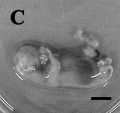
Dog Development
| Embryology - 10 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The domestic dog (Canis lupus familiaris) has been derived from an ancestoral wolf and now consists of a breed family of more than 300 worldwide, with extensive variations in morphology (size, shape and weight). The modern dog breeds show high phenotypic diversity and are thought to have arisen from this first population bottleneck associated with wolf domestication (7,000–50,000 generations ago) and a second from more recent intensive selection to create the breed (50–100 generations ago).[1]
The average canine gestation period from ovulation to birth (parturition) is approximately 64 days and there have been identified about 400 congenital disorders relating to dog development. Many of these developmental abnormalities are common to human development.
| Dog Links: Introduction | Estrous Cycle | Abnormalities | Category:Dog |
| Historic: 1958 Early Vascular Development | 1961 Foregut Development |
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Dog Development | Canine Development | Dog Embryology | Canine Embryology |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Taxon
NCBI Taxonomy Browser Canis lupus familiaris (Genbank common name: dog)
Synonyms: Canis familiaris, Canis domesticus, Canis canis
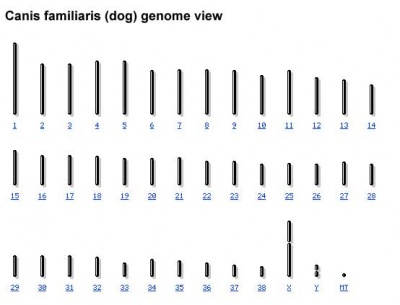
Chromosomes: 40 (38, X, Y)
Genetic code: Translation table 1 (Standard)
Mitochondrial genetic code: Translation table 2 (Vertebrate Mitochondrial) 16,700 bp
Lineage( full ):cellular organisms; Eukaryota; Fungi/Metazoa group; Metazoa; Eumetazoa; Bilateria; Coelomata; Deuterostomia; Chordata; Craniata; Vertebrata; Gnathostomata; Teleostomi; Euteleostomi; Sarcopterygii; Tetrapoda; Amniota; Mammalia; Theria; Eutheria; Laurasiatheria; Carnivora; Caniformia; Canidae; Canis; Canis lupus
Dog Estrous Cycle
- Proestrus (9 days) - Precedes estrus, estradiol concentration increases as ovarian follicules mature and the uterus enlarges. The vaginal epithelium proliferates accompanied by diapedesis of erythrocytes (most cells in vaginal smear) from uterine capillaries.
- Estrus (9 days) - Accompanied by female mating behaviour, glandular secretions increase, the vaginal epithelium becomes hyperemic, and ovulation occurs. Cycle is influenced mainly by estrogens and the interval between successive estrus cycles is about 7 months.
- Diestrus (70-80 days) - Accompanied by female non-mating behaviour, corpus lutea present and secretes progesterone. Uterine glands undergo hypertrophy and hyperplasia, vaginal secretions and the cervix constricts.
- Anestrus - Anestrus is a prolonged period of sexual rest where the reproductive system is quiescent.
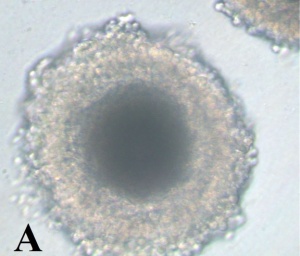
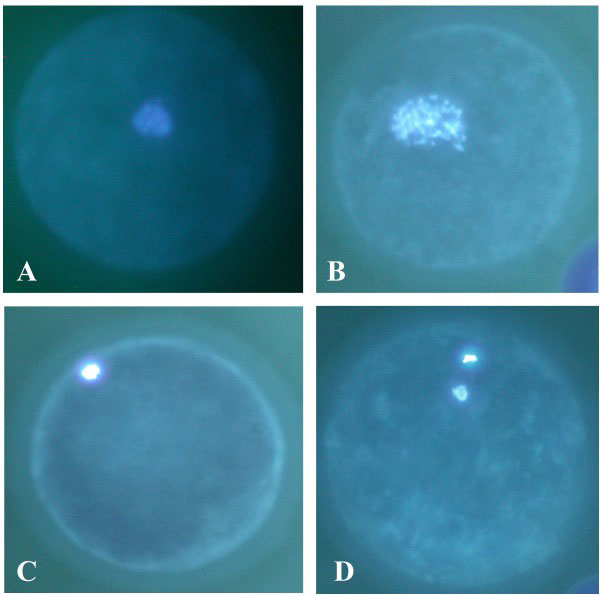
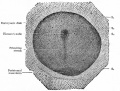
Oocyte Development
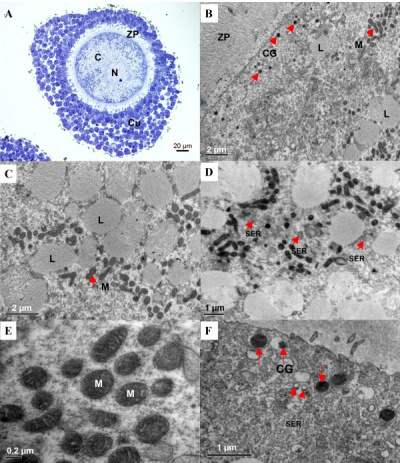
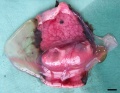
Canine geminal vesicle (GV) oocyte.[10]
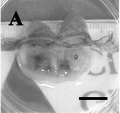
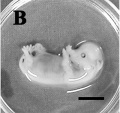
Dog oocyte development. (A) GV (B) GVBD (C) MI and (D) MII[10]
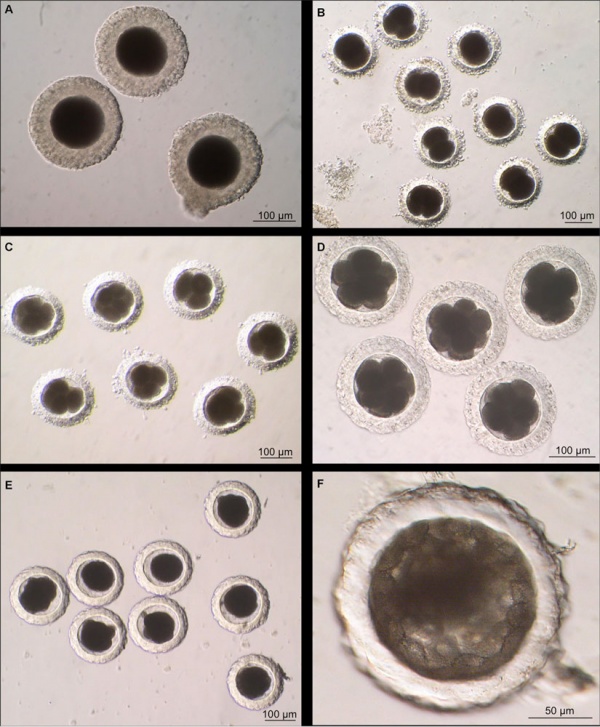
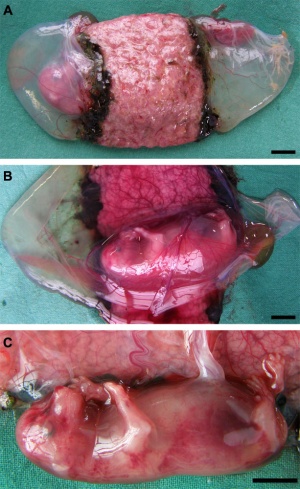
Oocyte to Blastocyst
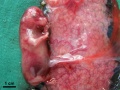
Canine oocyte to blastocyst (Image: Dr Karine Reynaud).
Development Overview
Days shown below relate to days after ovulation (day 0).
- 48-72 h - oocytes need to complete post-ovulatory maturation to the metaphase II stage in the isthmus of the oviduct[11]
- 2 to 5 days - fertilization
- 14 to 16 days - embryo attaches to uterus
- 22 to 23 days - heartbeat visible
- 62 to 64 days - parturition (birth or whelping)
See also Concannon 2001
Sexual differentiation begins early in the embryonic period prenatally and continues into early postnatal life.
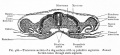
Caudal vena cava development- five theories to origin (right-sided supracardinal, caudal cardinal, sacrocardinal, lateral sympathetic or subcardinal veins).
Carnegie Stages
Gestational age timed from day 0 as the day of the preovulatory serum progesterone rise in the dam, should roughly correlate with fertilisation (+/- 1 day), data[12] *calculated staging only available.
- day 27 - carnegie stage 13.5
- day 28* - carnegie stage 14-15
- day 29* - carnegie stage 15-16
- day 30 - carnegie stage 17
- day 34 - carnegie stage 18.5 – 19
- day 36 - carnegie stage 19.5–20
- day 37 - carnegie stage 20
- Links: Carnegie Stages
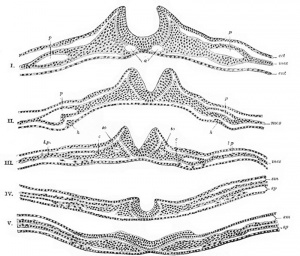
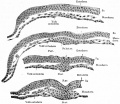
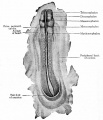
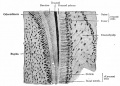
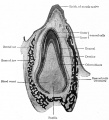
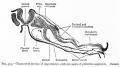
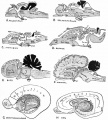
Historic Embryology
These images are from drawings by Charles Bonnet (1909), later republished in a 1921 textbook of embryology.[13]
- Links: Carnegie Stage Comparison

Estrous Cycle
Estrus, also called "in heat" is the time of sexually receptivity and occurs every 17 to 21 days.
- Ovulation occurs 5 to 6 days prior to the first day of diestrus and is indicated by plasma progesterone concentrations higher than 2 ng/mL. (Parturition (birth or whelping) occurs between 62 to 64 days after ovulation).
- Ovulated oocytes diameter[15]
- with the zona pellucida (167.5+/-12.7 microns)
- without zona pellucida (133.9+/-5.3 microns)
- Links: Estrous Cycle
Placenta
Classified as endotheliochorial placentation forming a zonary placenta, which is a complete girdle in dogs.
Three zones:
- girdle zone (endotheliochorial labyrinth)
- hemochorial hemophagous zone (marginal hematoma)
- polar zone (epitheliochorial free)
Trophoblast cell invasion continues after chorioallantois villous penetration and the materno–fetal interface is described as lamellar, with fetal projections interdigitating with maternal septa.
(Data from: Miglino MA, etal., 2006[16] and other sources)
Urogenital System
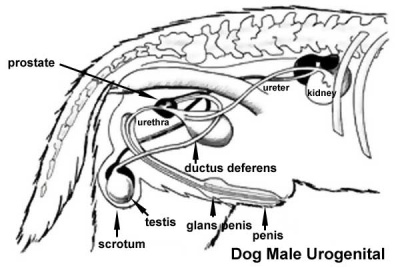
|
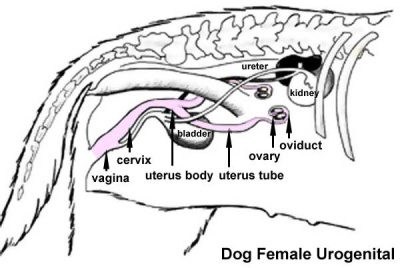
|
| Male Urogenital | Female Urogenital |
Male Gonad
Male sex differentiation is initially mediated by Sry expression then Leydig cell produced testosterone and anti-mullerian hormone (AMH, Mis), also called mullerian-inhibiting substance (MIS) or factor (MIF).
A study using timed pregnancies and male embryo development identified testis differentiation at 36 days gestation. At this time Mullerian duct regression also commenced and was completed by 46 days gestation. Immunohistochemistry also identified Mullerian Inhibitory Substance (MIS) was present during this period in testes and was absent in the undifferentiated testis.[17]
Genital Ridge Sry and Sox9[12]
Testis induction is associated with gonadal Sry and Sox9 expression in mammals, and also with Sox9 expression in vertebrates where Sry is absent. Timing was based upon the equivalent human carnegie staging and expression was measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
- Carnegie Stage 16-18 - Sry expression rose in genital ridge continuously, Sox9 expressed in both male and female genital ridge
- Carnegie Stage 17 - Sox9 expression tenfold greater than in the ovary
- Carnegie Stage 18 - Sry expression maximal
Chromosome 9 Sox 9
Genital Ridge Sf1 and Mis[18]
Mullerian-inhibiting substance - (Mis, Mif) Anti-mullerian hormone (AMH)
Splicing factor 1 - (Sf1) 623 amino acid protein containing a nuclear transport domain, a metal-binding or zinc finger motif, and glutamine- and proline-rich regions.
- Carnegie Stage 15 - Sf1 expression begins in genital ridges
- Carnegie Stage 17 - Sf1 expression pronounced in male and female gonads
- Carnegie Stage 18 - Mis expression only in male gonads
Chromosome 20 Anti-Mullerian hormone
- Links: Sry | Sox9 | Sox 9 Gene | Sf1 | Mis
Spermatogenesis
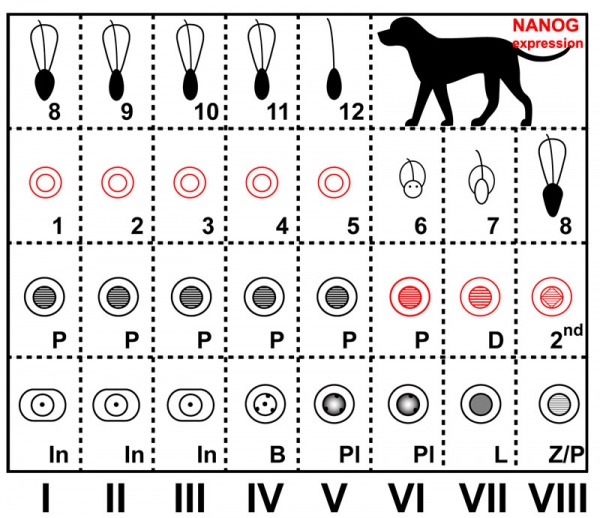
The cartoons below show nanog expression in the dog during spermatogenesis.PubmedParser error: The PubmedParser extension received invalid XML data. ()
Puberty
| Phase | Rat | Dog (beagle) | Primate (monkey) |
| Neonatal | Birth to postnatal Day 7 | Birth–3 weeks | Birth to 3–4 months |
| Infantile | Postnatal Days 8–21 | 3–5 weeks | Up to 29 months |
| Juvenile/prepubertal | Postnatal Days 22–37 | 5 weeks–6 months | Up to 43 months |
| Pubertal | Postnatal Days 37–38 | 6–8 months | 27–30 months |
Hair Development
Coat variation in the domestic dog is governed by variants in three genes.[20]</ref>
- "Coat color and type are essential characteristics of domestic dog breeds. Although the genetic basis of coat color has been well characterized, relatively little is known about the genes influencing coat growth pattern, length, and curl. We performed genome-wide association studies of more than 1000 dogs from 80 domestic breeds to identify genes associated with canine fur phenotypes. Taking advantage of both inter- and intrabreed variability, we identified distinct mutations in three genes, RSPO2, FGF5, and KRT71 (encoding R-spondin-2, fibroblast growth factor-5, and keratin-71, respectively), that together account for most coat phenotypes in purebred dogs in the United States. Thus, an array of varied and seemingly complex phenotypes can be reduced to the combinatorial effects of only a few genes."
Stem Cells
In 2009 a range of canine embryonic stem cell (ESC) lines were developed from preimplantation-stage embryos.[21]
- maintained a normal karyotype and morphology typical of undifferentiated ESCs after multiple in vitro passages and cryopreservation.
- embryoid bodies formed in the absence of a feeder layer in attachment or suspension culture.
- embryoid bodies differentiated into multiple cell types.
- ESCs introduced in vivo formed teratomas containing cell types of all three embryonic germ layers.
- Links: Stem Cells
Neural
A recent research paper has described a new online digital atlas of the dog brain based upon anatomical and functional magnetic resonance imaging (MRI).[22]
- Links: Database
Abnormalities
There are a number of dog developmental abnormalities that are used as models for human disease.
There are currently 566 abnormality links listed on the Online Mendelian Inheritance in Animals database.
Search OMIA: Canis familiaris
Genital
- Intersex
- Sex reversal - not due to SRY gene translocation to an X chromosome.
Hearing
Canine Congenital Sensorineural Deafness
Canine congenital sensorineural deafness (CCSD) is the most common cause of deafness in dogs occurring in more than 90 dog breeds.[23]
CCSD breed highest prevalence - dalmatian (most common), English setters, English cocker spaniels, bull terriers, Australian cattle dogs, whippets, catahoula leopard dogs, border collies and jack russell terriers.
This abnormality appears to be associated with intermediate cells (melanocytes, neural crest in origin) in the stria vascularis of the inner ear causing a cascade of inner ear degeneration (stria vascularis, then organ of Corti, then collapse of Reissner's membrane, finally collapse of cochlear duct.
Cardiac Defects
- Canine-dilated cardiomyopathy - not associated with canine desmin.[24]
Hip dysplasia
British Veterinary Association and the German Shepherd League scoring scheme
- scoring of nine different radiographic features of each hip
- scale from 0 (ideal) to 6 (worst)
- potential range of subjective scores from 0 to 108.
Other
- Congenital renal disease
- Canine Eclampsia - (puerperal tetany, hypocalcemia) develops mainly in small-breed dogs with large litters.
- Brucellosis - male and female can be carriers of this sexually transmitted disease.
References
- ↑ Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ, Zody MC, Mauceli E, Xie X, Breen M, Wayne RK, Ostrander EA, Ponting CP, Galibert F, Smith DR, DeJong PJ, Kirkness E, Alvarez P, Biagi T, Brockman W, Butler J, Chin CW, Cook A, Cuff J, Daly MJ, DeCaprio D, Gnerre S, Grabherr M, Kellis M, Kleber M, Bardeleben C, Goodstadt L, Heger A, Hitte C, Kim L, Koepfli KP, Parker HG, Pollinger JP, Searle SM, Sutter NB, Thomas R, Webber C, Baldwin J, Abebe A, Abouelleil A, Aftuck L, Ait-Zahra M, Aldredge T, Allen N, An P, Anderson S, Antoine C, Arachchi H, Aslam A, Ayotte L, Bachantsang P, Barry A, Bayul T, Benamara M, Berlin A, Bessette D, Blitshteyn B, Bloom T, Blye J, Boguslavskiy L, Bonnet C, Boukhgalter B, Brown A, Cahill P, Calixte N, Camarata J, Cheshatsang Y, Chu J, Citroen M, Collymore A, Cooke P, Dawoe T, Daza R, Decktor K, DeGray S, Dhargay N, Dooley K, Dooley K, Dorje P, Dorjee K, Dorris L, Duffey N, Dupes A, Egbiremolen O, Elong R, Falk J, Farina A, Faro S, Ferguson D, Ferreira P, Fisher S, FitzGerald M, Foley K, Foley C, Franke A, Friedrich D, Gage D, Garber M, Gearin G, Giannoukos G, Goode T, Goyette A, Graham J, Grandbois E, Gyaltsen K, Hafez N, Hagopian D, Hagos B, Hall J, Healy C, Hegarty R, Honan T, Horn A, Houde N, Hughes L, Hunnicutt L, Husby M, Jester B, Jones C, Kamat A, Kanga B, Kells C, Khazanovich D, Kieu AC, Kisner P, Kumar M, Lance K, Landers T, Lara M, Lee W, Leger JP, Lennon N, Leuper L, LeVine S, Liu J, Liu X, Lokyitsang Y, Lokyitsang T, Lui A, Macdonald J, Major J, Marabella R, Maru K, Matthews C, McDonough S, Mehta T, Meldrim J, Melnikov A, Meneus L, Mihalev A, Mihova T, Miller K, Mittelman R, Mlenga V, Mulrain L, Munson G, Navidi A, Naylor J, Nguyen T, Nguyen N, Nguyen C, Nguyen T, Nicol R, Norbu N, Norbu C, Novod N, Nyima T, Olandt P, O'Neill B, O'Neill K, Osman S, Oyono L, Patti C, Perrin D, Phunkhang P, Pierre F, Priest M, Rachupka A, Raghuraman S, Rameau R, Ray V, Raymond C, Rege F, Rise C, Rogers J, Rogov P, Sahalie J, Settipalli S, Sharpe T, Shea T, Sheehan M, Sherpa N, Shi J, Shih D, Sloan J, Smith C, Sparrow T, Stalker J, Stange-Thomann N, Stavropoulos S, Stone C, Stone S, Sykes S, Tchuinga P, Tenzing P, Tesfaye S, Thoulutsang D, Thoulutsang Y, Topham K, Topping I, Tsamla T, Vassiliev H, Venkataraman V, Vo A, Wangchuk T, Wangdi T, Weiand M, Wilkinson J, Wilson A, Yadav S, Yang S, Yang X, Young G, Yu Q, Zainoun J, Zembek L, Zimmer A & Lander ES. (2005). Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature , 438, 803-19. PMID: 16341006 DOI.
- ↑ 2.0 2.1 Parker HG & Ostrander EA. (2005). Canine genomics and genetics: running with the pack. PLoS Genet. , 1, e58. PMID: 16311623 DOI.
- ↑ Suzuki H, Watanabe H & Abe Y. (2021). Assisted reproductive techniques for canines: preservation of genetic material in domestic dogs. J Reprod Dev , , . PMID: 34840199 DOI.
- ↑ Orlandi R, Vallesi E, Boiti C, Polisca A, Troisi A, Righi C & Bargellini P. (2018). Contrast-enhanced ultrasonography of maternal and fetal blood flows in pregnant bitches. Theriogenology , 125, 129-134. PMID: 30414566 DOI.
- ↑ Pankowski F, Paśko S, Max A, Szal B, Dzierzęcka M, Gruszczyńska J, Szaro P, Gołębiowski M & Bartyzel BJ. (2018). Computed tomographic evaluation of cleft palate in one-day-old puppies. BMC Vet. Res. , 14, 316. PMID: 30342508 DOI.
- ↑ de Souza AF, Pieri NCG, Roballo KCS, Bressan FF, Casals JB, Ambrósio CE, Perecin F & Martins DS. (2018). Dynamics of male canine germ cell development. PLoS ONE , 13, e0193026. PMID: 29489867 DOI.
- ↑ Chastant-Maillard S, Chebrout M, Thoumire S, Saint-Dizier M, Chodkiewicz M & Reynaud K. (2010). Embryo biotechnology in the dog: a review. Reprod. Fertil. Dev. , 22, 1049-56. PMID: 20797342 DOI.
- ↑ Abe Y, Suwa Y, Asano T, Ueta YY, Kobayashi N, Ohshima N, Shirasuna S, Abdel-Ghani MA, Oi M, Kobayashi Y, Miyoshi M, Miyahara K & Suzuki H. (2011). Cryopreservation of canine embryos. Biol. Reprod. , 84, 363-8. PMID: 20926804 DOI.
- ↑ Tsutsui T, Takahashi F, Hori T, Kawakami E & Concannon PW. (2009). Prolonged duration of fertility of dog ova. Reprod. Domest. Anim. , 44 Suppl 2, 230-3. PMID: 19754575 DOI.
- ↑ 10.0 10.1 10.2 Turathum B, Saikhun K, Sangsuwan P & Kitiyanant Y. (2010). Effects of vitrification on nuclear maturation, ultrastructural changes and gene expression of canine oocytes. Reprod. Biol. Endocrinol. , 8, 70. PMID: 20565987 DOI.
- ↑ de Avila Rodrigues B & Rodrigues JL. (2003). Influence of reproductive status on in vitro oocyte maturation in dogs. Theriogenology , 60, 59-66. PMID: 12620580
- ↑ 12.0 12.1 Meyers-Wallen VN. (2003). Sry and Sox9 expression during canine gonadal sex determination assayed by quantitative reverse transcription-polymerase chain reaction. Mol. Reprod. Dev. , 65, 373-81. PMID: 12840810 DOI.
- ↑ Bailey, F.R. and Miller, A.M. (1921). Text-Book of Embryology. New York: William Wood and Co. online edition
- ↑ Huson HJ, Parker HG, Runstadler J & Ostrander EA. (2010). A genetic dissection of breed composition and performance enhancement in the Alaskan sled dog. BMC Genet. , 11, 71. PMID: 20649949 DOI.
- ↑ Lee HS, Yin XJ, Jin YX, Kim NH, Cho SG, Bae IH & Kong IK. (2008). Germinal vesicle chromatin configuration and meiotic competence is related to the oocyte source in canine. Anim. Reprod. Sci. , 103, 336-47. PMID: 17212978 DOI.
- ↑ Miglino MA, Ambrósio CE, dos Santos Martins D, Wenceslau CV, Pfarrer C & Leiser R. (2006). The carnivore pregnancy: the development of the embryo and fetal membranes. Theriogenology , 66, 1699-702. PMID: 16563485 DOI.
- ↑ Meyers-Wallen VN, Manganaro TF, Kuroda T, Concannon PW, MacLaughlin DT & Donahoe PK. (1991). The critical period for mullerian duct regression in the dog embryo. Biol. Reprod. , 45, 626-33. PMID: 1751638
- ↑ Meyers-Wallen VN. (2005). Sf1 and Mis expression: molecular milestones in the canine sex determination pathway. Mol. Reprod. Dev. , 70, 383-9. PMID: 15685633 DOI.
- ↑ Beckman DA & Feuston M. (2003). Landmarks in the development of the female reproductive system. Birth Defects Res. B Dev. Reprod. Toxicol. , 68, 137-43. PMID: 12866705 DOI.
- ↑ Cadieu E, Neff MW, Quignon P, Walsh K, Chase K, Parker HG, Vonholdt BM, Rhue A, Boyko A, Byers A, Wong A, Mosher DS, Elkahloun AG, Spady TC, André C, Lark KG, Cargill M, Bustamante CD, Wayne RK & Ostrander EA. (2009). Coat variation in the domestic dog is governed by variants in three genes. Science , 326, 150-3. PMID: 19713490 DOI.
- ↑ Vaags AK, Rosic-Kablar S, Gartley CJ, Zheng YZ, Chesney A, Villagómez DA, Kruth SA & Hough MR. (2009). Derivation and characterization of canine embryonic stem cell lines with in vitro and in vivo differentiation potential. Stem Cells , 27, 329-40. PMID: 19038794 DOI.
- ↑ Datta R, Lee J, Duda J, Avants BB, Vite CH, Tseng B, Gee JC, Aguirre GD & Aguirre GK. (2012). A digital atlas of the dog brain. PLoS ONE , 7, e52140. PMID: 23284904 DOI.
- ↑ Kluth S & Distl O. (2013). Congenital sensorineural deafness in dalmatian dogs associated with quantitative trait loci. PLoS ONE , 8, e80642. PMID: 24324618 DOI.
- ↑ Stabej P, Imholz S, Versteeg SA, Zijlstra C, Stokhof AA, Domanjko-Petric A, Leegwater PA & van Oost BA. (2004). Characterization of the canine desmin (DES) gene and evaluation as a candidate gene for dilated cardiomyopathy in the Dobermann. Gene , 340, 241-9. PMID: 15475165 DOI.
Reviews
Chastant-Maillard S, Chebrout M, Thoumire S, Saint-Dizier M, Chodkiewicz M & Reynaud K. (2010). Embryo biotechnology in the dog: a review. Reprod. Fertil. Dev. , 22, 1049-56. PMID: 20797342 DOI.
Poth T, Breuer W, Walter B, Hecht W & Hermanns W. (2010). Disorders of sex development in the dog-Adoption of a new nomenclature and reclassification of reported cases. Anim. Reprod. Sci. , 121, 197-207. PMID: 20537823 DOI.
Cerda-Gonzalez S & Dewey CW. (2010). Congenital diseases of the craniocervical junction in the dog. Vet. Clin. North Am. Small Anim. Pract. , 40, 121-41. PMID: 19942060 DOI.
Day MJ. (2007). Immune system development in the dog and cat. J. Comp. Pathol. , 137 Suppl 1, S10-5. PMID: 17560591 DOI.
Romagnoli S & Schlafer DH. (2006). Disorders of sexual differentiation in puppies and kittens: a diagnostic and clinical approach. Vet. Clin. North Am. Small Anim. Pract. , 36, 573-606, vii. PMID: 16564415 DOI.
Articles
Martins DS, Ambrósio CE, Saraiva NZ, Wenceslau CV, Morini AC, Kerkis I, Garcia JM & Miglino MA. (2011). Early development and putative primordial germ cells characterization in dogs. Reprod. Domest. Anim. , 46, e62-6. PMID: 20477984 DOI.
Hossein MS, Jeong YW, Park SW, Kim JJ, Lee E, Ko KH, Hyuk P, Hoon SS, Kim YW, Hyun SH, Shin T & Hwang WS. (2009). Birth of Beagle dogs by somatic cell nuclear transfer. Anim. Reprod. Sci. , 114, 404-14. PMID: 19059739 DOI.
Lee HS, Yin XJ, Jin YX, Kim NH, Cho SG, Bae IH & Kong IK. (2008). Germinal vesicle chromatin configuration and meiotic competence is related to the oocyte source in canine. Anim. Reprod. Sci. , 103, 336-47. PMID: 17212978 DOI.
Day MJ. (2007). Immune system development in the dog and cat. J. Comp. Pathol. , 137 Suppl 1, S10-5. PMID: 17560591 DOI.
Luvoni GC, Chigioni S & Beccaglia M. (2006). Embryo production in dogs: from in vitro fertilization to cloning. Reprod. Domest. Anim. , 41, 286-90. PMID: 16869883 DOI.
Aguirre GD, Rubin LF & Bistner SI. (1972). Development of the canine eye. Am. J. Vet. Res. , 33, 2399-414. PMID: 4641196
Holst PA & Phemister RD. (1971). The prenatal development of the dog: preimplantation events. Biol. Reprod. , 5, 194-206. PMID: 5165787
Sinowatz S, Wrobel KH, El Etreby MF & Sinowatz F. (1980). On the ultrastructure of the canine mammary gland during pregnancy and lactation. J. Anat. , 131, 321-32. PMID: 7462099
Online Textbooks
- National Research Council (US) Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research Committee National Academies Press (US); (2009)
- Proceedings of the November 2003 International Workshop The Development of Science-based Guidelines for Laboratory Animal Care National Academies Press (US); (2004)
Books
- Complete Book of Dog Breeding by DVM Dan Rice
- Practical Dog Breeding: Principles and Practice by William Haynes (1915)
Search Pubmed
Search Pubmed Now: dog development | canine development | Estrous Cycle |
Additional Images
Historic
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia.
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- University of Georgia College of Veterinary Medicine The Canine Estrous Cycle
- Comparative Placentation Dog
- A Digital Atlas of the Dog Brain PLoS ONE | Online Data
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 10) Embryology Dog Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Dog_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G