treatement (oral clomiphene citrate and/or injectable FSH with or without LH) aimed at stimulating development/release of more than one follicle during a single menstrual cycle.
Follicle Classification
The above images show the histological changes that occur with follicle development (folliculogenesis). In humans, this entire process occurs over the timecourse of at least 3 menstrual cycles. This means that within the ovary during each cycle (at any point in time) many follicles can be either developing (folliculogenesis), regressing (atresis) and only a single follicle will be selected as ready for release. The selected follicle readied for release, generally one of the largest antral follicle, and can be classifed or described as: an antral preovulatory follicle or Graafian follicle or type 8 follicle (depending upon the classification used).
Classification systems - There are several different nomenclatures for the stages of follicle maturation (shown below) all of which makes the literature very confusing. The simplest is primordial, preantral, antral, Preovulatory (Graffian). You can also use the 5 step follicle classification: Primordial, Primary, Secondary, Tertiary, Preovulatory. Note that some classifications refer to the antral follicle as a secondary follicle and do not use the term tertiary follicle.
Primordial Follicle
Alternative nomenclature: small follicle or type 1, 2, 3 (25 cells) less than 50 micron diameter
Preantral Follicle
Alternative nomenclature: preantral follicle or type 4 (26-100 cells), type 5 (101-300 cells) up to 200 micron diameter
Antral Follicle
Alternative nomenclature: small antral type 6 (301-500 cells), large antral type 7 (501-1000 cells) small antral 500 micron diameter, large antral 1000-6000 micron diameter
Preovulatory Follicle
Alternative nomenclature: largest antral follicle or Graafian follicle or type 8 (>1000 cells) greater than 6000 micron diameter
Atresia
At any one time the majority of follicles are destined not to complete maturation and at any stage (from type 4-7) degeneration of the follicle can occur, this process is called atresia.
Ovulation
Movie (click image to play) showing process of ovulation (release of oocyte and follicular fluid). Click on movie to start.
Note that following ovulation the remnant of the follicle will degenerate if implantation does not occur (non-pregnant) forming a corpus albicans or following implantation (pregnancy) a corpus luteum which provides endocrine support to the uterus.
An endocrine signal (hCG human Chorionic Gonadotropin) from the implanting conceptus syncitiotrophoblasts maintains the corpus luteum, which in turn supports the uterine functional lining, preventing menstruation.
Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 | Quiz
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Additional Information
The information below is not part of today's Practical.
Links: Female Reproductive Tract Histology
Terms
- antral follicle - the stage following preantral in the decription of the sequence ovarian follicle development.
- antrum - (L. a cave), cavity; a nearly-closed cavity or bulge. In the ovary this refers to the follicular fluid-filled space within the follicle.
- atretic follicle - An ovarian follicle that fails to mature and degenerates. Also called "atresia" refering to the process of degeneration of the ovarian follicle. This process can occur at any stage of follicle development (folliculogenesis).
- clomiphene citrate - drug taken orally to promote the process of follicle/egg maturation.
- corona radiata - Layer of follicle cells of cumulus oophorus remaining attached to zona pellucida of oocyte after ovulation. Also called granulosa cells.
- corpus albicans - (L. corpus = body, L. albicans = whitish); a degenerating corpus luteum in ovary.
- corpus luteum - (L. corpus = body, L. luteum = yellow) The remains of ovarian follicle after ovulation that acts as an endocrine organ supporting pregnancy and preventing menstruation (loss of the endometrial lining). de Graaf first observed it in the ovary of a cow as a yellow structure.
- cortical - (L. corticalis) at the outside (like the bark of a tree), usually combined with medulla meaning the core.
- cumulus oophorus - (L. cumulus = a little mound G. oon = egg + phorus = bearing); part of the wall of an ovarian follicle surrounding and carrying the ovum (oocyte).
- follicle - (L. folliculus = little bag,dim. of L. follis). A structure which develops in the ovary and contains a developing egg (oocyte).
- follicular fluid - the fluid found in the antrum of a secondary follicle. Secreted by cells in the wall of the follicle. This fluid is released along with the oocyte at ovulation.
- germinal epithelium - cellular component covering surface of ovary, it is continuous with mesothelium covering mesovarium. Note that it is a historical misnomer, as it is not the actual site of germ cell formation.
- Graafian follicle - named after Regnier de Graaf (1641-1673), an historic Dutch physician embryologist who studied pregnancy using rabbits.
- granulosa cells - the supporting cells that surround the developing egg within the follicle thecal layers.
- mesovarium - mesentry of the ovary formed from a fold of the broad ligament that attaches the ovary
- medullary - (L. medius = in the middle) relating to the medulla; pith, marrow, inner portion of an organ. Usually combined with cortex (cortical) meaning the outer layer.
- oocyte - (Greek, oo = egg, ovum) The term used to describe the haploid egg or ovum formed within the ovary (female gonad) and released to enter the uterine tube and be transported to the uterus. The mature oocyte is the cell released from the ovary during ovulation.
- oocyte retrieval - (egg retrieval) A clinical in vitro fertilization (IVF) procedure to collect the eggs contained in the ovarian follicles.
- oogenesis - (Greek, oo = egg + genesis = origin, creation, generation) process of diploid oogonia division and differentiation into an haploid oocyte (egg) within the ovary (female gonad). Mammalian meiosis will only be completed within the oocyte if fertilization occurs.
- oogonia - (Greek, oo = egg) diploid germ cells within the ovary (female gonad) which provide the primary oocytes for oocyte (egg) formation. In humans, all oogonia form primary oocytes within the ovary before birth.
- oophorus - (Greek, oo = egg + phorus = carrying, egg-bearing) cumulus oophorus, used to describe the granulosa cells within the follicle that tether or link the oocyte to the wall of the follicle.
- ovulation - release of the oocyte from the mature follicle. In humans generally a single oocyte is released from a cohort of several maturing follicles.
- preantral follicle - the stage following primordial in the description of the sequence ovarian follicle development.
- primary follicle - the stage following primordial in the description of the sequence ovarian follicle development.
- primordial follicle - the first stage in the description of the sequence ovarian follicle development. Present in the ovary from birth, located in the stroma of the ovary cortex beneath the tunica albuginea. The primordial follicle is the oocyte and the surrounding follicular cells.
- primordial germ cell - oocyte present in the primordial follicle ovary from birth, located in the stroma of the ovary cortex beneath the tunica albuginea. The primordial follicle is the oocyte and the surrounding follicular cells.
- secondary follicles - the stage following primary in the description of the sequence ovarian follicle development.
- stromal cells - in the ovary, cells surrounding the developing follicle that form a connective tissue sheath (theca folliculi). This layer then differentiates into 2 layers (theca interna, theca externa). This region is richly vascularized and involved in hormone secretion.
- superovulation therapy - a fertility drug treatement (oral clomiphene citrate and/or injectable FSH with or without LH) aimed at stimulating development/release of more than one follicle during a single menstrual cycle.
- tertiary follicle - the stage following secondary in the description of the sequence ovarian follicle development.
- theca folliculi - stromal cells in the ovary, cells surrounding the developing follicle that form a connective tissue sheath. This layer then differentiates into 2 layers (theca interna, theca externa). This region is vascularized and involved in hormone secretion.
- theca externa - stromal cells forming the outer layer of the theca folliculi surrounding the developing follicle. Consisting of connective tissue cells, smooth muscle and collagen fibers.
- theca interna - stromal cells forming the inner layer of the theca folliculi surrounding the developing follicle. This vascularized layer of cells respond to LH (leutenizing hormone) synthesizing and secreting androgens which are processed into estrogen.
- tunica albuginea - dense connective tissue layer lying between germinal epithelium and cortical region of ovary.
- uterus - site of embryo implantation and development. Uterine wall has 3 major layers: endometrium, myometrium, and perimetrium. Endometrium can be further divided into the functional layer (shed/lost during menstruation) and basal layer (not lost during menstruation).
- zona pellucida - extracellular layer lying directly around the oocyte underneath follicular cells. Has an important role in egg development, fertilization and blastocyst development. This thick extracellular matrix consists of glcosaminoglycans and 3 glycoproteins (ZP1, ZP2, ZP3).
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
This page covers the process of mammalian fertilization. A complex interaction between the two haploid gametes (oocyte and spermatozoa) resulting in a single diploid cell (zygote). Following entry of the spermatazoa into the oocyte a series of changes occur within the oocyte and zona pellucida that block further fertilization by additional bound spermatozoa (polyspermy).
Due to both scientific and medical research on this process, this can now occur outside the body (in vitro fertilization) as well as fertility therapies to aid normal (in vivo fertilization). In addition, our understanding of fertilization has led to the development of a number of alternative fertility control methods.
Fertilization Dynamics
Sperm Events
Capacitation - removal of glycoprotein coat and seminal proteins, alteration of sperm mitochondria
Binding - ZP3 acts as receptor for sperm
Acrosome reaction - exyocytosis of acrosome contents (Calcium ion mediated), enzymes to digest the zona pellucida, exposes sperm surface proteins to bind ZP2
Membrane fusion - between sperm and egg, allows sperm nuclei passage into egg cytoplasm
Egg Events
Sperm membrane fusion - causes depolarization of egg membrane, primary block to polyspermy
Cortical reaction - IP3 pathway elevates intracellular Calcium, exocytosis of cortical granules, enzyme alters ZP3 so it will no longer bind sperm plasma membrane (cortical reaction)
2nd meiotic division - completion of 2nd meiotic division, forms second polar body
Sperm Penetration
The animation shows:
|
Pronuclear Fusion
|
The animation shows:
|
| In the mouse zygote, separation of chromatin according to parental origin is preserved up to the four-cell embryo stage and then gradually disappears. |
Fertilization Overview
Capacitation
- The mammalian spermatozoa once released, must remain for a time in the female genital tract before having the capacity to fertilize the oocyte. This process involves modifying the spermatozoa.
Acrosome Reaction
- Penetration of egg by spermatozoa is initiated by the acrosome reaction which takes different forms in different species.
- Mammalian acrosomal lysins contain proteinases which lyse the glycoproteins of the zona pellucida.
- The central part of the acrosome elongates into a tube which extends form the head of the spermatozoon. On contact with the egg the acrosomal membrane fuses with the sperm plasma membrane thus opening the acrosomal vesicle and liberating the granules containing acrosomal lysins.
- The inner portion of the acrosomal membrane everts and lengthens to form the acrosomal tubule through which the sperm nucleus enters the egg.
Sperm Contact
The act of fertilization changes the egg from a stage of slow structural and metabolic decline to one of renewed activation. Morphologically egg activation is a series of surface changes immediately following sperm contact.
- Mammals - No phenomenon comparable to the raising of the fertilization membrane is displayed. Mammalian eggs are surrounded by the zona pellucida which undergoes a structural change known as the zonal reaction after sperm penetration. On sperm contact with the egg plasma membrane, cortical granules break down as in above forms, substances liberated into the perivitelline space rapidly modify the zona pellucida resulting in a block to further sperm penetration.
Sperm Activation of Egg
- During fertilization sperm activates the egg by induction of a calcium ion (Ca2+) oscillation within the egg's cytoplasm.
- Induction occurs by a sperm protein factor (unidentified) which can stimulate only once calcium ion oscillations in metaphase eggs.
- Another sperm derived factor is then responsible for the inactivation of this oscillation.
- The activation of the egg by this calcium ion oscillation is essential for entry of the egg into the first mitotic cycle.
Zygote - Sperm Contribution
What the fertilizing sperm contributes in addition to the genetic material to the zygote differes between species.
- Centriole - most mammalian species, sperm contribute a centriole to reconstitute the zygotic centrosome. In rodents, only a maternal centrosomal inheritance occurs.
- Sperm Mitochondria - may enter the zygote, but are eliminated by a ubiquitin-dependent mechanism.
Perinuclear Theca - located in the sperm head perinuclear region. Contains a cytoskeletal element to maintain the shape of the sperm head and functional molecules leading to oocyte activation during fertilization.
Note - ART intracytoplasmic sperm injection techniques may introduce sperm components normally lost during in vivo fertilization.
Zona Pellucida
The specialized extracellular matrix layer lying directly around the oocyte underneath follicular cells. The structure consists of glcosaminoglycans and three main glycoproteins (ZP1, ZP2, ZP3).
After fertilization, the zona pellucida:
- blocks polyspermic fertilization
- physically protects the preimplantation embryo during early embryonic development (aided by an initial "hardening" after fertilization)
- aids uterine tube transport
- impacts upon blastocyst development
Zygote Contributions
Maternal - mitochondria, nucleolus, oocyte contributes one centriole during fertilization
Paternal - spermatozoon contributes one centriole during fertilization
Both Parents - chromosomes (inherent epigenetic differences between the paternal and maternal pronuclei), plasma membranes spermatozoon/oocyte mingle to form a mosaic plasma membrane
Links: Dev Biol - Fertilization | MBoC - Fertilization
Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 | Quiz
Terms
- adplantation - Initial adhesion of blastocyst (released from zona pellucida) to uterine wall. Adplantation is followed by implantation.
- ampulla - longest segment (approximately 2/3 of overall length) of uterine tube (oviduct or Fallopian tube). Medial segment forming the remainder of the tube is called the isthmus.
- antrum- (L. a cave), cavity; a nearly-closed cavity or bulge. In the ovary this refers to the follicular fluid-filled space within the follicle.
- blastocyst - the developmental stage following morula, as this stage matures, the zona pellucia is lost allowing the coceptus to adplant and then implant into the uterine wall.
- capacitation - the process of activation of sperm, requires removal of surface glycoproteins and increased motility. The sperm now become capable of fertilizing an egg.
- cavitates- to form a space within a solid object.
- conceptus - the entire structure generated from the zygote.
- fertilization - (fertilisation) The process of penetration of the oocyte (egg) by the spermatozoa and the combining of their genetic material that initiates development of the embryo. The union of two haploid gametes to form the first diploid cell, the zygote. (More? Fertilization | Spermatozoa Development | Testis Development | Ovary Development | Lecture - Fertilization)
- fimbriae- ( L. = a fringe) fingerlike projections at the ovarian end of uterine tube.
- follicular fluid- (or follicular fluid) the fluid found in the antrum of a secondary follicle. Secreted by cells in the wall of the follicle. This fluid is released along with the oocyte at ovulation.
- infundibulum- funnel-shaped initial segment of uterine tube (oviduct or Fallopian tube) opening into peritoneal cavity and connected to the ampulla. The peritoneal opening sitting over the ovary.
- morula -(L. morus = mulberry) early stage of development (12-15 cells) when conceptus is a solid ball of cells, further cell division forms the blasocyst.
- oocyte - (egg or ovum) female germ cell.
- ovulation- release of the oocyte from the mature follicle.
- uterine tube- (also called oviduct or Fallopian tube) the laterally paired tubes that connect the ovary to the uterus. Is the site for oocyte fertilization and initial development of the conceptus.
- uterine wall - the site of normal blastocyst implantation.
- zona pellucida- glycoprotein shell that surrounds the oocyte through to blastula stage of development
- uterus- site of embryo implantation and development. Uterine wall has 3 layers; endometrium, myometrium, and perimetrium.
- zona pellucida- extracellular layer lying directly around the oocyte underneath follicular cells. Consists of glcosaminoglycans and glycoproteins (ZP1, ZP2, ZP3).
- zygote - The first diploid cell that forms following fertilization by fusion of haploid oocyte (egg or ovum) and [S#spermatozoa|spermatozoa]] (sperm), resulting in the combination of their separate genomes. This single cell will divide by mitosis to form all cells of the embryo, fetal membranes and the embryonic component of the placenta. The term conceptus is used to describe all these cells derived from the fertilization event forming the zygote.
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
This page covers the process of mammalian fertilization. A complex interaction between the two haploid gametes (oocyte and spermatozoa) resulting in a single diploid cell (zygote). Following entry of the spermatazoa into the oocyte a series of changes occur within the oocyte and zona pellucida that block further fertilization by additional bound spermatozoa (polyspermy).
Due to both scientific and medical research on this process, this can now occur outside the body (in vitro fertilization) as well as fertility therapies to aid normal (in vivo fertilization). In addition, our understanding of fertilization has led to the development of a number of alternative fertility control methods.
Fertilization Dynamics
Sperm Events
Capacitation - removal of glycoprotein coat and seminal proteins, alteration of sperm mitochondria
Binding - ZP3 acts as receptor for sperm
Acrosome reaction - exyocytosis of acrosome contents (Calcium ion mediated), enzymes to digest the zona pellucida, exposes sperm surface proteins to bind ZP2
Membrane fusion - between sperm and egg, allows sperm nuclei passage into egg cytoplasm
Egg Events
Sperm membrane fusion - causes depolarization of egg membrane, primary block to polyspermy
Cortical reaction - IP3 pathway elevates intracellular Calcium, exocytosis of cortical granules, enzyme alters ZP3 so it will no longer bind sperm plasma membrane (cortical reaction)
2nd meiotic division - completion of 2nd meiotic division, forms second polar body
Sperm Penetration
The animation shows:
|
Pronuclear Fusion
|
The animation shows:
|
| In the mouse zygote, separation of chromatin according to parental origin is preserved up to the four-cell embryo stage and then gradually disappears. |
Fertilization Overview
Capacitation
- The mammalian spermatozoa once released, must remain for a time in the female genital tract before having the capacity to fertilize the oocyte. This process involves modifying the spermatozoa.
Acrosome Reaction
- Penetration of egg by spermatozoa is initiated by the acrosome reaction which takes different forms in different species.
- Mammalian acrosomal lysins contain proteinases which lyse the glycoproteins of the zona pellucida.
- The central part of the acrosome elongates into a tube which extends form the head of the spermatozoon. On contact with the egg the acrosomal membrane fuses with the sperm plasma membrane thus opening the acrosomal vesicle and liberating the granules containing acrosomal lysins.
- The inner portion of the acrosomal membrane everts and lengthens to form the acrosomal tubule through which the sperm nucleus enters the egg.
Sperm Contact
The act of fertilization changes the egg from a stage of slow structural and metabolic decline to one of renewed activation. Morphologically egg activation is a series of surface changes immediately following sperm contact.
- Mammals - No phenomenon comparable to the raising of the fertilization membrane is displayed. Mammalian eggs are surrounded by the zona pellucida which undergoes a structural change known as the zonal reaction after sperm penetration. On sperm contact with the egg plasma membrane, cortical granules break down as in above forms, substances liberated into the perivitelline space rapidly modify the zona pellucida resulting in a block to further sperm penetration.
Sperm Activation of Egg
- During fertilization sperm activates the egg by induction of a calcium ion (Ca2+) oscillation within the egg's cytoplasm.
- Induction occurs by a sperm protein factor (unidentified) which can stimulate only once calcium ion oscillations in metaphase eggs.
- Another sperm derived factor is then responsible for the inactivation of this oscillation.
- The activation of the egg by this calcium ion oscillation is essential for entry of the egg into the first mitotic cycle.
Zygote - Sperm Contribution
What the fertilizing sperm contributes in addition to the genetic material to the zygote differes between species.
- Centriole - most mammalian species, sperm contribute a centriole to reconstitute the zygotic centrosome. In rodents, only a maternal centrosomal inheritance occurs.
- Sperm Mitochondria - may enter the zygote, but are eliminated by a ubiquitin-dependent mechanism.
Perinuclear Theca - located in the sperm head perinuclear region. Contains a cytoskeletal element to maintain the shape of the sperm head and functional molecules leading to oocyte activation during fertilization.
Note - ART intracytoplasmic sperm injection techniques may introduce sperm components normally lost during in vivo fertilization.
Zona Pellucida
The specialized extracellular matrix layer lying directly around the oocyte underneath follicular cells. The structure consists of glcosaminoglycans and three main glycoproteins (ZP1, ZP2, ZP3).
After fertilization, the zona pellucida:
- blocks polyspermic fertilization
- physically protects the preimplantation embryo during early embryonic development (aided by an initial "hardening" after fertilization)
- aids uterine tube transport
- impacts upon blastocyst development
Zygote Contributions
Maternal - mitochondria, nucleolus, oocyte contributes one centriole during fertilization
Paternal - spermatozoon contributes one centriole during fertilization
Both Parents - chromosomes (inherent epigenetic differences between the paternal and maternal pronuclei), plasma membranes spermatozoon/oocyte mingle to form a mosaic plasma membrane
Links: Dev Biol - Fertilization | MBoC - Fertilization
Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 | Quiz
Terms
- adplantation - Initial adhesion of blastocyst (released from zona pellucida) to uterine wall. Adplantation is followed by implantation.
- ampulla - longest segment (approximately 2/3 of overall length) of uterine tube (oviduct or Fallopian tube). Medial segment forming the remainder of the tube is called the isthmus.
- antrum- (L. a cave), cavity; a nearly-closed cavity or bulge. In the ovary this refers to the follicular fluid-filled space within the follicle.
- blastocyst - the developmental stage following morula, as this stage matures, the zona pellucia is lost allowing the coceptus to adplant and then implant into the uterine wall.
- capacitation - the process of activation of sperm, requires removal of surface glycoproteins and increased motility. The sperm now become capable of fertilizing an egg.
- cavitates- to form a space within a solid object.
- conceptus - the entire structure generated from the zygote.
- fertilization - (fertilisation) The process of penetration of the oocyte (egg) by the spermatozoa and the combining of their genetic material that initiates development of the embryo. The union of two haploid gametes to form the first diploid cell, the zygote. (More? Fertilization | Spermatozoa Development | Testis Development | Ovary Development | Lecture - Fertilization)
- fimbriae- ( L. = a fringe) fingerlike projections at the ovarian end of uterine tube.
- follicular fluid- (or follicular fluid) the fluid found in the antrum of a secondary follicle. Secreted by cells in the wall of the follicle. This fluid is released along with the oocyte at ovulation.
- infundibulum- funnel-shaped initial segment of uterine tube (oviduct or Fallopian tube) opening into peritoneal cavity and connected to the ampulla. The peritoneal opening sitting over the ovary.
- morula -(L. morus = mulberry) early stage of development (12-15 cells) when conceptus is a solid ball of cells, further cell division forms the blasocyst.
- oocyte - (egg or ovum) female germ cell.
- ovulation- release of the oocyte from the mature follicle.
- uterine tube- (also called oviduct or Fallopian tube) the laterally paired tubes that connect the ovary to the uterus. Is the site for oocyte fertilization and initial development of the conceptus.
- uterine wall - the site of normal blastocyst implantation.
- zona pellucida- glycoprotein shell that surrounds the oocyte through to blastula stage of development
- uterus- site of embryo implantation and development. Uterine wall has 3 layers; endometrium, myometrium, and perimetrium.
- zona pellucida- extracellular layer lying directly around the oocyte underneath follicular cells. Consists of glcosaminoglycans and glycoproteins (ZP1, ZP2, ZP3).
- zygote - The first diploid cell that forms following fertilization by fusion of haploid oocyte (egg or ovum) and [S#spermatozoa|spermatozoa]] (sperm), resulting in the combination of their separate genomes. This single cell will divide by mitosis to form all cells of the embryo, fetal membranes and the embryonic component of the placenta. The term conceptus is used to describe all these cells derived from the fertilization event forming the zygote.
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
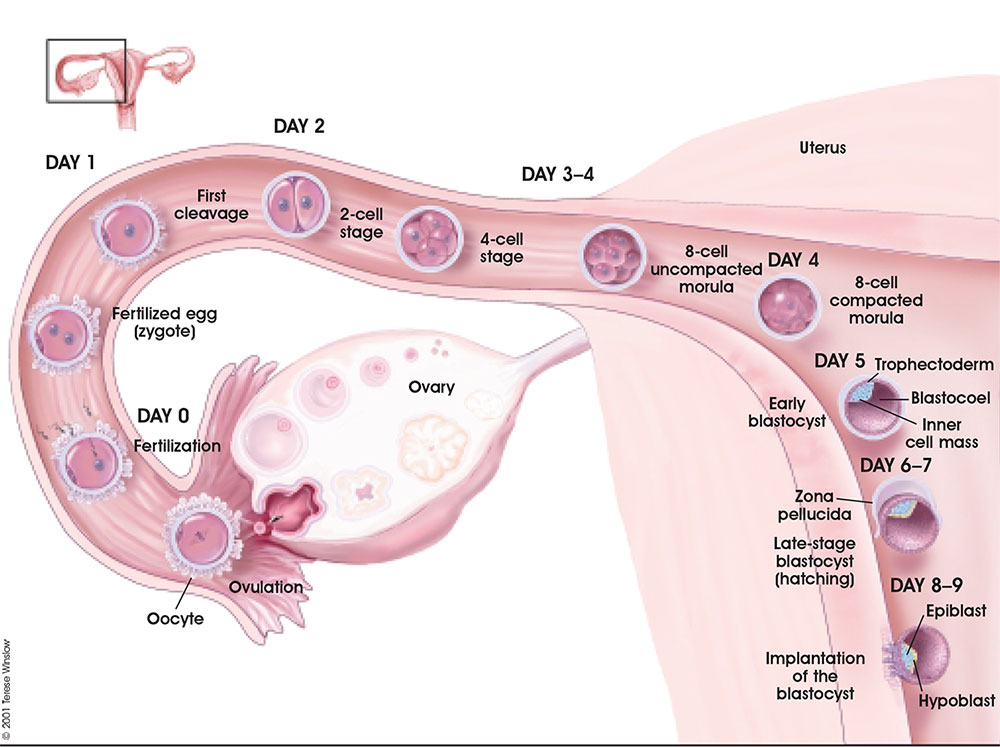
Following fertilization, the zygote undergoes a series of mitotic cell divisions during the first week of development leading to the formation of a hollow ball of cells, the 1-chambered conceptus or blastocyst (defined by the presence of this cavity the blastocoel).
From Oocyte to Blastocyst
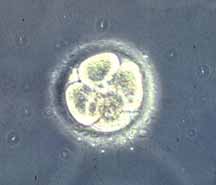
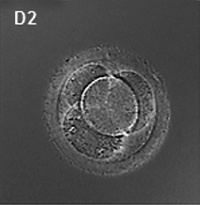
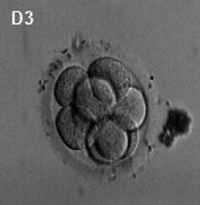
First cell divisions of the zygote forming initially 2 blastomeres and continuing to divide to form the morula.
This early mitosis is a unique embryonic cell cycle (M, S, M phases) compared to adult (M, G1, S, G2, M phase). With virtually no G1 or G2 phases this results in a reduction in cytoplasmic volume of each daughter cell with each cell division. See also Human oocyte to blastocyst
Facts: Week 1, 2-3 days, size 0.1-0.2 mm Features: zona pellucida, blastomeres
|
|
|
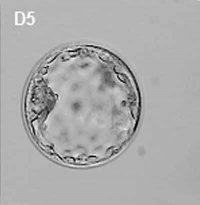
| Human Embryo (day 2) | Human Embryo (day 3) | Human Embryo (day 5) |
Blastocyst Hatching
By the end of the first week the blastocyst now consists of a ball of cells containing a large hollow fluid-filled (blastoceol) space.
Trophoblast Layer - Continued expansion of the blastoceol and cell division has led to the single layer of cells located at the periphery now being pressed against the inflexible zona pellucida wall and becoming flattened (squamous). This peripheral layer of cells is now called the trophoblast layer. Later in development this later will be involved in implantation and form part of the placenta.
Inner Cell Mass - On one wall of the blastoceol there is present a second layer of non-flattened cells. These inner cells are called the inner cell mass. Later in development from this layer the embryo will be formed.
Hatching - a combination of lysins (from the blastocyst or the uterus) and physical expansion, reduces the thickness and weakens the zona pelludica wall in preparation for hatching. Typically the blastocyst will hatch through a small opening (potentially at the site of fertilization) in the zona pellucida. The blastocyst is now ready to begin implantation.
Assisted Hatching - in vitro fertilization techniques use zona thinning, zona drilling, zona slitting or laser-assisted hatching to help blastocyst hatching and increase the probability of implantation occurring.
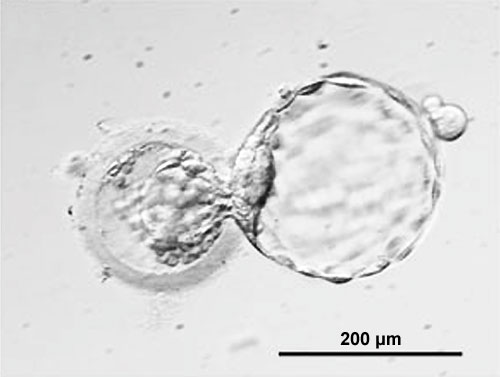 Zona pellucida (left) with Blastocyst (right) hatching through a small opening in the wall. (More? Carnegie stage 3)
Zona pellucida (left) with Blastocyst (right) hatching through a small opening in the wall. (More? Carnegie stage 3)
Links: MBoC - Mouse Blastocyst Development
Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 | Quiz
Additional Information
Dizygotic Twinning
Dizygotic twins (fraternal, non-identical) arise from separate fertilization events involving two separate oocyte (egg, ova) and spermatozoa (sperm).
Monoygotic Twinning
Monoygotic twins (identical) produced from a single fertilization event (one fertilised egg and a single spermatazoa, form a single zygote), these twins therefore share the same genetic makeup. Occurs in approximately 3-5 per 1000 pregnancies, more commonly with aged mothers. The later the twinning event, the less common are initially separate placental membranes and finally resulting in conjoined twins.
| Week | Week 1 | Week 2 | |||||||||||||
| Day | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Cell Number | 1 | 1 | 2 | 16 | 32 | 128 | bilaminar | ||||||||
| Event | Ovulation | fertilization | First cell division | Morula | Early blastocyst | Late blastocyst
Hatching |
Implantation starts | X inactivation | |||||||
|
|
|
|||||||||||||
| Monoygotic
Twin Type |
Diamniotic
Dichorionic |
Diamniotic
Monochorionic |
Monoamniotic
Monochorionic |
Conjoined | |||||||||||
Table based upon: Twinning. Hall JG. Lancet. 2003 Aug 30;362(9385):735-43. Review. PMID: 12957099
Terms
- bilaminar- having 2 layers
- blastocyst- the developmental stage following morula, as this stage matures, the zona pellucia is lost allowing the conceptus to adplant and then implant into the uterine wall.
- blastomeres-the cells resulting from the initial rounds of mitotic division of the zygote. These cells become smaller (in cytoplasmic volume) with each division.
- corona radiata- Layer of follicle cells of cumulus oophorus remaining attached to zona pellucida of oocyte after ovulation.
- inner cell mass- the clump of cells found inside the blastocyst. These cells will go in to form the embryo, these are the "stem cells" (we here about in the media) that are totipotential, they can form any tissue in the embryo. Mature oocyte-the female germ cell released at ovulation from the ovary.
- morula - (L. morus = mulberry) early stage of development (12-15 cells) Followed by formation of a cavity in the mass (blastocyst stage). (More? Week 1 Notes)
- parental genomes- the male (sperm) and female (oocyte) DNA which contributes to the embryo's cells.
- polar bodies- 3 exclusion bodies which contain the DNA not used by the embryo. Contributed to initially by the meiotic division of the oocyte.
- pronuclei- the male (sperm) and female (oocyte) nuclei within the fertilized oocyte, prior to their combination to form the new embryo's nuclei.
- trilaminar embryonic disc- the 3 layered embryo stage.
- Trophoblasts- (Gr. trophe = nutrition) outer layer of cells on blastocyst that will generate the embryonic part of the placenta.
- uterine wall - the site of normal blastocyst implantation.
- zona pellucida- glycoprotein shell that surrounds the oocyte through to blastula stage of development.
- Zygote- The first cell stage following fertilization of the oocyte by the sperm. This is the first cell of the conceptus which will divide into blastomeres.
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Week 1
Overview of development of the 1-chambered conceptus (blastocyst)
Ovulation is the initial release of oocyte.
Follicular fluid and fimbriae together aid oocyte movement into infundibulum then the ampulla of the uterine tube.
Sperm deposited in the vagina then enter the uterus, mature (capacitation), then actively migrate along the uterine tube.
Fertilization generally occurs in the ampulla region of the tube.
Following fertilization, repeated rounds of cell division occur without growth forming initially a solid ball of cells (morula), which cavitates to form the 1-chambered conceptus (blastocyst). Liberation of the blastocyst from the zona pellucida the allows attachment (adplantation) to the uterine wall.
Following ovulation the empty follicle within the ovary now forms the corpus luteum.
Note - the day timings shown above for the first week are approximate and may vary by several days for events following fertilization.
Blastocyst (right) hatching from zona pellucida (left)
Sex Determination
Mammalian sex determination is regulated by chromosomes.
- Females have two X chromosomes. (XX)
- Males have a single X and a small Y. (XY)
- The X and Y chromosome are morphologically and functionally different from each other.
- Evolutionary studies have shown that the Y was once the homologous pair for X.
- It is only in the last 5 years that we have some idea about how these two types of chromosomes may be regulated and genes of inportance located upon them.
- In females the main scientific problem was that of gene dosage, only one copy of X chromosome is needed to be active.
- In males the main scientific problem was what on the Y chromosome determined "maleness", and how did it do it.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 | Quiz
Additional Information
Terms
- adplantation - Initial adhesion of blastocyst (released from zona pellucida) to uterine wall. Adplantation is followed by implantation.
- ampulla - longest segment (approximately 2/3 of overall length) of uterine tube (oviduct or Fallopian tube). Medial segment forming the remainder of the tube is called the isthmus.
- antrum- (L. a cave), cavity; a nearly-closed cavity or bulge. In the ovary this refers to the follicular fluid-filled space within the follicle.
- blastocyst - the developmental stage following morula, as this stage matures, the zona pellucia is lost allowing the coceptus to adplant and then implant into the uterine wall.
- capacitation the process by which sperm become capable of fertilizing an egg, requires membrane changes, removal of surface glycoproteins and increased motility.
- cavitates - to form a space within a solid object.
- conceptus the product of conception, that is all the structures derived from the zygote. This includes not only the embryo, but also the placental and membrane components formed from the conceptus.
- fertilization The penetration of the egg by the sperm and the resulting combining of genetic material that develops into an embryo. The union of two haploid gametes to form a diploid cell or zygote.
- fimbriae (Latin, fimbria = a fringe) finger-like projections at the ovarian end of uterine tube. At ovulation they sit over the ovary to aid egg movement into the uterine tube.
- follicular fluid - the fluid found in the antrum of an antral follicle (secondary follicle). Secreted by cells in the wall of the follicle, this fluid is released along with the oocyte at ovulation.
- infundibulum - funnel-shaped initial segment of uterine tube (oviduct or Fallopian tube) opening into peritoneal cavity and connected to the ampulla. The peritoneal opening sitting over the ovary.
- morula (Latin, morula = mulberry) early stage in development (week 1) where the cells have divided to produce a solid mass of cells (12-15 cells) with a "mulberry" appearance. This stage is followed by formation of a cavity in the mass (blastocyst stage).
- oocyte - (egg or ovum) female germ cell.
- ovulation- release of the oocyte from the mature follicle.
- triploidy - in humans, three sets of 23 chromosomes instead of 2 (diploid) combine to form the embryo. This occurs mainly by fertilization of a single egg by two sperm and less frequently by a diploid egg or sperm. Most human triploids abort spontaneously, with very rare survival to term.
- tetraploidy - in humans, four sets of 23 chromosomes instead of 2 (diploid) due to a failure of the first mitotic division after fertilization, these fertilization events do not development.
- uterine tube - (also called oviduct or Fallopian tube) the laterally paired tubes that connect the ovary to the uterus. Is the site for oocyte fertilization and initial development of the conceptus.
- uterine wall - the site of normal blastocyst implantation.
- zona pellucida- glycoprotein shell that surrounds the oocyte through to blastula stage of development
- uterus- site of embryo implantation and development. Uterine wall has 3 layers; endometrium, myometrium, and perimetrium.
- zona pellucida- extracellular layer lying directly around the oocyte underneath follicular cells. Consists of glcosaminoglycans and glycoproteins (ZP1, ZP2, ZP3).
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
Hatching leaves the blastocyst now free of the zona pellucida and should have occured approximately at the end of the uterine tube or in the body of the uterus. It is now floating in the uterine glands rich mucus secretion and able to directly access this nutrition for continued growth.
The blastocyst initially weakly adheres to the endometrial wall rolling across its surface. Increased adhesion may lead to attachment, adplantation, on the inner cell mass side of the blastocyst. This will be the site where implantation will begin and the placenta will develop.
Trophoblast cells at the site of adplantation proliferate and form an additional layer the syncitiotrophoblast layer. This layer of cells rapidly divide, secrete enzymes that degrade the endometrial extracellular matrix and secrete human Chorionic Gonadotropin (hCG).
Implantation Dynamics
The uterine epithelium (white cells) are invaded by the trophoblast cells (green, syncitiotrophoblasts) with the inner cell mass now having 2 layers: an epiblast (blue) and hypoblast (yellow). The blastoceol is covered in cytotrophoblast cells (green).
Later in the movie the amniotic cavity forms adjacent to the epiblast layer(blue) and spaces in the syncitiotrophoblast layer are filled with maternal blood, lacunae.
<wikiflv height="220" width="248" autoplay="true" position="left">Week2_001.flv|File:Week2_001_icon.jpg</wikiflv>
This animation shows the process of implantation, occurring during week 2 of development in humans.
The beginning of the animation shows: the uterus lining (endometrium epithelium), the hatched blastocyst with a flat outer layer of trophoblast cells (green), the inner cell mass which has formed into the bilaminar embryo (epiblast and hypoblast) and the large fluid-filled space (blastocoel).
green cells - trophoblast layer of the conceptus
blue cells - epiblast layer of the bilaminar embryo
yellow cells - hypoblast layer of the bilaminar embryo
white cells - uterine endometrium epithelium
red - maternal blood vessel
Identify the embryoblast and trophoblast layers of the conceptus.
Carnegie Stage 4 represents the beginning of implantation. The blastocyst initially attached to the uterine endometrium (adplantation), syncitiotrophoblasts then secrete enzymes that digest extracellular matrix, allowing the blastocyst to sink into the uterine wall, eventually being completely enclosed within the uterine wall. Note the majority of growth occurs in the trophoblastic shell. The inner cell mass divides initially into 2 layers; epiblast and hypoblast (bilaminar embryo). Hypoblast cells migrate around the original blastoceol cavity forming the primary yolk sac. A second cavity (amniotic) forms between the inner cell mass and the cytotrophoblast shell; this cavity is lined by epiblast cells.
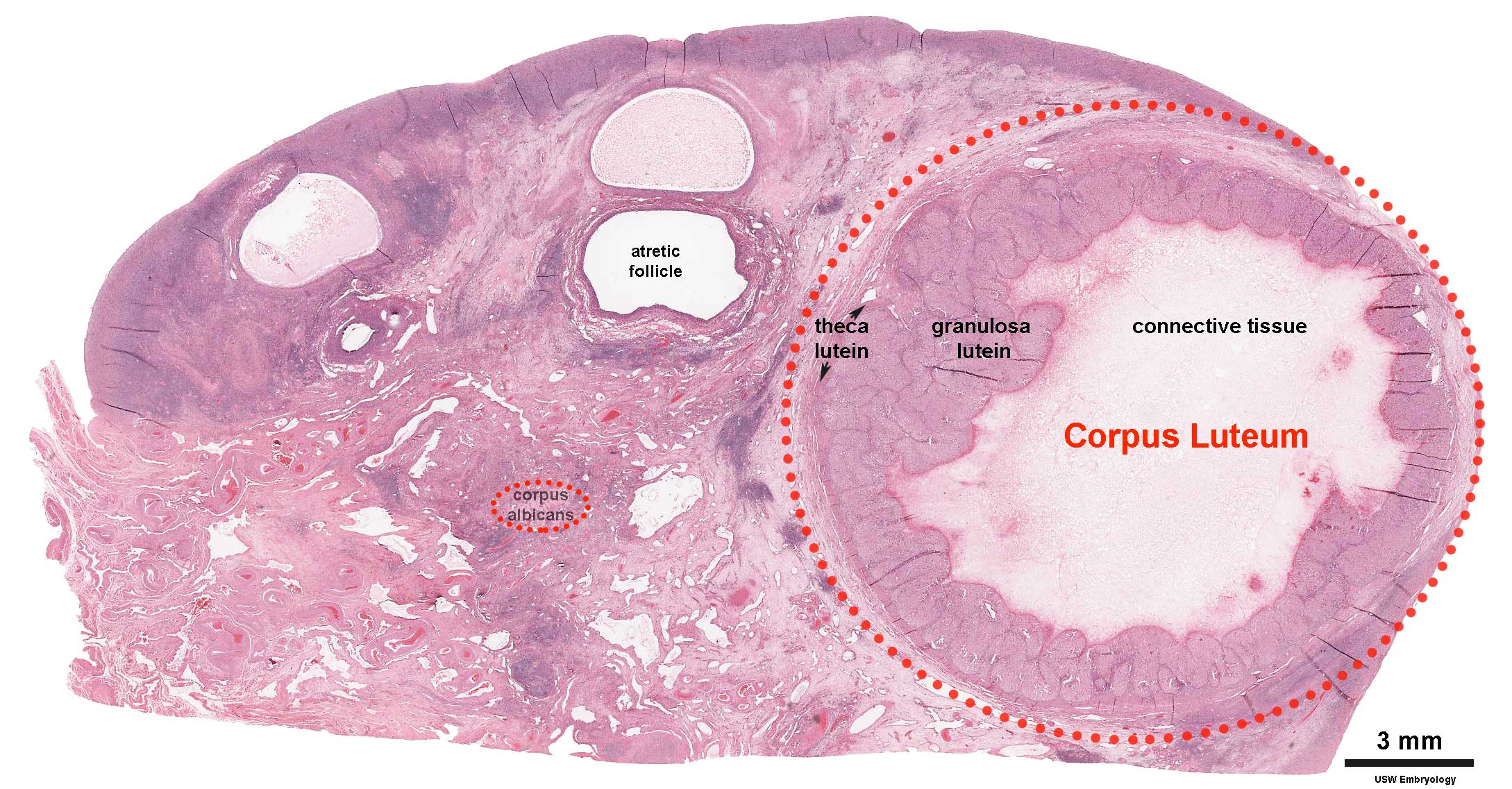
Corpus Luteum
An endocrine signal (hCG human Chorionic Gonadotropin) secreted from the implanting conceptus syncitiotrophoblast cells maintains the ovarian corpus luteum, which in turn provides hormonal support to the uterine functional lining, preventing menstruation. The corpus luteum is formed during the luteal phase (secretory phase) of the menstrual cycle by proliferation of both follicular granulosa cells (granulosa lutein cells) and thecal cells (theca lutein cells), which produce progesterone and oestrogens.
Following ovulation
- If implantation does not occur (non-pregnant), the remnant of the ovulating follicle will degenerate forming a corpus albicans.
- If implantation occurs (pregnancy), the remnant of the ovulating follicle will be maintained forming a corpus luteum.
If implantation does not begin until very late in the current menstrual cycle, or not at all, then that cycle will continue with loss of both the functional layer and the conceptus. Many human fertilization events never form an embryo or develop as a pregnancy.
Additional Information - Birth Control
There are a number of different chemical and mechanical methods of birth control. The most comon is the "birth control pill" taken daily and made up of two hormones, estrogen and progestogen and these stop a woman's ovaries from releasing an egg each month (ovulation), which means that a pregnancy cannot begin. Recently the drug RU486, which is an abortive rather contraceptive drug, has been the centre of political and medical discussions in Australia.
Chemical
- Estrogen - The hormone estrogen in birth control pills act on the pituitary gland (supress FSH and LH) which then blocks ovulation.
- Progesterone - The hormone progesterone in birth control pills act on the uterus to both alter the lining to prevent implantation and forms a cervical mucus plug that mechanically blocks acceess of sperm. There is also an inhibition of sperm capacitation.
- Injectable Control - There are commercial (Lunelle, USA) injectable estrogen/progestin contraceptives administered on a monthly basis.
- RU486 - Mifepristone (RU486) is a progesterone receptor antagonist (antiprogesterone) which can prevent between 92-100 % of pregnancies on oral intake of a 10-600 mg dose within 72 h of unprotected intercourse. (alternative commercial name: Mifegyne)
Links: Clinical Methods - Birth Control
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Terms
- bilaminar- having 2 layers
- blastocyst- the developmental stage following morula, as this stage matures, the zona pellucia is lost allowing the conceptus to adplant and then implant into the uterine wall.
- corpus albicans - (Latin, corpus = body, albicans = whitish) The histological structure formed by luteolysis of the corpus luteum in the ovary. If implantation does not occur and the hormone hCG is not released the corpus luteum degenerates and the structure is white, not yellow, because of the absence of steroid hormone synthesis/accumulation.
- corpus luteum - (Latin, corpus = body, luteum = yellow) The remains of ovarian follicle formed after ovulation that acts as an endocrine organ (produce progesterone and oestrogens) supporting pregnancy and preventing menstruation (loss of the endometrial lining). Formed during the luteal phase (secretory phase) of the menstrual cycle by proliferation of both follicular granulosa cells (granulosa lutein cells) and thecal cells (theca lutein cells), which produce progesterone and oestrogens. If fertilization and pregnancy does not occur, the corpus luteum degenerates to form the corpus albicans. Regnier de Graaf (1641 – 1673) first observed it in the ovary of a cow as a yellow structure, the yellow colour is caused by accumulation of steroidal hormones. (More? Menstrual Cycle | Ovary Development | Week 2 Ovary | Week 1 - Oogenesis)
- inner cell mass- the clump of cells found inside the blastocyst. These cells will go in to form the embryo, these are the "stem cells" (we here about in the media) that are totipotential, they can form any tissue in the embryo. Mature oocyte-the female germ cell released at ovulation from the ovary.
- trilaminar embryonic disc- the 3 layered embryo stage.
- Trophoblasts- (Gr. trophe = nutrition) outer layer of cells on blastocyst that will generate the embryonic part of the placenta.
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
Week 2 is about implantation and the endocrine signaling to block the normal menstrual cycle. We will also consider abnormal events that may occur in development during the second week. This involves abnormalities of implantation and conceptus development.
Note - Normal placentation will be covered in detail in a separate practical class.
Implantation Sites - Normal and Abnormal
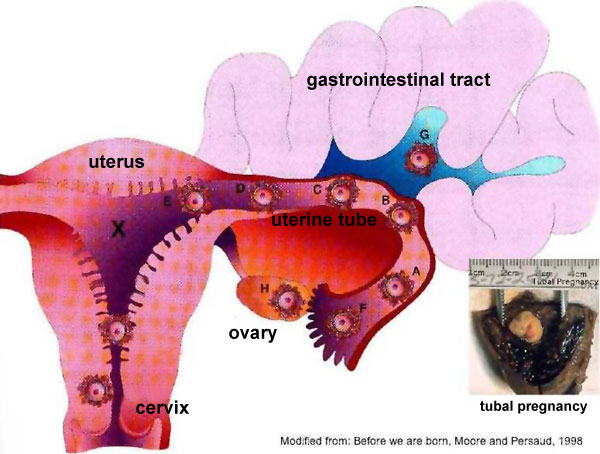
Sites of normal and abnormal blastocyst implantation.
Site of most common (normal) implantation is the posterior wall of uterus (shown by X). Abnormal implantation: tubal pregnancies (shown by A-F), ovarian (H), and abdominal (G). Implantation at the internal os generates the clinical condition placenta previa, (resulting in bleeding or placental separation during pregnancy). Note that spontaneous abortion of blastocysts is quite common and studies of blastocysts that do not implant indicate chromosomal abnormalities in many of these embryos.
Implantation Images Day 8 and 9
|
Overview of blastocyst implantation in uterine wall during the second week of development.
Identify the embryoblast and trophoblast layers of the conceptus. Carnegie Stage 4 (stages 1-23 describing key steps in embryonic development) represents the beginning of implantation. The blastocyst initially attached to the uterine endometrium (adplantation), syncitiotrophoblasts then secrete enzymes that digest extracellular matrix, allowing the blastocyst to sink into the uterine wall, eventually being completely enclosed within the uterine wall. Note the majority of growth occurs in the trophoblastic shell. The amniotic forms between the inner cell mass and the cytotrophoblast shell; this cavity is lined by epiblast cells. The inner cell mass divides initially into 2 layers; epiblast and hypoblast (bilaminar embryo). Hypoblast cells migrate around the original blastoceol cavity forming the primary yolk sac. These hypoblast cells are replaced by the endoderm during gastrulation. This endoderm lined cavity is the yolk sac. |
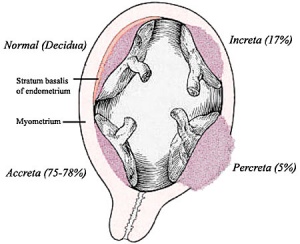
Placental Abnormalities
Placenta Accreta - Abnormal adherence, with absence of decidua basalis. The incidence of placenta accreta also significantly increases in women with previous cesarean section compared to those without a prior surgical delivery.
Placenta Increta - occurs when the placenta attaches deep into the uterine wall and penetrates into the uterine muscle, but does not penetrate the uterine serosa. Placenta increta accounts for approximately 15-17% of all cases.
Placenta Percreta - placental villi penetrate myometrium and through to uterine serosa.
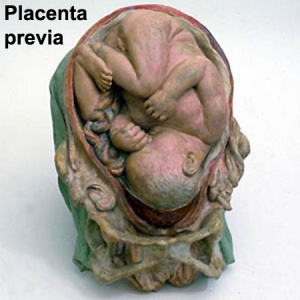
Placenta Previa
| In this placenatal abnormality, the placenta overlies internal os at the cervix of the uterus, essentially covering the birth canal. This condition occurs in approximately 1 in 200 to 250 pregnancies.
In the third trimester and at term, abnormal bleeding can require cesarian delivery and can also lead to abruptio placenta. Ultrasound screening programs during 1st and early 2nd trimester pregnancies now include placental localization. Diagnosis can also be made by transvaginal ultrasound. |
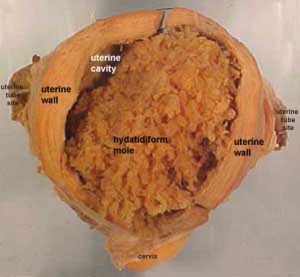
Hydatidiform Mole
| A placental tumor with no embryo development.
Several forms of hydatidiform mole: partial mole, complete mole and persistent gestational trophoblastic tumor. Many of these tumours arise from a haploid sperm fertilizing an egg without a female pronucleus (the alternative form, an embryo without sperm contribution, is called parthenogenesis). The tumour has a "grape-like" placental appearance without enclosed embryo formation. Following a first molar pregnancy, there is approximately a 1% risk of a second molar pregnancy. |
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
The conceptus is now fully implanted witin the endometrial wall and the uterine epithelium has reformed over the site of implanation.
Note that all future development will now occur within the wall of the uterus, not within the cavity of the uterine body.
As the conceptus continues to implant and grow a series of fluid-filled cavities form both outside and inside the implanting conceptus.
- Outside the Conceptus - maternal blood-filled lacunae
- Inside the Conceptus - 3 separate spaces - amniotic sac, yolk sac, chorionic sac
Conceptus Cavities Week 2 and Week 3
Outside
Continued expansion of syncitiotrophoblasts within the endometrial wall opens both uterine glands and uterine blood vessels. These spaces outside the conceptus fill with uterine gland secretions and maternal blood forming maternal blood-filled lacunae. These lacunae provide the initial nutrition to the growing conceptus, which will later be provided through the placenta.
Inside
A cavity forms now between the inner cell mass and the trophoblast (cytotrophoblasts) wall. This cavity is the amniotic cavity.
A new cell layer forms from cells proliferating and lining the inside of the blastoceol cytotrophoblastic layer (extraembryoic mesoderm). This extraembryoic mesoderm layer continues to proliferate and then vacuolates, splitting into two separate cavities.
Overview of blastocyst implantation in uterine wall during the second week of development. (Image: Moore & Persaud, 1998)
Beginning of Carnegie Stage 6, blastocyst fully implanted in endometrial wall.
Continued expansion of the cavities in the extraembryonic mesoderm, allow the primary yolk sac to collapse. The collapsed space later becomes lined with endodermal cells and forms the yolk sac.
The space formed outside is lined with extraembryonic mesoderm and forms the third cavity the chorionic cavity.
A section of extraembryonic mesoderm links the now bilaminar embryonic disc to surrounding shell and is called the connecting stalk.
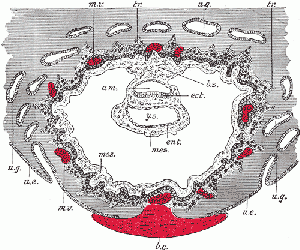
Human Conceptus Implantation 2nd and 3rd Week of Development
| Identify chorionic sac, yolk sac and amniotic sac, all containing different fluids.
Identify secondary chorionic villi, maternal blood "spaces" (empty) and uterine tissue (maternal decidua). The embryo lies between the yolk sac fluid and the amniotic sac fluid. Note that the ends ("bases") of some long secondary chorionic villi are attached to the maternal (decidual) tissue by dense clusters of trophoblast cells (the cytotrophoblastic column) - these are anchoring secondary villi. Other shorter, secondary villi are "free-floating" in the maternal intervillous spaces, from which the blood has drained into the large endometrial veins. (Image: Nishimura etal., 1977) |
 Carnegie Stage 6-7, human conceptus approximately 16-18 days. (about the time of the first missed menstrual period). Carnegie Stage 6-7, human conceptus approximately 16-18 days. (about the time of the first missed menstrual period).
|
--Mark Hill 15:34, 16 June 2010 (UTC) These 2 missing images Carnegie Stage 6-7 from the original practical class page have been added later and are not examinable.
Human Conceptus (high power view)
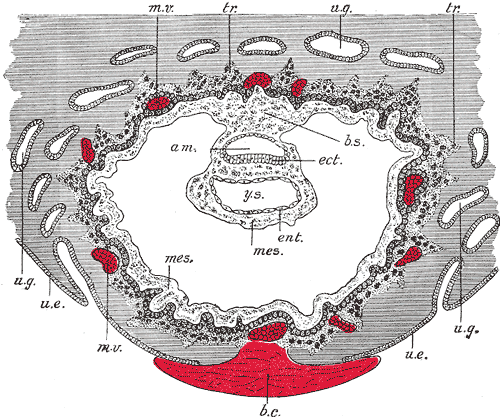
| Identify the three fluid-filled sacs, the embryo, and the body (connecting) stalk indicating the caudal end of the embryo. Note the thin amnion and the thicker wall of the yolk sac and conversely, the thick ectoderm and thin endoderm. Vascular channels in connecting stalk. The broad elevation in the ectoderm is the expansion dome (primitive single brain bulge) and the cluster of cells at the caudal end of the embryonic disc is the primitive node region. Note the precipitated protein in the extra-embryonic coelom and in the yolk sac, and the start of the formation of the intra-embryonic coelom between ectoderm and entoderm, at the cranial (rostral) end of the expansion dome. | 
|
--Mark Hill 15:34, 16 June 2010 (UTC) These 2 missing Carnegie Stage 6-7 images from the original practical class page have been added later and are not examinable.
Human Conceptus (high power view)
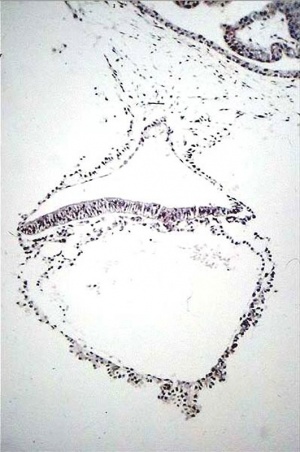
|
Approximate cross-section of an 18 day human conceptus.
(Image: Nishimura etal., 1977)
|
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Terms
- bilaminar- having 2 layers
- blastocyst- the developmental stage following morula, as this stage matures, the zona pellucia is lost allowing the conceptus to adplant and then implant into the uterine wall.
- inner cell mass- the clump of cells found inside the blastocyst. These cells will go in to form the embryo, these are the "stem cells" (we here about in the media) that are totipotential, they can form any tissue in the embryo. Mature oocyte-the female germ cell released at ovulation from the ovary.
- parental genomes- the male (sperm) and female (oocyte) DNA which contributes to the embryo's cells.
- trilaminar embryonic disc- the 3 layered embryo stage.
- trophoblasts- (Gr. trophe = nutrition) outer layer of cells on blastocyst that will generate the embryonic part of the placenta.
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction

|
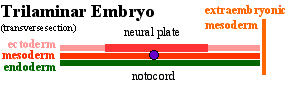
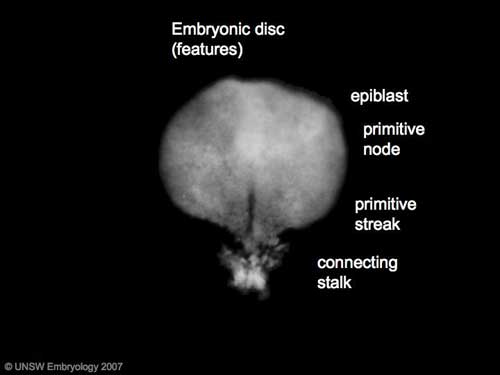
Gastrulation means "gut forming" and converts the inner cell mass which then formed the bilaminar embryo (epiblast, hypoblast) into the trilaminar embryo (ectoderm, mesoderm, endoderm).
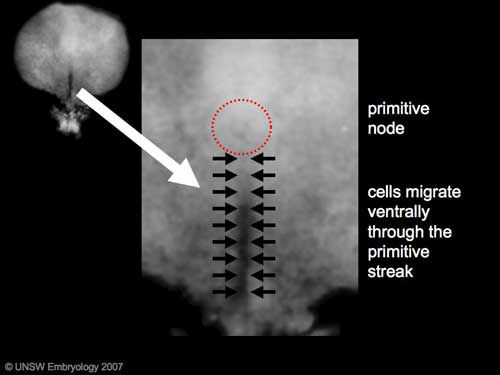
The process involves the migration of cells from the epiblast layer through the primitive streak to form first the endoderm layer and then a second intermediate layer the mesoderm layer. Once all cells have left the epiblast layer it now becomes the ectoderm layer. These three germ cell layers (ectoderm, mesoderm, endoderm) will form in a layer specific manner all the future tissues of the developing embryo.
Approximate cross-section of an 18 day human conceptus. Identify the 3 layers of the trilaminar embryo: ectoderm (columnar cells), intraembryonic mesoderm (mesenchymal cells, endodermal cells (cuboidal single layer). Identify primitive groove with dense cluster of primitive streak cells below it.
|
Stage 7
|
|
| Features: embryonic disc, primitive node, primative streak, primitive groove, yolk sac
Facts: Week 3, 15 - 17 days, 0.4 mm View 1: embryonic disc, showing the epiblast viewed from the amniotic (dorsal) side. Events: Gastrulation is continuing as cells migrate from the epiblast, continuing to form mesoderm. Mesoderm lies between the ectoderm and endoderm as a continuous sheet except at the buccopharyngeal and cloacal membranes. These membranes have ectoderm and endoderm only and will lie at the rostral (head) and caudal (tail) of the gastrointestinal tract.
|
Gastrulation: Through the primitive streak cells migrate continuously through week 3 into week 4. Initial cells replace hypoblast as an epithelial layer the endoderm. Later migrating cells spread between the two epithelial layers to form mesoderm. |
|
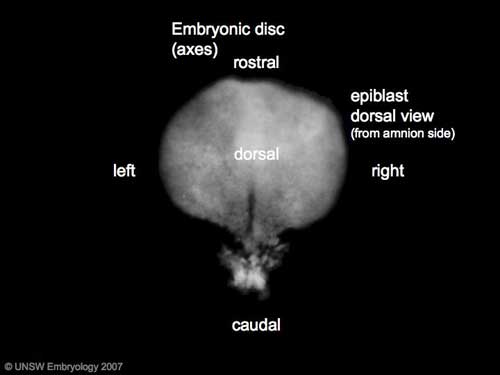
|
| Axes: embryonic disc is shown rostral (head) to top and caudal (tail) to bottom. Left and right are the lateral margins of the disc as shown. |
Gastrulation
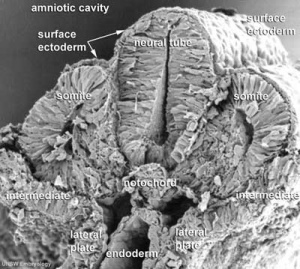
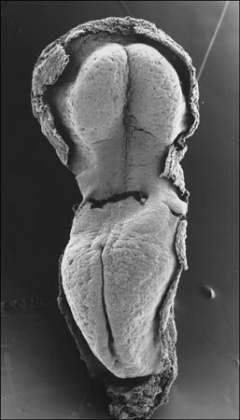
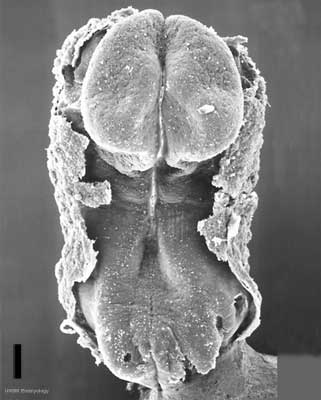
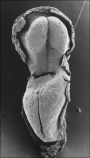
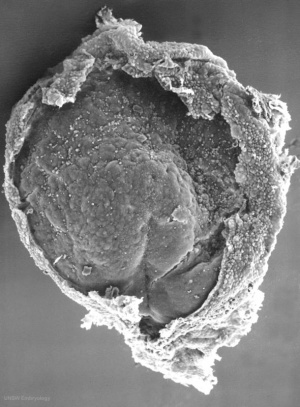
Carnegie Stage 7 and 8, gastrulation, migration of cells through the primitive streak to form endoderm and mesodermal layers of embryo.
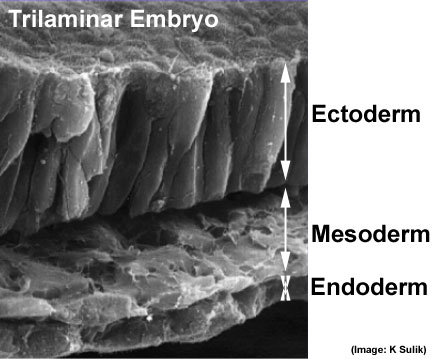 Scanning electron micrograph showing the early forming 3 layers: ectoderm, mesoderm and endoderm.
Scanning electron micrograph showing the early forming 3 layers: ectoderm, mesoderm and endoderm.
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
The embryonic structure which establishes body axes and patterns surrounding tissues is called the notochord.
The notochord is a midline column of cells running in a rostrocaudal direction (head-tail) within the mesoderm layer. It exists as a transient developmental patterning structure with a role in molecular signaling (patterning) and controlling the direction of embryonic disc folding (mechanical). These images are of the embryonic disc in week 3 (stage 7).
The notochordal process begins as a fold of ectoderm extending cranially toward the prechordal plate region. The sequence of differentiation: notochordal process -> notochordal plate -> notochord.
- Elongation of the notochordal process cranially from the primitive pit as a hollow tube (notochordal canal) in the midline of the embryonic disc underlying the ectoderm.
- The notochordal canal may appear to break down on the endodermal side forming a notochordal plate continuous with the endodermal layer.
- Notochordal plate folds to form notochord. The notochord (also called axial mesoderm) is an embryonic structure that regulates differentiation of surrounding structures including the overlying ectoderm (neural plate) and mesoderm (somites).
Disc Folding
Folding: all edges of the embryonic disc will fold ventrally, forming a rostro-caudal "C" shaped tube.
| Chorionic Cavity | Amniotic Cavity | Week 3 |
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
| Practical 3: Oogenesis and Ovulation | Gametogenesis | Fertilization | Early Cell Division | Week 1 | Implantation | Week 2 | Extraembryonic Spaces | Gastrulation | Notochord | Week 3 |
Introduction
This page is a overview of events that occur in human development up to week 3 post-fertilization. From this Practical understand concepts of: fertilization, blastocyst development, implantation, bilaminar and trilaminar embryo formation, development of embryonic cavities and brief understanding of early placenta development.
By the end of week 3, segmentation of the trilaminar embryo 3 germ layers has begun:
- Ectoderm - central neural plate and lateral parts form epidermis
- Mesoderm - midline notochord, adjacent somites, formation of the internal embryonic space (intraembryonic ceolom)
- Endoderm - epidermal lining of gastrointestinal tract and yolk sac lining
Note
Use the links to Carnegie stage 7, Carnegie stage 8 and Carnegie stage 9 to see a number of different views of the human embryo in the third week of development.
The timeline at the bottom of this page should give you a better perspective of the sequence of early developmental events. You would not be expected to know exact days (as they are only approximate anyway) it is more important to get the weeks and sequence right.
Stage 7
Facts
Human embryonic stage 7 occurs during week 3 between 15 to 17 days.
The embryo is now 0.4 mm diameter in size.
The initial images are displayed unlabeled to allow you to explore the embryo for yourself, linked labeled versions are also available for some images.
Events
Gastrulation is continuing as cells migrate from the epiblast, continuing to form mesoderm.
Mesoderm lies between the ectoderm and endoderm as a continuous sheet except at the buccopharyngeal and cloacal membranes. These membranes have ectoderm and endoderm only and will lie at the rostral (head) and caudal (tail) of the gastrointestinal tract.
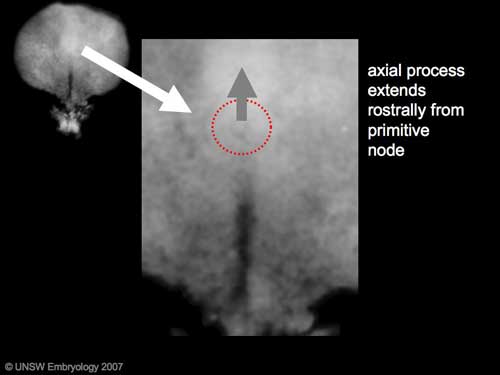
From the primitive node a tube extends under the ectoderm in the opposite direction to the primitive streak. This tube forms first the axial process then notochordal process, then finally the notochord.
The notochord is a key to embryonic folding and regulation of ectoderm and mesoderm differentiation. It lies in the rostrocordal axis and the embryonic disc will fold either side ventrally, pinching off a portion of the yolk sac to form the lining of the gastrointestinal tract.
Carnegie Stage 8
Facts
Human embryonic stage 8 occurs during week 3 between 17 to 19 days.
The embryo is now 1.0 - 1.5 mm in size.
Events
Gastrulation is continuing as cells migrate from the epiblast, continuing to form mesoderm.
Mesoderm lies between the ectoderm and endoderm as a continuous sheet except at the buccopharyngeal and cloacal membranes. These membranes have ectoderm and endoderm only and will lie at the rostral (head) and caudal (tail) of the gastrointestinal tract.
From the primitive node a tube extends under the ectoderm in the opposite direction to the primitive streak. This tube forms first the axial process then notochordal process, then finally the notochord.
The notochord is a key to embryonic folding and regulation of ectoderm and mesoderm differentiation. It lies in the rostrocordal axis and the embryonic disc will fold either side ventrally, pinching off a portion of the yolk sac to form the lining of the gastrointestinal tract.
Identify
- embryonic disc
- primitive node, primative streak, primative groove
- connecting stalk
- cut amniotic membrane
Carnegie Stage 9
Facts
Human embryonic stage 9 occurs during week 3 between 19 to 21 days.
The embryo is now 1.5 to 2.5 mm in size and somites have begun to form and number between 1 to 3 somite pairs during this stage.
The initial images are displayed unlabeled to allow you to explore the embryo for yourself, linked labeled versions are also available for some images.
Events
Ectoderm - Neural plate brain region continues to expand, neural plate begins folding over the notochord. Gastrulation continues through the primitive streak region.
Mesoderm - Paraxial mesoderm segmentation into somites begins (1 - 3 somite pairs). Lateral plate mesoderm begins to vacuolate, dividing it into somatic and splanchnic mesoderm and to later form the intra-embryonic coelom. Prechordal splanchnic mesoderm begins to form the cardiogenic region, from which the primordial heart will develop.
Endoderm - Notochordal plate still visible which will form the notochord. Endoderm is still widely open to the yolk sac and germ cells form part of this layer. Extra-embryonic mesoderm on the yolk sac surface begins to form "blood islands".
Identify
- Neural groove and neural folds, the mesoderm showing first somite bulges, that segments beside the neural groove to form somites but extends laterally to margin of embryonic disc lateral plate mesoderm, where it merges with the covering extraembryonic mesoderm.
- The intra-embryonic coelom develops in the middle of the lateral plate mesoderm.
- Carnegie Stages: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | About Stages | Timeline
Human Development Timeline
The table below shows human development features and approximate timing during the menstrual cycle to fertilization and the first 3 weeks of development.
The timing assumes fertilization the day after ovulation and the "weeks" refer to embryonic development and differ from clinical weeks (shown in brackets, from last menstrual period) and "stages" refer to Carnegie stages of development.
Week -2
(Clinical Week 1)
| Event | ||
| Menstrual Phase |
Menstrual Cycle changes: Uterine endometrium (loss), Ovary (Follicle Development) | |

| ||
| Proliferative Phase |   Menstrual Cycle changes: Uterine endometrium (proliferation), Ovary (Follicle Development) Menstrual Cycle changes: Uterine endometrium (proliferation), Ovary (Follicle Development)
| |
Week -1
(Clinical Week 2)
| Menstrual cycle | Event | |
| Proliferative Phase | ||
    Menstrual Cycle - Mid proliferative Menstrual Cycle - Mid proliferative
| ||
   Menstrual Cycle - Late Proliferative Menstrual Cycle - Late Proliferative
| ||
| Ovulation
Capacitation |
 |
Week 1
Week 1 (Clinical Week 3)
| Event | ||
| Secretory PhaseStage 1 |    Fertilization, Secretory Phase Fertilization, Secretory Phase
| |
| Stage 2 |  | |
| Stage 3 |  Blastocyst Hatching (zona pellucida lost) Blastocyst Hatching (zona pellucida lost)
| |
  Late Secretory, Blastocyst (free floating) Late Secretory, Blastocyst (free floating)
| ||
| Stage 4 | Adplantation | |
| Stage 5 |
Week 2
Week 2 (Clinical Week 4)
| Event | ||
| Stage 6 | ||
Week 3
Week 3 (Clinical Week 5)
| Event | ||
| Stage 7 | 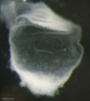 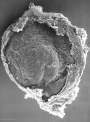 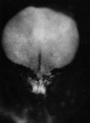
| |
| Stage 8 | 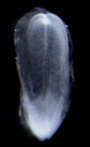 | |
| Stage 9 | 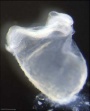 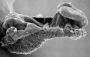 Musculoskeletal somitogenesis, first somites form and continue to be added in sequence caudally Musculoskeletal somitogenesis, first somites form and continue to be added in sequence caudally
Neural the three main divisions of the brain, which are not cerebral vesicles, can be distinguished while the neural groove is still completely open Neural Crest mesencephalic neural crest is visible PMID: 17848161 | |
| Heart cardiogenesis, week 3 begins as paired heart tubes. |
Next
Finished Lab 3 !
If you have finished and would like to apply your knowledge, I have also included some Clinical Questions based around this period of development.
If you have finished and need some more help understanding this period of development, I have included some links to Online References.
If you have finished and are interested in looking at tissues involved in this period of development, I have included some links to Histology Images.
Note that this Practical has discussed mainly development of the embryo as placental development will be covered in detail in another practical (Practical 8 - Placenta and Fetal Membranes).
The next Practical will continue on through embryonic development (Practical 6 - Implantation to 8 Weeks).
Terms
The selected list of terms below are extracted from the original UNSW Embryology Glossary, many of the links go to those original website pages. The full list of glossary terms should be used for any new terms not listed below.
acroplaxome
The sperm structure which forms the acrosome plate with intermediate filament bundles of the marginal ring at the leading edge of the acrosome.
(More? Spermatogenesis | Fertilization)
acrosin
A spermatazoa acrosomal protein has a role in fertilization including that of lysis of the zona pellucida (a serine protease) and in secondary zona pellucida (ZP) binding. Stored in mature spermatazoa as proacrosin.
(More? Spermatogenesis | Fertilization | OMIM Entry)
acrosome
The spermatazoa cellular structure containing a packet of enzymes located that allows it to dissolve a hole in the specialized extracellular matrix (zona pellucida, egg coat) surrounding the oocyte (egg). This enzymic digestion then allows the spermatazoa to penetrate and fertilize the egg. This structure is formed from the normal cellular organelle the [Golgi apparatus.
(More? Spermatogenesis | Fertilization)
acrosome reaction
The chemical change that enables release of acrosomal contents and allow a sperm to penetrate an egg.
(More? Spermatogenesis | Fertilization)
adnexa
(Latin, adnexae = appendages) Term used to describe any anatomical appendage (accessory structure, extension or outgrowth from the body). In reproductive anatomy used to describe appendages of the [U.htm#uterus uterus] "body"; ovaries, uterine tubes and uterus supporting ligaments.
Artificial Insemination
(AI) Fertility treatment, using placement of a sperm sample inside the female reproductive tract that can be carried out by a number of different techniques: intracervical insemination, intrauterine insemination, intratubal insemination.
(More? Week 1 Notes)
amenorrhea
The absence of a menstrual period, it can be either primary (not yet had a period by age 16) or secondary (regular period that has now stopped for 3 months).
(More? Human Menstrual Cycle)
androgens
The male sex hormones, eg testosterone.
(More? Genital System - Male)
aneuploidy
Term used to describe an abnormal number of chromosomes mainly (90%) due to chromosome malsegregation mechanisms in maternal meiosis I.
(More? Trisomy 21 | Meiosis)
antral follicle
(secondary follicle) Term used to describe the developmental stage of ovarian follicle development following preantral (primary) in describing the sequence (primordial, preantral, antral) of follicle development within the ovary. In humans, a number of primordial follicles will begin to develop into primary follicles, some of which will then form antral follicles (secondary), with only a single antral follicle developing into the ovulating follicle (Graafian) each menstrual cycle.
(More? Week 1 - Oogenesis)
antrum
(Latin from Greek, antron = a cave, cavity; a nearly-closed cavity or bulge). Identified anatomically in many structures (ovarian follicle, bone, cardiac, gastric). In the ovary this refers to the follicular fluid-filled space within the follicle.
(More? Week 1 - Oogenesis)
ART
acronym for Assisted Reproductive Technology. All treatments or procedures that involve the handling of human eggs and sperm for the purpose of helping a woman become pregnant. Types of ART include in vitro fertilization, gamete intrafallopian transfer, zygote intrafallopian transfer, embryo cryopreservation, egg or embryo donation, and surrogate birth.
(More? Week 1 - In Vitro Fertilization)
ART cycle
A process in which 1) an ART procedure is carried out, 2) a woman has undergone ovarian stimulation or monitoring with the intent of having an ART procedure, or 3) in the case of frozen embryos, embryos have been thawed with the intent of transferring them to a woman. A cycle begins when a woman begins taking fertility drugs or having her ovaries monitored.
(More? Week 1 Notes)
atresia
(Greek, a = without + tresis = perforation) Term used for anatomical closing or absence of a cavity or opening that should exist. Used as an antomical, pathological and clinical term: esophageal atresia, biliary atresia, duodenal atresia, jejunal atresia, choanal atresia, vaginal atresia, urethral atresia, pulmonary atresia, bronchial atresia, tricuspid atresia.
atretic follicle
An ovarian follicle that fails to mature and degenerates. Also called "atresia" referring to the process of degeneration of the ovarian follicle. At any one time the majority of follicles are destined not to complete maturation and degeneration can occur at any stage (from type 4-7).
(More? Week 1 Notes | Ovary Notes)
Balbiani body
(mitochondrial cloud) A collection of cell organelles (mitochondria, ER, and granulofibrillar material) asymmetrically located beside the nucleus in very young oocytes in some species. Appears similar to germinal granule precursors seen some species that contain a definitive germ plasm (flies, worms, and frogs).
(More? PNAS - Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body)
Bulbourethral Gland
(= Cowper's Gland) A male genital tract gland which secretes a small amount of a thick clear mucous fluid prior to ejaculation, the alkaline content apparently buffers acidity of the urethra. The equivalent female gland are Bartholin's glands.
(More? Urogenital Notes)
Call-Exner bodies
A feature seen in the developing ovarian follicle granulosa layer of some species, including human. Appears as a spherical space staining as an eosinophilic region and contains basal lamina components (type IV collagen and laminin) similar to thiose of the follicular basal lamina.
(More? Week 1 - Oogenesis)
centriole
A pair of small cylindrical structures each about 0.2 micron in diameter and 0.4 micron long, that lie at right angles to one another; present at each pole of the mitotic spindle in animal cells and in some other eukaryotes.
ciliated epithelium
(Latin, cilium = eyelid) An epithelium named on the basis of the cells having surface hair-like appearance of a cilium; singular, cilium. In many tissues, cilia are found as epithelial cell apical surface motile specializations. In the uterine tube epithelium, after ovulation used to move the unfertilized egg, then the fertilized zygote, then blastocyst during the first week of development.
clomiphene citrate
(CC) A fertility drug taken orally to promote the process of follicle/egg maturation in superovulation therapy. (CC) an anti-estrogen (MRL-41) therapy for WHO group II (eu-oestrogenic) infertility associated with polycystic ovary syndrome. Used for more than 40 years it is a simple, cheap treatment, with low side effects and yields a 25% live birth rate. Alternative therapeutics being considered are metformin, aromatase inhibitors and low-dose FSH.
(More? Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. Preliminary report. JAMA. 1961 Oct 14;178:101-4. | Homburg R. Clomiphene citrate--end of an era? A mini-review. Hum Reprod. 2005 Aug;20(8):2043-51.) | Notes - Ovary | Week 1 - In Vivo Fertilization | Week 1 - In Vitro Fertilization | Week 1 - Abnormalities)
cumulus oophorus
(Latin cumulus = a little mound G. oon = egg + phorus = bearing) The granulosa cells that form a column of cells that attaches the oocyte to the antral follicle wall within follicles of the ovary. This column of cells is broken or separates during ovulation to release the oocyte from its follicle attachment. Other granulosa cells within the follicle include: membrana granulosa and [#corona_radiata corona radiata].
(More? Week 1 - Oogenesis)
DAX1
Acronym for "D"osage sensitive sex reversal (DSS), "A"drenal hypoplasia congenita (AHC) critical region on the "X" chromosome, gene "1" , (gene NR0B1) is a nuclear hormone receptor involved in female ovary development.
(More? Urogenital Notes | OMIM Entry DAX1)
DAZL
Acronym for DAZ-like due to homology to DAZ (Deleted in AZoospermia), a gene on the long arm of the Y chromosome that is frequently deleted in infertile men with nonobstructive azoospermia.
(More? Spermatogenesis | OMIM Entry DAZL)
DET
Acronym for Double-Embryo Transfers, two embryos transferred when women undergo Assisted Reproduction Technology compared to single-embryo transfer (SET).
(More? Week 1 - In Vitro Fertilization)
ductuli efferentes
testis (male gonad) series of tubular structures which arise from the rete testis and conduct spermatazoa into the ductus epididymidis. Their columnar epithelium lining consisting of both absorptive and ciliated cells (giving rise to "cogwheel appearance) which removes much of the fluid associated with the spermatazoa leaving the testes (also by the upper epididymis) thereby increasing the spermatazoa concentration. (Spermatozoa Duct Pathway: seminiferous tubule‚ straight tubule‚ rete testis‚ ductuli efferentes‚ ductus epididymidis‚ ductus deferens)
(More? Spermatogenesis | Genital - Male | Genital Notes)
ductus epididymidis
(epididymidis) The male testes tubular structure which arise from the ductuli efferentes and conduct spermatazoa into the ductus deferens (vas deferens). The long duct is lined by a tall pseudostratified columnar epithelium.
(More? Genital - Male | Genital Notes)
dysmenorrhoea
A period pain which can be primary (increased sensitivity to the prostaglandins) or secondary (pathological), can be common with no associated abnormality or in association with ovarian cysts or endometriosis.
(More? Human Menstrual Cycle)
ectopic pregnancy
(Greek, ektopos = out of place) A pregnancy in which the fertilized egg implants outside of the [U.htm#uterus uterus] usually in the fallopian tube, but also on the ovary, or the abdominal cavity. Ectopic pregnancy is a dangerous condition that must receive prompt treatment.
(More? Week 2 - Abnormalities)
egg
([O.htm#oocyte oocyte] or ovum) An alternative term used to describe the haploid female reproductive cell Germ Cell). The term is also used to describe the avian and reptilian shell enclosed structure.
(More? Week 1 Notes | Chicken Development | Frog Development)
egg retrieval
(also called oocyte retrieval) A clinical in vitro fertilization (IVF) procedure to collect the eggs contained in the ovarian follicles.
(More? Week 1 - In Vitro Fertilization)
egg transfer
(also called oocyte transfer) A clinical in vitro fertilization (IVF) technique to transfer of retrieved eggs into a woman's fallopian tubes through [L.htm#laparoscopy laparoscopy]. This procedure is used only in gamete intrafallopian transfer (GIFT) (see definition).
(More? Week 1 - In Vitro Fertilization)
embryo
(Greek, en = in + bryein = to be full of) An egg that has been fertilized by a sperm and undergone one or more divisions. Also the set of early developmental stages in which a plant or animal differs from its mature form.
(More? [h.htm#human_embryo human embryo])
embryo transfer
Placement of an embryo or embryos into a woman's [U.htm#uterus uterus] through the cervix after in vitro fertilization (IVF) or in the case of zygote intrafallopian transfer (ZIFT) (see definition), into her fallopian tube.
(More? Week 1 Notes)
endocrine gland
(Greek, endon = within) A gland (organ, tissue) that is specialized for secretion of a hormone into the bloodstream for general circulation.
(More? Endocrine Notes)
endometrial gland
The mucous secreting gland associated with the epithelium lining the [U.htm#uterus uterus]. These glands develop and secrete each menstral cycle and are thought to provide initial blastocyst nutrition prior to implantation.
(More? Week 1 Notes)
endometriosis
Endometrium found incorrectly located possibly in the muscular layer of the uterus. Can also be seen as the presence of tissue similar to the uterine lining in locations outside of the uterus, such as the ovaries, fallopian tubes, and abdominal cavity.
endometrium
The epithelium lining of the non-pregnant [U.htm#uterus uterus]. During pregnancy this epithelium undergoes changes described as the decidual reaction and is renamed the "decidua".
(More? Week 1 Notes | Human Menstrual Cycle)
epigenetics
(Greek, epi = above, upon) gene silencing that occur without changes in the genes (DNA) sequence, this changes can also be inherited.
(More? Molecular Mechanisms - Epigenetics | DNA Introduction | Science - Epigenetics: A web tour | The Welcome Trust - Epigenetics)
estrogens
Sex hormone found in both male and female. In the female, this hormone is produced by the ovaries and is responsible for development of secondary feminine sex characteristics. Together with progesterone these hormones also regulate changes that occur each menstral cycle. In the male, Leydig cells produce estrogen into the rete testis fluid at variable levels in different species. During male embryonic development exposure to high levels of estrogen can lead to genital abnormalities.
estrous cycle
The cyclic alterations in animal female tract and in sexual receptivity related to hormone changes. When searching both American (estrous) and British (oestrous) spellings are used in the literature.
(More? Menstrual Cycle - Estrous Cycle | Mouse estrous cycle)
gametes
(Greek, gamos = marriage) A specialized reproductive cell through which sexually reproducing parents pass chromosomes to their offspring; a sperm or an egg.
(More? Week 1 Notes)
gamete intrafallopian transfer
see GIFT
(More? In Vitro Fertilization Notes)
gameteogenesis
The production of either the haploid germ cells of spermatazoa (male) or eggs (female).
(More? Week 1 Notes)
genitalia
(Latin, genitalia = ) The term used to describe either the external or internal male and female sexual and reproductive organs.
(More? Urogenital Notes)
haploid
(Greek, haploos = single) Having a single set of chromosomes as in mature germ/sex cells (egg, spermatazoa). Normally cells are diploid, containing 2 sets of chromosomes.
(More? DNA Notes)
human chorionic gonadotrophin
(hCG) Placental hormone initially secreted by cells (syncitiotrophoblasts) from the implanting conceptus during week two, supporting the ovarian corpus luteum, which in turn supports the endometrial lining and therefore maintains pregnancy. Hormone can be detected in maternal blood and urine and is teh basis of many pregnancy tests. Hormone also stimulates the onset of fetal gonadal steroidogenesis, high levels are teratogenic to fetal gonadal tissues.
(More? Placenta Notes | Week 2 Notes)
last menstrual period
(LMP) Clinical term used to describe the menstrual period that occurs before a pregnancy and is used as the date to calculate clinical pregnancy development (gestational age). Note that in humans this is approximately two weeks different from embryonic development, which begins at [F.htm#fertilization fertilization] around the mid-point of the menstrual cycle.
Leydig cells
(interstitial cells) Testis (male gonad) cell which secrete testosterone, beginning in the fetus. These cells are named after Franz von Leydig (1821 - 1908) a German scientist who histologically described these cells.
(More? Testis Development | Spermatozoa Development)
male factor
Any cause of infertility due to deficiencies in sperm quantity, function, or motility (ability to move) that make it difficult for a sperm to fertilize an egg under normal conditions.
(More? Week 1 Notes)
manchette
A transient microtubule structure formed in spermatids involved in the process of assembly of the mammalian sperm tail and mechanical shaping and condensation of the sperm nucleus.
(More? Spermatozoa Development)
mediastinum testis
(Latin, medialis = middle) A single conical mass of connective tissue within the testis (male gonad) which extends from the tunica albuginea (cortical thick capsule surrounding the testis) into the seminiferous tubule region (medullary). Embedded within this connective tissue are the rete testis component of the duct conduction system for spermatazoa (Spermatozoa Duct Pathway: seminiferous tubule ‚ straight tubule ‚ rete testis ‚ ductuli efferentes ‚ ductus epididymidis ‚ ductus deferens)
(More? Testis Development | Spermatozoa Development)
medullary
(Latin, medialis = in the middle) Term relating to the medulla; pith, marrow, inner portion of an organ. Usually combined with cortex (cortical) meaning the outer layer.
meiosis
The cell division that occurs only in production of germ cells where there is a reduction in the number of chromosomes (diploid to haploid) which is the basis of sexual reproduction. Note all other non-germ cells divide by mitosis.
(More? Week 1 Notes)
meiotic sex chromosome inactivation
(MSCI) The process of transcriptional silencing of the X and Y chromosomes that occurs only during male meiotic spermatogenesis. This is a specialised form of [#MSUC meiotic silencing of unsynapsed chromatin]. This specific silencing has also be called the second form of X chromosome inactivation, the first form occurs in all female embryo cells.
(More? Meiotic sex chromosome inactivation. Turner JM. Development. 2007 May;134(10):1823-3
membrana granulosa
The granulosa cells that line the developing follicles of the ovary. These cells proliferate to form the stratum granulosa and other granulosa cells are given specific names based upon their position within the follicle. In the antral follicle, membrana granulosa sits on the follicular basal lamina and lines the antrum as a stratified epithelium. The [C.htm#cumulus_oophorus cumulus oophorus] is a column of granulosa cells that attaches the oocyte to the follicle wall. The [C.htm#corona_radiata corona radiata] are the granulosa cells that directly surround the oocyte, and are released along with it at ovulation. Following ovulation the corona radiata provide physical protection to the oocyte and granulosa cells within the ovulating follicle contribute to [C.htm#corpus_luteum corpus luteum].
(More? Week 1 - Oogenesis)
menopause
(Greek, mene = moon, men = month, pause = end or cessation) The decrease in ovarian production of estrogen and progesterone leading to cessation of menstrual cycles, decrease in fertility, and end of female reproductive life. Usually occurs in the mid-40's, the term was first used by the French physician, de Gardanne in 1812.
(More? Human Menstrual Cycle | Medline Plus - Menopause)
menorrhagia
Term used to describe heavy menstrual bleeding, is common in women of reproductive age (WHO data, affects 1011 out of 5322 women).
(More? Human Menstrual Cycle)
menstrual age
The gestation time calculated from the first day of the last menstrual period (LMP) prior to [F.htm#fertilization fertilization]. In humans, this differs from embryonic age by approximately two weeks.
(More? Week 1 Notes)
menstrual cycle
The human reproductive cycle, an endocrine regulated change in female anatomy and physiology that occur over 28 days (4 weeks, a lunar month) during reproductive life (between puberty and menopause). This cycle ceases during pregnancy and differs from other non-primate vertebrates (eg rats, mice, horses, pig) females that have a reproductive cycle called the estrous cycle (oestrous, British spelling).
(More? Human Menstrual Cycle | Estrous Cycle)
mitosis
The normal division of all cells, except germ cells, where chromosome number is maintained. Note germ cell division (egg, sperm) is reductive meiotic division.
(More? Week 1 Notes)
Mullerian Inhibiting Substance
(MIS, Anti-Mullerian Hormone, AMH, Mullerian inhibiting hormone, MIH). A sertoli cell secreted glycoprotein (transforming growth factor-beta, TGF-beta superfamily) that regulates gonadal and genital tract development. The main role is to inhibit paramesonephric (Mullerian) duct development in males. Postnatally, after puberty it is also expressed in females by ovarian granulosa cells and has a role in follicle development.
(More? OMIM - AMH)
oocyte
(Greek, oo = egg, ovum) The term used to describe the haploid egg or ovum formed within the ovary (female gonad) and released to enter the uterine tube and be transported to the [U.htm#uterus uterus]. The mature oocyte is the cell released from the ovary during ovulation.
(More? Week 1 - Oogenesis)
oogenesis
(Greek, oo = egg + genesis = origin, creation, generation) process of diploid oogonia division and differentiation into an haploid oocyte (egg) within the ovary (female gonad). Mammalian meiosis will only be completed within the oocyte if [F.htm#fertilization fertilization] occurs.
(More? Week 1 - Oogenesis)
oogonia
(Greek, oo = egg) diploid germ cells within the ovary (female gonad) which provide the primary oocytes for oocyte (egg) formation. In humans, all oogonia form primary oocytes within the ovary before birth.
(More? Week 1 - Oogenesis)
oophorus
(Greek, oo = egg + phorus = carrying, egg-bearing) cumulus oophorus, used to describe the granulosa cells within the follicle that tether or link the oocyte to the wall of the follicle.
(More? Week 1 - Oogenesis)
ovarian factor
A cause of infertility due to problems with egg production by the ovaries.
(More? Week 1 - Oogenesis)
ovarian hyperstimulation syndrome
(OHSS) Condition associated with fertility drugs for in vitro fertilization and other reproductive abnormalities.
(More? Week 1 - In Vitro Fertilization | Medlineplus - OHSS)
ovarian monitoring
The use of ultrasound and/or blood or urine tests to monitor ovarian follicle development and hormone production.
(More? Week 1 Notes | Week 1 - Oogenesis)
ovarian stimulation
The use of drugs to stimulate the ovaries to develop follicles/eggs. (More? Week 1 - Oogenesis | Human Menstrual Cycle)
ovary
The two female gonads where female germ cells (oocytes, eggs) are generated and also the source of estrogen and progesterone the female hormones regulating secondary sex characteristics and menstrual cycle uterine changes. The ovary is embryonically formed from primordial germ cells entering region of the paired mesonephric ducts (Wolffian ducts) which are lost in females.
(More? Week 1 Notes | Week 1 - Oogenesis | Human Menstrual Cycle)
oviduct
(uterine horn, fallopian tube, oviduct, salpinx) see [#uterine_tube uterine tube]. A pair of tubular structures designed to transport the oocyte (egg) from the ovary to the [U.htm#uterus uterus] body.
(More? Week 1 - Oogenesis | Week 1 Notes | Blue Histology - Female Reproductive System)
ovulation
The term used to describe the process of the mature follicle releasing the oocyte or ovum (and support cells) from the ovary surface into the peritoneal cavity. In humans, generally a single oocyte is released from a cohort of several maturing follicles. More than one follicle may be released (superovulation) following reproductive therapeutic treatment.
(More? Week 1 - Oogenesis | Week 1 Notes)
rectouterine pouch
(Pouch of Douglas or rectovaginal) Anatomical description of the female peritoneal cavity lying between the back wall of the [U.htm#uterus uterus] and rectum.
reductive division
Term describing meiosis where diploid DNA content becomes haploid (halved).
rete ovarii
A group of epithelial tubules located at the hilum of the ovary possibly mesonephric origin.
(More? Urogenital Notes)
rete testis
The duct (epithelial tubules) conduction system for spermatazoa embedded within the mediastinum (connective tissue) located in the center of the testis (male gonad) derived from the mesonephric duct, and allow spermatazoa to travel from the seminiferous tubules to the vasa efferentia. (Spermatozoa Duct Pathway: seminiferous tubule ‚Üí straight tubule ‚Üí rete testis ‚Üí ductuli efferentes ‚Üí ductus epididymidis ‚Üí ductus deferens)
(More? Testis Development | Spermatozoa Development)
RU 486
RU 486 (or Mifepristone) is a steroid hormone similar in structure to the natural hormone progesterone, which is used as a birth control drug.
(More? RU486)
Sertoli cells
The supporting cells in the testes (male gonad) that induce primordial germ cells to commit to sperm development. Support is nutritional and mechanical, as well as forming a blood-testis barrier. In development these cells secrete anti-Müllerian hormone, which causes the Müllerian (paramesonephric) duct to regress, and help to induce other somatic cells to differentiate into Leydig cells. The cells are named after Enrico Sertoli (1842 - 1910), and italian physiologist and histologist.
(More? Testis Development | Spermatozoa Development)
sperm
The male haploid reproductive cell, often used generically (and incorrectly) to describe these cells and the fluid of the ejaculate. Term is a shortened form of scientifically correct term #spermatazoa spermatazoa.
(More? Testis Development | Spermatozoa Development)
spermatazoa
The male haploid reproductive cell, produced by meiosis in the testis (male gonad).
(More? Testis Development | Spermatozoa Development)
spermatogenesis
(Greek, genesis = origin, creation, generation) The term used to describe the process of diploid spermatagonia division and differentiation to form haploid spermatazoa within the testis (male gonad). The process includes the following cellular changes: meiosis, reoorganization of DNA, reduction in DNA content, reorganization of cellular organelles, morphological changes (cell shape). The final process of change in cell shape is also called [#spermiogenesis spermiogenesis].
(More? Week 1 - Spermatogenesis)
spermiogenesis
(Greek, genesis = origin, creation, generation) The maturation process of the already haploid spermatazoa into the mature sperm shape and organization. This process involves reorganization of cellular organelles (endoplasmic reticulum, golgi apparatus, mitochondria), cytoskeletal changes (microtubule organization) and morphological changes (cell shape, acrosome and tail formation).
(More? Testis Development | Spermatozoa Development)
spermatogonia
The cells located in the seminiferous tubule adjacent to the basal membrane that either divide and separate to renew the stem cell population, or they divide and stay together as a pair (Apr spermatogonia) connected by an intercellular cytoplasmic bridge to differentiate and eventually form spermatazoa.
(More? Testis Development | Spermatozoa Development)
spermatogonial stem cells
The spermatagonia cells located beside the seminiferous tubule basal membrane that either divide and separate to renew the stem cell population, or they divide and stay together as a pair (Apr spermatogonia) connected by an intercellular cytoplasmic bridge to differentiate and eventually form spermatazoa.
(More? Testis Development | Spermatozoa Development)
sry
(Sry, human; Testis-Determining Factor, TDF; Testis-Determining Factor on Y, TDY ) Gene name ====s====ex-determining ====r====egion of ====Y====, the gene locus on the Y chromosome encoding the male "testis determining factor", a protein transcription factor and a member of the high mobility group (HMG)-box family of DNA binding proteins. See also the transcription factor SRY-related protein, SOX9 (SRY-related high-mobility group (HMG) box 9)
(More? Molecular Notes | Week 1 Notes | OMIM)
stimulated cycle
An ART cycle in which a woman receives drugs to stimulate her ovaries to produce more follicles.
(More? Week 1 Notes)
stroma
(Greek, stroma = "a cover, table-cloth, bedding") Histological term used to describe supportive cells within an organ, tissue or structure. The term is often paired with [P.htm#parenchyma parenchyma], which describes the functional cells of an organ, tissue or structure. All organs can therefore be functionally divided into these 2 components, stromal/parenchymal.
stromal cells
(Greek, stroma = "a cover, table-cloth, bedding") Descriptive term in the ovary, for cells surrounding the developing follicle that form a connective tissue sheath (theca folliculi). This layer then differentiates into 2 layers (theca interna, theca externa). This region is vascularized and involved in hormone secretion.
testes
(Latin testis = "witness") The two male gonads (singular testis) where male germ cells (spermatozoa) are generated and also the source of testosterone (male hormone). Embryonically formed from primordial germ cells entering region of the paired mesonephric ducts (Wolffian ducts) which are preserved in male gonad development and lost in females.
(More? Testis Development)
testis
(Latin testis = "witness", plural testes) The male gonad where male germ cells (spermatozoa) are generated and also the source of testosterone (male hormone). Embryonically formed from primordial germ cells entering region of the paired mesonephric ducts (Wolffian ducts) which are preserved in male gonad development and lost in females.
(More? Testis Development)
testis-determining factor
(TDF, Sry, Testis-Determining Factor on Y, TDY ) Protein name for the protein transcription factor product of the Sry gene on the Y chromosome responsible for maleness. This protein is a member of the high mobility group (HMG)-box family of DNA binding proteins. See also the transcription factor SRY-related protein, SOX9 (SRY-related high-mobility group (HMG) box 9)
(More? Molecular Notes | Week 1 Notes | OMIM)
BGDA: Lecture 1 | Lecture 2 | Practical 3 | Practical 6 | Practical 12 | Lecture Neural | Practical 14 | Histology Support - Female | Male | Tutorial
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, May 7) Embryology BGDA Practical 3 - Quiz. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Talk:BGDA_Practical_3_-_Quiz
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G