Paper - Two presomite human embryos
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
West CM. Two presomite human embryos. (1952) J. of Gynaecol. Geat Brit. Emp., 59: 336-351.
| Online Editor Note | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This historic 1952 paper by West describes two early embryos similar to a Carnegie stage 7 (26 - 30 days), caudal neuropore closes, Somite Number 21-29.
Modern Notes:
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Two Presomite Human Embryos
Cecil M. West
Department of Anatomy, University College, Cardiff
History and Technique
I have recently had the good fortune to be presented with two well-preserved early human embryos, both at about the same stage of development.
For the first specimen I am indebted to Mr. R. G. Maliphant, of the Department of Obstetrics and Gynaecology of the Welsh National School of Medicine, and in recognition of his kindness I propose to designate the specimen “ Embryo Mal”.
For the second specimen I am indebted to Dr. C. V. Harrison, of the Department of Pathology of the British Postgraduate Medical School, who in turn received the specimen from Mr. V. B. Green-Armytage, of the West London Hospital, and as a token of appreciation I shall call this embryo the “ Gar Embryo ”. I should like here to thank all these gentlemen for their generous gifts and for the clinical information that they have given me about the specimens.
The patient from whom “ Embryo Mal ” was obtained, by hysterectomy, aflirmed repeatedly that she had for years menstruated regularly for 10 days every 28, and her last period began 22nd / 23rd December and continued till about 1st January. Coitus occurred on several occasions before and immediately after this period. If she had continued to menstruate regularly every 28 days her next period would have been due on 19th / 20th January, and assuming that ovulation occurred 14-15 days before this next expected flow it would happen on 3rd—5th January. Coitus took place “ on several occasions after 1st January ” and fertilization, if it occurred within.48 hours of ovulation, would be on 5th—7th January. Operation was on 19th January, and thus the age of the embryo would be 14 to 12 days.
The patient from whom “ Embryo Gar ” was obtained, by hysterectomy, had her last menstrual period from l0th~20th December. She, also, stated that her periods lasted 10 days and that they were at 24-day intervals. Her next period was therefore due on 3rd January, and she would have ovulated on 20th December, the last day of her previous period. Coitus was on 24th and 31st December, and on 5th January, and hysterectomy was performed on 11th January. From the stage of development of the embryo there seems no doubt that the fertile coitus was that of 24th December and not the 31st, and certainly not 5th January. The embryo would thus be 18 days old, which is about its stage compared with other specimens.
The two specimens “ Mal ” and “ Gar ” have thus rather similar menstrual histories, but while “Gar” fits well an age of 18 days “Mal” seems too far advanced for an age of 15 days, and is indeed in some respects more advanced than “ Gar ”.
In each case the specimen that I received consisted of the opened uterus, which had been fixed in formalin. In embryo “ Mal ” the endometrium had been damaged and the chorionic sac was torn at its superficial pole and thus had collapsed owing to the escape of the material (whatever that may be) that normally keeps it distended, but the specimen was otherwise undamaged. The site of implantation was on the left dorsal wall about half-way down the cavity of the body of the uterus.
Embryo “ Gar ” was embedded in the anterior wall close to the ostium of the left uterine tube. It appeared as a hemispherical haemorrhagic swelling 5 mm. in diameter, and raised 4 mm. above the surface of the adjacent endometrium; around the periphery of the swelling there floated a thin wisp of fibrous material (Plate 1, figs.1, 2).
In each case a block of tissue including the implantation area and the whole thickness of the uterus was removed. Most of the muscularis was cut off and the block was then embedded in paraflin and a complete series of sections was cut at 10 u by Mr. A. Welch of this Department. Quite by chance “ Mal” was cut in an almost perfect transverse plane and “ Gar ” in an almost perfect sagittal plane. The sections were mounted, 10 on a slide, and stained with haematoxylin and eosin. Some slides were stained by my colleague, Dr. D. B. Moffat, with silver by a modification of the Wilder-Gomori technique devised by Dr. F. Jacoby, of this Department. Sections were also made of the whole thickness of the uterine wall from blocks taken from the uterus opposite the implantation site. Wax plate reconstructions were also made.
Observations
General
In both cases the ovum consisted of a chorionic sac, with numerous branching villi sprouting from all its surface, and containing an embryo, connected by a thickened area of chorionic or primary mesoblast to the inner surface of the most deeply embedded part of the sac (Plate 1, figs. 3, 4). The state of preservation can be gauged by the numbers of mitotic figures, which are very numerous in “ Gar ” and not quite so numerous in “ Mal ”.
In “ Gar ” (Plate 1, fig. 3; Plate 2, fig. 9) the chorionic sac is not quite circular in the sections but rather triangular, tapering to a blunt point at its deepest part. In “ Mal” (Plate 1, fig. 4; Plate 5, fig. 10) the sac is collapsed and its original form cannot be determined. The internal diameter from side to side of the chorionic sac taken on the middle section is 2 mm. in “ Gar ” and 1.8 mm. in “ Mal ”. The cavity of the sac in “ Mal ” appears in about 290 sections each l0,u thick, though it is difficult to say exactly where the sac ends and villi begin, and thus it measures nearly 3 mm. in this plane. In “ Gar ” the cavity of the sac appears in about 300 sections and thus measures about 3 mm. The measurement from the surface inwards also can be made in “ Gar ” and is, in the middle section, 2.6 mm., and thus the sac is roughly spherical. It contains only the finest coagulum, compared with the darkly staining “ coagulable proteid ” in Herzog’s (1909) case, and the contents of the yolk sac appear to be similar. The chorionic sac is lined with primary mesoblast which is rather more clearly seen in “ Gar ” than in “ Mal” and is rather. thinner towards the superficial pole of the sac. It is continued into‘ the villi, of which it forms the core. The mesoblast cells are more robust and thicker in the villi than in the chorionic sac, where they are very thin and elongated, probably as a result of the greater pressure exerted on them by the contents of the sac. The nuclei are rather clear and granular and are rod-like, oval or nearly round, but some of the round ones are much more darkly stained than the great majority and resemble rather some nuclei of the cytotrophoblast, and it is possible that these are nuclei preparing for division. In many cases the cells of the mesoblast appear to be in continuity with the cytotrophoblast of the chorion from which they are presumably derived. The mesoblast cells lie in various planes but mostly parallel to the walls of the chorion or the villi.
The villi are rather more branched in “ Mal ” than in “ Gar ” and are more luxuriant over the embryonic than the abembryonic pole of the chorion sac.
Uterine Wall
The following description refers mainly to a section of the non-pregnant part of the uterus.
It happens that in both cases the whole thickness of the uterine wall measures 30 mm., of which 10 mm. in “ Mal ” and 6 mm. in “ Gar ” is endometrium (Plate 2, figs. 6, 7). In “ Ma ” the endometrium appears to be 13 mm. thick in the sections owing to obliquity of the plane of section. Both specimens have an endometrium typical of the late pre-menstrual phase. The division of the mucous membrane into the three zones—stratum compactum, and stratum spongiosum with its pars functionalis and pars basalis is very clear in “ Gar ”, the saw-tooth appearance of the glands in the pars functionalis standing out very sharply from the smooth outline of those in the pars basalis (Plate 1, fig. 3). In several places glands dip right down into the muscularis, where they form a nidus for regeneration. The muscularis as a whole is composed of two parts, an outer looser part and an inner, more compact, part. The outer, loose part contains many obvious blood-vessels. A layer of large arteries appears about 5 mm. from the peritoneal surface, and running parallel to it. Between these large arteries and the peritoneal surface there is a considerable plexus of thinwalled veins; then, at about 10 mm. from the surface, large vessels are cut running at right angles to the surface towards the endometrium. These vessels become rapidly smaller and give place to the small spiral arteries characteristic of the endometrium. These can be traced almost to the surface of the mucous membrane and adjacent to them are rather larger thin-walled veins. Capillaries are found close under the surface epithelium. Some of the spaces are almost certainly lymphatics, and as there are said to be no lymphatics in the placenta it is interesting to speculate as to the fate of these vessels in the implantation site. Though the large arteries mentioned above are found in the outer part of the muscularis, small coiled arteries also are found at this level and are more plentiful here than elsewhere in the muscularis. The same general arrangement is found in both specimens, but in “ Mal ” the compact part of the muscularis is reduced and the mucous membrane is increased, while the opposite is the case in “ Gar ”. There is more general vascular engorgement in “ Mal”, which renders the veins more easily visible.
In the pars basalis of the stratum spongiosum the glands have smooth, non-serrated walls; they are widely separated from one another by a stroma composed of closely packed cells with oval nuclei and with here and there smooth muscle cells, prolongations of the muscularis. Incidentally, the pars basalis is more deeply stained and, owing to its numerous closely packed nuclei, it stands out as a dark band compared with the pars functionalis.
In the pars functionalis of the stratum spongiosum the glands are much convoluted, very serrated and closely packed, with only a little inter-glandular stroma separating them. Owing to the large amount of gland and the small amount of stroma this part does not appear so dark as the pars basalis. The distinction between the two parts is clearer in “ Gar ” than in “ Mal ”. The great increase in thickness of the endometrium in “ Mal ” is due to a greater thickness of the stratum compactum, the stratum spongiosum being of about equal thickness in both specimens.
In the stratum compactum there are fewer glands and more stroma, but this zone does not appear dark like the pars basalis because the stroma, though extensive, is loose and there is some oedema; it is in this zone that decidual cells appear (Plate 2, fig. 8). These are large cells with very clear-cut walls and looking like squamous epithelium. They are more numerous in “ Mal” than in “ Gar ”, being widely distributed in “ Mal”, but being restricted in “ Gar ” to the tissue just subjacent to the ovum. The oedema is only slight in “ Gar ”, but it is very marked in “ Mal ”, where it occurs rather in patches and not quite under the surface but at a little distance deeper in the stratum compactum, and to a lesser degree in the stratum spongiosum. It is seen as a series of vacuoles which are presumably degenerative in nature, for, owing to the absence of nuclei, one cannot say whether the vacuoles are inter- or intracellular. The decidual cells, which are seen so well in “ Mal” are found in the stratum compactum close to the surface, and are present also in some of the wide interglandular septa in the depths of the pars functionalis of the stratum spongiosum. Scattered throughout the mucous membrane of “ Mal” are several patches of “ round cells ”, and there is a good deal of general leucocytic infiltration. This, and the increased thickness of the whole endometrium are suggestive of an inflammatory condition.
Implantation Site
A section through the middle of the implantation site is strikingly similar in the two specimens. In each case the area is bounded on its deep surface and at each side by large blood-spaces, and superficially it bulged into the uterine cavity, though this is not seen in “ Mal” owing to the collapse of the chorionic sac already referred toIt is difficult to make accurate measurements of the implantation site, with its irregular boundaries, but the distance from undisturbed maternal tissue on one side to the other in the middle sections measures 4.7 mm. in “ Mal” and 4.1 mm. in “ Gar”. The blood-spaces are large venous channels which drain from the sides rather than the deep surface of the implantation site into the uterine veins. The greatly increased vascularity is one of the main differences between sections through the implantation site and those far distant from it. The two specimens are quite different in regard to the condition of the uterine glands. In “ Gar ” many of them contain blood, but in “ Mal” none of them does. It is interesting to notice how, with the growth of the ovum, the relationship to glands changes. In Plate 1, fig. 5, is shown a section of the Dible-West (1941) ovum, about 12 days old, for comparison with “ Mal ” and “ Gar ” at the same magnification. It is seen that in the younger specimen only 3 or 4 glands are intimately related to the ovum in the sections, while in the older there are many more. Those at the periphery become bent outwards and do ultimately open on the surface, but those directly subjacent to the ovum never succeed in reaching the surface. I have not been able to find a gland being actually opened by the invading syncytiotrophoblast, They just seem to fade away at the necrotic zone of the endometrium, and it is these glands which are so distended, suggesting a damming-up of their secretion. Nevertheless, it seems probable that their more superficial parts are actually disrupted by the invading syncytium and that some of their secretion must enter the implantation site, where it will be available as a source of nutriment for the young ovum. It is, however, surprising to find how few glands do not succeed in reaching the surface. It is interesting, too, to notice that at this stage room for the growing ovum is obtained chiefly by its bulging into the uterine cavity, and it is only later that it burrows deeper into the endometrium, a placenta of the end of the second month having penetrated almost completely through the stratum spongiosum. The blood-vessels, both the spiral arteries and the veins, show rather better in “ Mal ” than in “ Gar ”. Plate 2, fig. 9, and Plate 3, fig. 10, show sections through the implantation site of “ Mal” and “ Gar ” in which the vessels have been marked with Indian ink, and they give a‘ good idea of the great vascularity. The spiral arteries are clearly seen approaching the 1mplantation site and, like the glands, they seem to fade away at the necrotic zone; I have not been able to find an artery in the process of being opened, but they obviously must open into the implantation site. The veins are large spaces, and those immediately bordering the implantation site constitute the blood sinuses. In many places they have lost the endothelium from the wall next to the growing ovum, while it is still clearly seen on the more distant wall.
As is the case with the glands, so the arteries at the sides of the implantation site are rather bent, but they do arrive at the superficial part of the endometrium, while those subjacent to the ovum fail to do so and are opened up, to pour their blood into the intervillous spaces. In a middle section there are about three or four spiral arteries so involved.
The Trophoblast
One of the most difiicult tasks in studying ova at this and earlier stages is the difierentiation of foetal and maternal tissues and the identification of the various cells of the implantation site.
In embryo “ Gar ”, of which the chorionic sac is undamaged, there are seen in a median section about 20 villi attached to the chorion and evenly distributed round it, though rather more luxuriant in their growth at the deeper surface of the ovum. Some of these villi are branched three or four times, and of course the cut surfaces of numerous adjacent villi appear in any section. The walls of the chorionic sac and of the villi are composed of the usual two layers, cyto- and syncytiotrophoblast, and are lined with primary mesoblast. Over most of the chorion and villi there is no distinction, except a topographic one, between the two layers of cells of which the walls are composed, and cell boundaries are not any more clear in the inner than in the outer layer, and the outer layer is not any more syncytial than the inner; but every here and there sprouts grow out from the outer layer (Plate 5, fig. 15) to form multinucleated masses in which cell boundaries cannot be seen and which form a true syncytium. Theoretically, if the syncytiotrophoblast is the outer layer of the wall of a villus, it should be the layer making contact with the maternal tissues in the case of the “ anchoring villi”, and though (Plate 3, fig. 12) the cells which form the actual anchoring cell column are cytotrophoblast, the column does have a thin covering of dark syncytial cells along its sides.
There are two main varieties of syncytium; one (a) consisting of rather pale multinucleate masses with clear, granular, oval nuclei, that sometimes grow out as sprouts from the syncytiotrophoblast layer of the chorion and of the villi, and sometimes appear (Plate 3, figs. 11, 12; Plate 4, fig. 14) as detached masses of a rather foam-like consistency with fine vacuoles in the cytoplasm, lying free in the intervillous spaces. These correspond to the “ resorpti0nsplasmodium ” of florian (1928) and have been often described as showing a brush border. Though these masses do frequently show a clean-cut edge I have not been able to satisfy myself that any brush border is present.
The second variety (b) appears (Plate 3, figs. 11, 12; Plate 4, fig. 14) as darkly staining, multinucleate wisps or shreds of cytoplasm, of which the nuclei are very darkly stained, pycnotic, and irregular in shape. This variety of syncytium is widely spread. It occurs as a thin covering for the more distal parts of the villi, it lies against the maternal tissue and even in among the maternal cells and appears to be the invasive agent and corresponds to florian’s “ Proliferationsplasmodium ”.
There is a third variety of syncytium (c) (Plate 3, figs. 11, 13) that consists of very pale, clear, cytoplasmic masses with well-defined borders and very clear, bright, granular nuclei. This variety usually appears as free masses adjacent to maternal tissue, sometimes lying free in the maternal sinuses, but sometimes it seems to be in direct continuity with maternal tissue, and I believe it to represent a stage in the degeneration and destruction of the maternal cells.
It is an extremely diflicult matter to distinguish foetal and maternal tissue. Brewer (1937) has shown that this can be done by recognizing that the maternal tissue always shows, in silver preparations, reticular fibres, which are absent from the foetal tissue. It is true that reticular fibres are prominent in the maternal tissues (Plate 5, fig. 17), but where the maternal tissues are being disintegrated by the growing ovum one comes across areas that are clearly maternal but do not show any reticular fibres. The difliculty is to distinguish such areas from sheets of cytotrophoblast which often occur adjacent to maternal tissues and which do not show reticular fibres. However, in some silver sections (Plate 5, fig. 17) one can see maternal tissue which has lost its reticular fibres and which I believe to be in a process of dissolution. In haematoxylin and eosin sectionsthe transitional zone between foetal and maternal tissues appears, under low magnification, as a dark blue band between the main implantation area and the unaltered maternal tissue.
In “ Mal ” the “ Resorptionsplasmodium ” is not so foam-like as in “ Gar ” but recalls the “ Plasmodium ” of Bryce and Teacher (1908). Otherwise the appearances are very similar in the two specimens.
There remains to be mentioned a curious condition, seen only in “ Gar ”, in the cells of the syncytiotrophoblast of the chorionic vesicle, and particularly of the villi, which show a vacuolation of their cytoplasm which pushes the nucleus to the periphery of the cell and gives it a crescentic shape, so that the cell comes rather to resemble a fat-cell. This condition is found only in those cells of the villi and chorion that lie towards the superficial pole of the ovum (Plate 5, fig. 16). It may represent a rather similar condition that Wislocki and Streeter (1938) have referred to in the 19-day macaque as an example of water imbibition, or pinocytosis.
Embryonic Rudiment
The embryonic rudiment consists of embryonic disc, yolk-sac and amnion joined to the inner surface of the deep part of the chorionic sac by the connecting stalk.
The embryonic disc in both specimens, seen from the dorsal surface, is wider than it is long, particularly in “ Mal”, the measurements being in “ Mal ” 0.45 mm. antero-posteriorly and 0.6 mm. from side to side, and in “Gar” 0.56 mm. antero-posteriorly and 0.69 mm. from side to side. There has been some deformation in “ Mal”, the anterior end of the disc having been bent ventrally on the right side. The yolk sac too has been pushed caudally and in this process has been peeled from off the ventral surface of the anterior end of the disc, which thus projects in front of the yolk-sac. The displacement is probably a good deal less than it appears to be and is partly due to the obliquity of section dependent on the arched character of the disc; this makes the interpretation of the structures at the anterior end of the embryo rather difficult.
In both specimens the dorsal surface of the embryonic disc is convex and the ventral surface concave from side to side and particularly from front to back.
As seen in a wax model, each embryonic disc shows a prominent elevation at about the middle of its dorsal surface overlying the primitive node, and towards the posterior end it is marked by the primitive groove. The arrangement of the ectoderm cells is better seen in a transverse section of “ Mal ” than in a sagittal section of “ Gar ”. Plate 5, fig. 15, shows a section through the primitive streak and groove of “ Mal”. The ectoderm cells are set in a palisade arrangement and at the margin of the disc there is an abrupt change to the flattened cells of the amniotic ectoderm.
The arrangement of the axial structure of the embryo can be most easily followed first in “ Gar ”, and it is again seen how abrupt is the change from the columnar ectoderm cells of the disc to the flat amniotic ectoderm (Plate 5, fig. 18). The sections are not quite in the sagittal plane, the middle of the anterior end of the disc being cut three sections before the middle of the posterior end.
On looking through the series of median and paramedian sections (Plate 6, fig. 21, to Plate 8, fig. 29) it is obvious that the disc is markedly convex dorsally and concave ventrally, and the summit of the curve can be used to divide the disc into an anterior and a posterior part. In the anterior part there is a wide and almost empty space between the ectoderm and endoderm occupied by only a few scattered cells. Projecting forwards into this space is the headprocess, which on its ventral side is closely fused with the endoderm, but on its dorsal side is separated from the ectoderm by a backward continuation of the space already mentioned. Traced caudally the head-process is seen to be continuous ‘with a mass of cells which, in its turn, is fused with the endoderm and with the ectoderm also. This is Hensen’s node. Though it is fused with the ectoderm dorsally the cells of the node and of the head-process are distinguishable from the ectoderm by their less regular arrangement and by their rather lighter staining (Plate 6, fig. 20). The ectoderm overlying the node is much thinner than elsewhere (Plate 6, fig. 20), and when traced either cranially or caudally it thickens rapidly, particularly cranially. The ectoderm caudal to the node which in the middle line is fused with the endoderm constitutes the primitive streak (Plate 7, figs. 23-25). Owing to its fusion with the endoderm there is not the same wide space caudal to the node that there has been noticed cranial to it. The head-process is much shorter than one would expect at this stage. It gradually fades away into the endoderm when traced forwards, and there is no thickening of the endoderm in front of it to indicate the presence of a prochordal plate.
In many of the sections of “ Gar ” small spherical blebs are seen projecting, like a secretion, from the dorsal surface of the ectoderm into the amniotic cavity (Plate 11, fig. 40).
The transverse sections of “ Mal” form a useful check on the appearances in “ Gar ”, and one can see more clearly than in “ Gar ” the difference in the characters of the ectoderm of the node and of the primitive streak and of the general body ectoderm.
Plate 8, fig. 30 and Plate 9, fig. 34, show sections through the primitive node in “ Mal ”. Passing caudo-cranially (i.e. from fig. 30 to fig. 34) one sees that the general body ectoderm in the middle line is arched over a mass of cells, from which it is quite distinct, and that it becomes thinner as it is traced cranially. The ectoderm cells in the middle line do not have the columnar character of those more laterally placed. As one proceeds cranially the mass of cells ventral to the ectoderm becomes more difficult to distinguish from the latter. It is throughout fused with the endoderm as well as with the ectoderm. It is the primitive node and as a whole its cells are arranged radially round what appears as a small cavity, hardly sufficient to warrant the name chorda canal. In this connexion it may be pointed out that it is not possible to differentiate Hensen’s node and head344
process, parfly due to the bending over that has aifected the anterior end of the embryo and partly to the very close connexion that, as Streeter has pointed out, exists between node and head process. The appearance in “ Mal” is very similar to that shown by Jones and Brewer (1941) in their Plate 5, figs. 13-17. The radial arrangement of the cells is also similar to what Heuser (1932) described in a beautiful embryo of about this age, but at a slightly more advanced stage of development, and, as Heuser pointed out, the nuclei of the cells are situated away from the centre of the circle that they form (Plate 9, fig. 33). Traced cranially the nodal tissue loses this radial arrangement and forms a thick wide sheet of cells, associated with the endoderm rather than the ectoderm, and which I interpret as the head-process though, as just mentioned, it is not easily distinguishable from the node. Traced caudally the tissue of the node gradually gives place to the primitive streak. Throughout its length the node is associated laterally with mesoblast, but in view of Streeter’s (1927) findings in the pig the association may be assumed to be only topographical.
Caudal to the node the ectoderm is continuous from one side to the other across the middle line forming a uniform curve convex dorsally, but after 7 or 8 sections a groove appears in the mid-dorsal line and soon the ectoderm on each side of this makes a prominent dorsal bulge and the whole ectodermal sheet appears in transverse section like a Cupid’s bow (Plate 5, fig. 15). This groove, the primitive groove, extends back to the caudal end of the disc, covering 180,11. One would expect to find ventral to it the primitive streak.
What one finds is that the ectoderm cells on each side of the middle line are arranged in a rather regular palisade fashion, but in the actual middle line the groove is formed by a turning-in ventrally of the ectoderm, and the nuclei become round rather than long and occupy the ventral part of the cell, with the result that the floor of the groove is formed by the cytoplasm of the dorsal part of the cells. The turned-in cells in the mid-line form a prominent V-shaped mass the apex of which just fails to make contact with the endoderm. Between the prominence of the node cranially and the primitive groove caudally, the surface ectoderm in the middle line is flat "on the dorsal surface. An ingrowth of cells from the ventral surface gives rise to a rather wide, flat plate. This is the “ neck ” that Streeter (1927) has referred to as being a transitional structure between primitive node and primitive streak.
Embryonic Mesoblast
In “ Mal” it is quite clear that the mesoblast is connected with the cells of the primitive streak and of the node. From these it spreads out on each side between the ectoderm and endoderm. Near the middle line it seems fairly thick, but further laterally it is represented by only a few scattered cells, either free or connected by protoplasmic strands to either ectoderm or endoderm. When their distribution is plotted on the embryonic disc the mesoblast cells are seen, particularly in “ Gar ”, to spread from the region of the primitive streak forwards in two horns, having an area adjacent to the middle line almost free of mesoblast. In “ Mal” the mesoblast cells are more evenly distributed.
It is of course difficult in the sections of “ Gar ” to trace any continuity between the primitive streak and the mesoblast. Indeed I rather get the impression that the connexion of the mesoblast is with endoderm rather than with ectoderm. The few cells that are found in the wide space between ectoderm and endoderm anterior to the node, and, indeed, posterior to the node also, seem to be more closely connected to the endoderm. Yet in many places the mesoblast cell nuclei are seen crowded against the dorsal surface of the endoderm but show attenuated processes extending towards the central surface of the ectoderm as if they had been derived from that layer and their connexion was just being severed. One must also bear in mind that the appearance may be due to shrinkage. At the margin of the embryonic disc the mesoblast cells form a sheet which splits to pass round the amniotic ectoderm dorsally and the yolk-sac endoderm ventrally. Over the amnion it blends with the primary chorionic mesoblast to form the connecting stalk.
In three sections of “ Gar ”, towards the caudal end of the embryo and 20 sections from the middle line (Plate 6, fig. 19), there appears a space in the embryonic mesoblast very like that referred to, and illustrated by Johnston (1940) which_ he considers to be a precociously developed coelomic space. In the present specimen it appears to be in continuity with the extraembryonic coelom and to represent a portion of this space that has become included within the embryo. There are so many spaces, artificial and otherwise, in the mesoblast that it seems unwise to attach any particular significance to this one, but I mention it on account of its similarity to the condition seen in J ohnston’s embryo.
The Yolk-sac
In “ Mal ” the yolk-sac has suffered slightly from the general collapse of the chorionic sac and is a thin-walled sac compressed ventrodorsally. In “ Gar ” it is thicker and almost spherical. In both specimens it is in general smooth, but the covering mesoblast is thickened in places, and here and there small protrusions of the endoderm occur, which get cut off as isolated islands of endoderm cells with a covering of mesoblast (Plate 8, fig. 29). It is tempting to regard these masses as foci of early blood formation. In many places in the mesoblast covering the yolk-sac there are small spaces with an endothelial lining (if one can speak of such a thing at this stage.) They are very similar to other spaces in the chorionic mesoblast which are early blood capillaries. The endoderm cells are largest in the ventral part of the yolk-sac and smallest in the dorsal part. They almost look as if they were destined for a different fate ventrally from what they are dorsally. They are also particularly enlarged just caudal to the evagination of the allanto-enteric diverticulum (Plate 8, figs. 27, 28).
Allantois
The allanto-enteric diverticulum is very striking in “ Gar ”. It has been out nearly along its whole length in one section (Plate 8, fig. 27; Plate 10, fig. 37). It arises from the yolk-sac just posterior to the attachment of the amnion to the embryonic disc and then runs dorsally and slightly forwards. It is of considerable length and at its distal end divides in a Y-shaped manner into two widely separated vesicles (Plate 10, figs. 35-38), one of which, however, has become detached from the stem. Both vesicles and the duct have a distinct lumen. The endoderm of the yolk-sac just caudal to the site of the evagination of the diverticulum is considerably thickened, and the walls of the duct and vesicles are composed of large, deeply staining cells. The diverticulum projects into the connecting stalk and is covered by a thick layer of connecting-stalk mesoderm on its anterior surface, but by only a very thin layer on its posterior surface. Several early blood capillaries are present in the mesoderm adjacent to the duct.
In “ Mal” the allantois is not so large. It arises from the yolk-sac quite a long way (0.2 mm.) caudal to the posterior edge of the disc ectoderm, and this is related to the caudal displacement of the yolk-sac which has been mentioned, and is associated too with obliquity of section resulting from the arched shape of the embryonic disc. From its origin it turns sharply in a cranial direction and its extremity is slightly dilated into a small vesicle. As in the case of “ Gar ”, the endoderm of the yolk-sac caudal to the diverticulum is thickened and stands out sharply in this respect from the adjacent endoderm.
Cloacal membrane
In “ Gar ” the cloacal membrane is recognizable as a caudal outgrowth of the amniotic ectoderm which comes into contact with the cells of the base of the allantois without any mesoblast intervening (Plate 10, figs. 37, 38). The membrane does not seem to involve either true embryonic ectoderm or yolk-sac endoderm. In “ Mal ”, on the other hand, the cloacal membrane is represented by an area well forward of the allantois (Plate 11, fig. 39) where disc ectoderm is in contact with yolk-sac endoderm with no mesoblast intervening.
Blood-vessels and connecting stalk
Early blood-vessels are numerous in the mesoblast of the chorion and its villi (Plate 11, fig. 41) and appear as extremely thin-walled empty spaces. The cells that bound these spaces are rather more slender than the general mesoblast cells and have smaller, darker, and less granular nuclei. These early vessels can sometimes be seen turning from the chorion into the villus. They are particularly large in the connecting stalk (Plate 11, fig. 42), about which a word may here be said.
It is seen best in “ Gar ” and is constituted by a union of the mesoblast covering the outer surface of the amnion with that lining the inner surface of the chorion. But it is not the whole antero-posterior length of the amnion that is so connected but only about the posterior twothirds. The connexion is not restricted to that between chorion and amnion, but the caudal part of the yolk-sac also is so connected, and it is into this mesoblast that the allantois grows. There is no definite anterior or posterior surface to the connecting stalk and one cannot speak of there being any mesothelial covering for it. The mesoblast is indeed rather loosely arranged caudally. Throughout the connecting stalk and particularly in its caudal part near the allantois primitive blood-vessels are large and numerous, but the absence of any true mesothelial covering excludes the origin of blood-vessels, as Bremer (1914) suggested, by the ingrowth of the cells of such a mesothelial covering. Hertig (1935) came to the same conclusion with regard to early blood-vessels in Stieve’s “ Hugo ” embryo. In the caudal part of the connecting stalk of “ Gar ”, and lying up against the amniotic ectoderm, there appears in three sections (Plate 6, figs. 21, 22) a curious compact mass of darkly staining cells with large, round nuclei, which I can only suppose to be early blood-cells, though they do not lie in any closed vessel.
Another curious structure appears in the connecting stalk in “ Gar ”, again on the dorsal side of the amniotic ectoderm and about half-way between the anterior and posterior ends of the amnion (Plate 11, fig. 40). It consists of a concentric arrangement of cells in which it is diflicult to distinguish amniotic ectoderm and mesoblast. All the cells have a degenerate look and scattered around this nest of cells there are many dark granules. Similar granules and cellular debris lying free in the amniotic cavity appear in several sections. I have little doubt that these and the concentric nest of cells represent different stages in the enlargement of the amniotic cavity
by breakdown of its dorsal wall, as suggested by florian (1930).
Discussion
The two specimens are at much the same stage of development, though the villi are rather more advanced in “ Mal”, but this is the younger specimen according to the history, which goes to show that the classification of ova by age does not necessarily give a true picture of their stage of development. The Heuser (1932) embryo, for example, is regarded as being 18% days old, but is considerably more advanced than both of the present specimens. There must be many different environmental factors alfecting the development of the ovum.
It is of interest that the two patients both showed exceptionally long periods of menstrual flow. In this connexion attention may be called to the haemorrhagic appearance of the ovum in the fresh condition in S“ Gar ” and the bearing of this on Brewer’s (1937) remarks on placental sign bleeding.
The two specimens show particularly well the structure of the whole thickness of the uterine wall and it is interesting to compare the appearance of the endometrium at, and distant from, the implantation site.
Both specimens are at the stage when the villi growing into the decidua basalis are beginning to exceed those at the opposite pole.
Krafka (1941) in describing an ovum a good deal younger than the present specimens refers to the operculum of earlier stages and says: “ Silver stain supports the contention that the operculum contains some stroma. It also includes some syncytium.” This raises the interesting question of the wayin which the ovum in later stages, e.g. “ Gar ”, bulges into the uterine cavity. What is it that really constitutes the decidua capsularis? An early-stage, such as the Dible-West (1941), ovum is hardly raised at all above the level of the adjacent endometrium; it has an operculum, and there is a gap in the epithelium and very little tissue at all, other than degenerate endometrium, covering the ovum, the distance from the anterior end of the chorionic sac to the surface being 0.2 mm. In “ Gar ” the similar measurement is 0.6 mm., and the ovum as a whole bulges into the uterus 3 mm. more than in the younger specimen. The thickness of 0.6 mm. in “ Gar ” is made up mostly of chorionic villi and the thickness of the actual covering tissue is only 0.1 mm., made up of a layer of fibrin next to the ovum and then of cellular tissue material, outside of which is a thin fibrinous layer, but there is no epithelium to be seen. The covering layer (decidua capsularis) is lined on its deep surface with patches of darkly staining syncytium, as elsewhere in the chorionic sac, and one wonders what prevents the erosion of the covering tissue and why it is not afiected by the growing ovum more than to be pushed into the uterine cavity.
Krafka suggests the use of the term “ stroma reaction ” rather than decidual reaction, and regards as part of this reaction the appearance of decidual cells, which may have become enlarged as the result of imbibition of oedema fluid or of storage of glycogen. This is not to be interpreted as meaning that all oedematous cells are decidual cells. The two, at least in “ Mal ”, are quite dilferent.
It would seem that the development of oedema would be for assisting in the implanting of the ovum rather than for the limitation of the advance of the syncytium. It is hard to see how a degenerate and fluid medium could well serve as an obstruction. Further, if some of the decidual cells are oedematous and some contain glycogen, and if both are easily disrupted, this would put the glycogen at the service of the ovum. But we know that decidual cells develop in the course of the ordinary menstrual cycle. Is their appearance yet another of the astonishing hopeful provisions made for a possible pregnancy?
Attention has been called to the outward bending of glands and blood-vessels around the ovum. Grosser (1910) regards this as an expression of the dilatation of the egg-chamber caused by the growth of the ovum. Greenhill (1927) also refers to the curvature of the glands. The absence of blood in the glands in the one case and its presence in the other is of interest, and is linked with the whole process of growth of the ovum. Krafka (1941) in his Plate 1, fig. 4, shows four zones of the endometrium which he calls, from the surface inwards, stratum compactum, oedema zone, spongy zone, and stratum basale. I get the impression from his picture and from comparison with “ Mal ” and “ Gar ” that his “ stratum compactum ” is continuous beneath the ovum from one side to the other and has been pushed in towards the stratum spongiosum by the growing ovum. In “ Gar ” in particular it looks as though the stratum spongiosum was being gradually pushed out of the way, and that the ovum is advancing as a wedge, partly pushing tissues forward in front of it and partly dividing them into two waves, and that there is as much a pushing apart as an erosion or digestion of the tissues. When one comes to later stages-—-—for example a pregnancy of about 2 months—one finds that the chorionic villi almost reach the stratum basale, but that between them and this stratum there is a thick layer of tissue looking just like the stratum compactum of earlier stages, and that at the periphery of the placental area the glands are bent out of the way.
Returning to the question of blood in the glands: Krafka deals at some length with this, and gives examples of ova belonging to Streeter’s (1927) Group I (i.e. embryos with no primitive streak), the earlier of which have no blood in the glands while the later have. He regards the blood-in-the-glands reaction as the final phase in what he calls the sinusoidal reaction, by which he appears to mean a venous dilatation associated with a circulation round the ovum. I do not know how long the blood remains in the glands nor what happens to it, but these two specimens suggest some variation in the time of its appearance. I assume that its fundamental interest is as evidence of erosion of the glands by the growing ovum.
In the implantation site, either among the maternal cells along the walls of blood-vessels or lying free in the intervillous spaces, there are syncytial masses of various kinds. It is difiicult to find in the literature an account of all the difierent varieties, though many authors have described some.
To sum up what has been said in earlier pages, there are in “ Mal” and] or “ Gar ”:
- Syncytial sprouts attached to the walls of chorion and villi, sometimes lying free in the intervillous spaces and having a foam-like appearance as described by Johnston (1941) and corresponding to the syncytiotrophoblast of Hamilton and Gladstone (1942) and to the Resorptionsplasmodium of florian (1928). I have not been able to satisfy myself that these masses attached to the ovum have a brush-border as shown by Brewer (1937) and by Hamilton and Gladstone (1942) and described by Herzog (1909).
- Very darkly staining wisps covering the surface of the villi and particularly obvious where the villi are spreading out to form the large cytotrophoblast sheets. It is this type of tissue which seems to “ stream out ” against the walls of maternal veins and among the cells of maternal stroma, as Johnston (1941) and Latta and Tollman (1937) describe. Hamilton and Gladstone (1942) confirm the opinion held by florian (1928) that such dark-staining masses lying deep in the maternal stroma are connected together. This may be so, but I have not always been able to find connexion between these conjoined masses and the syncytium in the implantation cavity, and one can only suppose that the previous connexion has broken. Though these wisps and shreds of syncytium are found far afield in the maternal tissue and are, one would therefore suppose, invasive in nature yet their nuclei are small, flat, and fragmented and appear degenerate. This material does not stain so darkly in “ Mal” as in “ Gar ” and occurs in rather broader masses.
- Pale, clear, and bright syncytial masses, with only one or two, or with many nuclei, lying free in the intervillous spaces or sometimes in the lumen of opened maternal veins. These masses always occur nearer the maternal than the foetal side of the implantation cavity but always in areas where there are no reticular fibres, and thus, according to some authorities, in foetal and not maternal tissue. Nevertheless, there surely must be an area where maternal tissue is becoming affected by the presence of the ovum, but has not yet completely disintegrated. and such an area might look to be composed of tissue that is maternal in all respects except that it has no reticular fibres. ,Delporte (1912) refers to these bright, clear, syncytial masses and derives them from the cytotrophoblast sheets. and regards them as an expression of the vitality of the tissue that they form.
Grosser (1910, p. 95) quotes Bonnet (1903) as defining syncytia and plasmodia as “living and active formations, endowed with especially energetic metabolism ” and symplasmata as masses “ which show unmistakable signs of commencing degeneration ”. Grosser goes on to say that “ syncytia and plasmodia are chiefly formed by foetal tissues ” and that “ symplasmata, on the contrary, arise from the maternal tissues ”. This may all be true and it may be that some of the detached syncytial or plasmodia] masses have got some particular metabolic function and may be responsible for altering maternal tissues. One is so accustomed to seeing these detached masses in sections that one is apt to forget what they are like in life. They are presumably floating freely in maternal blood; they are too large to pass into the maternal veins, but one does wonder why they do not flow; if they are free, with the blood into the mouths of the maternal veins. Grosser (1910, p. 147) actually does refer to the syncytial masses (“ proliferation-nodes ”) being “ carried in the circulation far from the intervillous space ”. Presumably these isolated masses receive nourishment from the blood in which they lie.
They cannot assist in the nourishment of the ovum unless they alter in some way the maternal blood so as to make it more easily utilized by the chorionic villi. Grosser (1910) refers (P. 126) to the absorbing syncytium possessing prickleprocesses, after the development of a circulation in the blood lacunae, but these, which I take to be synonymous with brush-border are described by several authors as found on the free syncytial masses which cannot possibly have any useful resorptive function. It may be, as he suggests later on, that these free syncytial masses are important in preventing coagulation of the maternal blood.
Mossman (1937) suggests that in the rabbit multinuclear giant-cells may secrete some enzymatic material that would help in dissolving the tissue debris found in this animal at the placental margin. In this connexion it is interesting to study the types of syncytial tissue found at later stages. In a placenta of about 2 months one finds villi attached to maternal tissue by spread-out sheets of cytotrophoblast. The cytotrophoblast of the villi is covered by the darkly staining wisps of the degenerate-looking syncytium as in earlier stages. These penetrate also into the maternal tissue. Free rnultinucleated syncytial masses are numerous, and I get the impression that they contain many more nuclei, more closely packed, than the corresponding masses of earlier stages. In many cases there is no doubt that they are detached portions of sprouts that have grown out from the syncytiotrophoblast of the chorion and villi. The bright, clear, pale, syncytial masses of earlier stages are absent, but there are places where it looks as if decidual cells were in the process of being converted into such masses. One wonders whether there is a continual degeneration and replacement of syncytium going on.
My conclusion is that all the darkly staining syncytial masses, whether sprouts from the chorion, shreds on the villi, or deeper in the maternal tissue or free in the intervillous spaces, are of foetal origin, while the bright, clear, masses are of maternal origin. The latter must be in a state of degeneration, and I believe that some of the former are also, though since in later stages it is only the syncytial layer of the villi that persists and since at the present stage some of the villi have no, or only a degenerative, syncytial covering it is clear that there must later be a renewal of the syncytiotrophoblast at the expense of the cytotrophoblast. Grosser (p. 150) refers to the way in which the cytotrophoblast (Langhans’s layer) becomes used up partly by being stretched out over the ever-increasing villi and partly in the formation of syncytium, and to the absence here and there of even a syncytial covering to the villi, the deficiency then being made up by a coating of fibrin.
Fibrin is not prominent in these two specimens, but a thin film of fibrin can be seen here and there, particularly at the superficial pole of the ovum and better in “ Gar ” than in “ Mal ”.
Embryo
Theoretically one would expect the dorsal surface of the embryonic disc to be circular in outline in its earlier stages and to acquire later on an elliptical form. Hill and florian (1931) have called attention to the long and wide varieties of disc and suggest that these may be fundamentally different types in man. They are no indication of different developmental stages.
The sections through the more caudal part of “ Mal” are very like those through the same region of the “ Dobbin ” embryo shown in Hill and florian’s figs. 22 and 23 (1931), but the “ Dobbin ” embryo as a whole is more advanced than “ Mal ” since it shows a chorda-canal. The authors call attention to the active proliferation of mesoderm from the primitive streak in these sections and regard the area as being a little anterior to the cloacal membrane. The appearance in “ Mal ” is very similar.
The middle sections through “ Gar ” are very like the picture shown by Heuser and Streeter (1941) of a 19-day macaque in their Plate 28, fig. 210, and even more like the drawing of the same specimen in their text-fig. 4E.
Streeter (1927), in his account of the development of the mesoblast and notochord in pig embryos, describes the earliest stages of the prochordal plate as being represented by a compact, orderly arrangement of the endoderm cells and he emphasizes the individuality of Hensen’s node, its function as a source of the notochord, and its tendency to a separation from the anterior end of the primitive streak and to fusion with the endoderm. All these characters are seen in “ Mal ” and “ Gar ”, but the arrangement of the endodermal cells preparatory to the formation of the prochordal plate has not yet taken place.
Another specimen that “ Gar ” resembles is Waldeyer’s (1929) “ Soho ”. It, too, has a wide embryonic disc. The sections are not quite sagittal, but those through the primitive streak are quite similar to “ Gar ”; but “ Scho” is undoubtedly older.
Hill and florian (1931) give in their text-fig. 15 an instructive series of reconstructed sagittal sections through several embryos. The one most closely resembling “ Gar ” in all respects is Stieve’s (1926) embryo “ Hugo ”, but this is only 13% days old. The dorsal view that these authors give of the embryo “ Hugo ” also is very like “ Gar ”, and is like “ Mal ” too.
Johnston (1940) shows in his embryo H.R.l an allantois rather like what is seen in “ Gar ”, though it is not cut so completely along its length. He shows also a mass of cells in the connecting stalk like what I have regarded as a bifurcation of the distal end of the allantois, but which he refers to (p. 19) as “ suggestive of a large blood island ”. I think I am right in my assumption that the similar mass of cells in “ Gar ”’ is allantois, since the cells are radially arranged round a central lumen. There is, however, in “ Gar ” a solid clump of cells in the connecting stalk, rather like that described by Johnston, that I think may well be a blood island. The cloacal membrane area, too, in H.R.1 is quite like what is seen in “ Gar ”.
The embryo of Jones and Brewer (1941) is very like “ Mal ” in the relationship of the “ primitive ectoderm ” to the node (their ‘Plate 1, fig. 3). Incidentally, the stage of development of the villi is very similar in the two. Their embryo is cut transversely and they define as headprocess cells that do not show a radial arrangement, but that are irregularly heaped up and are smaller than the cells of the node and distinctly separated from both the overlying ectoderm and the underlying endoderm. They regard the condition seen in their specimen as representing the earliest stage of the head-process seen in man. “ Mal” seems to be at a very similar stage of development, though I cannot be certain as to the neurenteric canal which Jones and Brewer describe. Their medium sagittal reconstruction and their dorsal view of the embryonic disc are both very like “ Gar ”. They estimate the age to be 18.5 days.
The connecting stalk in the two specimens hardly merits this name and resembles the area of contact between embryo and chorion seen in younger stages, and represented by florian’s (1930) text-fig. 2 of Von M<'5llendorff’s embryo “ O.P. ”. It is from florian’s work described in the above paper that the solution has come of the meaning of the concentric arrangement of cells in the connecting stalk just dorsal to the amnion. florian, in his turn, refers to the description by von Mollendorfl of a degenerative process occurring in the amniotic ectoderm.
With regard to the angiogenesis in the chorion and yolk-sac, Hertig (1935) suggested that, so far as the macaque is concerned, the appearance of angiogenic foci in the yolk-sac is delayed until after vascular primordia are prominent in the chorion, amnion, and villi, and that angiogenic foci in the yolk-sac are derived by a process of growth and migration of elements derived from angiogenic foci arising from trophoblast cells. In the present specimens angiogenesis is clearly seen in the mesoblast of chorion and villi, and seems more definite there than in the wall of the yolk-sac. Here and there in the latter there are spaces with apparently an endothelial lining, which I interpret as early blood-vessels. Blood islands also occur in the wall of the yolk-sac and in the connecting stalk, but I can see no evidence that angiogenic foci in the wall of the yolk-sac have been derived from elsewhere. The empty state of all these early vessels should be mentioned, as was noted by Gladstone and Hamilton (1941) in their embryo “ Shaw ”. These authors referred to the groups of rounded cells that lie between the endoderm and the mesoderm of the yolk-sac wall (the blood islands) and they suggest that the cells comprising these groups may be primarily derived from endoderm. This sometimes looks to be the case in “ Gar ”, and when outpocketings of the endoderm occur and are cut oil from the yolk-sac the centre of such groups of cells is clearly of endodermal origin.
Summary
- Two well-preserved human ova of about the 18th day are described. One is cut transversely and the other sagittally.
- An account is given of the endometrium at. and distant from, the implantation site.
- One specimen shows blood in the glands, the other does not.
- An account is given of the varieties of syncytium. It is suggested that maternal tissues in the process of being destroyed can be recognized.
- The embryonic discs measure 0.45 mm. and I0.56 mm. long, and 0.6 mm. and 0.69 mm. wide. Primitive streak and node can be recognized in both specimens, and a short head-process in one. The allantois is very well developed in one and much smaller in the other.
- Early blood-vessels are seen in the mesoblast of the chorion, villi and connecting stalk.
- A good example is seen of the method of enlargement of the amniotic cavity.
I am indebted to Mr. A. Welch, of this Department, for the photographs illustrating this paper. They are all untouched.
References
Bremer JL. The earliest blood-vessels in man. (1914) Amer. J Anat. 16(4): 447-475.
Brewer JI. A normal human ovum in a stage preceding the primitive streak (The Edwards-Jones-Brewer ovum). (1937) Amer. J Anat., 61: 429-481.
Bryce TH. Observations on the early development of the human embryo. (1924) Trans. Roy. Soc. Edinburgh, 53: 533-567.
Bryce, T. H., and Teacher, J. H. (1908): Contributions to the study of the early deveiopmemt and imbeciding of the human ovum. MacLehose, Glasgow.
Delporte, F. (1912): Contributions ii Fétude de la nidation de Foeuf humain et de la physiologie du trophoblast. Lamertin, Bruxelles.
Dible JH. and West CM. A human ovum at the previllous stage. (1941) J Anat. 75(3): 269–281. PMID 17104860
Florian, J. (1928): Anat. Anz., 66, Ergc'inzungsh., 211.
Florian, J. (1930): J. Anat., Lond., 64, 454.
Gladstone, R. J., and Hamilton, W. J. (1941): J. Anat., Lond., 76, 9.
Greenhill, J. P. (1927): Amer. J. Anat., 40, 315.
Grosser, O. (1910): in Keibel and Mall, Human embryology. Lippincott, Philadelphia. p. 91.
Hamilton, W. J., and Gladstone, R. J. (1942): J. Ana:., Lond., 76, 187.
Hertig, A. T. (1935): Contr. Embryol. Carneg. Insm., 25, 37.
Herzog, M. (1909): Amer. J. Anat., 9, 361.
Heuser, C. H. (1932): C ontr. Embryol. Carneg. Instn., 23, 251.
Heuser, C. H., and Streeter, G. L. (1941): Conrr. Embryol. Carneg. Insm., 29, 15.
Hill, J. P., and florian, J. (1931): Phiios. Trans., B., 219, 443.
Johnston, T. B. (1940): J. Anat., Lond., 75, 1.
Johnston, T. B. (1941): J. Anat., Lond., 75, 153.
Jones, H. 0., and Brewer, J. I. (1941): Contr. Embryol. Carneg. Instn., 29, 157.
Krafka, J., jr. (1941): Contr. Embryol. Carneg. Instn., 29, 167.
Latta, J. S., and Tollman, J. P. (1937): Anat. Rec., 69, 443.
Mossman, H. W. (1937): Contr. Embryol. Carneg. Instn., 26, 171.
Stieve, H. (1926): Z. mikr.-anat. Forsch., 7, 295.
Streeter, G. L. (1927): Contr. Embryo}. Carneg. Instn., 19, 73.
Waldeyer, A. (1929): Z. ges. Anat. I. Z. Anal‘. Entw~ Gesch., 90, 412.
Wislocki, G. B., and Streeter, G. L. (1938): Contr. Embryol. Cameg. Instn., 27, 1.
Explanation of Plates
Plate 1
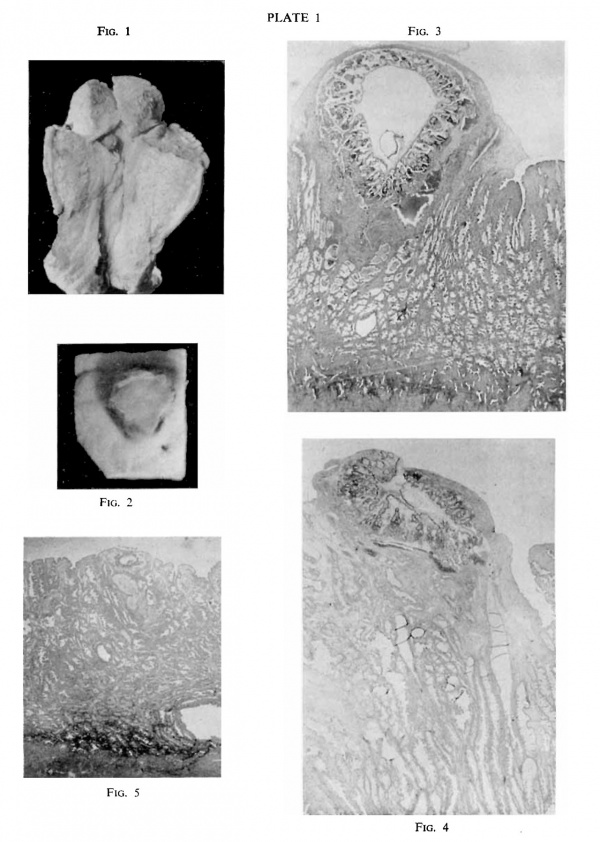
“Gar.” The specimen as received. The ovum is seen as a dark projection at the upper end of the uterine cavity. x 0.5
“ Gar.” Block of tissue containing the ovum removed for sectioning. This photograph should be turned 90° to the right, to correspond with its position in fig. 1. x 2.25
“Gar.” Slide 51, sec. 3. Through the middle of the ovum. A small part of the muscularis is seen at the lower edge of the photograph; projecting into it are some of the glands in the pars basalis of the stratum spongiosum of the endometrium. Passing towards the surface are seen the pars functionalis of the stratum spongiosum and then the stratum compactum. The embryo is seen in the deepest part of the chorionic sac. x 9
“ Mal.” Section through the middle of the ovum. The chorionic sac is seen to be collapsed. The endometrium is so much thicker than in “ Gar” that no muscularis is seen in this photograph. The stratum compactum and dilated glands of the stratum spongiosum are well seen. The embryo is at the middle of the lower border of the sac. x 9
“Dible-West” ovum. The ovum is seen as a small elliptical cavity just within the endometrium; part of the muscularis is seen below. Above this there is one large dilated gland. x 9
Plate 2
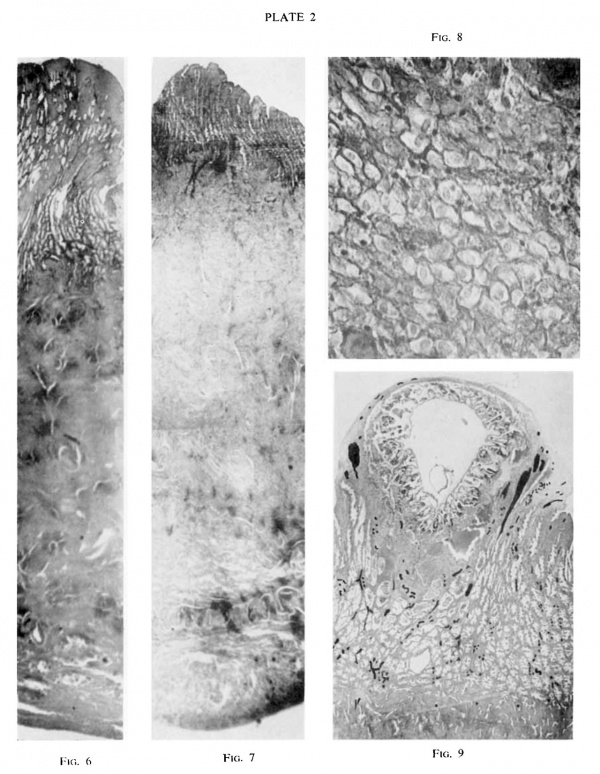
fiGS. 6 and 7. Section through the whole thickness of the uterine wall of “ Mal” (6) and
The difference in thickness x 6
Decidual cells, from the upper right corner of fig. 6.
“ Gar” (7) at some distance from the implantation site. of the endometrium in the two specimens is well seen.
“ Mal.” X 385
“ Gar.” The same section as Plate 1, fig. 3. All the vessels have been marked with Indian ink. Many coiled spiral arteries are seen. Larger vessels nearer the ovum are veins. x 10
Plate 3
“ Mal." Slide 30, sec. 2. All the blood-vessels have been marked with Indian ink. The large size of the veins and the great vascularity is well seen. ><1l
“ Gar.” Slide 51, sec. 3. Part of fig. 9 just to the right of the apex of the chorionic sac. The photograph shows part of the border zone between foetal (top left) and maternal (bottom right) tissues. Part of an anchoring cell column composed of cytotrophoblast, with a small piece of syncytium of type (b) in it, is seen in the top left corner. Part of a maternal sinus with varieties of syncytium (b) and (c) is at the bottom right. Several large sheets of cytotrophoblast and varieties (a) and (b) of syncytium are seen in the middle of the photograph. ><200
“Gar.” Slide 52, sec. 1. Part of the wall of the chorionic vesicle, with chorionic mesoblast, cyto~ and syncytiotrophoblast, is seen in the top left corner. In the bottom right corner is a large amount of maternal blood lying in a maternal sinus from the wall of which the endometrium has completely disappeared. Sheets of cytotrophoblast are seen lying free in the intervillous spaces, also the two varieties, (a) and (b), of the syncytium. ><94
“ Gar” Slide 47, sec. 3. Part of the ab-embryonic wall of a maternal sinus, with maternal decidual cells in the lower part of the photograph, undergoing transformation into the third variety of syncytial tissue (c). Above the syncytium IS a mass of serum. x 184
Plate 4
fiG. 14. “Gar.” Slide 41, sec. 3. The border zone between foetal and maternal tissue. The ends of two chorionic villi appear in the top right corner projecting into the intervillous space which contains a few blood-cells. Part of a maternal blood sinus with many red cells stretches across the lower left corner. Sheets of cytotrophoblast and varieties (a) and (b) of syncytium are seen. X164
Plate 5
fiG. 15. “ Mal.” Slide 30, sec. 10. Transverse section through the embryonic disc at the level of the cranial part of the primitive streak. The wall of the chorionic vesicle with its primary mesoblast and two layers of trophoblast stretches across the top left corner. Syncytial sprouts, variety (a), are seen growing from the syncytial trophoblast. Next to the primary mesoblast is the cavity of the amnion; to the right is the larger yolk-sac.
A little embryonic mesoblast is seen spreading laterally from the primitive streak between ectoderm and endoderm. x184
fiG. 16. “ Gar.” Slide 52, sec. 1. Section of two chorionic villi, showing their core of primary mesoblast and the vacuolated syncytiotrophoblast, suggestive of pinocytosis. Part of the intervillous space with a few blood-vessels is seen below and to the right. x 320
fiG. 17. “ Gar.” Slide ,62, sec. 1. Silver preparation showing reticular fibres in the maternal tissue below and to the r_ight, and also on the left. In the upper part of the photograph is part of a maternal blood sinus with a few cells in it, and in the middle of
the photograph is a heaped~up mass of degenerative maternal cells which have lost their reticular fibres. x 320
fiG. 18. “ Gar.” Slide 52, sec. 4. Part of the anterior end of the embryonic disc where it is turned up to join the amnion. There is a sudden change from the large cells of the disc to the thin, flattened cells of the amnion. Note also the characters of the mesoblast cells seen to the right of the amnion. X 700
Plates 6, 7 and 8
fiG. 19. “ Gar.” Slide 48, sec. 8. Part of the caudal end of the embryonic disc where it is turning up to join the amnion. At the lower left corner is seen some of the endoderm: between this and the embryonic disc is a mass of embryonic mesoblast in which two
cavities are seen; they may represent a commencing development of intra-embryonic coelom. x 700
fiG. 20. “ Gar.” Slide 50, sec. 8. The primitive node and head-process are seen in the middle of the photograph, the node being covered over by much thin embryonic ectoderm. x 320
fiG. 21. This photograph and PLATE 6, fiG. 22, PLATE 7, fiGS. 23-26, and PLATE 8, fiGS. 27-29, are serial sections of “ Gar”, being Slide 50, secs. 7-10, and Slide 51, secs. 1-5. They show the general shape of the embryonic disc with its dorsal convexity overlying the primitive node (figs. 22, 23, 24). The cranial end of the embryonic disc is towards the right, the caudal end to the left. The large yolk-sac is seen below and the smaller amnion above. The latter is joined to the whole of the chorionic sac by primary mesoblast which constitutes the connecting stalk. The allantois is seen growing out from the caudal end of the yolk-sac into the connecting stalk (fig. 27). In figs. 21 and 22 there is seen a small darkly staining mass of cells which is believed to be a blood island. In figs. 28 and 29 a small diverticulum of the yolk-sac projects in a caudal direction. Note that there is very little embryonic mesoblast between the ectoderm of the disc and endoderm of the yolk-sac cranial to the head-process. The bases of some villi are seen growing out from the wall of the chorion. fiG. 30.
Plates 8, 9
This photogra_ph and PLATE 9, fiGS. 31-34, are of serial sections through the primitive node of “ Mal The photographs are arranged in a caudo-cranial direction. fig. 33 shows some indication of a cavity in the node around which the cells are radially arranged. The wall of the chorionic sac is seen in the top right corner with a few syncytial sprouts growing from it. It is lined with primary mesoblast. Below this is the cavity of the amnion, then the embryonic disc. lts asymmetry is due to the bending down of this part of the disc referred to in the text. The yolk-sac becomes reduced in size as one passes through the sections. ‘There is a little mesoblast between the yolk-sac and the embryonic disc. X150
Plate 10
fiGS. 35-38 represent a series of sections through the allantois of “ Gar ”, Slide 51, secs. 1*4.
fiG. 39. “ Mal.”
fiG. 40. “Car.” Slide 5], sec. 3.
fiG. 4].
Mal.” The allantois is seen dividing at its distal end into two vesicles. That on the right has become separated from the main allantoic duct. A small part of the caudal end of the embryonic disc is seen below and to the right in each photograph. It is continued into the amnion which, in figs. 37 and 38, seems to make contact with the allantois constituting the cloacal membrane. An early blood-vessel is well seen in the top right corner of fig. 36. ><32O
Plate 11
Slide 32, sec. 1. A section through the caudal end of the embryonic disc. The cavity of the yolk—sac is below and its endoderm makes contact with the ectoderm of the embryonic disc forming the cloacal membrane. Above the embryonic disc
is the cavity of the amnion. Embryonic mesoblast is seen between yolk-sac endoderm and embryonic disc. x 385
At the bottom of the photograph is the embryonic disc, with yolk-sac endoderm and a few mesoblast cells below it. A few small blebs are seen on the middle of its dorsal surface. Above the disc is the cavity of the amnion, and above this again is the mesoblast of the connecting stalk in which is a concentric arrangement of cells and a number of dark granules. This is considered to represent the method by which the amniotic cavity is enlarged, as is described in the text. x 385
Slide 42, sec. 2. The wall of the chorionic vesicle is shown above, with some free masses of syncytium lying in the intervillous space. Below is the primary mesoblast in which are seen early blood-vessels. X320
fiG. 42. “ Gar." Slide 49, sec. 10. Early blood-vessels in the connecting stalk. The amniotic ectoderm and amniotic mesoblast are the two layers of cells in the lower part of the photograph. x 320 PLATE 1
- Carnegie Stages: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | About Stages | Timeline
Carnegie Stage Table
Weeks shown in the table below are embryonic post ovulation age, for clinical Gestational Age (GA) measured from last menstrual period, add 2 weeks.
(not to scale) |
||||
 |
fertilized oocyte, zygote, pronuclei | |||
 |
morula cell division with reduction in cytoplasmic volume, blastocyst formation of inner and outer cell mass | |||
 |
loss of zona pellucida, free blastocyst | |||
| attaching blastocyst | ||||
(week 2) |
 |
implantation | ||
 |
extraembryonic mesoderm, primitive streak, gastrulation | |||
| gastrulation, notochordal process | ||||
| primitive pit, notochordal canal | ||||
 |
Somitogenesis Somite Number 1 - 3 neural folds, cardiac primordium, head fold | |||
| Somite Number 4 - 12 neural fold fuses | ||||
| Somite Number 13 - 20 rostral neuropore closes | ||||
| Somite Number 21 - 29 caudal neuropore closes | ||||
| Somite Number 30 leg buds, lens placode, pharyngeal arches | ||||
| lens pit, optic cup | ||||
| lens vesicle, nasal pit, hand plate | ||||
| nasal pits moved ventrally, auricular hillocks, foot plate | ||||
| finger rays | ||||
| ossification commences | ||||
| straightening of trunk | ||||
| upper limbs longer and bent at elbow | ||||
| hands and feet turned inward | ||||
| eyelids, external ears | ||||
| rounded head, body and limbs | ||||
The embryos shown in the table are from the Kyoto and Carnegie collection and other sources.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - Two presomite human embryos. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Two_presomite_human_embryos
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G