Paper - Origin of the pulmonary vessels in the chick (1922)
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Buell CE. Origin of the pulmonary vessels in the chick. (1922) Contrib. Embryol., Carnegie Inst. Wash. Publ. 66 14: 11-26.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Origin of the Pulmonary Vessels in the Chick
By Charles Elbert Buell Jr. (2 plates) 11-26 (1922).
Introduction
This paper deals with the origin and early stages of the pulmonary vessels of the chick, as demonstrated by stained serial sagittal and cross-sections of fixed specimens and by injecting living embryos with dilute india ink. The serial sections begin at the stage of 20 somites, in which the first evidence of a pulmonary system is seen in the proliferation of angioblasts from endothelial walls of established vessels, although it is possible that a few of these cells may differentiate from mesothelium. From these sections I have been able to show that this proliferation of angioblasts gives rise to both the common pulmonary vein and the left valve of the sinus venosus. The angioblasts spread over the ventral surface of the gut, acquire a lumen, and form a capillary mesh from which the vessels of the lung are evolved. After this plexus is patent and connected to the systemic vessels, the changes leading to the formation of the earliest pulmonary system may be followed by injections. By means of a modified technique for injection I have been able to demonstrate earlier stages in these vessels than have heretofore been shown and to trace the metamorphosis which this capillary plexus undergoes in forming the rudimentary pulmonary vessels. The study ends at the stage of 85 hours' incubation, at which time the pulmonary system is definitely laid down in its earliest complete form.
Concerning the origin of the pulmonary vessels, not only is our present knowledge meager, but the views are conflicting and based on observations of embryos of different forms, made with varying technique. Wax reconstruction of small blood-vessels, while a valuable asset to the embryologist, is open to manifold errors, and where possible should be checked up by injections. Confusion has arisen from the efforts to prove or disprove the probable course of events in one embryonic form from observations on another embryonic form. In recent studies of the pulmonary vessels, guinea-pig, rabbit, cat, and chick embryos have been represented. The finer details in the development of separate structures might follow quite different courses in these several forms. Although it is to be remembered that any attempt to draw conclusions for one on the basis of another is open to error, this study is presented in the hope that demonstrating the developmental steps of the vessels of the lung in the chick may by comparison prove of value in working out the embryology of similar structures in other forms.
In an investigation of this kind some obstacles are sure to be encountered, even in so simple an embryo as the chick. In mammalian embryos these are harder to overcome and offer a possible explanation for our present inadequate knowledge of the origin of these vessels. The period of origin, from the beginning of the proliferation of the angioblasts until the establishment of a lumen in the pre-pulmonic capillaries, represents a relatively short period of incubation and exact stages are not easily defined. The delicate collapsing capillaries are difficult to make out in serial sections unless they are injected. Wax reconstruction of such minute vessels is more or less impracticable because there are no bloodcorpuscles in the small capillaries. Angioblast and mesenchyme cells are not easily differentiated and hence interpretation is often difficult. The location and continuity of angioblastic cells with endothelium or other angioblasts are of great value in their identification. The angioblast is larger than the mesenchyme cell, the cytoplasm contains more basophilic substance, and the nucleus is more oval, larger, and more vesicular. In injected specimens mounted in toto the small capillaries are concealed by large systemic vessels packed with granules of ink. This drawback has been overcome by a simple method of paraffin dissection for the younger embryos and direct dissection of the older ones.
Methods
Three methods were used in this study: (1) Injecting living embryos and clearing, by the Spalteholz technique, for dissection in oil of wintergreen ; (2) embedding injected chicks in paraffin for dissection; (3) cutting serial sagittal and cross-sections (10 to 15 microns) for staining. A summary of the development of the technique of injections is found in the work of Sabin (1915). The injection method used in this work is a modification of that devised by Popoff. The injections were made by blowing ink into the vitelline vein of the living embryo by means of a fine glass canula. Popoff (1894) first described this method of injecting small vessels. In his work on the yolk-sac he found that injections of prussian blue greatly facilitated the study of the capillaries. He did not apply the method to vessels within the embryo proper, but used it for the vessels of the yolk-sac by injections made into the marginal sinus. At that time he noted the influence of the heart and the direction of the blood-flow upon the completeness of his injections.
My injections were made into the right vitelline vein, which lies over the artery and lends itself readily to injection. The tributaries of this vein join at an angle just before entering the body of the embryo. The point of the canula was introduced into the vein at the vertex of this angle, which acts as a guide and offers sufficient resistance to allow the entry of the needle into the vein. The tip of the canula is visible and the extent of the injection under perfect control. MacCallum simplified the injection of small vessels by following its course under a compound microscope. A binocular microscope is of great help in making very dilute injections where danger lies in blowing too much ink into the blood-stream. A small amount of ink diluted with physiological saline does not embarrass the circulation. The heart action mixes the ink thoroughly with blood plasma and gives a complete injection. The ink granules adhere to the endothelium of the vessels, due either to the sticky surface of the endothelium or to direct phagocytosis. Care should be observed that no vessels are torn in preparation for the injection.
To make a suitable glass canula, select a piece of soft glass tubing 12 cm. long and 5 cm. in diameter. Using a Bunsen flame, mold the tubing into the shape of a U . Hold an arm of the U in each hand and draw out quickly until the base measures 5 cm. ; now substitute a small flame (1 cm.) for the Bunsen burner. Gently heat the base of the U near either arm, and as it softens a quick drawing motion completes the canula. A second one can be made from the other arm. Trim the tip to the desired size with a pah of small scissors and the canula is ready for use. Equip with a piece of rubber tubing of convenient length and a glass mouthpiece.
Injection Mass
It has been found that india ink is more suitable for injection than prussian blue, because of its finer granulation. At first I diluted the ink 1 to 1 with water. This was freshly filtered and used at once. These injections, however, were too intense and seemed to embarrass the circulation. Better injections were obtained by diluting the ink 1 to 5 with physiological saline and filtering several times through the same paper; the ink is still further diluted in the blood-stream. This gives excellent injections of the capillaries and at the same time renders the large overlying veins transparent, so that they do not obscure the lung- vessels.
To inject a chick, draw up a small quantity of freshly filtered dilute ink into
the canula, followed by a drop of physiological saline to prevent soiling of the field
of injection. Prepare a dish of warm Locke's solution, about 37° C, and another
dish containing 10 per cent nitric acid for fixation. Place the egg in a shallow
glass jar packed with cotton. Remove a sufficient quantity of shell to expose the
embryo and permit free access to it. Add a few cubic centimeters of warm Locke's
solution to prevent drying. Place the preparation under a low-power binocular
microscope and remove the vitelline membrane over the site of the proposed
injection. Introduce the tip of the canula into the angle formed by the tributaries
of the right vitelline vein and blow the ink into the vein. The heart action completes the injection. In fixing, add the 10 per cent nitric acid first directly to
the embryo, then remove the embryo from the shell and place it for 5 minutes in
a cover-glass containing the acid solution. The acid fixative makes the tissues
more transparent and prevents diffusion of the ink through the vessel-walls. The
fixed specimens are washed in several changes of water to remove the excess acid.
Some of my specimens were given a light lavender tint with Ehrlich's haematoxylin,
but this is not necessary. The embryos are dehydrated with graded alcohols —
absolute alcohol, absolute alcohol and xylol, xylol — then (on an electric stove)
through xylol and paraffin, and finally paraffin for embedding and dissection.
The larger embryos are not embedded, but are put through benzine into oil of
wintergreen for direct dissection.
Paraffin Dissection
In using whole mounts of injected embryos for the study of the early lung vessels, a difficulty is encountered in the large overlying cardinal veins that obscure the delicate vessels beneath. This difficulty increases with older stages and more complete injections. In efforts to overcome it I have had good results with the following simple method of dissection : The embedded embryo is trimmed into a block so that the broad surface is parallel to the sagittal plane of the embryo. With gentle heat the block is fixed upon a glass slide. In good light, under the high power of a binocular microscope, holding a sharp scalpel lightly in the fingers of the right hand, successive layers of paraffin are shaved off until the overlying injected vessels are removed. The block is then reversed, exposing the other side of the embryo, and the procedure repeated. The block is then removed, the paraffin dissolved off in xylol, and the block mounted in balsam. By this method the early pulmonary capillaries are brought out clearly and their development can be readily followed. Figures 7 and 8 were drawn from dissections of this sort.
Direct Dissection
This method was more practical with the older embryos. The chick was injected as above described, dehydrated in graded alcohols, and passed through benzine into oil of wintergreen. The preparation was then placed under the high power of a binocular microscope, and held in position with a fine camel's-hair brush. With needle-pointed forceps the large limb-buds on both sides were carefully removed before attempting the more delicate structures. The cardinal veins, duct of Cuvier, and sinus venosus were opened and brushed free of ink granules. This procedure exposes the pulmonary vessels in situ while preserving their anatomical relations. Such a technique was used for specimens shown in figures 9 and 10.
Serial Sections
In the early stages in which the splanchnic plexus can not be injected, i. e., before the pre-pulmonary capillaries are patent, I resorted to serial sections. The embryos were fixed with Bouin's mixture (75 parts picric acid, 20 parts 40 per cent formalin, and 5 parts glacial acetic acid). After removal from the shell the chick was fixed for one hour in this mixture, then passed directly through several changes of 60 per cent alcohol to remove the excess of the fixative, and finally through the graded alcohols to paraffin, as above described. Sections 10 to 15 microns in thickness were cut by the water-knife method of Huber and stained in hematoxylin and erythrosin. Both sagittal and cross sections were used, so as to serve as a check in either series and to give a more exact localization of the anatomical structures. By using both types of sections the left valve of the sinus venosus can be assigned to its correct position in relation to the mass of cells giving rise to the common pulmonary vein. Sagittal sections have a close relation to the injected specimens, which are used as guides. Erythrosin is used as a cytoplasmic stain, although in the early stages the cells show a marked affinity for basic dyes. Cochineal carmine may be used alone after cells have been identified.
Pulmonary Vein
The present status of our knowledge of the origin of the pulmonary vein is embodied in the seemingly opposed views of Fedorow and Brown. The former holds that the vein is derived from an endothelial proliferation of the dorsal wall of the sinus venosus, while Brown thinks that it is a part of an indifferent plexus originally present in this region.
Fedorow (1910), studying embryos of four orders (amphibian, reptile, bird, and mammal), reports the origin of the vein as an outgrowth of endothelium from the dorsal wall of the sinus venosus. The cavity of the sinus extends into this proliferation for a short distance, forming the pulmonary vein, then breaks up into two vessels which in turn ramify into capillaries that unite with a similar capillary outgrowth formed by the pulmonary arteries.
Brown (1913), from his observations on embryos of the domestic cat, together with reference to sections of chick embryos, questions the work of Fedorow. He states that the pulmonary system is simply a specially developed part of an indifferent plexus originally present in this region, and that the proliferation of endothelium described by Fedorow is the left valve of the sinus venosus, which occupies that position.
In considering these two views it must be remembered that the investigators were using embryos of different forms and that the course of development may vary in these types. My work on the chick can do no more than establish the process as it occurs in that embryo and is designed only for that end. At the same time I feel that this paper tends to show that the views of Brown and Fedorow are mutually exclusive only so far as their interpretations are concerned, not in any actual differences in the mode of development of the pulmonary vein in their respective embryos. That there is a proliferation of endothelium from the dorsal wall of the sinus venosus is apparent. Equally so is the fact that the pulmonary vein is not established at that time. In slightly older stages the pulmonary vein is seen opening into the sinus venosus at that point, and yet in the same section is a mass of endothelium readily recognizable as the left valve of the sinus venosus. Fedorow did not recognize the left valve of the sinus venosus or the dual character of the mass of endothelium giving rise to both the endothelial
lip of the left valve and the common pulmonary vein. Brown, from his catembryo material, does not exclude the possibility of this origin of the pulmonary
vein. He says:
- "It is the purpose of this paper to follow the development of the pulmonary vein of the domestic cat from the early stage in which it empties into the cephalic portion of the sinus venosus in the median line to the stage in which it attains its definitive connections with the left auricle."
From his work it is clear that in his earliest stage the pulmonary vein is already established and that, instead of offering proof as to the origin of the vein, he is merely describing a stage in its development. Earlier stages might show that in the formation of the pulmonary vein the cat follows the same process as the chick. At least Brown's observations do not exclude such a probability and suggest further work on the cat embryo.
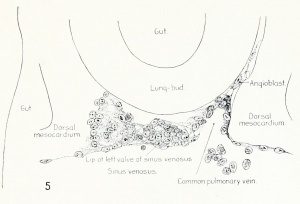
Brown raised a legitimate objection to Fedorow's work so far as the left valve of the sinus venosus is concerned, in that the latter observer did not recognize the left valve as such nor show its relation to or origin with the common pulmonary vein. On the other hand, Brown is in error in rejecting Fedorow's work upon the origin of the pulmonary vein, since he based his contention upon findings in a different embryo and at stages that are plainly older than those described by Fedorow. Brown probably saw the endothelial lip of the left valve of the sinus (fig. 5) and the pulmonary vein opening into the sinus and concluded that this was what Fedorow described.
Common Pulmonary Vein and its Tributaries
The first indication of the common pulmonary vein is a proliferation of angioblasts from the dorsal wall of the sinus venosus extending dorsally toward the gut at the level of the lung-bud. This occurs in the chick at the stage of 20 somites and is best seen in sagittal section (fig. 1). There is no venous opening into the sinus at this time, indicating that the pulmonary vein is not established. This primary proliferation of angioblasts soon shows a differentiation into a right and left portion having distinct histological differences (figs. 2 to 5). The right twothirds forms a compact mass of endothelium of the lip of the left valve of the sinus venosus (fig. 5), into which the mesothelium of the dorsal mesocardium extends. On the left the angioblasts are larger and more loosely connected; they extend dorsally to the surface of the gut and spread out in all directions over its ventral surface in the plane of tissue between the endoderm of the gut and the dorsal mesocardium. At the same time, angioblasts can be seen to differentiate from both sides of the dorsal aorta and from the bulbus of the ventral aorta, until the whole ventral surface of the gut is covered with a plexus of angioblasts which have not yet formed the capillaries. It is possible that some of the angioblasts may differentiate in situ from mesoderm, but I have not found any isolated clumps of these cells that would indicate that this does actually occur. At this stage the thickness of the embryo precludes the study of living cells in this region, which is necessary for direct proof of such a process. The loosely meshed clump of angioblasts tying between the tip of the lung-bud and the sinus venosus on the left side (figs. 2 and 3) undergoes central liquefaction and opens secondarily into the sinus venosus. This is the common pulmonary vein, which at this stage is a blind pouch, as the plexus of angioblasts covering the ventral surface of the gut is not patent but is merely a network of cells connecting the common pulmonary vein with the ventral and dorsal aortse. This plexus of angioblasts acquires a lumen and forms a capillary net, the splanchnic plexus, which connects the lumen of the sinus venosus, through the common pulmonary vein, to the dorsal and ventral aorta? and cardinal veins.
I am not prepared to state whether the lumen of this plexus of capillaries is an
extension of the lumen of the common pulmonary vein or of the ventral or dorsal
aorta?, or whether, as in the case of the common pulmonary vein, it is produced by
central liquefaction. In the case of the pulmonic arches (sixth) there is definitely
an extension of the lumen through a cord of angioblasts, while the common pulmonary vein is formed by central liquefaction. Both processes occur in early
blood-vessel formation and are probably dependent upon the hydrodynamics of
circulation in any given area. This would explain the different processes seen in the
case of the pulmonic arches in contrast to the common pulmonary vein. Fedorow thought that the lumen of the sinus venosus extended into this endothelial proliferation. In my sections the reverse seems to be true; the mass of endothelial cells undergoes central liquefaction, forming a lumen that opens secondarily into the sinus venosus. Figure 3 shows a stage in which central liquefaction has occurred but there is no opening into the sinus. Figure 5 shows this process slightly older and there is now an opening into the sinus at that point.
That this outgrowth of endothelium or angioblasts is the first indication of the
common pulmonary vein is supported by the following facts: (1) There is no venous
opening into the sinus at this point, either before or during the proliferation of
angioblasts from the dorsal wall of the sinus venosus. (2) This mass of cells occupies the exact position at which, in a later stage, the common vein opens into the sinus venosus. (3) Liquefaction can be seen in this mass of angioblasts before the
vein has opened into the sinus. (4) The orifice of the common pulmonary vein
in later stages can be seen at this point, the mass of cells having disappeared.
(5) The lip of the left valve of the sinus venosus is also derived from these cells and
is present throughout the process, having distinct histological differences that
render its identification a simple matter (figs. 4, 5).
Some confusion may arise from the fact that the pulmonary vein opens into
the sinus at the left of the left valve of the sinus ; in other words, the left valve lies
to the right of the opening of the vein. A study of the early development of the
heart shows this to be the case. Later, however, when the left valve fuses with the
dorsocaudal extremity of the septum superius (Brown), the opening of the vein is
assigned to its final position in the left auricle.
The pulmonary circulation goes through two phases of development, ascending and retrograde. The former reaches its maximum at the stage of 90 hours' incubation. At this time the system consists of two pulmonary arches, two pulmonary arteries, and a common pulmonary vein with four main branches plus connections to both anterior cardinal veins. From this time on, the system may be said to undergo retrograde changes leading to the adult structure. It is beyond the scope of this paper to consider more than the origin of these vessels and the first step in their retrogression, i. e., the loss of two of the branches of the common vein.
With the formation of the common pulmonary vein and its connection with
a patent splanchnic plexus of capillaries over the ventral surface of the gut, a new
path of blood-flow is established between the arterial and venous portions of the
heart through this plexus. The axis of the common vein is perpendicular to that
of the plexus and divides the plexus into two portions, the cephalic and post-caval,
both of which drain into the common vein. A change occurs, due to dynamics
of circulation and growth, in which the capillaries in each of the four directions about
the common vein are replaced by individual vessels that take over the function of
the plexus. On the right and left sides of the gut, at the level of the lung-bud, the
right and left lateral branches are formed. These are the true pulmonic branches,
in that each drains its respective artery in the right and left lung rudiment. They
persist and develop with the lungs.
The capillaries caudal to the common vein begin to disappear early, decreasing
in size, number, and importance. They are merely the connections between the
cephalic and post-caval portions of the splanchnic plexus. At the stage of 90
hours of incubation they are represented by only one or two small twigs which soon
disappear. It is of interest to note that the persistence of one of these vessels may
give rise to a very unusual anomaly of the pulmonary circulation. Brown gives an
excellent description of such a case.
A most interesting vessel is derived from the capillaries cephalad to the common vein, i. e., the cranial tributary of the pulmonary vein. This lies in the midline of the ventral surface of the gut and drains a system of anastomoses between
the two pulmonary arteries, receiving also small twigs from the pulmonary arches.
Figure 10 shows this vessel at the height of its development. It, also, is a temporary
structure and begins to degenerate at the stage of 100 hours. Squier has shown
a later stage in which it has lost its rich arterial connections and stands out like a
dead branch of a tree, finally disappearing. Squier used a method of wax reconstruction and described a stage 10 hours older than that shown in figure 10. During this period several changes take place. The cranial tributary loses its connections
with the pulmonary arteries and disappears. The distal communications with the
post-caval plexus have disappeared. The lung rudiments begin to show definite
signs of lobulation and the vascular picture has accommodated itself to that change.
In summary, then, the formation of the pulmonary circuit falls into three main
periods:
- Precirculatory - A proliferation of angioblastic cells from established embryonic endothelium, with the possibility also that some of the vasoformative cells may differentiate from mesoderm and join in the process. This mesh of angioblasts undergoes cytoplasmic liquefaction, forming a capillary net over the surface of the primitive gut. From this plexus the pulmonary vessels are evolved.
- Circulatory - After the capillary plexus is patent, a new route is established between the arterial and venous portions of the heart. The plexus undergoes a change in pattern with the establishment of new lines of blood-flow and the formation of definite vessels, such as the pulmonary arches, arteries, capillaries, and veins.
- Adaptive - With the development of the lung, new patterns of vessels are evolved to accommodate the circulation to this change. This leads to the formation of a true pulmonary circulation. The arteries increase in length, the capillaries over the lung rudiments increase in number, and the remnants of the indifferent plexiform stage disappear. The cranial tributary has reached its highest development and is about ready to disappear. The post-caval connections have already disappeared except for one or two small remaining twigs.
Streeter (1915), in a study of the vascular system of the brain of the human embryo, divides the stages of development of the brain vessels into five periods, showing the various adaptive changes which the circulation goes through in accommodating itself to the ever-changing environment of embryonic development.
Pulmonary Artery
The recent views on the origin of the pulmonary artery have undergone a complete change from the old concepts that still dominate the text-books, based on the works of His, Zimmermann, Rathke, and others. The old idea that the pulmonary artery is derived as a branch from the pulmonary arch was the accepted one until the recent work of Fedorow, Bremer, and Huntington. Even Bremer (1902, 1909) adhered to this conception in his first two articles, but corrected it in a third paper on the rabbit embryo. He describes the origin as a blind extension of a capillary net from the ventral aorta. Unknown to Bremer, Fedorow, in a Russian publication, antedated the former's work by a similar description of the origin of the pulmonary artery in the embryo of the guinea-pig. Bremer (19126), in a fourth paper, generously acknowledged the priority of Fedo row's work. Huntington, basing his observations on reconstructions from the cat embryo, holds that the artery is formed by the "organization of a distinct arterial channel in the ventral portion of the post-branchial plexus." Thus far his observations coincide with my own on the chick. Concerning the origin of the original plexus, he states that it is derived from the dorsal aorta and links up secondarily with the ventral aorta:
"The so-called outgrowth from the pulmonary sixth arch serves merely as the point of junction, at which after coalescence with the pulmonary plexus, the blood is carried from the ventral segment of the sixth arch into this prepared channel of the pulmonary artery. The outgrowth would be more correctly defined as the pulmonary arterial tap or approach of the sixth arch."
Huntington's description of the origin of the splanchnic plexus in the cat is
quite different from the condition met with in embryos of the guinea-pig, rabbit,
and chick. It may be possible that the cat is individual in this respect. Fedorow,
using guinea-pig embryos, described an extension of capillaries from the ventral
aorta. A similar observation is made by Bremer in rabbit embryos. My chick
embryos show an extension of angioblasts from the ventral aorta. However, this
is but a part of the whole process and there are other factors which contribute to
the formation of the splanchnic plexus. In considering this we must realize that
the splanchnic plexus consists of more than merely that portion giving rise to the
pulmonary arteries; it lies caudal to the fourth aortic arch and includes the developing" hepatic system as well. In the chick the different parts of the plexus are derived from different structures. The cephalic (pre-pulmonic or post-branchial)
portion of the plexus is formed from angioblasts derived from the endothelium
of the dorsal aorta, ventral aorta, and sinus venosus. The post-caval portion is
largely from the dorsal aorta and partially from the sinus venosus. The cardinal
veins may also contribute to both parts of the plexus, although I have not seen any
direct proliferation from them. They are joined to the plexus at a very early stage,
namely, at 35 somites. It is also possible that certain of the angioblasts may differentiate from mesenchyme and contribute to this formation.
In order to understand the origin of the pulmonary artery, it is necessary to
consider that portion of the splanchnic plexus lying between the fourth aortic arch
and the sinus venosus at the level of the lung-bud. The pulmonary artery, and the
pulmonary arch (sixth) as well, are persisting channels in this capillary bed.
As to the origin of the capillary plexus, it is derived from angioblasts that
proliferate from endothelium of established vessels. From the dorsal aorta angioblasts spread out ventrally over the surface of the gut. From the ventral aorta
they extend caudally under the surface of the gut. From the sinus venosus, as a
part of the common pulmonary vein, the angioblasts spread laterally, caudally, and
cranially, so that the ventral surface of the primitive gut is covered with a network of angioblasts. This sheet of angioblasts later forms a network of capillaries connecting the dorsal and ventral aortae to the sinus venosus through the common pulmonary vein. There are also connections to anterior and posterior cardinal veins.
This capillary plexus, meeting the fate of all embryonic capillaiy meshes,
is changed into individual vessels, certain ones of which increase in size and take
over the function of the smaller capillaries, leading to the atrophy and loss of the
latter. This process is followed in the splanchnic plexus. I have already shown
how the tributaries of the pulmonary vein are evolved in this manner. In a similar
way the arteries are formed. In figure 8, along the junction of the lateral and
ventral surface of the gut on each side, is a capillary vessel which arises from the
ventral aorta, extends caudalward, following a diagonal course to the laterodorsal
surface of the lung rudiment, where it connects with other capillary vessels, the
forerunners of the corresponding branches of the common pulmonary vein. It
is possible to inject the vessel at 60 hours' incubation (35 somites).
It is interesting to note that the lumen of the artery can be injected before
the pulmonary arch is patent, showing that the artery antedates the arch. This
does not agree with the observations of Huntington in cat embryos, in which he
states the arches are formed before the arteries.
Pulmonary Arches
The pulmonary arches (sixth) arise in a manner slightly different from that of the other aortic arches. The difference is largely chronological. The fact that the arches are formed in conjunction with the splanchnic plexus and hence may be regarded as a part of that capillary net does not cover the whole process, as there are certain differences in origin that must be considered. The arches are formed later than the pulmonary artery and vein and other capillaries in the splanchnic plexus. It is possible to inject these vessels before a lumen is established in the arches, although the dorsal and ventral primordia can be seen. Figure 7 shows such a stage.
The pulmonary arch on each side arises from two sources. The first or dorsal
rudiment often has a double origin, part from the dorsal aorta and part from the
fourth aortic arch at the angle formed by the union of these two vessels. This is
the most constant relation, although some injections show it coming almost entirely
from the fourth arch near its junction with the dorsal aorta. It curves ventrally
around the last pharyngeal pouch and is connected with a similar process extending
dorsally from the ventral aorta. The lumina of the dorsal aorta and fourth arch
penetrate the dorsal angioblastic cord from above, often separately for a short distance, then uniting and extending ventrally. In a similar manner the lumen of the ventral aorta extends dorsally into the ventral angioblastic cord. The two lumina
meet behind in the fourth pharyngeal pouch, completing the pulmonary arch.
This occurs in chicks of 35 somites.
It is possible to inject both the dorsal and ventral primordia before the arch
is complete (fig. 7). In embryos a few hours older it is possible to inject the whole
arch, the large, pouch-like lumina of the two rudiments being connected by a delicate capillary filament. I have injected such a stage, which is earlier than that
shown in figure 8, and in which there is a complete arch, in the form of an extremely
fine capillary, connecting the large dorsal and ventral pouches of the arch. This
is the earliest stage at which it is possible to inject the arch by this method. The
specimen was not used for illustration because other structures, due to faulty dissection, did not show clearly.
As soon as the arch is complete it undergoes a rapid increase in size until it is
equal in importance to the other arches. Its position, connecting the ventral
aorta to the dorsal aorta, puts it in the direct line of arterial blood-flow. The
dynamics of increased pressure, rate of flow, and action of the heart are undoubtedly
responsible for this rapid increase in size. The pulmonary artery, lying in an indirect path connected with the venous circulation, has no such stimulus to growth
and remains a small, unimportant-looking vessel. The early connection of the
pulmonary artery with the ventral aorta, adjacent to the pulmonary arch, is soon
altered. The arch during its rapid growth actually carries the small artery along
with it, until in later stages the artery is seen to come off at the junction of the
ventral and middle third of the arch. This early disproportion in size, together
with the relation of the artery to the arch at this stage, gave rise to the former
erroneous view that the pulmonary artery arises as a small branch from the arch.
In reality the two arise independently of each other, the artery actually antedating the arch.
I wish to take advantage of this opportunity to acknowledge the generous assistance and encouragement of Dr. F. R. Sabin, under whose supervision this work was done.
Summary
- The first phase of the vascular system of the lung consists of masses of solid angioblasts, rather than of a plexus of vessels, but although the origin of the pulmonary system falls well within the period in which vasoformative cells are seen to differentiate out of mesoderm, I have in my material no positive evidence that the angioblasts giving rise to this system do actually differentiate in situ from mesenchyme. No isolated clumps of these cells indicating such a process are seen in my sections. A study of the cells of this region in a living blastoderm is impracticable because of the dense intervening tissues. The angioblasts seen are connected to other angioblasts, and the earliest cells are in continuity with and lie near the endothelium of established vessels, and the zone between the gut and the dorsal mesocardium is almost acellular before the spread of angioblasts into that area.
- The first indication of the common pulmonary vein is a proliferation of angioblastic cells from the dorsal endothelial wall of the sinus venosus at the level of the developing lung-bud, seen in chicks of 20 somites.
- This mass of cells extends between the folds of the dorsal mesocardium until the solid wall of the ventral surface of the gut is encountered. They then grow out in all directions over the ventral surface of the gut, contributing to the formation of the splanchnic plexus (20 to 30 somites).
- The core of angioblasts between the primitive gut and the sinus venosus becomes differentiated into two parts. The right two-thirds is a compact mass of endothelium forming the left valve of the sinus venosus; the left third undergoes central liquefaction and opens into the lumen of the sinus venosus. This is the common pulmonary vein in the form of a blind pouch connecting the sinus venosus with the angioblasts on the surface of the gut (24 somites).
- The angioblasts on the ventral surface of the gut in the region of the developing lung-bud acquire a lumen and form the splanchnic plexus (30 to 35 somites). The four tributaries of the pulmonary vein are surviving vessels in this plexus of capillaries. The veins from the right and left lobes persist and develop with the lungs. The post-caval connections disappear at about 90 hours of incubation. The cranial tributary loses its arterial connections and disappears at about 100 hours of incubation.
- The pulmonary arteries are persisting longitudinal vessels in the cephalic portion of the splanchnic plexus of capillaries. The angioblasts giving rise to these capillaries begin as a caudal extension of angioblasts from the endothelium of the ventral aorta.
- The pulmonary arches (sixth) arise in the cephalic portion of the splanchnic plexus at the stage of 35 somites. The angioblastic precursors of the arches are derived from two sources, the dorsal rudiment from the junction of the dorsal aorta and fourth aortic arch, the ventral rudiment from the ventral aorta.
- The pulmonary arches and arteries arise in the same plexus of capillaries, but independently of each other. The arteries are patent before the arches are complete. As a result of unequal rates of growth, the arch increases more rapidly in size than the artery and includes the mouth of the artery within its wall. This relation and early disproportion between the arteries and arches led to the former erroneous view that the artery is derived as a small branch from the arch.
Bibliography
Bremer, J. L., 1902. On the origin of the pulmonary arteries in mammals. Amer. Jour. Anat., vol.1, pp. 137-144.
- 1909. On the origin of the pulmonary arteries in mammals. Anat. Record, vol. 3, pp. 334-340.
- 1912a. The development of the aorta and aortic arches in rabbits. Amer. Jour. Anat., vol. 13, pp. 111-128.
- 1912b. An acknowledgment of Fedorow's work on the pulmonary arteries. Anat. Record, vol. 6, pp. 491-493.
Brown, A. J., 1913. The development of the pulmonary vein in the domestic cat. Anat. Record, vol. 7, pp. 299-329.
Duval, M., 1889. Atlas d'Embryologie. Paris, G. Masson.
Evans, H. M., 1909. On the development of the aorta, cardinal, and umbilical veins, and other bloodvessels of vertebrate embryos from capillaries. Anat. Record, vol. 3, pp. 498-518.
Fedorow, V., 1910. Cber die Entwickelung der Lungenvene. Anat. Hefte, Erste Abth., Bd. 40, pp. 529-607.
- 1911. Communications of the Military Med. Acad., St. Petersburg, Russia, vol. 22. (After Bremer, 19126.)
Flint, J. M., 1906-07. The development of the lungs. Amer. Jour. Anat., vol. 6, pp. 1-137.
Huntington, G. S., 1919. The morphology of the pulmonary artery in the mammalia. Anat. Record, vol. 17, pp. 165-201.
Lillie, F. R., 1919. The Development of the ChickHenry Holt and Co.
Mall, F. P., 1906. A study of the structural unit of the liver. Amer. Jour. Anat., vol. 5, pp. 227-308.
MacCallum, W. G., 1902. Die Beziehung der Lymphgefasse zum Bindegewebe. Arch. f. Anat. u. Physiol., Anat. Abth., pp. 273-291. Also translated in Johns Hopkins Hosp. Bull., Baltimore, 1903, vol. 14, pp. 1-9.
Popoff, D., 1894. Die Dottersack-Gefasse des Huhnes. C. W. Kreidel's Verlag.
Sabin, F. R., 1915. On the fate of the posterior cardinal veins and their relation to the development of the vena cava and azygos in the embryo pig. Contributions to Embryology, vol. 3, Carnegie Inst. Wash. Pub. No. 223, pp. 5-32.
- 1917. Origin and development of the primitive vessels of the chick and of the pig. Contributions to Embryology, vol. 6, Carnegie Inst. Wash. Pub. No. 225, pp. 61-124.
Squier, T. L., 1916. On the development of the pulmonary circulation in the chick. Anat. Record, vol. 10, pp. 425-438.
Streeter, G. L., 1915. The developmental alterations in the vascular system of the brain of the human embryo. Contributions to Embryology, vol. 8, Carnegie Inst. Wash. Pub. No. 271, pp. 5-38.
Description of Plates
Plate 1
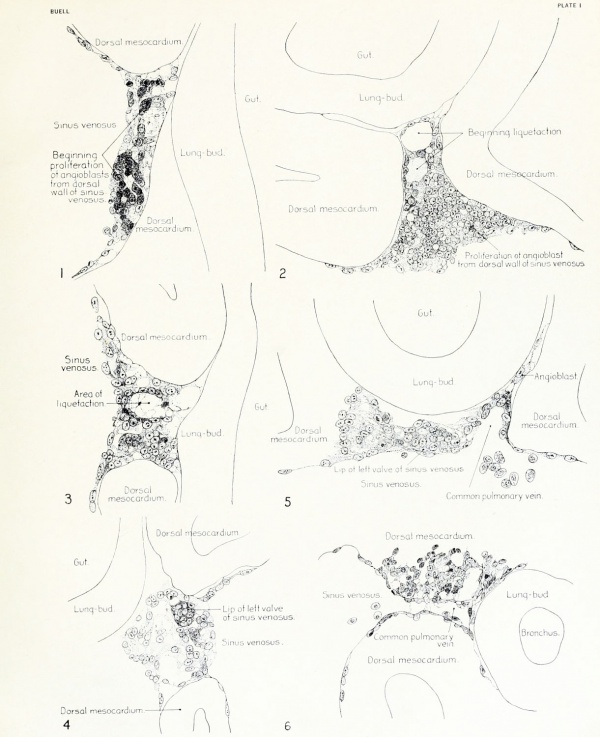
|
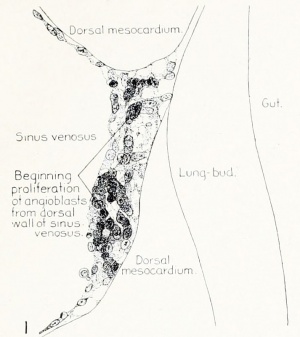
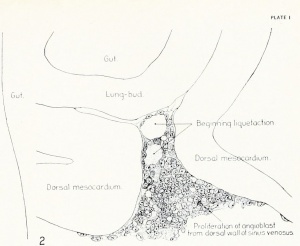
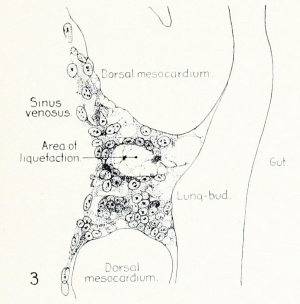
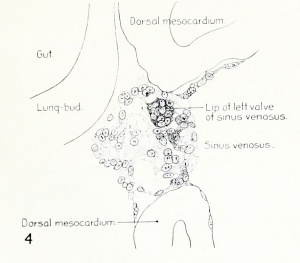
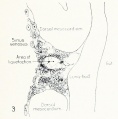
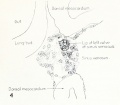
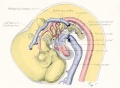
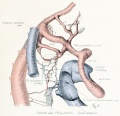
Fig. 1. Median-sagittal section (10 micron in thickness) through tip of lung-bud of a 20-somite chick, 40 hours' incubation; hematoxylin and eosin slain, series B. Angioblasts, forerunners of pulmonary system, are seen proliferating from and near dorsal endothelial wall of sinus venosus. They extend dorsally toward the ventral surface of the gut, which shows a slight ventral swelling - the primary lung rudiment. This is about the earliest stage in which there is any evidence of the formation of a pulmonary vascular system. Fig. 2. Cross-section (10 micron) through tip of primary lung rudiment of a 21-somite chick, 48 hours' incubation; carmine stain, series 0. A slightly later stage in the angioblastic proliferation from the dorsal sinus wall. The right portion of the cell-mass has begun to form a matted group of cells, (lie tip of the left valve of the sinus venosus. The left portion of the proliferation shows signs of liquefaction by which the common pulmonary vein is formed. Fig. 3. Sagittal section (10 micron) through left third of proliferation of angioblasts, to show process of liquefaction extending toward sinus venosus. It is about to open into the sinus, thus forming the common pulmonary vein. Hematoxylin and eosin stain; chick of 23 somites, 48 hours' incubation, series D. Fig. 4. Median-sagittal section (10 micron) through right two-thirds of mass of angioblasts, to show matting together of cells to form left sinus valve tip. The pulmonary vein is not established. This embryo (22 somites, 44 hours' incubation) is slightly younger than that shown in figure 3. Hematoxylin and eosin stain; series II. Fig. 5. Cross-section (10 micron) through tip of primary lung rudiment of a chick of 24 somites, 48 hours' incubation; carmine stain, series E. The right two-thirds of the mass of angioblasts has formed the tip of the left valve of the sinus. The common pulmonary vein is now established, opening into the sinus to the left of the valve. Angioblasts can be seen spreading over the ventral surface of the gut. This is the earliest stage in which the common vein is complete. Fig. 6. Sagittal section (15 m) through plane of common pulmonary vein, showing it complete, from the sinus venosus to tip of lung rudiment. There is no pulmonary circulation at this stage Angioblasts can be seen over the surface of the lung-bud. Embryo of 31 somites, 50 hours' incubation; hematoxylin and eosin stain; series X. |
Plate 2
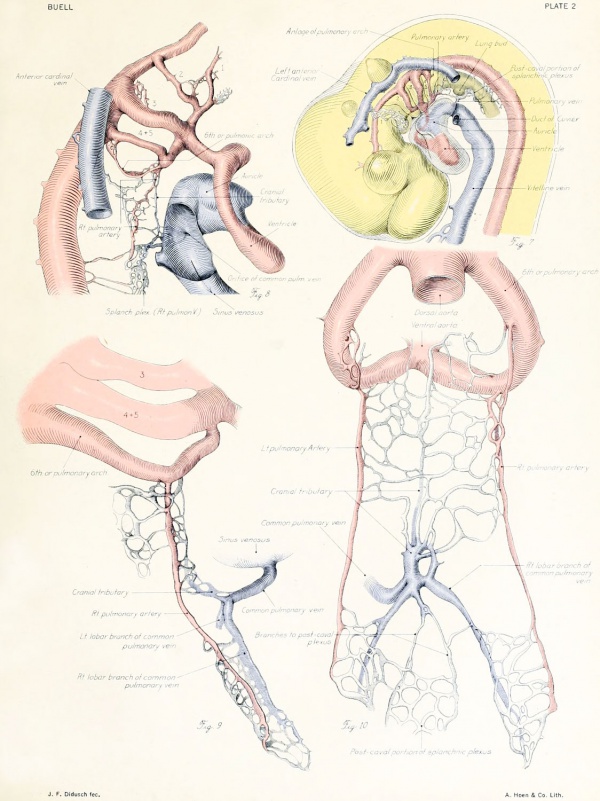
|
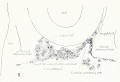
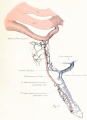
Fig. 7. From an injected embryo of 35 somites, 55 hours' incubation, dissected by the paraffin method. The lung
consists of a simple ventral diverticulum beginning to show lateral swellings into right and left primary buds. The common pulmonary vein opens into the sinus at the level of the lung rudiment. It drains the capillaries of both cephalic and post-caval portions of the splanchnic plexus. The anastomoses between the plexus and the cardinal veins are established. The pulmonary arches are not formed, although the dorsal and ventral primordia of the arch are indicated by the blind pouches. The cranial end of the pulmonary artery is now easily recognized in the capillary plexus.
|
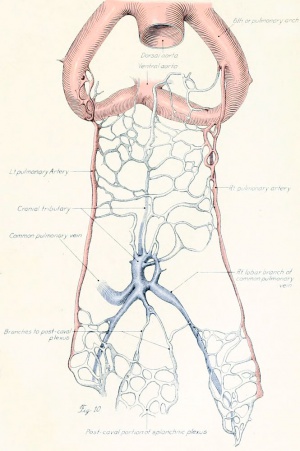
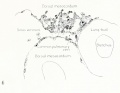
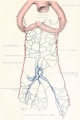
Figs. 9-10. Dissections of injected chick embryos of 85 hours' incubation. Figure 9 shows the right side of the pulmonary system. In figure 10 the spinal cord, dorsal aorta, and dorsal surface of the gut have been removed, exposing the pulmonary system in a coronal plane from a dorsal view. The lung is in a simple stage of right and left primary buds which do not show further lobulation. The left bud is more ventral than the right and is parallel to the gut. The right bud tends more toward a horizontal position in relation to the plane of the gut. The pulmonary vessels bear a constant relation to the bronchi of the buds, even at this early stage. The artery lies dorsal and lateral to the bronchus; the vein, ventral and medial to the bronchus, the lung capillaries lying between the two on the dorsal surface of the buds. The pulmonary artery comes off from the arch at the junction of its middle and proximal third, and passes directly back to the tip of the lung-bud, where it joins freely, in a capillary net, with the corresponding tributary of the pulmonary vein. Very near the arch a capillary connection is given off to the anterior cardinal vein. The two arteries extend parallel to each other and in their proximal third are joined by numerous capillary anastomoses which are drained by the cranial tributary of the common vein. The middle third of the artery has no branches. The entire distal third is connected with the vein by a rich plexus of capillaries over the dorsal surface of the lung-bud. A few twigs are still present, connecting with the post-caval portion of the plexus. The pulmonary vein is made up of several tributaries which unite in a common trunk; this in turn empties into the sinus venosus. Considerable variation is encountered in the pattern of these branches in different specimens. The right and left lobar branches to the lung-buds drain their respective arteries. In figure 9 a vessel connects the right lobar vein to the cranial tributary. This is not constant and is absent in figure 10. A few small branches to the post-caval plexus are seen caudal to the lobar branches. The cranial tributary of the common vein drains the anastomotic vessels between the two pulmonary arteries and arches. It extends directly caudad on the ventral surface of the gut and, with the other tributaries, empties into the common vein. It may have but one opening into the common vein, as in figure 10. This stage is about the oldest in which the cranial tributary is seen complete and represents its highest development. In a later stage, as described by Squier, the cranial tributary loses its arterial connections and disappears. The pulmonary arches (sixth) have undergone rapid growth and have included the arteries within their walls.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - Origin of the pulmonary vessels in the chick (1922). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Origin_of_the_pulmonary_vessels_in_the_chick_(1922)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G