Some Recent Findings
- Review - Specification of sensory placode progenitors: signals and transcription factor networks[2] "Sensory placodes contribute to much of the sensory nervous system in the vertebrate head. They give rise to parts of the eye, ear and nose, as well as to the sensory ganglia that innervate the face, tongue, oesophagus and visceral tissues. Despite their diversity, during development placodes arise from a population of common progenitor cells, which are first specified at the border of the neural plate. The chick has been particularly instrumental in dissecting the timing of these events, and recent evidence has highlighted the close relationship of placode progenitors and precursors for neural crest cells and the central nervous system. This review focuses on the induction of placode progenitors by localised signalling events, and the transcriptional networks that lead to their specification."
- Nasal and otic placode specific regulation of Sox2 involves both activation by Sox-Sall4 synergism and multiple repression mechanisms[3] "Transcription factor gene Sox2 is expressed throughout sensory development, but the enhancers that regulate the gene vary depending on the developmental stages and tissues. To gain new insights into the gene regulatory network in sensory placode specification, regulation of the nasal-otic bispecific NOP1 enhancer of Sox2 was investigated in chicken embryos. Deletion and mutational analyses using electroporation showed that transcriptional repression mechanisms in combination with activation mechanisms determine placodal specificity. Activation of the NOP1 enhancer involves synergistic action by Sall4 and SoxB1/SoxE factors that bind to the adjacent sites. Deletion of repressive elements resulted in widening of the tissue area for enhancer activity to a region where the expression of Sall4 and SoxB1/E overlaps, e.g., the CNS and neural crest. Among multiple repressive elements that contribute to the placodal confinement of the NOP1 enhancer activity, CACCT/CACCTG motifs bound by Zeb/Snail family repressors play important roles. Overexpression of δEF1 (Zeb1) or Snail2 (Slug) strongly inhibited NOP1 activity. These data indicate that both activation by Sall4-Sox synergism and multiple repression mechanisms involving Zeb/Snail factors are essential for Sox2 regulation to be confined to the nasal and otic placodes."
- Anosmin-1 is essential for neural crest and cranial placodes formation in Xenopus[4] "During embryogenesis vertebrates develop a complex craniofacial skeleton associated with sensory organs. These structures are primarily derived from two embryonic cell populations the neural crest and cranial placodes, respectively. ...Anos1 was identified as a target of Pax3 and Zic1, two transcription factors necessary and sufficient to generate neural crest and cranial placodes. Anos1 is expressed in cranial neural crest progenitors at early neurula stage and in cranial placode derivatives later in development. We show that Anos1 function is required for neural crest and sensory organs development in Xenopus, consistent with the defects observed in Kallmann syndrome patients carrying a mutation in ANOS1." Frog Development
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Placode Otic Placode | Optic Placode | Nasal Placode |
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- Review - Transcriptional regulation of cranial sensory placode development[5] "Cranial sensory placodes derive from discrete patches of the head ectoderm and give rise to numerous sensory structures. During gastrulation, a specialized "neural border zone" forms around the neural plate in response to interactions between the neural and nonneural ectoderm and signals from adjacent mesodermal and/or endodermal tissues. This zone subsequently gives rise to two distinct precursor populations of the peripheral nervous system: the neural crest and the preplacodal ectoderm (PPE). The PPE is a common field from which all cranial sensory placodes arise (adenohypophyseal, olfactory, lens, trigeminal, epibranchial, otic). Members of the Six family of transcription factors are major regulators of PPE specification, in partnership with cofactor proteins such as Eya. Six gene activity also maintains tissue boundaries between the PPE, neural crest, and epidermis by repressing genes that specify the fates of those adjacent ectodermally derived domains. As the embryo acquires anterior-posterior identity, the PPE becomes transcriptionally regionalized, and it subsequently becomes subdivided into specific placodes with distinct developmental fates in response to signaling from adjacent tissues. Each placode is characterized by a unique transcriptional program that leads to the differentiation of highly specialized cells, such as neurosecretory cells, sensory receptor cells, chemosensory neurons, peripheral glia, and supporting cells. In this review, we summarize the transcriptional and signaling factors that regulate key steps of placode development, influence subsequent sensory neuron specification, and discuss what is known about mutations in some of the essential PPE genes that underlie human congenital syndromes."
- Setting appropriate boundaries: Fate, patterning and competence at the neural plate border[6] "The neural crest and craniofacial placodes are two distinct progenitor populations that arise at the border of the vertebrate neural plate. This border region develops through a series of inductive interactions that begins before gastrulation and progressively divide embryonic ectoderm into neural and non-neural regions, followed by the emergence of neural crest and placodal progenitors. In this review, we describe how a limited repertoire of inductive signals-principally FGFs, Wnts and BMPs-set up domains of transcription factors in the border region which establish these progenitor territories by both cross-inhibitory and cross-autoregulatory interactions."
- Graded levels of Pax2a and Pax8 regulate cell differentiation during sensory placode formation[7] "Pax gene haploinsufficiency causes a variety of congenital defects. Renal-coloboma syndrome, resulting from mutations in Pax2, is characterized by kidney hypoplasia, optic nerve malformation, and hearing loss. ..We sho.w that differential levels of zebrafish Pax2a and Pax8 modulate commitment and behavior in cells that eventually contribute to the otic vesicle and epibranchial placodes."
- Mutual repression between Gbx2 and Otx2 in sensory placodes reveals a general mechanism for ectodermal patterning[8] "In the vertebrate head, central and peripheral components of the sensory nervous system have different embryonic origins, the neural plate and sensory placodes. This raises the question of how they develop in register to form functional sense organs and sensory circuits. Here we show that mutual repression between the homeobox transcription factors Gbx2 and Otx2 patterns the placode territory by influencing regional identity and by segregating inner ear and trigeminal progenitors. Activation of Otx2 targets is necessary for anterior olfactory, lens and trigeminal character, while Gbx2 function is required for the formation of the posterior otic placode. Thus, like in the neural plate antagonistic interaction between Otx2 and Gbx2 establishes positional information thus providing a general mechanism for rostro-caudal patterning of the ectoderm."
- An effective assay for high cellular resolution time-lapse imaging of sensory placode formation and morphogenesis[9] "This new imaging assay provides a powerful method to analyze directly development of placode-derived sensory neurons and subsequent ganglia formation for the first time in amniotes. Viewing placode development in a head cross-section provides a vantage point from which it is possible to study comprehensive events in placode formation, from differentiation, cell ingression to ganglion assembly. Understanding how placodal neurons form may reveal a new mechanism of neurogenesis distinct from that in the central nervous system and provide new insight into how cells acquire motility from a stationary epithelial cell type."
- Epibranchial Placodes[10] "The inner ear and the epibranchial ganglia constitute much of the sensory system in the caudal vertebrate head. ...However, recent studies indicate that both systems arise from a morphologically distinct common precursor domain: the posterior placodal area. This review summarises recent studies into the induction, morphogenesis and innervation of these systems and discusses lineage restriction and cell specification in the context of their common origin."
- Otic Placode[11] "The inner ear epithelium, with its complex array of sensory, non-sensory, and neuronal cell types necessary for hearing and balance, is derived from a thickened patch of head ectoderm called the otic placode. ...Collectively, our results suggest that Wnt8a provides the link between FGF-induced formation of the pre-otic field and restriction of the otic placode to ectoderm adjacent to the hindbrain."
- Postotic Placode[12] "The (zebrafish) embryonic line originates from a postotic placode that produces both a migrating sensory primordium and afferent neurons. Nothing is known about the origin and innervation of the larval lines. Here we show that a "secondary" placode can be detected at 24 h postfertilization (hpf), shortly after the primary placode has given rise to the embryonic primordium and ganglion."
|
Movies
- Links: Movies
Preplacodal Development
The neural plate and mesoderm together release fibroblast growth factors (FGFs) that, together with local down-regulation of BMPs and Wnts, act to induce the "pre-placodal" region.[13] This initial original ectodermal thickenings arising from the anterior neural plate border area.
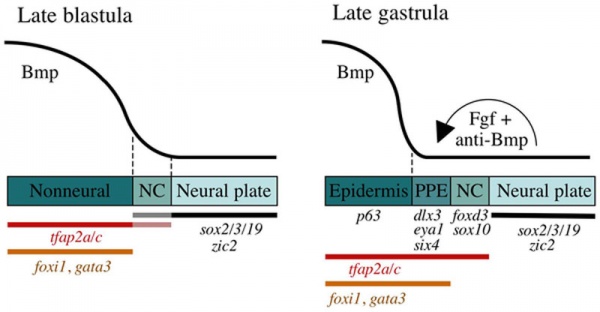
Preplacodal development model[14]
Late Blastula Stage
- Bmp acts as a morphogen that specifies neural crest (NC) within a narrow but low range of signalling.
- Higher levels of Bmp signaling establish the non-neural ectoderm as a broad zone of uncommitted cells with potential to form epidermal or preplacodal ectoderm (PPE).
- Within the non neural ectoderm
- changing levels of Bmp do not distinguish preplacodal from epidermal potential.
- preplacodal competence factors are uniformly induced throughout this domain.
- expression of tfap2a/c overlaps with the lateral edges of the neural plate where, perhaps in combination with neural markers, they cell-autonomously specify NC fate.
|
Late Gastrula Stage (9–10 hpf)
- preplacodal ectoderm (PPE) fate is specified in competent cells near the neural-nonneural border by dorsally expressed Bmp antagonists, Fgf and Pdgf.
- Complete attenuation of Bmp is required for PPE specification.
Relevant markers for each ectodermal domain are shown. Experiments carried out in zebrafish.
(Above text from figure legend[14])
|
Otic Placode
The otic placode is the first of the sensory placodes visible on the surface of the developing human embryo. This placode will differentiate to contribute almost entirely the components of the inner ear. The images below show the first appearance on the embryo surface during week 4 and the eventual disappearance from the surface by week 5. This is only the beginning of the complex development of this structure, influenced by the surrounding epidermis, neural tube and neural crest.
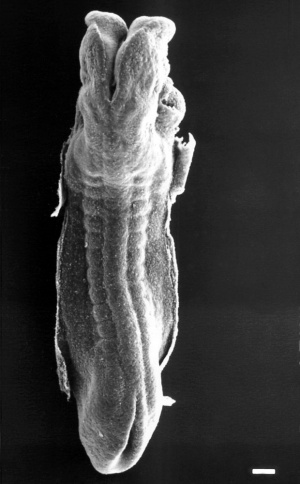
Stage 11
The scanning EM of the week 4 human embryo Carnegie stage 11 shown below is a superior dorsal view of the paired otic placodes sinking into the surface at the level of the hindbrain between day 24 and day 25.
 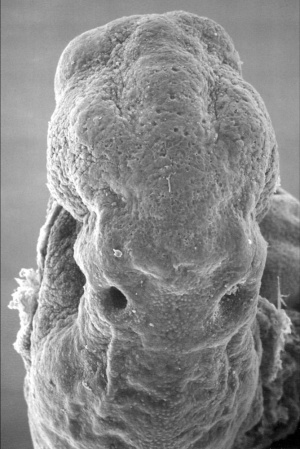
Stage 12
By Carnegie stage 12 26 days, only a small opening of the developing otic vesicle (otocyst) remains visible on the embryo surface located behind the second pharyngeal arch.
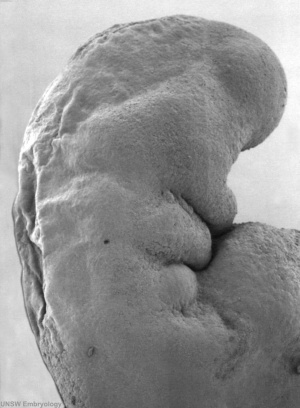 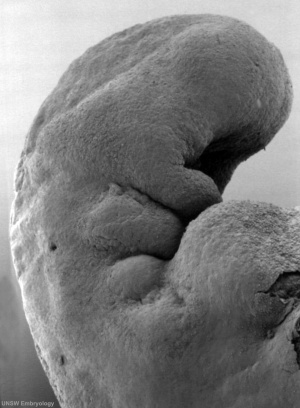
Stage 13
By week 5 Carnegie stage 13 the otic vesicle (otocyst) is completely formed and is no longer visible on the embryo surface.
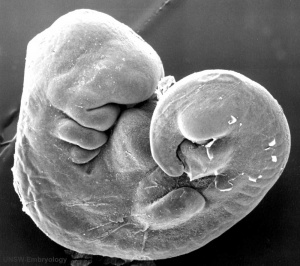
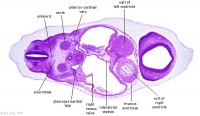
Cross-sections of the embryo head at this stage show the otocyst now lies within the embryo as a hollow fluid-filled epithelial "ball", located between the epidermis and the neural tube (hindbrain).
| Carnegie stage 13 - serial sections serial labeled images
|
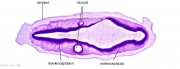
|
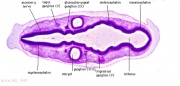
|
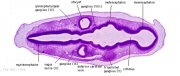
|
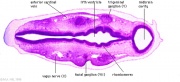
|
| A2L
|
A3L
|
A4L
|
A5L
|
- Links: inner ear | hearing | balance
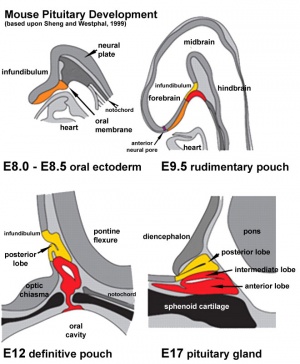
Adenohypophyseal Placode
Adenohypophysis placodal precursors arise within the midline rostral preplacodal cranial ectoderm.[15] This placode is unique, in being unpaired (only single placode) and also generating a structure endocrine endocrine character.
The hypophysis, or pituitary, is an endocrine gland that links the brain to peripheral endocrine organs and systems of the body through several specific hormones. There is a dual developmental origin of the hypophysis, with epithelial origins from neural ectoderm (posterior) and from surface ectoderm (anterior) the adenohypophyseal placode.
In the mouse, gonadotropin-releasing hormone-1 neurones control the release of gonadotropins from the anterior pituitary and were thought to originate from the adenohypophyseal placed. A recent study has shown that they are really associated early with the formation of the nasal placode.[16]
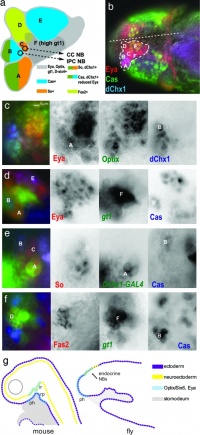
Drosophila and mouse placode similarity.[17]
- Links: pituitary
Olfactory Placodes
(Nasal)
The olfactory epithelium develops from the paired nasal placodes, each develop initially with two components, a medial and lateral region.
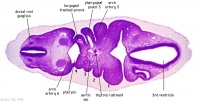
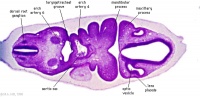
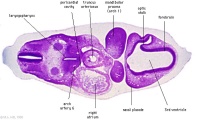
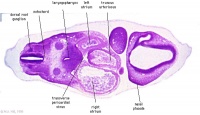
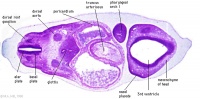
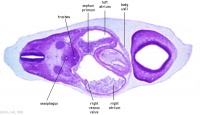
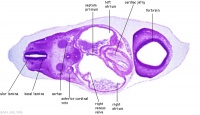
| Carnegie stage 13 - serial sections nasal placodes
|
|
|
|
|
|
|
|
|
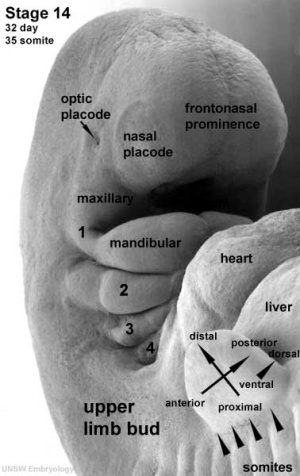
| Nasal Placode - Human Embryo stage 14
|
|
In the mouse, gonadotropin-releasing hormone-1 neurones control the release of gonadotropins from the anterior pituitary and were thought to originate from the adenohypophyseal placed. A recent study has shown that the real origin is associated with the formation of the nasal placode.[16]
- Links: nasal placode | {{smell}
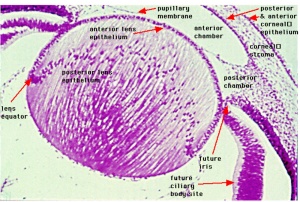
Optic Placodes
Optic placodes (Lens) lie on the embryo surface, adjacent to the out-pocketing of the nervous system (forms the retina) and will form the lens.
surface ectoderm -> lens placode -> lens pit -> lens vesicle -> lens fibres -> lens capsule and embryonic/fetal nucleus.
- Links: Lens Development | Vision Development
Trigeminal Placodes
(Profundal)
- Links: Profundal/trigeminal placodes
Epibranchial Placodes
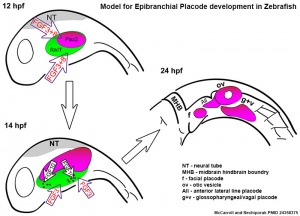
Zebrafish placode model[18]
|
Epibranchial ganglia sensory neurons formed by the facial, glossopharyngeal, and vagal placodal regions. These ganglia neurons relay from the sensory organs such as gustatory taste buds, heart baroreceptors, gut sensory enteric nerves.
|
Embryo Week: Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9
- Carnegie Stages: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | About Stages | Timeline
Paratympanic Organ Placode
The paratympanic organ is a mechanoreceptive sense organ in the middle ear of birds and other tetrapods. It develops from the paratympanic organ placode that is probably developmentally independent of the ventrally adjacent first epibranchial (or 'geniculate') placode.
References
- ↑ Washausen S & Knabe W. (2019). Chicken embryos share mammalian patterns of apoptosis in the posterior placodal area. J. Anat. , 234, 551-563. PMID: 30734277 DOI.
- ↑ Streit A. (2018). Specification of sensory placode progenitors: signals and transcription factor networks. Int. J. Dev. Biol. , 62, 195-205. PMID: 29616729 DOI.
- ↑ Sugahara S, Fujimoto T, Kondoh H & Uchikawa M. (2018). Nasal and otic placode specific regulation of Sox2 involves both activation by Sox-Sall4 synergism and multiple repression mechanisms. Dev. Biol. , 433, 61-74. PMID: 29137924 DOI.
- ↑ Bae CJ, Hong CS & Saint-Jeannet JP. (2018). Anosmin-1 is essential for neural crest and cranial placodes formation in Xenopus. Biochem. Biophys. Res. Commun. , 495, 2257-2263. PMID: 29277616 DOI.
- ↑ Moody SA & LaMantia AS. (2015). Transcriptional regulation of cranial sensory placode development. Curr. Top. Dev. Biol. , 111, 301-50. PMID: 25662264 DOI.
- ↑ Groves AK & LaBonne C. (2014). Setting appropriate boundaries: fate, patterning and competence at the neural plate border. Dev. Biol. , 389, 2-12. PMID: 24321819 DOI.
- ↑ McCarroll MN, Lewis ZR, Culbertson MD, Martin BL, Kimelman D & Nechiporuk AV. (2012). Graded levels of Pax2a and Pax8 regulate cell differentiation during sensory placode formation. Development , 139, 2740-50. PMID: 22745314 DOI.
- ↑ Steventon B, Mayor R & Streit A. (2012). Mutual repression between Gbx2 and Otx2 in sensory placodes reveals a general mechanism for ectodermal patterning. Dev. Biol. , 367, 55-65. PMID: 22564795 DOI.
- ↑ Tehindrazanarivelo A, Massiou H & Bousser MG. (1990). [What is new in the treatment of migraine?]. Rev Prat , 40, 407-10. PMID: 2155472
- ↑ Ladher RK, O'Neill P & Begbie J. (2010). From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development , 137, 1777-85. PMID: 20460364 DOI.
- ↑ Urness LD, Paxton CN, Wang X, Schoenwolf GC & Mansour SL. (2010). FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Dev. Biol. , 340, 595-604. PMID: 20171206 DOI.
- ↑ Sarrazin AF, Nuñez VA, Sapède D, Tassin V, Dambly-Chaudière C & Ghysen A. (2010). Origin and early development of the posterior lateral line system of zebrafish. J. Neurosci. , 30, 8234-44. PMID: 20554875 DOI.
- ↑ Adameyko I & Fried K. (2016). The Nervous System Orchestrates and Integrates Craniofacial Development: A Review. Front Physiol , 7, 49. PMID: 26924989 DOI.
- ↑ 14.0 14.1 Kwon HJ, Bhat N, Sweet EM, Cornell RA & Riley BB. (2010). Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. , 6, e1001133. PMID: 20885782 DOI.
- ↑ Sanchez-Arrones L, Sandonís Á, Cardozo MJ & Bovolenta P. (2017). Adenohypophysis placodal precursors exhibit distinctive features within the rostral preplacodal ectoderm. Development , 144, 3521-3532. PMID: 28974641 DOI.
- ↑ 16.0 16.1 Metz H & Wray S. (2010). Use of mutant mouse lines to investigate origin of gonadotropin-releasing hormone-1 neurons: lineage independent of the adenohypophysis. Endocrinology , 151, 766-73. PMID: 20008041 DOI.
- ↑ Wang S, Tulina N, Carlin DL & Rulifson EJ. (2007). The origin of islet-like cells in Drosophila identifies parallels to the vertebrate endocrine axis. Proc. Natl. Acad. Sci. U.S.A. , 104, 19873-8. PMID: 18056636 DOI.
- ↑ McCarroll MN & Nechiporuk AV. (2013). Fgf3 and Fgf10a work in concert to promote maturation of the epibranchial placodes in zebrafish. PLoS ONE , 8, e85087. PMID: 24358375 DOI.
Online Textbooks
Search Bookshelf placode development
Reviews
Streit A. (2018). Specification of sensory placode progenitors: signals and transcription factor networks. Int. J. Dev. Biol. , 62, 195-205. PMID: 29616729 DOI.
Adameyko I & Fried K. (2016). The Nervous System Orchestrates and Integrates Craniofacial Development: A Review. Front Physiol , 7, 49. PMID: 26924989 DOI.
Schlosser G, Patthey C & Shimeld SM. (2014). The evolutionary history of vertebrate cranial placodes II. Evolution of ectodermal patterning. Dev. Biol. , 389, 98-119. PMID: 24491817 DOI.
Patthey C, Schlosser G & Shimeld SM. (2014). The evolutionary history of vertebrate cranial placodes--I: cell type evolution. Dev. Biol. , 389, 82-97. PMID: 24495912 DOI.
Graham A & Shimeld SM. (2013). The origin and evolution of the ectodermal placodes. J. Anat. , 222, 32-40. PMID: 22512454 DOI.
Schlosser G. (2010). Making senses development of vertebrate cranial placodes. Int Rev Cell Mol Biol , 283, 129-234. PMID: 20801420 DOI.
Ladher RK, O'Neill P & Begbie J. (2010). From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development , 137, 1777-85. PMID: 20460364 DOI.
Schlosser G. (2006). Induction and specification of cranial placodes. Dev. Biol. , 294, 303-51. PMID: 16677629 DOI.
Begbie J, Brunet JF, Rubenstein JL & Graham A. (1999). Induction of the epibranchial placodes. Development , 126, 895-902. PMID: 9927591
Articles
Washausen S & Knabe W. (2018). Lateral line placodes of aquatic vertebrates are evolutionarily conserved in mammals. Biol Open , 7, . PMID: 29848488 DOI.
Abitua PB, Gainous TB, Kaczmarczyk AN, Winchell CJ, Hudson C, Kamata K, Nakagawa M, Tsuda M, Kusakabe TG & Levine M. (2015). The pre-vertebrate origins of neurogenic placodes. Nature , 524, 462-5. PMID: 26258298 DOI.
Mazet F. (2006). The evolution of sensory placodes. ScientificWorldJournal , 6, 1841-50. PMID: 17205191 DOI.
Bhattacharyya S & Bronner-Fraser M. (2004). Hierarchy of regulatory events in sensory placode development. Curr. Opin. Genet. Dev. , 14, 520-6. PMID: 15380243 DOI.
Köster RW, Kühnlein RP & Wittbrodt J. (2000). Ectopic Sox3 activity elicits sensory placode formation. Mech. Dev. , 95, 175-87. PMID: 10906460
Search Pubmed
June 2010 "placode development" All (852) Review (90) Free Full Text (285)
Search Pubmed placode development | otic placode development | optic placode development | nasal placode development | adenohypophyseal placode development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Placodes. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Placodes
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
|