Cardiovascular System - Lymphatic Development
| Embryology - 26 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
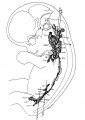
An important part of the cardiovascular system is the lymphatic vasculature, which functions to both return interstitial fluid (lymph) to the bloodstream and also as part of the immune system. In the embryo, lymphatic development begins at the cardinal vein, where venous endothelial cells differentiate (express Prox1) to form lymphatic endothelial cells that out-pocket and bud to form lymph sacs. During development these lymph sacs remodel to form both the lymphatic space within future nodes, formed by engulfed connective tissue, and the associated afferent and efferent vessel network.
This system was first identified by Aselli G. (1627) in a paper "De Lacteibus sive Lacteis Venis", Quarto Vasorum Mesarai corum Genere novo invento. Milan: Mediolani. Then postulated by Sabin (1902)[1] as venous in origin, it required a recent 2007 lineage tracing study to confirm this theory.[2] Only vertebrates possess a true lymphatic vascular system, with primitive fish possessing a lymphatic-like secondary vascular system that also contains blood. Clinically, important for roles in immune surveillance and oncogenic (cancer) processes.
| Historic Embryology |
|
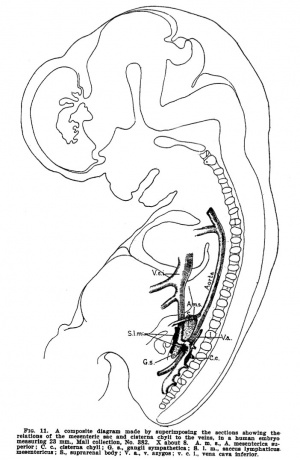
Florence Rena Sabin (1871-1953) was an early USA researcher of the embryological development of the lymphatic system. She studied human embryos (from the Carnegie Collection) and used injected ink studies of the pig embryo, A 1912 textbook chapter on "The Development of the Lymphatic System" was one of the earliest reviews of this system. |
| Cardiovascular Links: cardiovascular | Heart Tutorial | Lecture - Early Vascular | Lecture - Heart | Movies | 2016 Cardiac Review | heart | coronary circulation | heart valve | heart rate | Circulation | blood | blood vessel | blood vessel histology | heart histology | Lymphatic | ductus venosus | spleen | Stage 22 | cardiovascular abnormalities | OMIM | 2012 ECHO Meeting | Category:Cardiovascular | ||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Lymphatic Development | Lymphatic Vessel Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
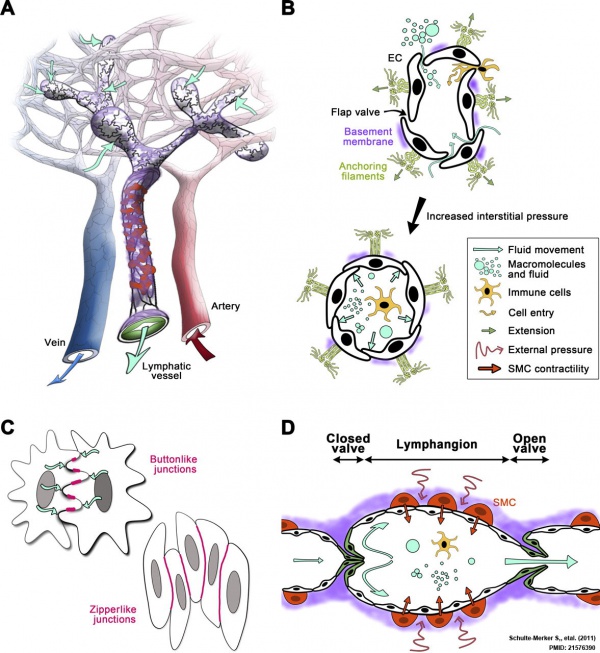
Lymphatic Vessels
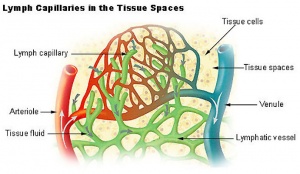
- Lymph capillaries - begin as blind-ending tubes in connective tissue, larger than blood capillaries, very irregularly shaped.
- Lymph collecting vessels - larger and form valves, morphology similar to lymph capillaries.
- Lymph ducts - 1 or 2 layers of smooth muscle cells in wall.
- (Remember the anatomy acronym NAVL = Nerve, Artery, Vein and Lymph)
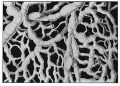
Lymphatic Capillaries
- single-cell layer of overlapping endothelial cells
- lack a basement membrane
- lack smooth muscle cells or pericytes (pre-collecting and collecting trunks contain both)
- linked by discontinuous endothelial cell-cell junctions (button-like).
- junctions open in response to increased interstitial fluid pressure.
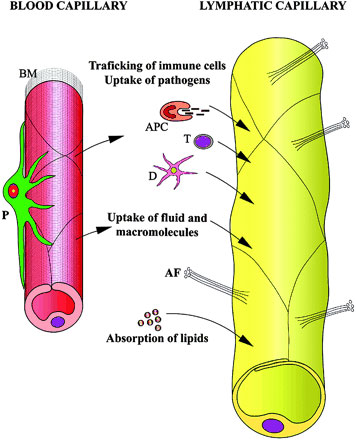
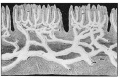
Lymphatic microvasculature model[9]
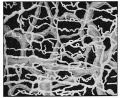
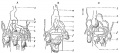
Lymphatic Vessel Development
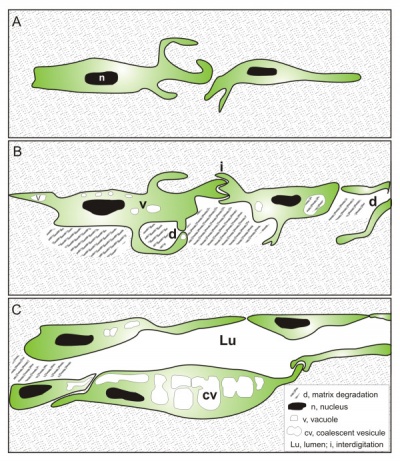
Tunneling model of lymphatic vessel formation. |
The model shown here is from a recent paper[10] and is based on ultrastructural observations performed in in vitro and in vivo models of lymphangiogenesis. Lymphatic endothelial cells (LEC) display tight junctions and interdigitations, and are connected to the surrounding collagen fibers by anchoring filaments.
Note that this postnatal model may differ from developmental lymphatic vessel development.
|
Lymphatic Vessel Contraction
Lymphatic vessels undergo spontaneous rhythmic contractions which aid lymph flow. This is most easily demonstrated in models based upon mesentry lymphatics of the gastrointestinal tract. Contractile activity is regulated by physical factors (transmural pressure) and neurological (alpha-adrenergic, histamine, bradykinin) acting on lymphatic smooth muscle. Contractility and receptor expression may also be different in different parts of the lymphatic system.
Alpha-adrenergic - alpha 1- and not alpha 2-adrenoceptors.
Histamine - lymphatic smooth muscle via stimulation of H(1) (and in some vessels H(2)) receptors.
Bradykinin - chronotropic but not inotropic effects on lymphatic pump activity via stimulation of B1 receptors.
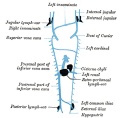
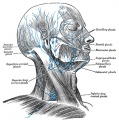
Lymphatic Vasculature Organization[11]
Molecular Development
Angiopoietins (Ang1–Ang4)
Notch probably mediates choice of fate between arterial and venous.
Prox1 Prospero-related Homeobox 1 - expressed in a subpopulation of blood endothelial cells that then generate, by both budding and sprouting, cells of the lymphatic vascular system. Triggers the molecular program leading to the formation of the lymphatic system. (OMIM - PROSPERO-RELATED HOMEOBOX 1; PROX1)
Tie (Tie1 and Tie2) tyrosine kinase receptors.
Vascular endothelial growth factor (VEGF) family of proteins and angiopoietin/Tie, Notch, and ephrin/Eph pathways play major roles in eary vessel development. (VEGFR-3)
LYVE-1
Podoplanin
Abnormalities
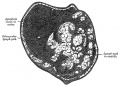
Lymphangioma
Dysplasia of childhood form lymphatic capillaries or collectors, which form fluid-filled cysts.
- lymphatic spaces lined by endothelium
- smooth muscle fascicles in the septa between the lymphatic spaces
- lymphoid aggregates in the delicate collagenous stroma
References
- ↑ Sabin FR. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig (1902) Amer. J Anat. 1(3): 367-389.
- ↑ 2.0 2.1 Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM & Oliver G. (2007). Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. , 21, 2422-32. PMID: 17908929 DOI.
- ↑ Rondon-Galeano M, Skoczylas R, Bower NI, Simons C, Gordon E, Francois M, Koltowska K & Hogan BM. (2020). MAFB modulates the maturation of lymphatic vascular networks in mice. Dev. Dyn. , , . PMID: 32525258 DOI.
- ↑ Stone OA & Stainier DYR. (2019). Paraxial Mesoderm Is the Major Source of Lymphatic Endothelium. Dev. Cell , , . PMID: 31130354 DOI.
- ↑ Jha SK, Rauniyar K & Jeltsch M. (2018). Key molecules in lymphatic development, function, and identification. Ann. Anat. , 219, 25-34. PMID: 29842991 DOI.
- ↑ Yang Y & Oliver G. (2014). Development of the mammalian lymphatic vasculature. J. Clin. Invest. , 124, 888-97. PMID: 24590273 DOI.
- ↑ Del Giacco L, Pistocchi A & Ghilardi A. (2010). prox1b Activity is essential in zebrafish lymphangiogenesis. PLoS ONE , 5, e13170. PMID: 20976189 DOI.
- ↑ Oliver G & Alitalo K. (2005). The lymphatic vasculature: recent progress and paradigms. Annu. Rev. Cell Dev. Biol. , 21, 457-83. PMID: 16212503 DOI.
- ↑ Pepper MS & Skobe M. (2003). Lymphatic endothelium: morphological, molecular and functional properties. J. Cell Biol. , 163, 209-13. PMID: 14581448 DOI.
- ↑ Detry B, Bruyère F, Erpicum C, Paupert J, Lamaye F, Maillard C, Lenoir B, Foidart JM, Thiry M & Noël A. (2011). Digging deeper into lymphatic vessel formation in vitro and in vivo. BMC Cell Biol. , 12, 29. PMID: 21702933 DOI.
- ↑ Schulte-Merker S, Sabine A & Petrova TV. (2011). Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. , 193, 607-18. PMID: 21576390 DOI.
Journals
- Lymphology - Journal Homepage
Reviews
Oliver G & Srinivasan RS. (2008). Lymphatic vasculature development: current concepts. Ann. N. Y. Acad. Sci. , 1131, 75-81. PMID: 18519960 DOI.
Bellini C, Boccardo F, Bonioli E & Campisi C. (2006). Lymphodynamics in the fetus and newborn. Lymphology , 39, 110-7. PMID: 17036631
Oliver G & Alitalo K. (2005). The lymphatic vasculature: recent progress and paradigms. Annu. Rev. Cell Dev. Biol. , 21, 457-83. PMID: 16212503 DOI.
Takahashi M, Yoshimoto T & Kubo H. (2004). Molecular mechanisms of lymphangiogenesis. Int. J. Hematol. , 80, 29-34. PMID: 15293565
Oliver G. (2004). Lymphatic vasculature development. Nat. Rev. Immunol. , 4, 35-45. PMID: 14704766 DOI.
Oliver G & Harvey N. (2002). A stepwise model of the development of lymphatic vasculature. Ann. N. Y. Acad. Sci. , 979, 159-65; discussion 188-96. PMID: 12543725
Articles
Gancz D, Perlmoter G & Yaniv K. (2019). Formation and Growth of Cardiac Lymphatics during Embryonic Development, Heart Regeneration, and Disease. Cold Spring Harb Perspect Biol , , . PMID: 31818858 DOI.
Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL & Oliver G. (2008). Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. , 22, 3282-91. PMID: 19056883 DOI.
Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM & Oliver G. (2007). Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. , 21, 2422-32. PMID: 17908929 DOI.
Bäckhed F, Crawford PA, O'Donnell D & Gordon JI. (2007). Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc. Natl. Acad. Sci. U.S.A. , 104, 606-11. PMID: 17202268 DOI.
Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M & Hong YK. (2006). Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol. Biol. Cell , 17, 576-84. PMID: 16291864 DOI.
Karpanen T, Wirzenius M, Mäkinen T, Veikkola T, Haisma HJ, Achen MG, Stacker SA, Pytowski B, Ylä-Herttuala S & Alitalo K. (2006). Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am. J. Pathol. , 169, 708-18. PMID: 16877368 DOI.
Oliver G & Detmar M. (2002). The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. , 16, 773-83. PMID: 11937485 DOI.
Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity Nicole C. Johnson, Miriam E. Dillard, Peter Baluk, Donald M. McDonald, Natasha L. Harvey, Sharon L. Frase, and Guillermo Oliver Genes Dev. 2008;22 3282-3291
Search Pubmed
Search NLM Bookshelf: lymphatic
Search PubMed: lymphatic development | lymphology
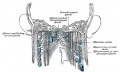
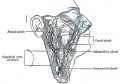
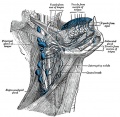
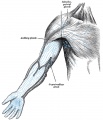
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
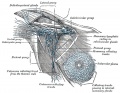
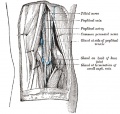
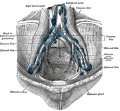
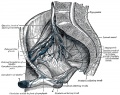
- Gray's Anatomy - Lymphatic
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- International Society of Lymphology ISL | Lymphology Journal
- Australian Lymphology Association ALA
- American Society of Lymphology ASL
- British Lymphology Society BLS
- Embryology original page
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 26) Embryology Cardiovascular System - Lymphatic Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cardiovascular_System_-_Lymphatic_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G