Neural - Ventricular System Development
| Embryology - 5 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
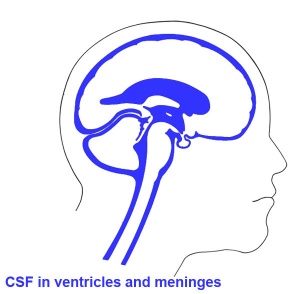
The ventricular system develops from the single cavity formed from the hollow neural tube. This fluid-filled space is separated from the amnion following fusion of the neural tube and closure of neuropores. At different regions sites within the wall (floor of lateral ventricle and roof of the third and fourth ventricles) differentiate to form choroid plexus a modified vascular structure which will produce cerebrospinal fluid (CSF)
In development and the space within the spinal cord (central canal) and the brain (ventricles) was derived from the same space within the neural tube. In the adult these 2 spaces remain connected containing the same CSF.
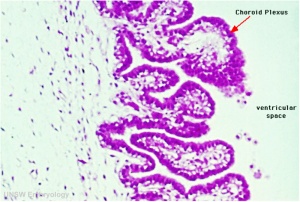
Early in development the cavity within the neural tube (which will form the ventricular space) is filled with amniotic fluid. As the brain and spinal cord grow, this fluid filled space makes up the majority of the nervous system (by volume). Upon closure of the neuropores and development of the embryonic vasculature, this fluid is then synthesized by the choroid plexus, a specialized vascular epithelium. In mammals, the choroid plexuses develop at four sites in the roof of the neural tube shortly after its closure, in the order fourth (IV), lateral, and third (III) ventricles.
The choroid plexuses form one region of the blood-brain barrier that regulates the brain's internal environment.
In the adult, the choroid plexus produces about two thirds of the CSF, the rest is produced by ventricular ependymal cells and cells lining the subarachnoid space. Normal CSF contains high amounts of salts, sugars and lipids and low amounts of protein (0.3-0.7 microg/microL), though there appears to be 60+ proteins as identified by 2D gel. Presence of some protein in the CSF can be indicative of disruption of or incomplete blood/brain barrier.
- Links: hydrocephalus | Category:Ventricular System
| Historic Papers - Ventricular |
|---|
|
Heuser CH. The development of the cerebral ventricles in the pig. (1913) Amer. J Anat. 15(2): 215-251. Streeter GL. The development of the venous sinuses of the dura mater in the human embryo. (1915) Amer. J Anat.18: 145-178. Weed LH. The development of the cerebro-spinal spaces in pig and in man. (1917) Contrib. Embryol., Carnegie Inst. Wash., 5, No. 14 . Sensenig EC. The early development of the meninges of the spinal cord in human embryos. (1951) Contrib. Embryol., Carnegie Inst. Wash. Publ. 611. |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Neural Ventricular System Development | Choroid Plexus Development | Cerebrospinal fluid Development | lateral ventricles Development | third d+ventricle Development | cerebral aqueduct Development | central canal Development | hydrocephalus | Subarachnoid Space Development | arachnoid villi development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
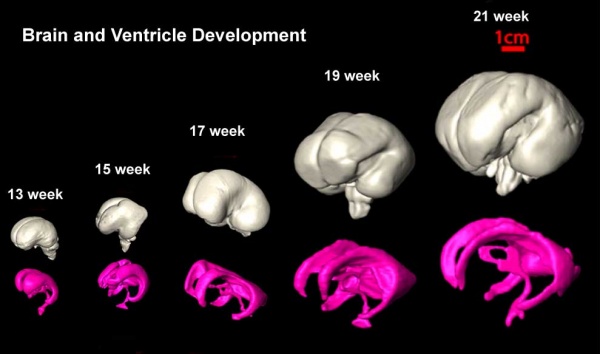
Development Overview
The initial neural grove and tube, with open neuropores, is filled with amniotic fluid. By stage 13 (4 weeks, GA week 6) the neuropores are closed and the neural tube is no longer directly connected to the amniotic cavity. Initially during early 3 and 5 vesicle neural stages and prior to choroid plexus development, the "ventricular space" is reliant upon overall tube growth and directional fluid transport to maintain the fluid-filled space. There is research suggesting that hydrostatic pressure[6] and a functioning heart are required to maintain the vesicle spaces, and there are several models as to how pressure and osmotic gradients may be established. See also zebrafish model studies[7] and a recent review.[8]
Ventricular Timeline
| Week | Carnegie Stage | Event | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 4 | 11 | appearance of the optic ventricle. The neural groove/tube space is initially filled with amniotic fluid. | ||||||||||||||||||
| 12 | closure of the caudal neuropore, onset of the ventricular system and separates the ependymal from the amniotic fluid. | |||||||||||||||||||
| 13 | cavity of the telencephalon medium is visible. | |||||||||||||||||||
| Week 5 | 14 | cerebral hemispheres and lateral ventricles begin, rhomboid fossa becomes apparent. | ||||||||||||||||||
| 15 | medial and lateral ventricular eminences cause indentations in the lateral ventricle | |||||||||||||||||||
| Week 6 | 16 | hypothalamic sulcus is evident. | ||||||||||||||||||
| 17 - 18 | interventricular foramina are becoming relatively smaller, and cellular accumulations indicate the future choroid villi of the fourth and lateral ventricles. | |||||||||||||||||||
| Week 7 | 18 | areae membranaceae rostralis and caudalis are visible in the roof of the fourth ventricle, and the paraphysis is appearing. | ||||||||||||||||||
| 19 | choroid villi are visible in the fourth ventricle, and a mesencephalic evagination (blindsack) is visible | |||||||||||||||||||
| Week 8 | 20 | choroid villi are visible in the lateral ventricle. | ||||||||||||||||||
| 21 | olfactory ventricle is visible. | |||||||||||||||||||
| 21 - 23 | lateral ventricle has become C-shaped (anterior and inferior horns visible). Recesses develop in the third ventricle (optic, infundibular, pineal). | |||||||||||||||||||
| ||||||||||||||||||||
| Links: ventricular | neural | timeline | Category:Timeline Table Data Reference[9] | ||||||||||||||||||||
Embryonic
Subarachnoid space, choroid plexus, and arachnoid villi studied in 60 normal human embryos.[10] and other studies.[11][12]
- stage 14 - primitive subarachnoid space (future subarachnoid space) cavity formation within the meninx primitiva in the areas ventral to the middle brain vesicle. Primitive subarachnoid space precedes the appearance of the choroid plexus. The primitive subarachnoid space appears earlier in the region ventral to the rhombencephalon than in the region posterior to the fourth ventricle.
- Stage 18 - dura mater is formed and spaces surround the circumference of the spinal cord, which coalesce and contain many blood vessels.
- stage 20 - primitive subarachnoid space almost completely surrounds the neural tube. Abnormal human embryo findings imply that the extra-ventricular spread of fluid of choroid plexus origin is not an essential factor for the development of the subarachnoid space.
- stage 23 - arachnoid villi do not appear even at the end of the embryonal stage. Absorption of the cerebrospinal fluid in an embryo is done by the way other than the arachnoid villi.
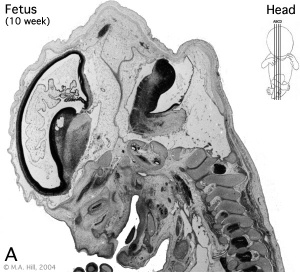
Fetal
Fetal Period - posterior horn of the lateral ventricle, choroid plexus of the third ventricle, suprapineal recess, interthalamic adhesion, aqueduct, and apertures in the roof of the fourth ventricle.
Anatomy
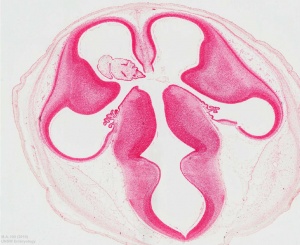
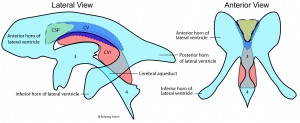
Lateral Ventricles
The paired lateral ventricles lie with the cerebral hemispheres.
Third Ventricle
The third ventricle lies midline in the diencephalon between the two thalami connecting the lateral ventricles to the cerebral aqueduct.
Cerebral Aqueduct
The cerebral aqueduct connects the third ventricle with the fourth ventricle and allows cerebrospinal fluid
Fourth Ventricle
The fourth ventricle lies within the hindbrain connected by the cerebral aqueduct to the third ventricle and with the central canal of teh spinal cord.
Central Canal
The central canal (ependymal canal) lies within the centre of the spinal cord along its length and is continuous with the brain ventricular system.
Choroid Plexus
The choroid plexus along with ventricular ependymal cells and cells lining the subarachnoid space synthesise CSF. In humans, the choroid plexuses develop at four sites in the roof of the neural tube shortly after its closure, in the order fourth (IV), lateral, and third (III) ventricles.

|
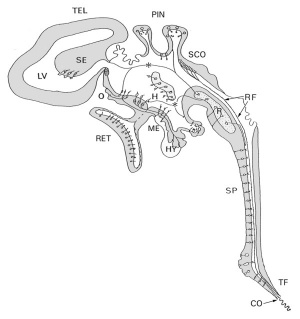
Human Ventricular System
A schematic diagram of structures and specialized cell types bordering the different parts of the mammalian ventricular system, and in contact with the cerebrospinal fluid (CSF)[13] Abbreviations:
|
Epithelium from the neural tube epithelium.
Mesenchyma from the meninges.
Enzymes required for CSF production are Na+/K+ ATPase and carbonic anhydrase.
CSF Synthesis
Two key enzymes are required to produce CSF they are the Na+/K+ ATPase and carbonic anhydrase.
Other known chorid plexus enzymes include: alkaline and acid phosphatases, magnesium-dependent ATPase, glucose-6-phosphatase, thiamine pyrophosphatase, adenylate cyclase, oxidoreductase, esterases, hydrolases, cathepsin D, and glutathion S-transferase. (More? Catala M., 1998)
- "The epithelial cells of the choroid plexus secrete cerebrospinal fluid (CSF), by a process that involves the movement of Na(+), Cl(-) and HCO(3)(-) from the blood to the ventricles of the brain. This creates the osmotic gradient, which drives the secretion of H(2)O. The unidirectional movement of the ions is achieved due to the polarity of the epithelium, i.e., the ion transport proteins in the blood-facing (basolateral) are different to those in the ventricular (apical) membranes."[14]
CSF Reabsorption
The main reabsorption (absorption) of CSF is thought to occur in arachnoid villi (arachnoid granulations, Pacchionian granulations, Pacchionian body), into the superior sagittal sinus, into the venous system. CSF in the subarachnoid space extends into the villi, which then project through the dura into the superior sagittal sinus.
Recently, in the rabbit model, extra-cranial reabsorption of CSF has been identified with direct venous connections between the subarachnoid space and the perispinal veins.[15] In addition, clusters of arachnoidal villi have been identified in the dorsal root of the spinal nerves and the absorptive surface areas of microvessels have been suggested to serve a putative role in reabsorption.
- Antonio Pacchioni (1665-1726) in Dissertatio Epistolaris de Glandulis Conglobatis Durae Meningis Humanae (1705) first described these arachnoid granulations.[16]
Adult CSF Normal Values
Lumbar CSF
- Opening pressure: 50–200 mm H2O CSF
- Color: Colorless
- Turbidity: Crystal clear
- Mononuclear cells: less than 5 / mm3
- Polymorphonuclear leukocytes: 0
- Total protein: 22–38 mg/dl Range 9–58 mg/dl (mean ± 2.0 SD)
- Glucose: 60–80% of blood glucose
(Data from: Clinical Methods, 3rd ed, Table 74.1)
CSF Circulation
Information below is for the adult and is based upon data from a radiologic investigation using MR imaging and radionuclide cisternography.[17]
- CSF-circulation is propelled by a pulsating flow, which causes an effective mixing. Flow is produced by the alternating pressure gradient, which is a consequence of the systolic expansion of the intracranial arteries causing expulsion of CSF into the compliant and contractable spinal subarachnoid space.
- No bulk flow is necessary to explain the transport of tracers in the subarachnoid space.
- Main absorption of the CSF is not through the Pacchionian granulations (arachnoid granulations), but a major part of the CSF transportation to the blood-stream is likely to occur via the paravascular and extracellular spaces of the central nervous system. (MH- Note this statement conflicts with previous CSF Reabsorption in literature)
- The intracranial dynamics may be regarded as the result of an interplay between the demands for space by the four components of the intracranial content (arterial blood, brain volume, venous blood and CSF).
- Interaction has a time offset within the cerebral hemispheres in a fronto-occipital direction during the cardiac cycle (the fronto-occipital "volume wave").
- Outflow from the cranial cavity to the cervical subarachnoid space (SAS) is dependent in size and timing on the intracranial arterial expansion during systole.
Abnormalities
Dandy Walker Syndrome
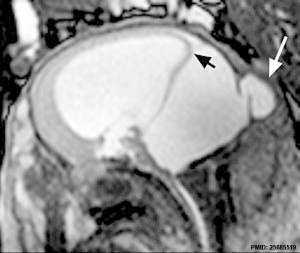
The vermis of the cerebellum can be small or absent, the fourth ventricle enlarges due to cyst formation.
An ultrasound study[19] of fetuses with Dandy-Walker malformation 13 to 16 weeks (GA 15-18 weeks) identified the fourth ventricle widely open posteriorly, even in the standard transcerebellar view, and the brainstem-vermis (BV) angle was > 45°, significantly increased compared to that in normal fetuses (P < 0.0001). Note that at this age, an open fourth ventricle can also found in about 10% of normal fetuses.
- Links: Dandy-Walker | hydrocephalus | Genetics Home Reference
Hydrocephalus
See hydrocephalus
References
- ↑ Sainz LV, Zipfel J, Kerscher SR, Weichselbaum A, Bevot A & Schuhmann MU. (2019). Cerebro-venous hypertension: a frequent cause of so-called "external hydrocephalus" in infants. Childs Nerv Syst , 35, 251-256. PMID: 30474714 DOI.
- ↑ Boardman JP, Ireland G, Sullivan G, Pataky R, Fleiss B, Gressens P & Miron V. (2018). The Cerebrospinal Fluid Inflammatory Response to Preterm Birth. Front Physiol , 9, 1299. PMID: 30258368 DOI.
- ↑ Koshida R, Oishi H, Hamada M, Takei Y & Takahashi S. (2017). MafB is required for development of the hindbrain choroid plexus. Biochem. Biophys. Res. Commun. , 483, 288-293. PMID: 28025141 DOI.
- ↑ Khazanov S, Paz Y, Hefetz A, Gonzales BJ, Netser Y, Mansour AA & Ben-Arie N. (2017). Floor plate descendants in the ependyma of the adult mouse Central Nervous System. Int. J. Dev. Biol. , 61, 257-265. PMID: 27528042 DOI.
- ↑ Jain N, Lim LW, Tan WT, George B, Makeyev E & Thanabalu T. (2014). Conditional N-WASP knockout in mouse brain implicates actin cytoskeleton regulation in hydrocephalus pathology. Exp. Neurol. , 254, 29-40. PMID: 24462670 DOI.
- ↑ Desmond ME, Levitan ML & Haas AR. (2005). Internal luminal pressure during early chick embryonic brain growth: descriptive and empirical observations. Anat Rec A Discov Mol Cell Evol Biol , 285, 737-47. PMID: 15977221 DOI.
- ↑ Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, Yang H & Driever W. (1996). Mutations affecting the development of the embryonic zebrafish brain. Development , 123, 165-78. PMID: 9007238
- ↑ Gato A & Desmond ME. (2009). Why the embryo still matters: CSF and the neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histiogenesis. Dev. Biol. , 327, 263-72. PMID: 19154733 DOI.
- ↑ O'Rahilly R. and Müller F. Ventricular system and choroid plexuses of the human brain during the embryonic period proper. (1990) Amer. J Anat. 189(4): 285-302. PMID 2285038
- ↑ Osaka K. Matsumoto S. and Yasuda M. The development of cerebro-spinal fluid pathway in human embryos. (1977) (Article in Japanese) No Shinkei Geka. 5(10): 1047-1055. PMID 909616
- ↑ Patelska-Banaszewska M & Woźniak W. (2005). The subarachnoid space develops early in the human embryonic period. Folia Morphol. (Warsz) , 64, 212-6. PMID: 16228957
- ↑ Patelska-Banaszewska M & Woźniak W. (2004). The development of the epidural space in human embryos. Folia Morphol. (Warsz) , 63, 273-9. PMID: 15478101
- ↑ Veening JG & Barendregt HP. (2010). The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res , 7, 1. PMID: 20157443 DOI.
- ↑ Speake T, Whitwell C, Kajita H, Majid A & Brown PD. (2001). Mechanisms of CSF secretion by the choroid plexus. Microsc. Res. Tech. , 52, 49-59. PMID: 11135448 <49::AID-JEMT7>3.0.CO;2-C DOI.
- ↑ Biceroglu H, Albayram S, Ogullar S, Hasiloglu ZI, Selcuk H, Yuksel O, Karaaslan B, Yildiz C & Kiris A. (2012). Direct venous spinal reabsorption of cerebrospinal fluid: a new concept with serial magnetic resonance cisternography in rabbits. J Neurosurg Spine , 16, 394-401. PMID: 22243405 DOI.
- ↑ Brunori A, Vagnozzi R & Giuffrè R. (1993). Antonio Pacchioni (1665-1726): early studies of the dura mater. J. Neurosurg. , 78, 515-8. PMID: 8442786 DOI.
- ↑ Greitz D. (1993). Cerebrospinal fluid circulation and associated intracranial dynamics. A radiologic investigation using MR imaging and radionuclide cisternography. Acta Radiol Suppl , 386, 1-23. PMID: 8517189
- ↑ Saleem SN. (2014). Fetal MRI: An approach to practice: A review. J Adv Res , 5, 507-23. PMID: 25685519 DOI.
- ↑ Contro E, Volpe P, De Musso F, Muto B, Ghi T, De Robertis V & Pilu G. (2014). Open fourth ventricle prior to 20 weeks' gestation: a benign finding?. Ultrasound Obstet Gynecol , 43, 154-8. PMID: 24151160 DOI.
Journals
Online Textbooks
Reviews
Sakka L, Coll G & Chazal J. (2011). Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis , 128, 309-16. PMID: 22100360 DOI.
Gato A & Desmond ME. (2009). Why the embryo still matters: CSF and the neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histiogenesis. Dev. Biol. , 327, 263-72. PMID: 19154733 DOI.
Johansson PA, Dziegielewska KM, Liddelow SA & Saunders NR. (2008). The blood-CSF barrier explained: when development is not immaturity. Bioessays , 30, 237-48. PMID: 18293362 DOI.
Dziegielewska KM, Ek J, Habgood MD & Saunders NR. (2001). Development of the choroid plexus. Microsc. Res. Tech. , 52, 5-20. PMID: 11135444 <5::AID-JEMT3>3.0.CO;2-J DOI.
Catala M. (1998). Embryonic and fetal development of structures associated with the cerebro-spinal fluid in man and other species. Part I: The ventricular system, meninges and choroid plexuses. Arch. Anat. Cytol. Pathol. , 46, 153-69. PMID: 9754371
Articles
Desmond ME, Levitan ML & Haas AR. (2005). Internal luminal pressure during early chick embryonic brain growth: descriptive and empirical observations. Anat Rec A Discov Mol Cell Evol Biol , 285, 737-47. PMID: 15977221 DOI.
Patelska-Banaszewska M & Woźniak W. (2004). The development of the epidural space in human embryos. Folia Morphol. (Warsz) , 63, 273-9. PMID: 15478101
Shotland LI & Hecox KE. (1990). The effect of probe tube reference placement on sound pressure level variability. Ear Hear , 11, 306-9. PMID: 2210106
Oteruelo FT. (1986). On the cavum septi pellucidi and the cavum Vergae. Anat Anz , 162, 271-8. PMID: 3813041
Osaka K, Handa H, Matsumoto S & Yasuda M. (1980). Development of the cerebrospinal fluid pathway in the normal and abnormal human embryos. Childs Brain , 6, 26-38. PMID: 7351160
Osaka K, Matsumoto S & Yasuda M. (1977). [The development of cerebro-spinal fluid pathway in human embryos (author's transl)]. No Shinkei Geka , 5, 1047-55. PMID: 909616
Search PubMed
Search Pubmed: ventricular system development | ventricular development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Additional Images
Terms
- aqueduct of Sylvius - (cerebral aqueduct) acts as a tubular communication between the third and fourth ventricles.
- cerebral aqueduct - (aqueduct of Sylvius) acts as a tubular communication between the third and fourth ventricles.
- cerebrospinal fluid -
- choroid plexus
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 5) Embryology Neural - Ventricular System Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Ventricular_System_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G