Paper - A three weeks human embryo with especial reference to the brain and nephric system (1905)
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Gage SP. A three weeks' human embryo, with especial reference to the brain and nephric system. (1905) Amer. J Anat. 4: 409-443.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Three Weeks’ Human Embryo, with Especial Reference to the Brain and the Nephric System
By Susanna Phelps Gage
Embryologic Laboratory, Cornell University.
With 5 plates
Introduction
The specimen under consideration was loaned to me sometime ago by Dr. Mall for the investigation of the evidence of segmentation in the brain of the higher mammals.
While the study of this embryo is of necessity incomplete in the present state of knowledge, certain points were shown so clearly by it, that they are here presented.
These points are :- For the central nervous system (a) the approximate location of the morphologic front of the brain, and (b) the location of folds and lobules of the fore- and hind-brain which may have segmental significance; for the nephric system, (a) the presence of a pronephros and (b) a generalized state of the mesonephros, illustrating well-known conditions in the lower as well as in the higher forms.
Other facts which agree with or differ slightly from those generally accepted are presented incidentally.
The specimen is No. 148 of the Johns Hopkins University Medical School Collection gathered by Dr. Mall, sectioned under his direction, and most generously opened for inspection to students. In the catalogue of this collection[1] is found a list of nine papers which use this specimen to illustrate special points. These will be referred to under the proper headings.
The embryologic collections of Cornell University and of the Harvard and Johns Hopkins Medical Schools were also freely placed at my command for study and comparison.
Models and Drawings
A model of the entire specimen was made by the Born method,[2] modified as described by Bardeen.[3] As the sections were not equally perfect, the best of each group ofthree was drawn at a magnification of 66%. The sections are 10p. thick, and as but one-third of them was drawn, each wax plate of which the model was made, represents the thickness of three sections, or 30a. The reconstruction was laid out on an outline enlarged from the photograph made by Dr. Mall.‘ The completed model has a length of nearly 300 mm. A comparison of the model with the photograph taken before embedding shows that in the process, there had been a shrinkage of 10 to 12%.
The model was used as a basis for the drawings, the contour line of every fifteenth or thirtieth section being indicated. In this Way the different levels can be correlated throughout the set of drawings which represent the model sectioned at different planes. The drawings in each case, were corrected by repeated comparison with the specimen.
The form of the coelom was obtained by building up the parts cut from the entire model.
Models at a much greater magnification were made of various details, as the fore-brain, the mesonephros and mesonephric tubules.
A part of the details are illustrated by reproductions of photographs of sections of the embryo.
External Form
This embryo was photographed by Dr. Mall at three times enlargement. The photograph, published in an article by him,[4] shows the specimen from the right side, lying upon the opened chorion and is described as “ An embryo three weeks old.” He also mentions that the umbilicus is at the right instead of the left, as is the more usual position. In another paper, he shows an enlarged outline drawing.[5]
The general form is shown in Figs. 1 and 1a, the body being about once and a half as long as the head and at an angle of about 65° with it. The face is featureless except for the wide slit-like mouth (Fig. 2). At the corner of the mouth (Figs. 2, 5, 6) is a depression with a thin gill-cleft like membrane. The four gill-clefts are irregularly spaced giving the second arch great prominence while the third and fourth are in a common depression, the sinus precervicalis. On the right the gill-clefts are less crowded and the sinus not quite so deep. The first gill-cleft is wider at its dorsal end indicating probably a beginning of the external meatus.
The heart is prominent. The yolk sac is extensive turning to the right instead of the left as is more usual, thus making the umbilicus more apparent in a view from the right than from the left side (Figs. 5, 6).
The limb-buds are remarkably prominent in comparison with other specimens supposed to be of the same age.
Epithelial Thickenings
In Fig. 1 are mapped out the regions having a thickened epithelium. Details of these thickenings as shown by individual sections are found in many of the figures, as the arm (Fig. 10, 11), the leg (Fig. 5), anal plate (Fig. 1), gill-arches (Figs. 1, 11), mouth (Fig. 5), olfactory region (Fig. 2), lens (Figs. 1, 2, 5, 8, 16), and the neuropore (Figs. 1-8, 16). In Fig. 1 the thickness of the epithelium corresponds with the density of the dots. The portions left white represent one layer of cells which become flattened over the heart and near the dorsimesal line. Over the entire oblongata this layer of flattened epithelium coalesces with the wide, thin roof of. the brain (Fig. 4). Mall‘ calls attention to the thickening of the neuropore in this specimen. Especially noticeable is the H-shape of the thickening over the olfactory and cerebral regions (Fig. 2). The continuity of leg-bud thickening with that of the anal plate (Fig. 1) is comparable to the condition in amphibian embryos.
Age of the Specimen
The exact age of this specimen cannot be determined any more closely than has been done by Dr. Mall, who considers that it is of about twenty one days development. Its relative age is somewhat important since it presents certain features not hitherto fully described. It is necessary to determine whether it may be a transitional stage or a pathologic or arrested condition which is under observation.
Though it presents one feature (the small number of thoracic myotomes) not universal, and others (the umbilicus turning to the right) which are not common, and still others rare or not previously observed, still on the whole, it so well fits into the series which has been described by various authorities or examined by the author that the weight of evidence seems to point in the direction that this is in general a normal transitional condition, though possibly somewhat exaggerated in a few particulars or retarded in others.
Comparing this specimen with the His models and Atlas,[6] it is seen that there are some resemblances to his specimen Lr estimated at twenty one days, but in external appearance, it seems to lie between a and R estimated by him to be from 21 to 25 days.
The specimens No. 164, 209, 1-18, and 80 in the Johns Hopkins collection show that in certain features of development they form a progressive series (see Nephric System, below). The formula devised by Mall,[7] for determining the age (days : \/ 100 x length of embryo), approximately worked out gives the following:
| Table I | ||||
|---|---|---|---|---|
| No | 209 | 164 | 148 | 80 |
| Length in mm | 3 + | 3.5 | 4.3 5 or 4.5 | |
| Myotomes | 19 | 28 to 29 | ||
| Days | 17 + | 19 — | 21- | 22 + |
The Alimentary Canal and its Appendages
Mouth
The mouth (Figs. 1-4) is simply a cleft between the forebrain region and the mandible, extending laterally to the just forming maxilla and at its tips having a thin membranous portion (Fig. 5) in section strongly resembling the membranous tips of the gill-pouches.
No remnant of the oral plate was found. The position of the dorsal limit of the ectodermic portion of the oral plate is indicated by the hypophysis (Figs. 9, 3, -1). The latter is a bi-lobed, widely opened pouch in contact with the infundibulum of the brain and partially surrounding it.
Pharynx
No signs of Sessel’s pocket, the most cephalic of the entodermic structures of the pharynx could be found since the sections are not favorable in this region.
The lateral or lingual folds, described by Kallius[8] as forming the first rudiment of the tongue, are represented in the mesal View (Fig. 4), as a ridge lying at the side of a mesal pit. The tuberculum impar has not yet arisen in front of the median thyroid. The latter body with the pit from which it arises is present, but the tubular connection between gland and pit has nearly disappeared (Figs. 3, -1).
The floor of the pharynx is partially exposed in Fig. 11, showing lateral extensions into the four gill pouches, each of which ends in a thin plate. The location of the membranous tips of the gill pouches is indicated upon the mesal view (Fig. 4). The gill pouches have also small ventrally projecting blind pouches ending in a somewhat thickened epithelium, beginnings or protons of the thymus and lateral thyroid bodies.
The larynx is represented by a slight depression, on the ventro-lateral borders of which is a pair of minute pouches (Fig. 11) similar in appearance to the ventral processes from the gill pouches. From their general relations it seems probable that they represent the rudiments of a 5th pair of gill pouches.
A tubular projection in the roof of the pharynx over the entrance to the esophagus is apparent in several sections. Killian[9] identified a mesal pouch occupying a position just caudal of the pharyngeal tonsils as the Bursa pharyngea of Meyer. He traced this back to the 11th week of embryonic development. The specimens of the 7th to the 12th week in Cornell University make it apparent that the pouch seen in Figs. 3, 4, at the left of the abbreviation ch, is this same Bursa.
Trachea
The trachea. (half a mm. long) ends in a pair of widely spreading bronchi, each with a single slightly enlarged end, the lung—bud (Figs. 3, 11), surrounded by a.n enlargement in the mesentery.
Alimentary Canal
The esophagus is small and practically closed through part of its length. It extends to section 107, where it merges gradually into the stomach which shows a spindle-shaped enlargement increasing at its caudal end and turning to the left (Figs. 3, 11). The lesser peritoneal cavity pushing the stomach to the left (Figs. 2, 17) is shown at its opening into the coelom (Fig. 11, crossed by line pointing to mesentery).
The stomach narrows again as it merges into the duodenum (Fig. 10). A minute dorsal enlargement of the duodenum is the rudiment of the pancreas (Fig. 3). On the ventral side is found the short bile duct (Figs. 3, 6). As somewhat diagrammatically shown in Figs. 6 and 10, the trabeculae of the liver are in a great sinusoid along the path of the vitelline vein. In Fig. 11 both lobes of the liver are shown from the dorsal side, and in Fig. 6 there is a section through it at the level of the bile duct. The duodenum is enlarged at this point of union with the bile duct, and continues as a tube to its wide (260;r) union with the vitelline sac (Fig. 5).
The caudal intestine within its free dorsal mesentery (Fig. 5) continues from the vitelline sac in a curve following the back. It enlarges again as it leaves the free mesentery and curves around the end of the coelom, and unites with the allantois to form the wide cloaca which is joined by the Wolifian duct (Figs. 5, 17).
The cloaca is closed by the anal membrane, a thickened plate with only a slight indentation (Fig. 17).
Allantois
The allantois extends as a narrow tube along the abdominal stalk and bending over the caudal end of the coelom (Fig. 5), enlarges to form the bladder as it unites with the cloaca (Fig. 17).
From the standpoint of the development of the diaphragm Mall[10] gives the following description of the organs of the thoracic and abdominal cavities :—“ Sections of the embryo 4.3 mm. long (No. 148) show the liver sprouts growing in all directions through the septum transversum, encircling and ramifying through the omphalo-mesenteric veins, making a condition slightly in advance of that in His’s embryo Lr. The sections of this embryo show clearly, that the heart, lungs, liver, and lower peritoneal cavity arise in tissues surrounded by that portion. of the coelom extending into the head in Embryo XII. . . . The lungs arise when the pericardial coelom goes over into the pleural, 12. (2., high up in the region of the head. Immediately on the dorsal side of them is the beginning of the lesser peritoneal cavity, and the intestinal tube struck in this section is the stomach. All these structures lie on the cephalic side of the first cervical myotome. Projecting into the peritoneal coelom, encircling and penetrating the omphalo-mesenteric veins, are the projections of the liver. The two lobes reach from the tip of the lungs and the foramen of Winslow to the point where, the entodermal cells of the liver arise from the alimentary canal, or in this case, the duodenum. The lobes of the liver lie entirely within the canals of the coelom on either side of the head. The caudal ends of these coelom canals have migrated from opposite the second cervical myotome in Embryo XII, to opposite the second thoracic myotome in Embryo 148. It has moved toward the tail, while the cephalic end of the canal, the pericardial coelom, has been kinked over to correspond with the growth of the heart. . . . We have in the embryo the necessary stage to locate the organs which arise in the neighborhood of the septum transversum, as well as to give the fate of the coelom in their immediate neighborhood.” The points of the above description illustrated by the figures are: the penetration of the septum transversum by liver substance and bloodvessels (Fig. 10); the continuity of pericardial and abdominal coelom (Figs. 2, 11) ; the position of lung, stomach, liver, and it may also be said, duodenum, in the caelom canals which lie dorsad of the septum transversum (Fig. 3) ; the caudal end of the coelom canals opposite the 2d or perhaps the 3d thoracic myotome (13th myotome) .
It seems to me that the most cephalic portion of the pericardial coelom might be said to be that surrounding the bulbus arteriosus, in which case it would be dificult to decide that it had retreated so far from its original position opposite the ear as Mall has mentioned.
The specimen and the model show more clearly than any of the drawings, the caudal traction of the organs which has taken place while their original cephalic attachment can still be traced. The furrows of the mesentery (Figs. 3, 6, 10, 11) show this more clearly than the varying caliber which indicates the division of the entodermal tube into organs. The furrow, for instance, separating duodenum and yolk sac extends cephalad to the 8th myotome (6th cervical), and the attachments of all the entodermal organs cephalad of it are spread out between this point and the 1st occipital myotome, that is in the neck. From this, it hardly seems correct to say that lung and stomach are really so far cephalad as mentioned by Mall (13. e. in the head).
Mesoderm
General
The general appearance of the mesodermic tissue is shown in Plate V, being entirely cellular. The condensations in the nephric region are described below. Other condensation is seen in the limbs, but without clear differentiation into separate masses. Condensation also occurs at the side of the pharynx and markedly so in the floor. This continues uninterruptedly into the thick mesentery surrounding lungs, esophagus, stomach, and intestines (Figs. 2, 3, 6, 10, 11), and in the region of the cloaca joins the condensation around the Wolffian duct and continues into and fills the leg-bud.
A distinct spindle-shaped condensation of cells occurs ventrad of the eye (Figs. 7, 8).
Myotomes
Bardeen and Lewis[11] give the following description: “Length, neck-breach, 4.3 mm.; age about three weeks. . . . Though more advanced in development than Lr (His), but twenty—seven myotomes are present (20, 8c, 10t, 51, 2s). This has been determined by careful counting of the myotomes in serial sections of the embryo. The base of the arm-bud appears to lie opposite the seventh to the eleventh myotomes. It is, therefore, probable that two occipital myotomes are present. But nine myotomes lie in the area between the arm-bud and the leg—bud. The base of the latter lies opposite the 21st to the 25th or 26th myotomes. If two myotomes be considered occipital myotomes, the leg, in this instance, lies two segments nearer the head than usual. It is therefore probable that this embryo has an unusually short body-wall.”
Twenty-nine myotomes were counted and modelled on the left side, and twenty-eight on the right, the discrepancy occurring in the caudal region. This count in general, agrees with Bardeen and Lewis; 2 occipital, 8 cervical, 10 thoracic, 5 lumbar, and 3 or 4 instead of 2 sacral, as noted by them.
The arm-pads have even a longer cephalo-caudal enlargement than noted by Bardeen and Lewis, and cover the 7th to the 13th myotomes, thus leaving only 7 complete myotomes between the arm- and leg-buds.
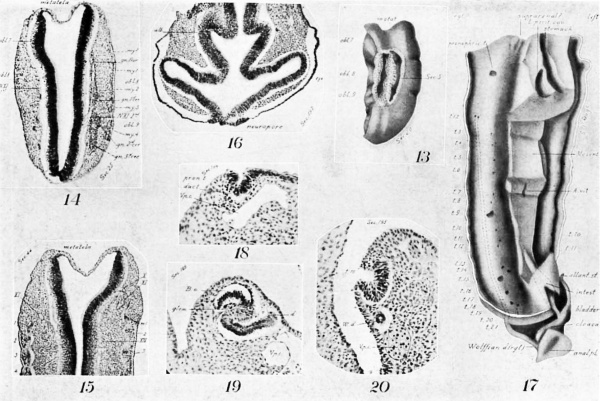
Many of the myotomes do not model in the regular forms usually shown. The first occipitals are small and imperfect (Figs. 14, 15). The 3d, 4th, and 5th are dorsally composed of two distinct, hollow horns (Fig. 14), which merge ventrally into a common cavity. More ventrally they become solid and are marked across the middle by a band of cells (Fig. 15, at left). The lumen is not large until the 11th (Fig. 11), from which point until near the end of the series it is a marked feature. The largest and most regular myotomes are opposite the legs, the most irregular among the cervical.
In several myotomes (Figs. 5, 11), careful examination could detect no limitations between a certain area at their ventral end and the condensed mesoderm of the limb-bud, in fact in these cases, the appearance would indicate the origin of limb tissue from myotomes.
Evidences of segmentation in the mesoderm are also seen cephalad of the two clearly recognizable, occipital myotomes 1 and 2, and immediately in line with them. Cephalad of the 1st is a minute area with apparently the identical structure of a myotome, including the familiar corrugation of the epidermis (Fig. 14). Still more cephalad are two other lesser condensations and corresponding epidermal corrugations (Fig. 15) . That is, there are indications of three more occipital myotomes than are distinctly figured.
Sclerotomes
On their mesal aspect the mesodermic tissue is shrunken away from the myotomes in loops (Fig. 15), and the tissue shows a condensation (sclerotome) corresponding with each loop down to the 18th myotome, In the cephalic region a slight condensation occurs ventrad of the notochord. That is, these last two points indicate a slight differentiation and segmentation of sclerotogenous tissue. In some sections, the continutiy of these sclerotomes with the myotomes can be seen.
Dermatomes
This specimen by itself does not throw much light on the question as to the fate of the outer wall of the myotome, i. e., whether it is in reality a dermatome or not.
Vascular System
Heart
In Figs. 1, 3, and 5 the position of the heart is seen, and in fig 12, a ventral view showing a compact, somewhat square form, reminding one of the His’ model from a 10 mm. (4 week) specimen rather than those from younger specimens. The greater length of the right auricular portion is similar to the His model of a 5 weeks’ embryo. A very young embryo, modeled and presented by J . L. Bremer[12] at the American Association of Anatomists, in December, 1904, shows’ an exaggeratedly long right auricle. In external form, the heart of this specimen resembles older, rather than younger stages, but the internal relations accord with the descriptions given for the 3 weeks’ stage. That is, the tubular heart is -already dividing into right and left (Figs. 2, 6) ; the entrance of the sinus venosus is decidedly to the right (Fig. 2, left of Fig.), the connection of the auricle with the ventricle is through a narrowed tube, the auriculo-ventricular canal (Fig. 10), entering the ventricle at the left (Fig. 6), while the bulbus arteriosus makes its exit at the right (Figs. 12, 6), turns sharply cephalad and extends along the ventral aspect of the heart.
The walls of the heart contain only undifferentiated muscular tissue. In parts the endothelial tube is closely applied to the walls; in parts, notably along the path of the auriculo-ventricular canal (Fig. 10), the ventral end of the right ventricle (Fig. 6) and of the entire bulbus (Figs. 10, 2), the endothelial tube is connected with the outer wall by only a delicate mesh-work of tissue.
Arteries
The endothelial tube of the bulbus plunges into the floor of the pharynx, expands into a wide sinus, giving off on each side near the middle line, a small branch which divides into the 1st and 2d gill arches and a mesh-work of capillaries supplying the mandible. The 1st and 2d arches join with the dorsal aorta by a very slender capillary connection. The 3d and 4th gill arches (Fig. 11) are given ofi? from the side of the sinus and unite dorsally to form the main portion of the dorsal aorta. The 5th gill arch is given off caudally near the middle line, divides into capillary branches, supplying the larynx and trachea (5th and 6th arches), reaching the dorsal aorta by very small branches. Each dorsal aorta sends forward a branch which can be traced in the roof of the pharynx nearly to the hypophysis. This portion of the vascular system resembles closely others described of the same age, but reminds one, in the extreme difference in size of the aortic arches, of the condition described by Miss Lehmann,[13] where in lower mammals the six arches are not all complete at any one time.
The two dorsal aorta; unite (Fig. 3) near the cephalic border of the liver and the caudal margin of the arms, giving ofi just before their union the brachial arteries and soon after, the small vitelline or omphalomesenteric artery (Figs. 10, 17). Branches could occasionally be traced to the tubules of the mesonephros (Fig. 6). In contrast to the other arteries the umbilicals are large and in the body stalk anastomose across the middle (Fig. 5), but continue as -a. pair for some distance in the body stalk.
Veins
The veins are remarkable for the great variation in caliber. The much branched jugular (precardinal) can be traced from a point lying between the eyes and cerebrum (Fig. 7), keeping near the surface (Fig. 8), sending branches through, and then passing mesad of the Gasserian ganglion (Fig. 9), laterad of the ear vesicle and the ganglia of the 7th and 8th nerves (Fig. 11), and by a breaking-up in the 9th and 10th ganglia, comes to lie mesad of the 10th ganglion as it passes over into the vagus nerve, then unites with the cardinal (Fig. 11, at left) and the umbilicals (Fig. 2, at left) to form the ducts of Cuvier. The course of the jugular in the head seems to illustrate one phase of the change of position of the bloodvessels with relation to the nerves as demonstrated by Dr. Mall[14] in a recent article.
The ducts of Cuvier unite across the middle to form the sinus venosus ; and this connects by a small opening with the great sinusoid[15] of the liver formed in the course of and by the union of the vitelline veins (omphalomesenteric) (Fig. 10).
The umbilical veins coming from the body stalk are joined by veins from the legs, become enlarged and break up in the body wall into a great sinus (Figs. 5, 6, 10, 11), and finally as they enter the duct of Cuvier become so small as to be scarcely traceable (Fig. 2, at left, and Fig. 10, at right).
The Nephric System
General
The nephric system (Fig. 17), as is usual at this age, consists of a. pair of Wolfliau ridges extending along the back from the arm pad nearly through the region of the leg-pad, and of Wolffian ducts opening into the cloaca. Sections of the ridges and their contained structures are shown at different levels, beginning cephalad, in Figs. 11, 18, 10, 19, 6, 20, and 5.
There is no definite thickening in the coelomic epithelium or the mesoderm which can be designated as a rudiment of the genital ridge or of the Miillerian duct.
In the ridges, the Wolffian duct is traceable as indicated by dots in Fig. 17 from the 1st mesonephric tubule along the lateral border of the ridge’ and extending beyond the coelom in a curve (Fig. 5), to open into a the lateral border of the cloaca (Fig. 17). Extending still farther laterad along the Wolifian ridge is the cardinal vein, the diameter of which varies greatly in different sections (Figs. 18, 19).
Adrenal
Near the cephalic end of the Wollfian ridge (Figs. 1'7, 11, suprarenal), on the mesenteric border are slight folds in the epithelium which remind one of the structures in the cephalic part of the mesonephros found by Aichel[16] in the rabbit to be associated with the development of the adrenal or suprarenal body.
Pronephros
A wide open funnel (Figs. 17, 18), opening to the coelom and connecting with a small, blind tube extending cephalad for a few sections, is here called a pronephric tubule on account of its position in the cephalic portion of the Wolffian ridge. It has no connection whatever with the Wolffian duct and is separated by a marked interval from the beginning of that duct. This is apparently only a further retrogression from the condition found by MacCallum[17] in which a separated portion of duct extends opposite the 6th, 7th, and 8th myotomes of an embryo 3.5 mm. long. MacCallum calls attention to the possibility that this separated tube with its cephalic opening through a funnel to the cualom may be a pronephric remnant. In an older embryo (5 mm. long) he finds such a disconnected remnant with a single tubule not opening to the ccnlom.
Handler[18] says that in human embryos from 5 to 20 mm. he found pronephric tubules in communication with the coelom eight times. These were at the level of the 5th to 6th segment.
In Embryo 148, the isolated pronephric tubule lies opposite the 11th myotome. Whether the difference in position relative to the myotomes from those reported by MacCallum and Tandler, indicates that this is not a true pronephric remnant, orwhether a caudal shifting of the Wolffian ridges has taken place, or whether there is an increase in the number of myotomes in the occipito-cervical region of No. 148 cannot be determined. But at present, it is unmistakable that in three embryos of 21/2 to 3 weeks in development (Nos. 164, 148, and 80 of the Mall collection) there exists a structure which has many resemblances to a pronephros.
In a 3 mm. human embryo, Janosik[19] found that there is a distinct glomerulus protruding into the coelomic cavity. The position of this glomerulus is similar to the tubules found in the embryos of the Mall collection.
Tandler“ found one glomerulus in eight cases. It seems, therefore, that in spite of the apparent discrepancy of position, there should be no hesitation in expressing the homology of the structure under consideration with the pronephros of lower forms.
Mesonephros
A preliminary report on the generalized constitution of the mesonephros of Embryo No. 148 was presented with models at the December, 1903, meeting of the American Anatomists.[20] The main facts noted were, that in the Wolffian ridges an open pronephric tubule occurs on each side followed by two groups of mesonephric tubules, the first group of eight being of the usual embryonic type, S—shaped, with Bowman’s capsule, rudimentary glomerulus, and union with the Wolflian duct; the second group of eleven or twelve, consisting of tubules, none of them uniting with the Wolffian duct and varying from solid aggregations of cells to hollow vesicles and finally to vesicles open to the coelom. The two sides present the same general arrangement although the tubules do not form symmetrical pairs in precisely the same stage of development (cf. Figs. 17-19).
McMurrich[21] says, page 391, “ One of the characteristics of the mammalian mesonephros is that it possesses no nephrostomes.” Minot[22] in his Embryology, page 237, says, “In all amniota the nephrostomes all become completely separated from both the myotomes and peritoneum throughout -the region of the Wolffian body, except that possibly in a few anterior segments, the connection with the peritoneum is retained as is suggested by Sedgwick’s observations (in birds).” In an embryo 4.25 mm. in length, Meyer[23] showed that in the cephalic half of the Wolflian ridge, certain of the tubules are directly continuous with the epithelium covering the ridge, but there are no hollow tubules opening into the coelom. In the caudal half the unsegmented blastema which he considers the fore-runner of the tubules, was not connected with the epithelium. From the description, this is probably a less advanced specimen than No. 1483“[24]
Janosik" found in an embryo, 3 mm. long when fresh, that in the cephalic part of the Wolflian ridge there are a Wolflian duct and a number of independent tubules; the concentrated blastema serially following the tubules, is not segmented, but connects with the coelomic epithelium at intervals.
MacCallum," the latest special investigator on the subject, shows in a. 3.5 mm. embryo (164. of the Mall collection) that extending from the 10th to the 19th or last formed myotome there are thirteen enlargements of the Wolflian duct, the 5th to the 8th being considerably elongated, but showing no glomoruli or Bowman’s capsules. In No. 80 of the same collection (4.5 to 5 mm. long), there are 17 to 18 tubules with the characteristic S-shaped curve, enlarged Bowman’s capsule, and union with the Wolffian duct. He showed in this case a‘ close" connection of the Wolffian duct at intervals with the coelomic epithelium. In none of these did the tubules open to the coelom.
It has been my privilege to look over the two specimens described by MacCallum and also a more recent specimen of the same collection (No. 209). These with No. 148 form a series which may throw light on the true development. The following apparently consecutive history is drawn from a study of these embryos ranging from the 17th to about the 23d day (see above, Age of Specimen), and from comparison with various embryos of the Cornell University collection as cat, shark, lamprey, etc.
In the mesonephric region of the least developed stage (No. 164), in respect to the nephric region, the Wolflian duct lying at the dorso-lateral part of the Wolffian ridge has enlargements, some of them, the 5th to the 7th, quite pronouncedly thorn-like. In the next stage (No. 209) in which the sections were thick and details difficult to determine there exists a series of about fourteen rounded tubules with thickened epithelial walls, and a lumen well-defined or in process of formation. In the ventral most prominent part of the Wolffian ridge some of these, as the 5th, are connected by a solid out-growth with the caalomic epithelium of the most protruding part of the ridge. At the other extremity of the tubule it joins the Wolflian duct.
In a cat from which a part of the mesonephros was modeled, the next stage apparently was found. Some of the centrally located sub-cylindrical tubules open from the coelom at the crest of the Wolffian ridge, and at the other end of the tubule into the Wolffian duct. The general appearance of these tubules and their connections is perfectly comparable to the condition found in the early shark, the tubules of which were also modeled and found to difier from those of the cat in the fact that they were more S-shaped.
No. 148 (Figs. 17-20) furnishes the next stage. It has apparently in its series of twenty or more tubules a complete recapitulation of the preceding stages co-existing with the commonly recognized type-form of tubule.
There are :— 1st, The Wolffian duct in the caudal part, having no connection with the tubules, but presenting a series of distinct enlargements; 2d, Aggregations of cells with no apparent lumen, and no connection with the coelomic epithelium or the duct; 3d, Cavities or vesicles deep in the Wolffian ridge surrounded by a several-layered epithelium, and having no connection with either coelomic epithelium or duct; 4th, Similar vesicles lying near to the coelomic epithelium of the crest of the Wolffian body, connected with that epithelium by (a) a solid string, (b) a narrow open channel, or (c) a wide open funnel (Fig. 20) ; 5th, In the cephalic half, one tubule, the 7th, with a slight opening from the Bowman’s capsule to the coelomic epithelium; 6th, The remainder (8th, 6th, 5th, 4th, 3d) of the cephalic group have the typical Bowman’s capsule, the S-shaped tube, and the connection with "the Wolffian duct but no connection with the coelom (Fig. 19) ; 7th, The 2d tubule having two Bowman’s capsules, one dorsad of the other, that is, an apparent beginning of the dorso-ventral division of the tubules; 8th, The 1st small tubule, appar ently consisting only of a Bowman’s capsule, sessile on the beginning of the Wolffian duct.
The artery and vein supplying the rudimentary glomerulus of these Bowman’s capsules (Fig. 6) could in a few places be clearly seen. The specimen not being favorable to the study of blood-vessels, the vascular supply could not always be made out.
Such a tubule as was found in the cat with both ends open (see above) did not occur in No. 148. The tubules which had attained connection with the duct had in this specimen apparently lost connection with the coelomic epithelium. In No. 80 (MacCal1um") the transitional forms of tubules described above are all lost. The 17 to 18 tubules are complete and like Fig. 19.
In brief, there is, in human embryos, of the 17th to the 23d day, the history of the origin of the tubules from a cellular blastema. This blastema segments into rounded masses, these masses develop cavities that first unite with the cualomic epithelium, then losing this connection, unite with the Wolflian duct which already had definite enlargements, and then finally each tubule forms the thin-walled Bowman’s capsule with its glomerulus.
The connection which probably existed between the myotomes and the coelom through the intermediate cell mass which gives rise to the blastema formingthe tubules, is of an earlier stage than these here considered. Dr. Minot in the discussion of this paper when presented at the meeting of the American Anatomists stated that in a very early rabbit in the Harvard University Medical School collection, such connection can actually be seen between myotome and nephrotome to the coelom. I have recently seen the same in the chick.
Metanephros
If any trace of the metanephros or true kidney exists in specimen No. 148, it consists merely of a slightly condensed portion of the blastema, caudad of the mesonephric region where the Wolflian duct extends beyond the coelom (Fig. 17).
Central Nervous System
General
Most of the figures illustrate some features of the central nervous system. The general outline follows closely the profile shown in Fig. 1. The lateral and mesal views are seen in Figs. 3, 4, and with segments of the model and photographs of the sections together give better than words, the idea of shape. The form more nearly approaches that of embryo Lr (4.2 mm. long, with 32 myotomes) shown in His’s° atlas (Plate IX, Fig. 13), and the model of the same than any other specimen figured.
The stage of development of eye, olfactory pit, and ear vesicle also put it in the same class, that is, the nervous system is like, in general features, this well-known specimen of three weeks. Details which bear upon the object of the present investigation are not shown by His nor, as far as I know, by other Writers on mammalian brains. Mall “ in an early presentation of this same embryo mentions the neuropore, which in the present article is fully illustrated and in a Way made the starting point for definite conclusions.
The evidence from human and mammalian as well as immammalian material has been accumulating in the form of specimens, models, drawings, and notes with preliminary papers,”° until it seems clear that the statements presented with regard to the central nervous system of this embryo are not artifacts due to shrinkage, nor abnormalities, but that the individual characteristics of this specimen may be depended upon to represent one phase of development. This phase is probably transitory because nothing exactly like the neuropore in this specimen has been found in any other specimen examined, though stages both older and younger are seen. On account of the clear demonstration of important facts in transitional stages it seems worth while to record them fully.
The cephalic end of the bram tube and its relation to a, serial order of parts —von Baer[25] in 1828 represented as perfectly obvious the original cephalic end of the body, including thetneural plate, at the point where the hypophysis arises. By more refined methods, Keibel[26] in 1889. showed with apparent conclusiveness, that in the rabbit the neural plate, the ehteron, and the notochord end in an undifferentiated mass of tissue which is the true cephalic end of the body, and corresponds with the point indicated by von Baer.
From another view point, the place of final closure of the neuropore has been considered by some a crucial test for determining the end of the brain tube. His[27] found it at the optic recess and found also that just before final closing, the neuropore is a slit, includes the recessus infundibuli, chiasma, recessus opticus and the olfactory lobes, and extends to the dorsal end of the lamina terminalis. That is, in fact, His really agrees with von Baer and Keibel as to the original condition of the cleft between the neural plates.
Kuplfer,[28] in sharks and some other fishes, found that the final closure of the neural tube occurs between the olfactory lobes a.t a point which he calls the lobus olfactorius impar. Herrick[29] in discussing these widely divergent views concluded that the final closure had no necessary relation to the morphologic front of the brain but that the recessus infundibuli is the primitive cephalic end. Herrick, therefore, also goes back to von Baer’s original location of this point.
Accepting then the von Baer-Keibel observations as correct, I believe that at all stages of development, the hypophysis and its corresponding fold of the brain-wall represent the morphologic cephalic end of the body and of the brain. It logically follows that the cephalic end is the point at which the dorsimesal and ventrimesal lines meet. The ventrimesal line is present from the beginning as the middle line between the neural plates. The dorsimesal line is that in which the original margins of the neural plates unite in closing the brain tube.
To redetermine the exact location of this point of union of the two lines, young specimens of both immammalia and mammals, including man as far as material was available, have been examined step by step from the open neural plates to the closed neural tube and until adult landmarks become unmistakable. From these observations reported in 1903[30] and 1904,[31] it becomes certain to me that the original margin or dorsimeson extends as far as the hypophysial fold of the brain (Figs. 3, 4). This is irrespective of the exact place where the final closure of the neural tube takes place. In all the higher forms examined this final closure is between the eye-stalks, its adult representative being the pre-optic recess. In torpedo, as Kupffer found in sharks, this point of final closure lies between the olfactory lobes. But even here, in earlier stages the Original margin of the neural plate extended as in mammals to the hypophysial region. The difficulties of determining the cephalic end of the tube in lamprey and other forms having an original solid neural plate are, that when the cavity does form, it has seemed somewhat uncertain as to the location of the front of the tube. From my observations it seems that even in the lamprey the front of the tube can be placed at the hypophysis. In amphibia the large size of cells and the consequent thickening produce an obscurity as to the exact formation, but here again, the weight of evidence seems to show that the original cleft between the two sides of the neural plate extends to the hypophysial region.
The human embryo here under special consideration is the most illuminating of any specimen examined for the purpose of determining the exact point at which final closure takes place, because that event is delayed until the surrounding parts are so well developed that identification is unmistakable. At the point recognized by Mall as the neuropore, lying between the eye-stalks, a connection of brain and skin tissues exists and extends through a number of sections (10011. or more). In parts the arrangement of cells indicates that the margins have only recently united (Fig. 16). Figs. 1-8 show the relations of the neuropore, and the extent of the epidermic thickening. The thickened epidermis, separated from the brain, extends cephalad from the point of contact toward the olfactory region and hence away from the hypophysis (Fig. 7). Following still farther away from the hypophysis the olfactory region is found (Figs. 3, 4, 5), separated by a sharp fold from the eye-stalk. From the above, the only logical conclusion seems to me that this specimen gives positive evidence that the olfactory region of the brain is not its morphologic cephalic part, but that the eyes are relatively to the original margin of the neural plate cephalad of the olfactory region, 13. e., nearer the hypophysis. This is in contradistinction to the arrangement that has always been accepted namely, that in the vertebrate brain the olfactory region is the most cephalic, forming the first of the series. Even His after his acceptance of the demonstration of Keibel and his own statements concerning the neuropore ignored the logical conclusion as to the order of the parts in the series.
Following von Baer, Beichert, and Giitte, Studniéka[32] has finally demonstrated that the olfactory lobes and the cerebrum are essentially dorsal and paired outgrowths from the neural tube. The present investigation confirms this and further places the eye-stalk and the retina in a similar category as dorsal paired organs serially in front, 13. e., toward the hypophysis from, the olfactory lobe. The natural corollary follows that the optic chiasma crosses the original margin or dorsimesal line; that it is in serial order with the pre-commissure, forni-commissure, callosum, supra-commissure, and post-commissure, each binding together paired dorsal organs, the chiasma being as truly dorsal as the post-commissure.
Nor is the conclusion above reached based merely upon logic. The study of a model made of a mouse in which the neural plates are cleft to the hypophysis show that in tracing the series of folds, which have relation to the margin, there are on each side: 1st, Hypophysial rudiments both in the skin and the brain, consisting of folds which reach the margin; 2d, A fold which ends in the outgrowing eye, and extends to the margin, while along the outer surface of this fold the skin is in direct contact with the brain; 3d, A flap or margin, still undifferentiated, lying between the 2d fold and the mesencephal. By comparison of this specimen with later stages of various specimens, it is seen that the flap (3d) becomes differentiated into olfactory, cerebral, and diencephalic rudiments.
A model made of the neural plates of the human embryo 12 of the Johns Hopkins University collection, lends strong confirmatory evidence to the above observations, while the comparative studies on lower forms lend their quota to the result.
These conclusions were stated by me in the above-mentioned abstracts.”” Johnston,[33] the latest reviewer of the serial order of segments of the head, does not agree with my view. His work is largely based on immammalian material which in my experience, as above stated, does not show the facts clearly being obscured by either thickened walls or secondary formation of a neural cavity. The crucial point on which he rests the conclusion that the olfactory is in front of the eye is dependent upon those observations which tend to show that; (1) The segmental mesoderm extends past the eye to the olfactory region; (2) The hypophysial thickening of the skin is continuous with the nasal epithelium in petromyzon. With regard to the first point attention is called to a recent paper by Froriep[34] in which he shows that the original mesoderm of the head does not primarily pass cephalad of the hypophysis upon the mesal line. As to the second point, Lubosch[35] shows that the thickened epithelium of hypophysis and olfactory plates is not continuous but is separated by an interval of thin epithelium. Moreover, it is shown incidentally in his figures, that it is in close connection with this thin mesal plate that the incipient cavity of the eyes originates, that is between hypophysis and olfactory.
In order to make his contention good, Johnston is driven to the conclusion that the eye is a dorsal organ lying between the olfactory region and the diencephal and in the course of development, is dragged ventrad to its final position, the cerebrum and the eye being portions of the same neuromere. The observation that the eye vesicle is originally at the edge of the neural plate between hypophysis and olfactory region seems to make this device unnecessary.
The question of the cephalic end of the brain is not, to what point the neural plate is cleft after the formation, and final growth of the mesoderm into the head, but to what point it was cleft at the outset. As above stated, in mammals and torpedo, the cleft originally extends to the hypophysis. The eye lies next to the hypophysis and distinctly intervenes between hypophysis and olfactory region.
More recent investigations on invertebrate brains,[36] seem to have established the fact that the lobe of the brain connected with the compound eyes is really cephalad of that connected with the antennae, now proved to function as organs of smell. This fact seems to fit into the finding given above on the serial order of parts.
Total Folds
In Figs. 3, 4, it is seen that the neural tube is divided more or less clearly into lobules and these again into folds. Those now under consideration are not the total folds of the cerebrum consi-dered by some authors as transitory fissures and by others as artifacts. Indeed, some of them antedate the distinct formation of a cerebrum, some being formed in early human specimens with open neural plates (No. 12 of Johns Hopkins University Collection, and No. 714 of the Harvard University Embryological Collection). In the present study, the cerebrum itself is the name applied to one of these total folds.
Nor are they newly recognized structures. Bischoff[37] in 1845 published a minute figure of the brain of an embryo dog showing such folds in the oblongata.
Total Folds in the Immammalta. — Orr,[38] in 1887, found in the lizard a. series of such total folds. McClure[39] followed with similar results. Lucy’s[40] remarkable dissections of shark and also of chick and of Amblystoma show the beginning of these folds as marginal structures before the mesoderm had reached the parts, and therefore indicating the segmentation of the epidermis antecedent to that of the mesoderm. Locy’s results have been questioned by Neal,[41] but in going over some of the same ground it seems to me that Locy’s observations are well founded.‘ "The careful confirmatory work done in Locy’s laboratory by Hill[42] seem to put the essential point beyond controversy. He shows 3 neuromeres in the forebrain, 2 in the mid-brain, and 6 in the hind-brain.
Orr, McClure, and Locy call the folds, neuromeres. This term is here avoided because it leads too far afield into a consideration of related questions of theory and fact concerning nerve distribution and mesodermic segments on which the literature is extensive and well-known.
Total Folds in Mammals
In mammals, since the time of Bischoff,” figures of such folds appear occasionally in literature. Mihalkovics[43] shows folds in the rabbit’s oblongata; Pregnant[44] in that of the pig.
Zimmermann[45] in rabbit and chick shows 2 in the fore-brain, 3 in the mid-brain, 8 in the hind-brain, and 4 in connection with the accessorius nerve. Froriep[46] found in the mole 3 in the diencephal, 3 in the midbrain, which disappear later, and 7 in the hind-brain, the last being connected with the vagus nerve. Schultz[47] figures folds in the pig. Lewis[48] and Minot[49] show four neuromeres in the oblongata of the pig. Bradley[50] is the latest investigator to study these folds in the pig, finding 7 in the hind-brain, the 1st belonging to the cerebellum and the 7th connected with the Xth nerve.
Total Folds in Homo
Kupffer[51] merely mentions in a human embryo of three weeks, five pairs of total folds in the oblongata. His[52] shows that in the region of the oblongata, certain folds in disappearing, leave behind cell-nests as in the olive. However, in his monumental work on human embryology,‘ he seems to have avoided giving any hint that such structures exist, while in his models of early specimens, a glittering smoothness occurs in regions which are really full of significant form. In models of human embryos made in Dr. Mall’s laboratory, certain facts shown in his specimens were avoided - since they did not bear on the subjects he was investigating. However, this seems to be a case where a little positive evidence more than counterbalances a vast amount of silence.
Granting the existence of total folds in the neural tube of mammals at certain stages of development, the question has been put, are they artifacts due to shrinkage of the mesoderm? In answer I would say :— 1st. The crowded cellular growth of the neural tissue and the scattered cells of the mesoderm would seem to indicate that though the latter might shrink away from the neural tube, it would not throw it into such sharp foldings as occur. Direct observation seems to corroborate this argument. 2d. The folds are found at the margins of the neural plate in man before the mesoderm has grown up to this margin. 3d. In lower mammals corresponding in general to this human specimen, the mesoderm as far as it has grown around the sides of the neural tube shows little indication of shrinkage and forms complete contact with the foldings of the tube, indicating that the folds give form to the mesoderm rather than the reverse. 4th. Many of the young mammalian specimens examined are cut so accurately, after such perfect preservation, that no question of asymmetry can be raised as might be the case with the human material examined. It may be said that in all the models made by me, no matter how twisted or imperfect the specimen, the evidence of essential symmetry is clear. 5th. As is natural to suppose, the folds arise step by step with the growth and development of the tube. In human specimens they are in their most typical condition during the third week, after which their external creases are bridged by the growth of white matter and later their internal sharp lines are gradually obliterated. To realize the existence and probable significance one should study them when most typical and when they all have approximately the same size as in this specimen (148).
Serial Arrangement of Folds
It must not be understood that the table given below represents a final conclusion as to the number of folds in the brain tube. It is an attempt to bring into as nearly definite relations as possible at this time, the early structures shown, with those of the adult. The lobules and folds cannot be said to accurately represent the definitive segments which Wilder[53] proposes for convenience in studying later stages, nor do they more nearly coincide with the divisions settled upon by the German committee on Anatomical terms. In fact could we start without so many preconceptions from the complicated adult structure and the names which have been applied to the parts our task would be simpler. As it is, in the figures, as few names as possible have been used and even these do not always agree with the customary usages; for instance, the term Diencephal is here used for a part of the roof and lateral wall, but does not, as usually understood, include the infundibulum and the eye-stalk. Perhaps the old and indefinite term thalamus would better fit the case.
The common characteristic of all these folds (except the albicantial, see below), is that each pair takes its origin ‘in a common pocket at the dorsimeson (see above Cephalic End of Brain Tube), or at the edge of the membranous roof or metatela and thence radiates a greater or less distance along the lateral wall of the brain. Each fold, no matter how obliquely, tends in general direction across the axis of the brain tube.
Table II
| Table 2 |
|---|
| To be formatted
E 5 4. 15’; 5 . - ' -1 _ 5 -E 1% :2 -7 E .7 -‘-3-3 § '33 .9 E: .33 .9 § :3 o 3 E 3 £4: g = 3:: ; 3% :3: . Alblcantial a alb. albi 3 4 6 8 9 16 I I f d b 1 . ’ ' ' ’ ’ ' ' ’ D “u 1 u at Hypophysml 1 infund. 3, 4, 6, 8 Hyp. . E - 1: 1k 2 II,V1sua1 {E;:_:e';ic,e 3} eye 3, 4, 5, 9, 16 .... Striatal 4 str., stri. III, Cerebral Olfactory 5 olf. Q 3, 4, 5, 7 Cerebral 6 cer. IV Th 1 ' Di h 1' 1 7 .
. Oblongata 1 13 obl. 1 VII Gasserian 0; Pom,“ % 3 3% 3, 4, 10, 11 Vth N.
|
- E 5 4. 15’; 5 . - ' -1 _ 5 -E 1% :2 -7 E .7 -‘-3-3 § '33 .9 E: .33 .9 § :3 o 3 E 3 £4: g = 3:: ; 3% :3: . Alblcantial a alb. albi 3 4 6 8 9 16 I I f d b 1 . ’ ' ' ’ ’ ' ' ’ D “u 1 u at Hypophysml 1 infund. 3, 4, 6, 8 Hyp. . E - 1: 1k 2 II,V1sua1 {E;:_:e';ic,e 3} eye 3, 4, 5, 9, 16 .... Striatal 4 str., stri. III, Cerebral Olfactory 5 olf. Q 3, 4, 5, 7 Cerebral 6 cer. IV Th 1 ' Di h 1' 1 7 .
- m,.
- .;".:::i:' °"°? 2 8} 4,5. V, Mesencephalic 4: Mesencfphalic ; lg r::el1l§,g_ 10_ g 4, 5-10 . . . . VI, Cerebellar -{ C°'°f’,°“’‘' ; C31’ 4, 11 . . . .
. Oblongata 1 13 obl. 1 VII Gasserian 0; Pom,“ % 3 3% 3, 4, 10, 11 Vth N.
VIII, Ofic Ob1o,ngata : obl. , } 3’ 4’ 10 E73, VIII Oblongata 618 obl. 6 3 4 11 IX N. xx‘ “'3'” i u 7 19 u 7 3: 4: 13.15 X N. . Obl t 8 20 bl. S , X, Accessorial { "fig" '1 9 21 0,, 9 3» 3, 4, 13, 14
Notes on the Total Folds
Albicantial
In early stages of the chick and amblystoma, the first folds to form in the cephalic region before the neural plates close, is this pair lying at either side of a middle piece which is molded over the cephalic tip of the blind cephalic end of the enteron. This connection is soon lost, a great mass of mesoderm filling the cephalic bend, and intervening between the albicans and the pharynx (Fig. 3, 4). The prominence of these folds in this specimen (Figs. 9, 16) is especially marked. In mammals, they seem to decrease in relative prominence as the gill region begins to transform.
In many studies which have been made of the brain, a fold from the infundibular region is shown to extend entirely across the brain tube to the roof of the diencephal. In this specimen, at first glance, it seemed that the only one from this region which could possibly extend to the roof of the diencephal, is the albicantial. The early history of the fold as seen above, does not make the interpretation seem probable and, moreover, a careful modeling of the region as shown in Figs. 6, 8, 9, seems to indicate that the albicantial and the diencephalic folds, originating at Widely separated parts of the brain in the middle line, end near each other in the lateral wall but are distinctly not continuous.
This albicantial fold is only tentatively put at the beginning of the series, since from its original close approximation to entodermal rather than ectodermal tissue, it does not seem to belong to a truly dorsal series nor to be the ventral end of a diencephalic fold.
1. Hypophysial
These folds are strongly developed in later human embryos. They are not sharply outlined in this specimen but the pair can be distinguished lying opposite to the pair of widely open pouches representing the hypophysis at this period (Fig. 9). There seems to be no fact thus far. found which might bar these folds from the -series. In a young mouse (see above), the folds as modeled show distinct relation to the margin of the neural plate, while the associated organ, the hypophysis, is a really paired organ[54] from the ectoderm as distinguished from the early entodermal relationship of the albicans. In this connection, the position of the hypophysial as the first in the series, it is significant that Boeke[55] finds in Amphioxus and certain fishes a ciliated pit in the region of the infnndibular process, having according to him, the physical appearance of an organ of sense. Should there be confirmation of this it would represent a lost sense organ, the first of a dorsal series.
2. Optic or Eye-stalk
In its earlier stages, this region is represented by a pair of wide furrows extending from the margin of the neural plate to the pouches forming the optic vesicles. With the closure of the plates, each furrow forms a wide vesicle connected in the present specimen, with the epidermis through the neuropore (Figs. 4, 6, 8, 16). As development proceeds, the portion of this vesicle toward the hypophysis, is constricted by a complicated folding to form the so-called optic nerves, the portion toward the olfactory remaining single as the pre—optic recess.
3. Eye-vvesicle or eye proper
As shown in Figs. 6, 8, and 9, the eye is distinctly _constricted off from its stalk. In Figs. 6-8, it shows secondary folding, but a typical cupping does not occur.
The eye-vesicle seems to have relationship with the original margin, only through the stalk, the two together forming a lobule. Even at the point where the eyes approach the skin, they are separated from it "by a thin layer of mesoderm (Fig. 16). The lens thickening has only a slight development, showing no tendency to bend towards the eye. Its borders are ill-defined.
4. Striatal
A deep fold separates the visual region from the cerebral. On the side of the fold toward the cerebmm is a smaller total fold crossing the middle line (Fig. 4). From a careful comparative study this is identified with the fold later bordering the triatum.
5, 6. Olfactory and Cerebral
In Figs. 4, 7, are shown the slight total folds, the forerunners of the olfactory and cerebral regions proper. Each pair of folds, begins in a mesal pocket but does not pass far across the brain tube. Mall[56] considers these among the artificial fissures of the cerebrum but in fact only one of them is cerebral and it represents the whole of that organ.
Relatively to the dorsimesal line, the three folds included in the cerebral lobule are seen to be caudad of the eye or as expressed by Studniéka,[57] they are dorsal.
While the neural plate is still open it was found both in man and mouse that the region of the cerebrum and also of the diencephal (see above, Cephalic End of Brain Tube) is comprised in a narrow undifferentiated flap beyond the eye and including a portion of the margin. The flap becomes relatively wide before closure and shows some total folds which need more careful identification.
The olfactory epithelium shown in Fig. 3 has an irregular H-shape with a bar across the meson. This -seems to agree with the idea that the olfactory epithelium shifts from the margin to its final, lateral position. Bedford“ has in the pig, found a certain amount of lateral shifting of the olfactory plate. van Wijhe[58] found that in shark, both olfactory organs and nerve arise out of the neuropore, thus lending confirmation to the fact shown in this specimen.
7, 8. Diencephalic
Are the two folds seen in the roof of the diencephal, each meeting its fellow of the opposite side, in the dorsimeson. 7, is in the region which ultimately forms the membranous roof and 8, is apparently to form the epiphysial outgrowth from its mesal pocket (cf. Minot[59]).
9, 10. Mesencephalic
The roof of the mesencephal or mid-brain is in this specimen so broken that details of form were impossible to work out, but in general, it is possible to see that there are two pairs of total folds, one, the 9th, beginning in a mesal pocket lying caudad ofthe deep notch dividing the diencephal from the mesencephal, or in other words, caudad of the future post-commissure and extending obliquely caud-ad for half the length of the mesencephal, the other, the 10th, arising near the cephalic border of the metatela and ending abruptly near the point where the IVth nerve will take its origin (4 of Fig. 10).
12. Cerebellar
These are two total folds represented clearly only in Fig. 11, rising at the cephalic border of the metatela and involving the part of the lateral wall which at this stage of development represents the cerebellum. Their history has not been traced.
14-18. Oblongata
Are folds which arise at the edge of the membranous roof of metatela, and extend across the brain wall near to the ventrimeson. If one remembers that the neural plate on closing in this region as well as in the fore-brain, at first, is a tube with as thick walls dorsally as elsewhere, it is easily seen how the origin of these folds at the edge of the membrane, may represent the dorsimesal pockets occurring farther cephalad, especially since Locy’s " work shows marginal folds in the early stages.
13. Oblongata 1
In this and several other specimens studied, this fold seems fully separated from the following, obl. 2, but the sections are so cut as to make it difficult to trace it with certainty to the dorsal edge. In other human specimens studied I was not certain of its presence. In this region some authors find a ventral representative of the cerebellum, but in this specimen, at least, there is no such relation.
14. Oblongata 2
This fold is one of the most strongly defined of the series. Its invariable connection with the roots of the Vth nerve and the Grasserian ganglion makes it avland-mark in the embryos of all vertebrates studied. The Gasserian ganglion is large but loosely formed and penetrated by branches of the jugular vein.
15. Oblongata 3
This is also a sharply defined fold and has been widely recognized though as yet no structure has been definitely associated with it.
16. Oblongata. 4
The roots of the VIIth and VIIIth nerve are as invariably associated with this fold as the Vth with its fold. In the present specimen, in its dorsal portion, it is divided into two folds, the more cephalic being connected with the roots of the VIIth, the more caudal with those of the VIIIth nerve. The roots are very short, soon uniting with their corresponding ganglia. The ganglia lie close together yet are for the most part distinguishable, the auditory following the thickening of the auditory vesicle on the cephalic and lateral portions, that of the VIIth extending without special differentiation into its nerve which forms a union with thickened epithelium at the dorsal end of the 1st gill-cleft (Figs. 1, 11).
17. Oblongata 5
In the chick and all mammals examined, this fold lies opposite the otic vesicle but has no connection with it, unless possibly at a very early stage while the ear is merely a thickened plate pushed close to the neural plate. Dr. Johnston called my attention to the fact that such a fold is wanting in Amblystoma and an examination of material at hand, confirms his observation that no fold exists between that to which the VIIIth and IXth nerves are attached, in early stages of Amblystoma. However, in the shark Sewertzoff[60] shows such a fold to exist and it seems probable that modelling of the region in Amblystoma might reveal its rudiment.
18. Oblongata 6
In all forms examined, this permanent fold has been found connected with the roots of the IXth nerve.
19. Oblongata. 7
This is a large fold arising at the caudal end of the metatela, extending obliquely cephalad and ending in the floor of the oblongata in close relation to the previous fold. It is connected with the roots of the Xth nerve. Apparently in other specimens, this fold cannot be so clearly defined as the others in the oblongata since it has rarely been recognized. Froriep "’ in one human specimen, and Bradley in the pig, have observed this fold.
20, 21. Oblongata. 8, 9
These two pairs of folds are really dorsal pockets extending only through the dorsal half of the neural tube. Opposite their ventral portion, roots of the XIth nerve arise (Fig. 14). This nerve is interrupted in its course to join the Xth nerve by masses of ganglionic cells.
The relations of the VIIth, IXth, Xth, and Xlth nerve of this specimen to their ganglia and the sensory epithelium have so recently been fully discussed by Streeter[61]that they will not be treated. The sensory epithelial thickenings to which he calls attention, are here figured (Fig. 1).
Beyond the clearly formed folds, above discussed, there occur several others each corresponding with an enlarged part of the ganglionic cord. As this cord has no further indication of dorsal nerve roots, the exact relations cannot be determined. Moreover, the following total folds in the myel are not strongly marked, and in other specimens it is only in favorable sections that they can be seen at all.
The Special Points Appearing from a Study of this Embryo
- Both external form and internal organs show with diagrammatic clearness a normal development but with individual diiferences from other specimens of about the same age, some of these differences indicating greater, some less development. It seems probable that a careful study of such embryonic peculiarities in man and higher mammals may throw light on very important questions of heredity and variation.
- Epithelial thickenings occur at the neuropore, olfactory region, lens, gill-clefts, and about the mouth, at the summit of the limbs, the thickening of the leg being continuous with that of the anal region.
- There are 29 myotomes, 2 being occipital, and also remnants of 3 other occipital myotomes.
- The nephric system is in a generalized condition presenting a re- * capitulation in one specimen of several distinct stages of development. This is shown by :——An open pronephric tubule on each side, independent of the Wolflian duct; each mesonephros having in its cephalic half, 8 rudimentary glomeruli opening by tubules into the duct; in its caudal half, 11 or 12 tubules not opening into the duct, but part of them opening to the coelom. The mesonephric tubules vary in structure from solid masses of cells to tubules with glomerulus and Bowman’s capsule.
- The developmental stage of the central nervous system shows with definiteness the position of the neuropore and its relation to the hypophysial region. In comparison with other specimens examined this makes it possible to determine the front end of the brain tube and of the body.
- I believe that the morphologic cephalic end of the body is as figured by von Baer, in the region of the hypophysis and, furthermore, I believe as a generalization, that in all stages of development the hypophysial region is at the morphologic, cephalic end of the body, and consequently that parts which in the exigencies of growth have gone beyond this point are morphologicly caudad of it, as the eye and olfactory region.
- The brain tube shows both at this stage and at earlier and later stages total foldings which are directly correlated with definite nerves or epithelial thickenings. Other foldings have not yet been correlated with definite organs. These foldings are so uniformly present in mammals, birds, and selachians that they cannot be conceived of as artifacts but are believed to be true morphologic features.
References
- ↑ Mall FP. Hosp. Bull., XIV, 1903. Catalogue of the collection of human embryos in the Anatomical Laboratory of the Johns Hopkins University. Johns Hopkins Hopkins University. Baltimore, 1904.
- ↑ Born, G., Morph. Ja.hrb., II, 1876; Arch. f. mikr. Anat., XXII, 1883..
- ↑ Bardeen, C. R., Johns Hopkins Hosp. Bull., XII, 1901.
- ↑ Mall, F. P., Welch Anniversary Volume, 1900; also Johns Hopkins Hosp. Reports, IX, 1900.
- ↑ Mall, F. P., Johns Hopkins Hosp. Bull., XII, 1901.
- ↑ His, W., Anatomie Menschlicher Embryonen. Text and Atlas. Leipzig, 1880-1885.
- ↑ Mall, F. P., Age of Human Embryos. Ref. Handb. Med. Sci., 2d Ed., III, 1901.
- ↑ Kallius, E., Anat. Gesell., Verhand, 1903.
- ↑ Killian, G., Morph. Jahrbuch, XLV, 1888.
- ↑ Mall, F. P., Johns Hopkins Hosp. Bu1l., XII, 1901.
- ↑ Bardeen CR. and Lewis WH. The development of the limbs, body-wall and back. (1901) Amer. J Anat. 1: 1-36.
- ↑ Bremer, J L., Amer. Jour. Anat., IV, No. 2, p. VIII.
- ↑ Lehmann, Harriet, Anat. Anz., XXVI, 1905.
- ↑ Mall, F. P., Amer. Jour. Anat., IV, 1904.
- ↑ Minot, C., Boston Soc. Nat. Hist, Proc. XXIX, 1900.
- ↑ Aichel, Otto, Arch. 1. mikr. Ana.t., LVI, 1900.
- ↑ MacCallum JB. Notes on the Wolffian body of higher mammals. (1902) Amer. J Anat. 1(2): -259.
- ↑ Tandler, J., Centralbl. f. Physiol., XVIII, 1904.
- ↑ Janosik, J., Arch. f. mikr. Anat., XXX, 1887.
- ↑ Gage, Susanna P., Amer. Jour. Anat., III, 1904, p. VI.
- ↑ McMurrich JP. The Development Of The Human Body. (1914) P. Blakiston's Son & Co., Philadelphia, Pennsylvania.
- ↑ Minot CS. Human Embryology. (1897) London: The Macmillan Company.
- ↑ Meyer, H., Arch. f. mikr. Anat., XXXVI, 1890.
- ↑ Meyer's specimen was 2 mm. long after being hardened, thus making the discrepancy in length less apparent than would be indicated from the measurement 4.25 mm., taken while fresh.
- ↑ von Baer, K. E_., Ueber Entwicklungsgeschichte der Thiere. Konlgsberg, 1828.
- ↑ Keibel, F., Arch. f. Anat. u. Phys. Abth., 1889.
- ↑ His, W., Arch. 1. Ant. u. Phys., Anat. Abth., 1892.
- ↑ Kupffer, C., Studien zur verglelchenden Entwicklungsgeschichte des Kop fes der Kranioten. Heft 1. Miinchen u. Leipzig, 1893.
- ↑ Herrick, C. L., Jour. Comp. Neur., III, 1893, p. XVI.
- ↑ Gage, Susanna. Phelps, Science, N. S., XVII, 1903.
- ↑ Gage, Susanna Phelps, Amer. Jour. Anat., IV, 1904, No. 2, 1). VIII.
- ↑ Studnléka, F. K., Kgl. biihm. Ges. Wiss., Math.-natur., XIV, 1901; Zool. Centra1bl., VIII, 1901.
- ↑ Johnston, J. B., Jour. Comp. Neurol. & Psychology, XV, 1905.
- ↑ Froriep, A., Anat. Gesell., Verhandl. 16, 1902.
- ↑ Lubosch, W., Morph. Jahrb., XXIX, 1902.
- ↑ Comstock, J. H. and Chujiro Kochi, Am. Nat., XXXVI, 1902. They summarize the work, including Patten’s, from 1775-1900.
- ↑ Von Bischoff, T. L. W., Entwlcklungsgeschichte des Hunde-eles. Brannschweig, 1845.
- ↑ Orr, Henry, Jour. Morph., I, 1887.
- ↑ McClure, Chas. F., Jour. Morph., IV, 1890.
- ↑ Locy, W. A., Jour. Morph., XI, 1895.
- ↑ Neal, H. V., Bull. Mu. Comp. Zool., Harvard Co1l., XXXI, 1898.
- ↑ Hill, C., Zool. Jahr-b., Abth. f. Anat. u. Ontog. d. Thiere, XIII, 1900.
- ↑ Mihalkovics, V. Von, Entwicklungsgeschichte des Gehirns. Leipzig, 1877.
- ↑ Prenant, A., Soc. de la. science de Nancy, Bu11., 'Ser. 2, IX, 1889
- ↑ Zlmmermann, W., Anat. GeseIl., Verhandl. 5, 1891.
- ↑ Frorterp, A., Anat. Gese1}., Verhandl. 6, 1892.
- ↑ Schultz, Oscar, Grundriss der Entwieklungsgeschlchte des Menschen u. der Siiugethiere. Leipzig, 1897.
- ↑ Lewis FT. The gross anatomy of a 12 mm pig. (1903) Amer. J Anat. 2: 221-225., F., Amer. Jour. Anat., II, 1903.
- ↑ Minot CS. A Laboratory Text-Book Of Embryology. (1903) Philadelphia:P. Blakiston's Son & Co.
- ↑ Bradley, 0. 0., Rev. Neurol. and Psychiatry, II, 1904.
- ↑ Kupffer, C., Konig. baierische Akad. der Wiss., Math.-Phys. 01., SM.XV, 1885.
- ↑ His, W., Kfinig. siichs. Ges. d. Wlss., Abhand. d. Math.-Phys. Klasse, XVII, 1890.
- ↑ Wilder, B. G., Reference Handbook of the Medical Sciences, 2d Ed., Vol. II, 1901. See also Bibliography in Wilder Quarter Century Book.
- ↑ Gaupp, E., Arch. 1. mlkr. Anat., XLII, 1893.
- ↑ “Boeke, J., Anat. Anz., XXI, 1902.
- ↑ Mall, F. P., Amer. Jour. Anat., II, 1903.
- ↑ Bedford, E. A., Jour. Comp. Neur. & Psych., XIV, 1904.
- ↑ van Wljhe, J. W., Zool. Anz., IX, 1886.
- ↑ Minot, C. S., Science, N. S., XIV, 1901.
- ↑ Sewertzoff, A. N., Anat. Anz., XXI, 1902.
- ↑ Streeter GL. Peripheral development of the cranial and spinal nerves in the occipital region of the human embryo. (1904) Amer. J Anat. 4: 84-116.
Explanation of Plates
Names and Abbreviations used on the Figures
- alb. or albi — —Albicans, albicantial fold.
- allant. st — Allantolc stalk.
- amn — Amnion.
- analpl — Anal plate.
- ao.ar.,1st-!,th — Aortic arches, 1st to 4th.
- A. umb.-Arteria umbilicalis.
- aur — Auricle, left, right.
- auric-vent. c — Auricu1o-ventricular canal.
- A. vit — —Arteria vltelllna.
- b. art. or b. arter — Bulbus arteriosus.
- B. c — Bowman’s capsule.
- bile d — Bile duct.
- cbl — Cerebellum. cbl..
- 1, 2 — Cerebellar fold, 1, 2.
- cer. — Cerebrum.
- lst-8th cer —1st to 8th cervical myotomes.
- ch. — Chorda.
- d. Cuvier — Duct of Cuvier.
- Dien — Diencephal.
- duod — Duodenum.
- g. c. 1st-4th — Ectodermal gill clefts.
- glom.- Glomerulus.
- gn — Ganglion.
- gn. Fror — Frorlep’s ganglion. (first spinal ganglion)
- gn. Gsn — Gasserlan ganglion.
- g.p.1st.-4th — Entodermal gill pouches.
- Hypoph:-Hypophysls.
- inf. or infund — Intundlbulum or infundibular fold.
- intest — lntestine.
- Lach.-—Lachrymal furrow.
- lens ep — -Lens epithelium.
- l. perit. cav — Lesser peritoneal cavity.
- mand — Mandible.
- max — Maxilla.
- Mesen — Mesencephal.
- mesent. —Mesentery.
- mesoneph — Mesonephros.
- metat — Meta.te1a.
- my. 1-32 — Myotomes.
- N. III-XII — Nerves III-XII.
- mzs. ep — Nasal epithelium.
- neur. or neurop — Neuropore. neph. or
- neph. t — Nephric tubule.
- obl.1-9 — -Oblongata folds, 1 to 9.
- ws — Esophagus.
- olf — -Olfactory epithelium or olfactory fold.
- pron. t — -Pronephric tubule.
- s. venosus.- Sinus venosus.
- sec — Section.
- sept. trans — Septum transversum.
- stom — Stomach. str.,
- stri — Striatum.
- supra-ren — Supra-renal capsule, adrenal.
- t. 1-21 — Mesonephric tubules, 1-21.
- V. jug.-—-Venn. jugularis.
- V. postcard. or V. pc — Vena cardinalis.
- V. umb — Vena umbilical.
- V. vit — Vena vitellina.
- Vent — Ventricle.
- vit. ves.-—Vitelline vesicle.
- W. (1., Wolff. d., Wolman d — Wolffian duct.
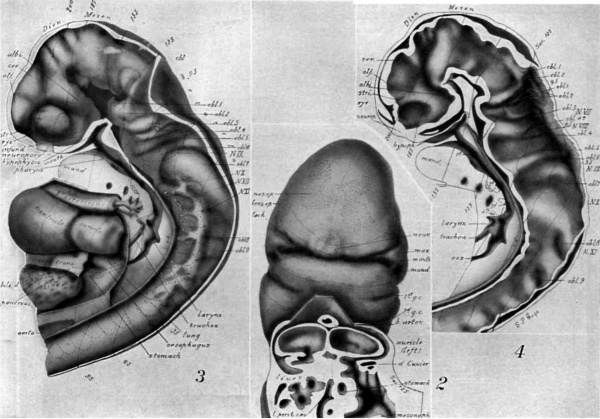
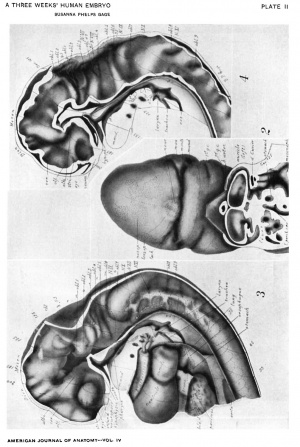
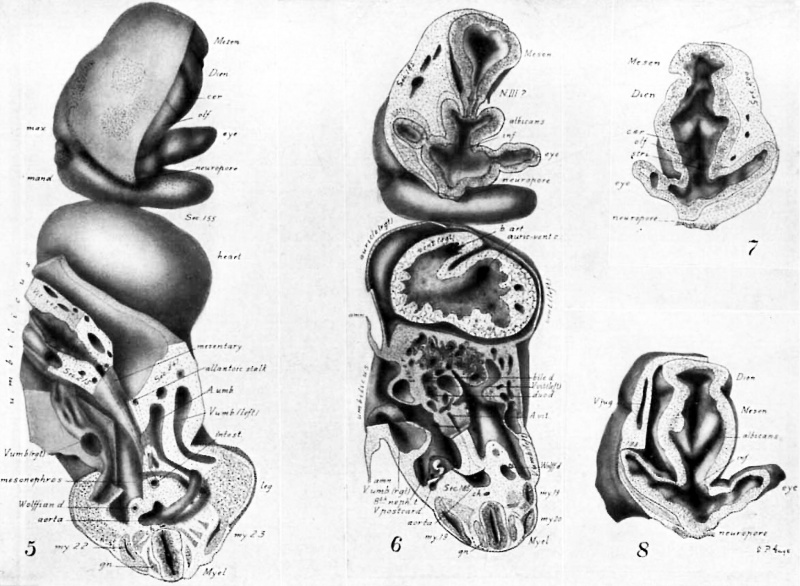
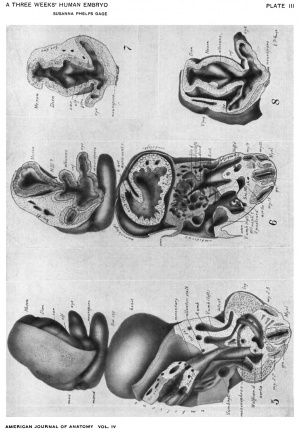
figures 1-13
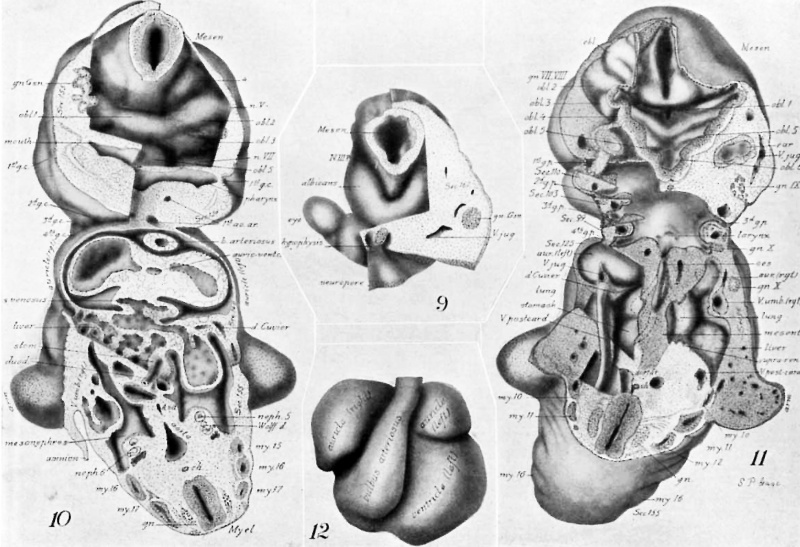
Drawings made from a model of Embryo 148 of the Mall collection, with sections and dissections of the same (see above, Models and Drawings). Magnification of the figures, X 33%.
Plate I
|
|
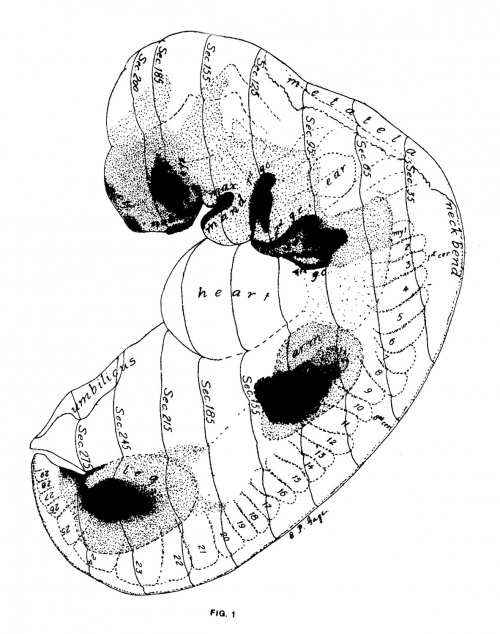
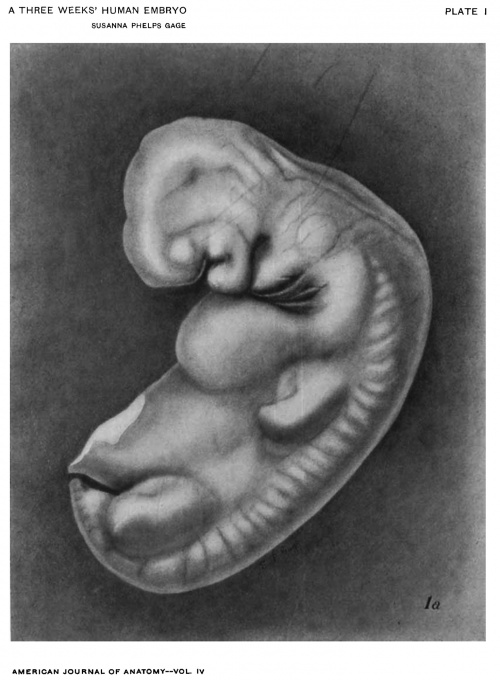
Figs. 1 AND 1A. View of the left side of the model, Compare with figures of this embryo in articles by Mall 1, 5.
It shows: The head comparatively small in diameter but great in length, and forming at the neck-bend an angle of 65° with the body; the position of the neuropore; the eye and ear scarcely apparent as external features; the "prominent heart, limb buds, and tail; the umbilicus turning to the right (cf. Fig. 5); the wide undeveloped mouth and small maxillary process; the crowding of the 2d, 3d, and 4th clefts into the precervical sinus; and 29 myotomes, the 3d being noted as the 1st cervical.
The density of the stippling on Fig. 1 indicates the relative thickness of the epithelium (see above, External Form).
The topographic lines show the direction of the sections, the numbers upon them indicate the corresponding sections of the eries. The following figures have either topographic lines or the section number at which they are cut, and hence can be located with reference to Fig. 1.
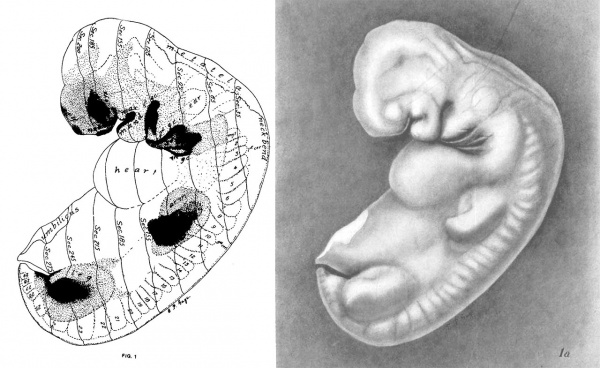
Plate II
Fig. 2. A face view of the head. As shown by the topographic lines, it is tilted to give a clear view of the parts about the mouth which is merely a wide slit between the hypophysial region of the head (cf. Figs. 3, 4) and the mandibular process.
There are seen: The small maxillary process with the depression at the corner of the mouth lying between maxilla and mandible; the H-shape of the nasal epithelium extending also over the cerebral region; the large neuroporic thickening; the lens epithelium with a tract extending along the lachrymal furrow.
The cut surface (see. 125) shows: The division in the dorsal part of the auricles (cf. Fig. 3); the entrance of the sinus venosus into the right part (left of Fig.); the liver lying in the septum transversum; the folds about the duct of Cuvier, pushing across the space to help form the diaphragm; the connection of pericardial and abdominal ccelom; the opening of the lesser peritoneal cavity into the abdominal ccelom.
Fig. 3. A view from the left side showing the central nervous system, pharynx, heart, lung, and liver. The lateral wall has been removed. Projections of the ear vesicle and myotomes 1-15 are indicated by dotted outlines.
The approximately uniform tube formed by the central nervous system and the strong cephalic flexure characteristic of this stage of development are evident. The mesoderm in the flexure has been removed.
The brain shows: The series of total folds; the great prominence of the albicantial; the relation of the neuropore to the epidermis and to the visual lobe or eye; the small size of the striatal, olfactory, and cerebral folds; the relation of the fold, oblongata 2, to the Vth nerve root (shown by a dotted circle); of the fold, obl. 4, to the VIIth and VIIIth nerves (dotted circles); of fold, obl. 6 to N. IX; the great size of fold, obl. 7 and its relation to N. X; the continuity and segmented character of the ganglionic chain in the neck region with the roots of the XIth nerve extending along its dorsal side; and the folds in the myel.
There are seen: The inner tube of the bulbus arteriosus as it enters the floor of the pharynx and divides into the aortic arches; the median thyroid cephalad of this branching; lateral folds just cephalad of the thyroid, the only rudiments of the tongue present; the bursa pharyngea, the dorsal pocket at the division of trachea and esophagus (at left of abbreviation ch.); dotted lines indicating the outline of the epithelial tubes forming lung and alimentary canal; the wide communication of the pericardial and abdominal ctelom dorsad of the septum transversum; the point of union of the aortae (aorta); the hypophysis cut to the left of the middle line.
Fig. 4. A mesal view of the brain, myel, and pharynx. The brain shows from the interior the same total folds as Fig. 3, but brings out somewhat more clearly the grouping of folds into lobules (cf. Table II, in the text). The elevations in Fig. 3 correspond to the depressions in Fig. 4.
Especially noticeable in this figure are: The short striatal folds; the apparent continuity of the albicantial fold with the roof fold (cf. Figs. 8, 9); the cerebellar folds 1 and 2 (cf. Fig. 11, cbl.); the cleft in the oblongata fold 4, on either side of which arise the roots of the Vllth and VIIIth nerves (shown by dotted circles); the cut ends of the ring of mesoderm surrounding the neuropore; the intrusion of mesoderm into the cephalic flexure; the fact that no mesoderm intervenes between hypophysis and infundibulum on the middle line; the union of all layers in the roof of the oblongata; the dark areas in the pharynx, indicating with their dotted extension the membranous parts of the 1st to the 4th gill pouches; the notochord touching the caudal wall of the hypophysis and coming in close contact with the roof of the pharynx between the level of the 1st and 3d gill pouches.
Plate III
Fig. 5. A ventral view of a segment of the model (Fig. 1) extending from section 247 to 155.
The left side of the head is dissected away to show: The relation of the visual lobe, the neuropore, the olfactory and cerebral regions, and the roof of the diencephal.
The right side of the head shows: The thickened epithelium of the neuropore; the olfactory region and its extension over the cerebrum; the future lens; the mandible and gill-cleft-like pocket at the corner of the mouth.
The caudal portion of the figure (Sec. 247) shows the 23d myotome apparently continuous with the mesoderm of the leg-bud; the division of the aorta into the umbilical branches, the left one looping over the caudal end of the coelom; the left umbilical vein with a branch from the leg; the right plexiform umbilical vein passing along the body-wall toward the heart.
The middle part shows: The wide umbilicus turning to the right and containing the thick-walled vitelline sac with its veins and arteries and its union with the caudal intestine inclosed in mesentery; the reappearance of the intestine in section near the union of the Wolflian duct and allantoic stalk (cf. Fig. 17).
Fig. 6. A ventral view of a deeper segment of the model. It extends from section 185 to 155. In the head region (of. Figs. 2-5) it is cut through; the neuropore; the eye vesicles, partially constricted off from the stalk; the albicantial folds (of. Fig. 9); and the cephalic end of the thick-walled mesencephal as it dips into the albicantial region. At N. III is a strand of mesodermic tissue but apparently no true nerve fibers.
The caudal part of the figure (Sec. 185) shows: On one side, the appearance of a. myotome cut through the middle; on the other side the overlapping ends of two myotomes; the 8th mesonephric tubule opening into the Wolflian duct and with its cap of thickened peritoneal epithelium (cf. Fig. 17) and its artery and vein.
In the middle portion are: The umbilical veins at either side, the left showing the greatly divided sinuses; the vitelline veins as they approach on either side of the alimentary canal; the vitelline artery; the liver near its caudal part embedded in the transverse septum and near the level where the bile duct unites with the duodenum; the umbilicus turning to the right of the specimen.
The heart (Sec. 168) cut near its middle shows: The undivided chamber of the ventricle; a strong fold arising between the two sides and separating the exit of the bulbus arteriosus (cf. Fig. 12) from the entrance of the auriculo-ventricular canal (cf. Fig. 10).
Fig. 7. From a segment of the model cut at section 200, near the edge of the neuropore, looking into the roof of the curved brain tube and showing that the striatal, olfactory, and cerebral folds, and those of the roof of the diencephal and of the mesencephal, meet in the mid-dorsal line, there being no division by a middle partition.
Fig. 8. A segment of the model extending from Sec. 198 to 155. It cuts the neuropore (cf. Fig. 16), showing a pit on its neural aspect, and looks into the visual lobe and eye vesicles in the opposite direction from Fig. 7. It shows the notch at the tip of the optic vesicle, apparently the beginning of the optic cup.
The deep projection of the floor of the mesencephal into the albicantial region is here shown and the independence of the albicantial folds from those dorsad of it (cf. Fig. 9).
Plate IV
Fig. 9. A segment of the model from section 162 to section 200 (cf. Figs. 1-4), showing: A caudal view of the eye vesicle and visual lobe; the V-shaped union of the albicantial folds and their independent dorsal ending; the mesencephal with its sharpened beak-like ventral ending between the albicantial folds; the strand of tissue, at the point where the III N. would later appear; the hypophysis forming a bi-lobed, ectodermic organ surrounding the end of the hypophysial fold ; part of the neuroporic thickening; and the Gasserian ganglion.
Fig. 10. A segment of the model extending from Sec. 155, through the cephalic flexure, to Sec. 96. With dissections at Secs. 130 and 140. It shows: The base of the mesencephal and oblongata, with the large protuberance (4) at the end of the second total fold of the mesencephal; the oblongata folds 1-5, and the relations to the Vth and VIIth Ns.; mouth; pharynx; the ending of the gill-clefts 2-4 in the precervical sinus; the entrance of the vitelline veins into the liver at the side of the duodenum and their union in the dorsal part of the liver with the sinus venosus; the vitelline artery; and the mesonephros.
Fig. 11. A View from the dorsal side of the same segment of the model as is shown from the ventral side in Fig. 10, i. 9., it extends from See. 96 to Sec. 155 (of. Figs. 1, 3, 4). It cuts the arm buds. looks into the door of the pharynx and cephalad into the pons, mesencephal and ear vesicles.
There are seen: A portion of the cerebellum with its folds; the mesencephal with its narrow opening cephalad and its floor protruding deeply into the pons region; the interior view of the pons lobule with its three folds, obl. 1, obl. 2, obl. 3; the otic lobe showing the ventral ends of folds, obl. 4, 5; obl. 4 connected with the VIIth and VIIIth nerve; at the right the relation of the ear vesicle to obl. 5; at the left the ganglion of the VIIIth lying next the otic vesicle, that of the VIIth crossing dorsad of the first gill-pouch; at the right the intimate union with the epidermis of the ganglion of the lXth nerve; the ventral ends of folds obl. 6.
On the left, dissections down to Secs. 99, 103, 110, and 128 show: The relations of the gill arches, and the four gill pouches to the pharynx, the larynx, esophagus; the coelomic cavities separated by the mesentery and only partially divided into pericardial and abdominal regions by the lateral infolding formed by the ducts of Cuvier; at the left the dissected cardinal vein arching over the coelom and uniting with the jugular vein to form the duct of Cuvier and thence dipping ventrad to join the sinus venosus (cf. Figs. 10, 2); the right and left aortae near their point of union; the right arm—bud with its thickened epithelium and the branches of the terminal blood-vessels; the 10th myotome merging into the mesoderm of the left armbud near its dorsal portion; well developed motor nerve roots.
Fig. 12. A ventral view of the heart showing the somewhat greater length of the right part of the common auricular chamber. The figure is labeled as though the right and left sides were separate. By comparison with Figs. 2-6, the‘ relations are seen of the bulbus arteriosus and the sinus venosus to the single tube forming the heart.
Plate V
Fig. 13. A segment of the model (Fig. 1) from Secs. 5 to 20, showing: The total foldings obl. 7, 8, 9 of the neural tube dorsad of the roots of the Xth, Xlth, and Ist cervical nerves (cf. Figs. 3-4); the foldings of the skin corresponding with those of the neural tube; the metatela rapidly widening from the cephalic end of the cut surface (cf. Fig. 14); the close connection of neural and epidermic epithelium.
Fig. 14. From a photograph (X 47%) of Sec. 25 of the human embryo 148 (cf. Figs. 1, 2, 4). It shows: The neural tube just at the ventral border of the folds, obl. 8, 9, represented in Fig. 13; the cephalic, Ist, root of N. XI, attached to the base of fold, obl. 8; the 2d root of N. XI, attached to obl. 9; the Xlth N. as it passes through the 1st cervical and Froriep’s ganglia; the intimate union of the 2d and 3d cervical ganglia.
The cilia lining the tube appear faintly and stop short of the dorsal margin of the neural tube.
At the right, the first four myotomes are seen, the 4th showing especially well the dorsal division into two separate horns. Noticeable is a continuation cephalad of similar cell-groups and epidermal corrugations representing remnants of still more cephalic, occipital myotomes.
Fig. 15. A photograph (x 473/2) of part of Sec. 44 (cf. Figs. 3, 4), showing the neural tube with its cilia, metatela, and the relations of the Xth, Xlth, and Xllth nerves.
At the left of Fig. 14, the fold, obl. 7, is seen. At the right, it has disappeared, to reappear as a more marked depression at the lower level of Fig. 15, where the attachment of the Xth N. is found.
At the left is seen the appearance of the 1st, 2d, and 3d myotomes at a lower level than in Fig. 14, and at the right, at a still lower level, as they recede farther from the skin and where the nuclei, related to the developing muscle fibers, form a band across the myotome. At the left is a continuation cephalad of the same segmented appearance of the epidermis as that which lies over the myotome.
Fig. 16. A photograph (x 47175) of Sec. 192, through the neuropore to "show: The point of most intimate union of the thickened epidermis with the neural epithelium (for the extent of the neuroporic thickening, (cf. Figs. 1-8); the total folds entering into the formation of the visual lobe; the sharp angle formed by the albicantial furrow at the right; the external filling up of this angle at the left, indicating its independent ending (see text).; the mesoderm continuous over the eye-vesicle, but interrupted at the neuropore (cf. Fig. 4). The cilia. present in this section do not show clearly here.
Fig. 17. Ventral view of a large model of the nephric region of Homo 148, extending from See. 94 to Sec. 295 (X 75). It shows the two Wolflian ridges, each including the pronephric remnant; the mesonephros; the dorsal portion of the mesentery; a part of the stomach and lesser peritoneal cavity; the union of the allantoic stalk with the intestine; the cloaca and the imperforate anal plate; the right Wolffian duct and its union with the cloaca.
The opening of the pronephric tubules (Fig. 18) on the cmlomic surface is shown. Crosses indicate the position of the first eight mesonephric tubules which do not open on the caelomic surface (Fig. 19) but connect with the Wolflian duct. The 9th to the 20th tubules have no connection with the Wolflian duct. The 9th and the 13th to the 18th are open to the cualom. The 10th to the 12th are hollow but connect neither with duct nor ouelom. The 19th to the 21st are thickenings touching the ctnlomic epithelium but showing no cavity. The general arrangement of the tubules of the other side is similar but not identical.
The slight furrows at the cephalic end may represent a rudiment of the supra-renal or adrenal body.
Fig. 18. From a photograph (X 160) of Sec. 104, Homo 148, showing the pronephric tubule of the right side (Fig. 17) with its opening into the coelom and its duct traceable for a few sections farther cephalad. The cardinal vein is also seen.
Fig. 19. From a photograph (X 160) of Sec. 166 through the 7th mesonephric tubule of the right side (Fig. 17), showing the small rudiment of the glomerulus; the typical S-shaped tubule connecting with the Wolifian duct and thin-walled Bowman's capsule which a few sections farther caudad unites with the cuelomic epithelium and forms a small opening. The remainder of the first eight tubules are of this ame general type but are completely separated from the cualomic epithelium.
Fig. 20. From a photograph (X 160) of Sec. 195, through the 10th meso nephric tubule of the left side (Fig. 17), showing a wide opening to the coelom and its independence of the Wolflian duct.
The photographs reproduced on this plate were made by Henry Phelps Gage and are part of a complete series of 96 taken from the sections.
Gage SP. A three weeks' human embryo, with especial reference to the brain and nephric system. (1905) Amer. J Anat. 4: 409-443.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - A three weeks human embryo with especial reference to the brain and nephric system (1905). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_three_weeks_human_embryo_with_especial_reference_to_the_brain_and_nephric_system_(1905)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G[[[Category:Carnegie Stage 13]]