Neural - Cerebellum Development
| Embryology - 2 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
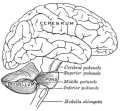
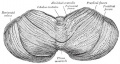
The cerebellum (Latin, ‘little brain’) major role is in role in sensory-motor processing that in the adult human contains more than half of all the brain's neurons. The adult cerebellum anatomy consists of three parts, the vermis (median) and the two hemispheres (lateral), which are continuous with each other.
The adult human cerebellum contains 69,030,000,000 ± 6,650,000,000 (sixty-nine billion thirty million) neurons and 16,040,000,000 ± 2,170,000 other cell types.[3]
- "For every neuron added to the cerebral cortex in evolution, four neurons are added to the cerebellum."[4]
| Historic Embryology | ||||
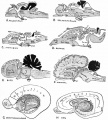
Much of the basic structure of the cerebellum comes the historic histological studies and staining of Ramón Cahal (1852 - 1934) and Camillo Golgi (1843 - 1926). Cahal was a Spanish researcher who used the then new histology Golgi staining techniques to identify the cerebellum cellular structure. His work was a turning point in our understanding of the structure of the brain, that until then had been described as a "syncytium" and not consisting of discrete cellular elements. For this research and other work on defining the structure of the brain he, along with Camillo Golgi (1843 - 1926), received the 1906 Nobel Prize in Medicine.
| ||||
| Historic Embryology |
|
During the 1940's to 50's Olof Larsell (1886-1964) in the USA described cerebellum development in several species[5][6][7][8][9][10], including man.[11][12] He also organized the terminology for the lobes and lobules of the cerebellum. Larsell was a professor and chair of anatomy at the University of Oregon Medical School. |
Neural development is one of the earliest systems to begin and the last to be completed after birth. This development generates the most complex structure within the embryo and the long time period of development means in utero insult during pregnancy may have consequences to development of the nervous system.
Within the neural tube stem cells generate the 2 major classes of cells that make the majority of the nervous system : neurons and glia. Both these classes of cells differentiate into many different types generated with highly specialized functions and shapes. This section covers the establishment of neural populations, the inductive influences of surrounding tissues and the sequential generation of neurons establishing the layered structure seen in the brain and spinal cord.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Cerebellum Development | Cerebellum Embryology | Purkinje cell Development | Bergmann glia | Dentate Nucleus Development | Fastigial Nucleus Development | Interposed Nuclei Development | Vestibular Nuclei Development | Dandy-Walker Syndrome | Foliation Defects | Joubert Syndrome | Pontocerebellar Hypoplasia | Medulloblastoma
|
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Development Overview
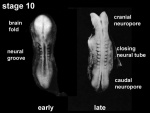
Neuralation begins at the trilaminar embryo with formation of the notochord and somites, both of which underly the ectoderm and do not contribute to the nervous system, but are involved with patterning its initial formation. The central portion of the ectoderm then forms the neural plate that folds to form the neural tube, that will eventually form the entire central nervous system.
- Early developmental sequence: Epiblast - Ectoderm - Neural Plate - Neural groove and Neural Crest - Neural Tube and Neural Crest
| Neural Tube | Primary Vesicles | Secondary Vesicles | Adult Structures |
|---|---|---|---|
| week 3 | week 4 | week 5 | adult |
| prosencephalon (forebrain) | telencephalon | Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles | |
| diencephalon | epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle | ||
| mesencephalon (midbrain) | mesencephalon | tectum, Cerebral peduncle, cerebral aqueduct, pons | |
| rhombencephalon (hindbrain) | metencephalon | cerebellum | |
| myelencephalon | medulla oblongata, isthmus | ||
| spinal cord, pyramidal decussation, central canal | |||
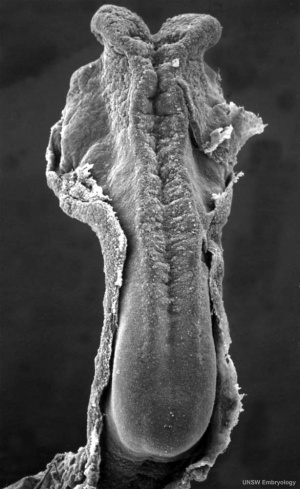
The following text description is from a study of 20 human embryos and fetuses between 6 weeks to 16 weeks from the Madrid Collection.[22]
- 6 weeks (CRL 12–16 mm) - anlage of the cerebellum was first identified as a pair of thickenings on the lateral site of the alar plate that faced the fourth ventricle.
- 7-9 weeks (CRL 28 mm) - rhombic lip (a pair of thickenings of the alar plate) protruded dorsally, bent laterally, extended ventrolaterally and fused with the medially located midbrain. During that process, the primitive choroid plexus appeared to become involved in the cerebellar hemisphere to form a centrally located eosinophilic matrix. At that stage, the inferior olive had already developed in the thick medulla. Thus, the term 'bulbo-pontine extension' may represent an erroneous labeling of a caudal part of the rhombic lip. The cerebellar vermis developed much later than the hemisphere possibly from a midline dark cell cluster near the aqueduct.
- 11–12 weeks (CRL 70–90 mm) - cerebellar hemisphere became as thick as the mid- brain. In the hemisphere, a laminar configuration became evident but the central eosinophilic matrix remained pres- ent. Fissures of the future vermis appeared in the midline area (Fig. 6): the developing fissures provided island-like structures in horizontal sections. The hemisphere and ver- mis, including the surfaces of the fissures, were covered by the external germinal cell layer.
- 15–16 weeks (CRL 110–130 mm) - cerebellar hemisphere contained the primitive dentate nucleus. The nodule and flocculus were identified, vermis became as thick as the hemisphere and it accompanied several deep fissures.
Early Brain Vesicles
| Primary Vesicles | Secondary Vesicles |
|---|---|
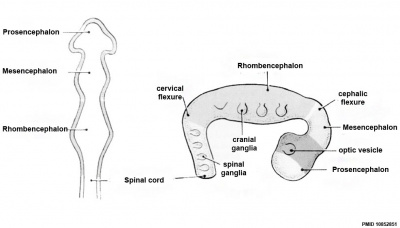
|
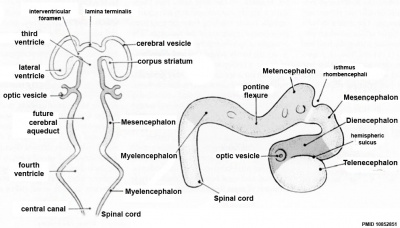
|
| week 3 to 4 | week 5 + |
Embryonic Cerebellum
Week 4
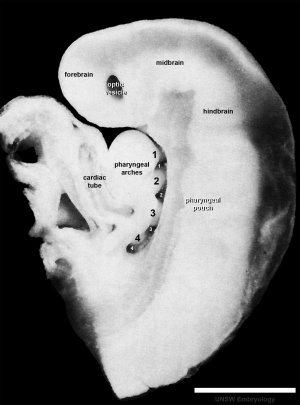
|
This midline section through the upper half of the embryo shows the 3 primary vesicle regions (unlabeled image).
The cerebellum will develop from the hindbrain (rhombencephalon) region. Adjacent to the forth ventricle (see as the enlarged dark dorsal region).
Human Embryo (Carnegie stage 13) 28 - 32 days. |
Week 8
| Carnegie stage 21 | |
|---|---|
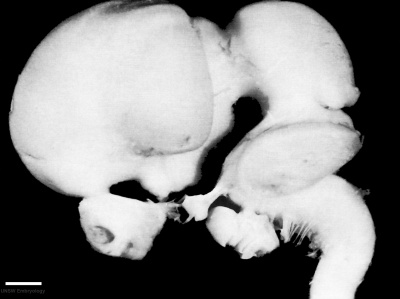
|
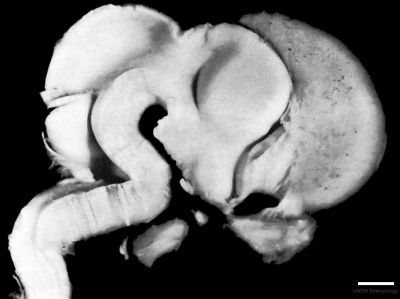
|
| lateral view (scale bar 1 mm) | Median view |
Fetal Cerebellum
Week 10
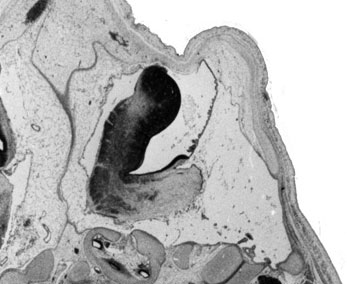
|
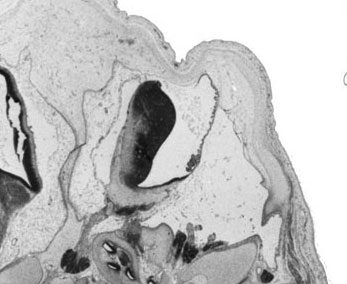
|
| Plane A (midline) | Plane B (medial) |
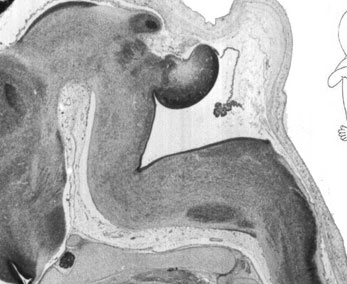
|
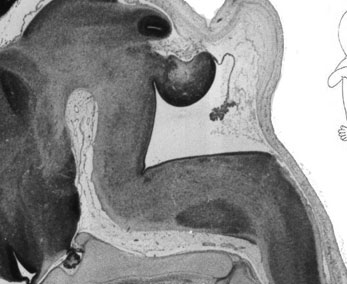
|
| Plane C (lateral) | Plane D (most lateral) |
- Links: 10 week Fetal | Fetal Development
Third Trimester
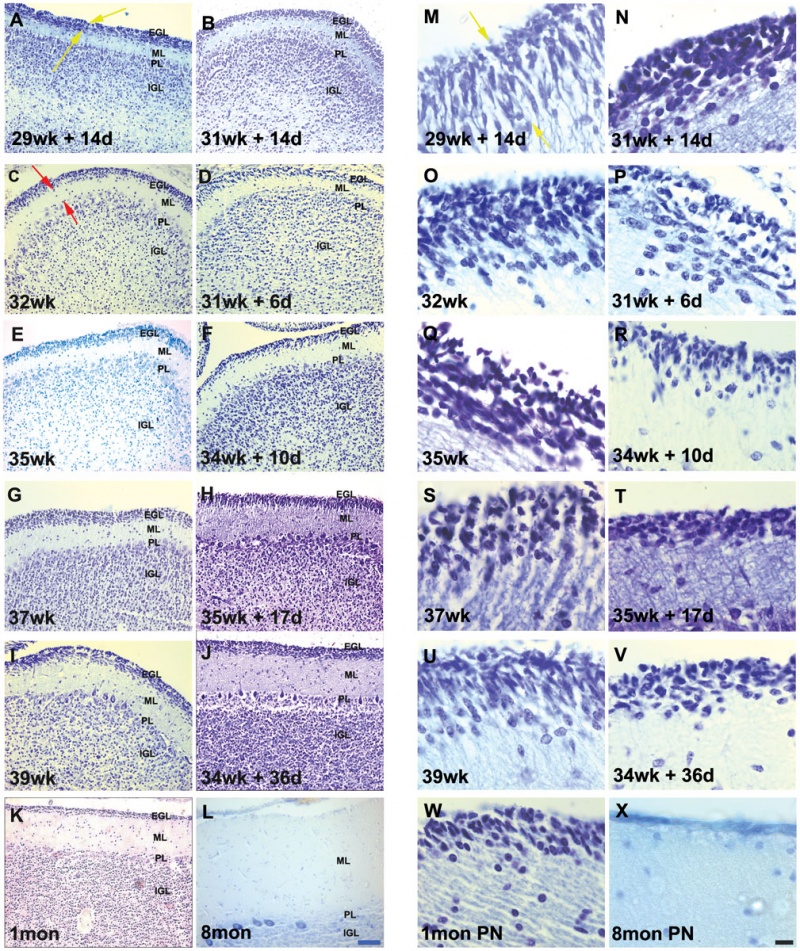
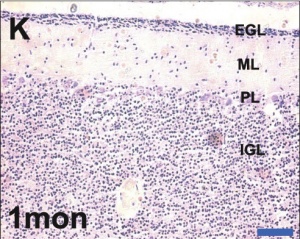
Developing human cerebellum preterm[1]
A greater EGL cell density and reduced EGL thickness were reported in preterms with ex-utero exposure, as compared to their age matched stillborn controls.
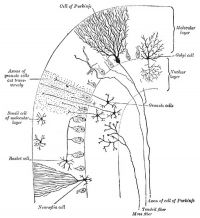
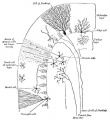
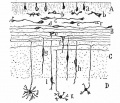
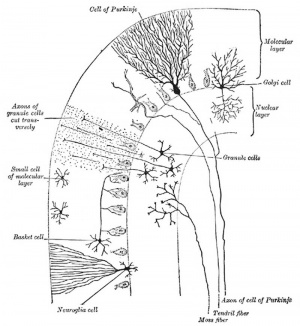
Cerebellar Cells
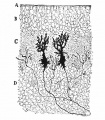
These drawings of cerebellar cells were based upon electron micrograph images from the rat cerebellum.[23]
Purkinje Cell
Search: Purkinje cell Development
Historic Description
Gray H. Anatomy of the human body. (1918) Philadelphia: Lea & Febiger.
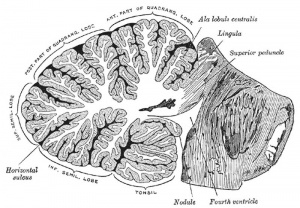
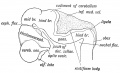
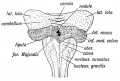
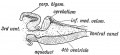
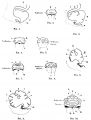
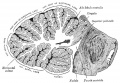
- "The cerebellum is developed in the roof of the anterior part of the hind-brain (Figs. 649 to 654). The alar laminæ of this region become thickened to form two lateral plates which soon fuse in the middle line and produce a thick lamina which roofs in the upper part of the cavity of the hind-brain vesicle; this constitutes the rudiment of the cerebellum, the outer surface of which is originally smooth and convex. The fissures of the cerebellum appear first in the vermis and floccular region, and traces of them are found during the third month; the fissures on the cerebellar hemispheres do not appear until the fifth month. The primitive fissures are not developed in the order of their relative size in the adult—thus the horizontal sulcus in the fifth month is merely a shallow groove. The best marked of the early fissures are: (a) the fissura prima between the developing culmen and declive, and (b) the fissura secunda between the future pyramid and uvula. The flocculus and nodule are developed from the rhombic lip, and are therefore recognizable as separate portions before any of the other cerebellar lobules. The groove produced by the bending over of the rhombic lip is here known as the floccular fissure; when the two lateral walls fuse, the right and left floccular fissures join in the middle line and their central part becomes the post-nodular fissure."
Researcher timeline based on a recent review on cerebellum[24]
- Rolando - role in movement control
- Flourens - role in movement coordination
- Purkinje (1837) - histology of the cerebellar cortex
- Luciani (1891) - cerebellum has a tonic facilitating effect on central structures
- Bolk (1906) - localization for coordinating action on the motor system (medio-lateral organization)
- Cajal - histology of cortex circuitry
- Eccles and Ito - inhibitory interneurons and the Purkinje cells, excitatory connections of mossy and climbing afferents and granule cells
- Babinski and Holmes - anatomoclinical insights
- Marr and Albus - theories involving cognition and emotion
- Leiners and Dow
- Magnus - cerebellum no role in body posture
Precerebellar Neurons
Precerebellar neurons (PCNs) are born in the hindbrain alar plate in the specific region called the rhombic lip (for review see[25]). From there they migrate by a process termed nucleokinesis[26], extending a cytoplasmic process then move their nucleus.
In recent years a number of different chemotactic positive and negative factors have been suggested to have a role in driving the guided migration of these cells. Many signals are thought to be mediated through the Rho family GTPases links to the cytoskeleton.
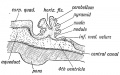
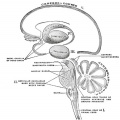
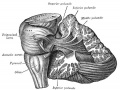
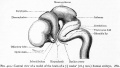
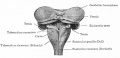
Cerebellar Nuclei
| Most medially located of the cerebellar nuclei. | Receives input from the vermis and cerebellar afferents that carry vestibular, proximal somatosensory, auditory and visual information. | |
| Consists of emboliform nucleus and globose nucleus. Interposed nuclei are situated laterally with respect to the fastigial nucleus. | Receives input from intermediate zone and cerebellar afferents that carry spinal, proximal somatosensory, auditory and visual information. | |
| Largest of the cerebellar nuclei. Lateral to interposed nuclei. | Receives input from lateral hemisphere and cerebellar afferents that carry information from cerebral cortex. | |
| Located outside cerebellum in the medulla. | Considered to be cerebellar nuclei as their connectivity patterns are identical to those of cerebellar nuclei. Receive input from flocculonodular lobe and from the vestibular labyrinth. | |
| Links: cerebellum |
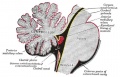
Cerebellar Pathways
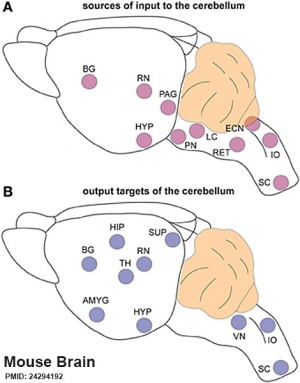
|
(A) Regions that send input to the cerebellum.
|
| Cerebellum connections to the brain and spinal cord (mouse).[28] |
Molecular
- Calm1 - signaling pathway is essential for the migration of mouse precerebellar neurons.[18]
- Merlin - impacts on cerebellar pre- and post-synaptic terminal organisation, not the overall cerebellar development.[29]
- sonic hedgehog - signaling by Bergmann glia is required for proper cerebellar cortical expansion and architecture.[15]
Abnormalities
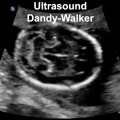
Dandy-Walker Syndrome
| Dandy-Walker Syndrome/Malformation (DWS) is a cerebellar hypoplasia and upward rotation of the cerebellar vermis with ventricular enlargement (cystic dilation of the fourth ventricle). Named in 1954 after the earlier identification by Walter Dandy (1914) and Arthur Earl Walker (1942), two USA neurosurgeons.
Walter Dandy (1886 – 1946) Arthur Earl Walker (1907 – 1995). International Classification of Diseases Q03 Congenital hydrocephalus Incl.: hydrocephalus in newborn Excl.: Arnold-Chiari syndrome (Q07.0) hydrocephalus: acquired (G91.-) due to congenital toxoplasmosis (P37.1) with spina bifida (Q05.0-Q05.4)
|
|
Foliation Defects
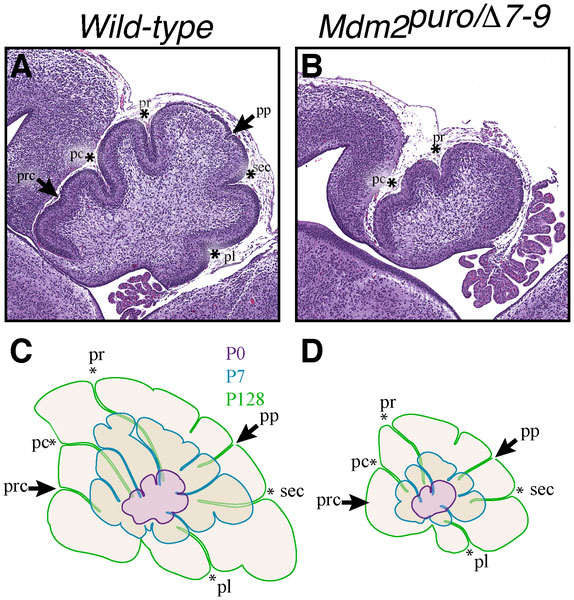
Mouse Cerebellar Foliation Defects[30]
(A–B) Midsagittal sections of newborn (P0) wild-type and Mdm2puro/Δ7-9 cerebella stained with H&E. (C–D) Superimposition of P0 (purple outline), P7 (blue outline), and adult (green outline) cerebella from wild-type (C) or Mdm2puro/Δ7-9 (D) mice. By P7, all four primary fissures, as well as two additional fissures, are evident in Mdm2puro/Δ7-9 mice. Moreover, even in adulthood, the mutant cerebellum does not reach the size or complexity of the wild-type cerebellum. Abbreviations are: prc, precentral; pc, pre-culminate; pr, primary; pp, prepyramidal; sec, secondary; pl, posterolateral fissures.
Joubert Syndrome
Joubert syndrome (Joubert-Boltshauser syndrome, Cerebelloparenchymal disorder 4, Cerebellar vermis agenesis) is a rare disease of the cerebellum. Identified as a ciliopathy, characterized by the absence or underdevelopment of the cerebellar vermis, that controls balance and coordination. There is also malformation of the stem, connecting the brain and spinal cord. A recent super-resolution microscopy study has shown that the syndrome is caused by disruption of the ciliary transition-zone architecture. [31] Ciliopathies are a class of cell abnormalities that can be caused by mutations in components of the cellular transition zone, a domain near the base of the cilium, that controls the protein composition of its membrane.
- hypotonia - weak muscle tone
- ataxia - difficulty coordinating movements
- hyperpnea - episodes of fast breathing (improves with age and usually disappears around 6 months of age)
- oculomotor apraxia - difficulty moving the eyes from side to side.
- language and motor skills
- mild to severe intellectual disability
- distinctive facial features - broad forehead, arched eyebrows, droopy eyelids (ptosis), widely spaced eyes, low-set ears, and a triangular-shaped mouth.
- Links: NIH - Rare Diseases
Pontocerebellar Hypoplasia
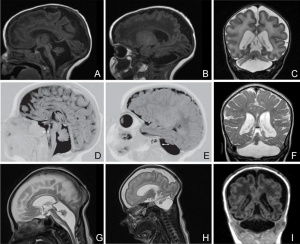
Pontocerebellar Hypoplasia (PCH) are very rare, inherited progressive neurodegenerative disorders with prenatal onset (for recent review see[32]). The major features are: hypoplasia or atrophy of cerebellum and pons, progressive microcephaly, and variable cerebral involvement. There is a further classification of 7 different subtypes (PCH1-7) and there is prenatal testing for the related inherited mutations.
- PCH2, PCH4, PCH5 - Mutations in the 3 tRNA splicing endonuclease subunit genes.
- PCH6 - Mutations in the nuclear encoded mitochondrial arginyl- tRNA synthetase gene.
- PCH1 - Mutations in the tRNA splicing endonuclease, the mitochondrial arginyl- tRNA synthetase and the vaccinia related kinase1.
Medulloblastoma
Medulloblastomas are the most common childhood primary central nervous system tumour (in children). They are thought to arise in the developing cerebellum from the precursors of the granule cell. A recent PNAS paper has suggested a role for SHH pathway in development of this disease.
Modeling SHH-driven medulloblastoma with patient iPS cell-derived neural stem cells DOI
"Here we describe and utilize a model of medulloblastoma, a malignancy accounting for 20% of all childhood brain cancers. We used iPS-derived neural stem cells with a familial mutation causing aberrant SHH signaling. We show that these cells, when transplanted into mouse cerebellum, form tumors that mimics SHH-driven medulloblastoma, demonstrating the development of cancer from healthy neural stem cells in vivo. Our results show that reprogramming of somatic cells carrying familial cancer mutations can be used to model the initiation and progression of childhood cancer."
Rhombencephalosynapsis
Rhombencephalosynapsis (RES) is a unique cerebellar malformation characterized by fusion of the cerebellar hemispheres with partial or complete absence of a recognizable cerebellar vermis.[33]
- craniofacial features - prominent forehead, flat midface, hypertelorism, ear abnormalities
- somatic malformations - heart, kidney, spine, and limb defects.
Brain Function
A recent consensus paper on experimental neurostimulation of the cerebellum suggests that this may be a target for symptomatic alleviation a number of neurological conditions.[34] These neurological and neuropsychiatric conditions include:ataxia, dystonia, essential tremor, Parkinson's disease (PD), epilepsy, stroke, multiple sclerosis, autism spectrum disorders, dyslexia, attention deficit hyperactivity disorder (ADHD), and schizophrenia.
References
- ↑ 1.0 1.1 1.2 Haldipur P, Bharti U, Alberti C, Sarkar C, Gulati G, Iyengar S, Gressens P & Mani S. (2011). Preterm delivery disrupts the developmental program of the cerebellum. PLoS ONE , 6, e23449. PMID: 21858122 DOI.
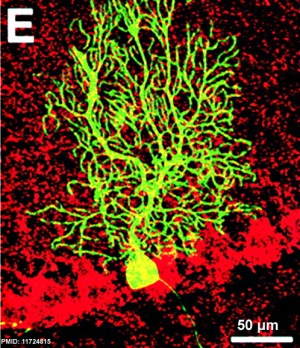
- ↑ Priller J, Persons DA, Klett FF, Kempermann G, Kreutzberg GW & Dirnagl U. (2001). Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J. Cell Biol. , 155, 733-8. PMID: 11724815 DOI.
- ↑ Herculano-Houzel S, Catania K, Manger PR & Kaas JH. (2015). Mammalian Brains Are Made of These: A Dataset of the Numbers and Densities of Neuronal and Nonneuronal Cells in the Brain of Glires, Primates, Scandentia, Eulipotyphlans, Afrotherians and Artiodactyls, and Their Relationship with Body Mass. Brain Behav. Evol. , 86, 145-63. PMID: 26418466 DOI.
- ↑ Herculano-Houzel S. (2010). Coordinated scaling of cortical and cerebellar numbers of neurons. Front Neuroanat , 4, 12. PMID: 20300467 DOI.
- ↑ LARSELL O. (1946). The cerebellum of cyclostomes. Anat. Rec. , 94, 478. PMID: 21020604
- ↑ LARSELL O. (1952). The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J. Comp. Neurol. , 97, 281-356. PMID: 12999992 DOI.
- ↑ LARSELL O & WHITLOCK DG. (1952). Further observations on the cerebellum of birds. J. Comp. Neurol. , 97, 545-66. PMID: 13034933 DOI.
- ↑ LARSELL O. (1954). The development of the cerebellum of the pig. Anat. Rec. , 118, 73-107. PMID: 13124788 DOI.
- ↑ LARSELL O. (1948). The development and subdivisions of the cerebellum of birds. J. Comp. Neurol. , 89, 123-89. PMID: 18889711 DOI.
- ↑ LARSELL O. (1947). The cerebellum of myxinoids and petromyzonts including developmental stages in the lampreys. J. Comp. Neurol. , 86, 395-445. PMID: 20239748 DOI.
- ↑ LARSELL O & STOTLER WA. (1947). Some morphological features of the human cerebellum. Anat. Rec. , 97, 352. PMID: 20341845
- ↑ LARSELL O. (1947). The development of the cerebellum in man in relation to its comparative anatomy. J. Comp. Neurol. , 87, 85-129. PMID: 20267600 DOI.
- ↑ Xu F, Ge X, Shi Y, Zhang Z, Tang Y, Lin X, Teng G, Zang F, Gao N, Liu H, Toga AW & Liu S. (2020). Morphometric development of the human fetal cerebellum during the early second trimester. Neuroimage , 207, 116372. PMID: 31751665 DOI.
- ↑ Yawno T, Sutherland AE, Pham Y, Castillo-Melendez M, Jenkin G & Miller SL. (2019). Fetal Growth Restriction Alters Cerebellar Development in Fetal and Neonatal Sheep. Front Physiol , 10, 560. PMID: 31191328 DOI.
- ↑ 15.0 15.1 Cheng FY, Fleming JT & Chiang C. (2018). Bergmann glial Sonic hedgehog signaling activity is required for proper cerebellar cortical expansion and architecture. Dev. Biol. , 440, 152-166. PMID: 29792854 DOI.
- ↑ Kalinichenko SG & Pushchin II. (2018). The modular architecture and neurochemical patterns in the cerebellar cortex. J. Chem. Neuroanat. , 92, 16-24. PMID: 29753860 DOI.
- ↑ Poretti A, Boltshauser E & Huisman TA. (2016). Pre- and Postnatal Neuroimaging of Congenital Cerebellar Abnormalities. Cerebellum , 15, 5-9. PMID: 26166429 DOI.
- ↑ 18.0 18.1 18.2 Kobayashi H, Saragai S, Naito A, Ichio K, Kawauchi D & Murakami F. (2015). Calm1 signaling pathway is essential for the migration of mouse precerebellar neurons. Development , 142, 375-84. PMID: 25519244 DOI.
- ↑ Hou C, Ding L, Zhang J, Jin Y, Sun C, Li Z, Sun X, Zhang T, Zhang A, Li H & Gao J. (2014). Abnormal cerebellar development and Purkinje cell defects in Lgl1-Pax2 conditional knockout mice. Dev. Biol. , 395, 167-81. PMID: 25050931 DOI.
- ↑ Huang GJ, Edwards A, Tsai CY, Lee YS, Peng L, Era T, Hirabayashi Y, Tsai CY, Nishikawa S, Iwakura Y, Chen SJ & Flint J. (2014). Ectopic cerebellar cell migration causes maldevelopment of Purkinje cells and abnormal motor behaviour in Cxcr4 null mice. PLoS ONE , 9, e86471. PMID: 24516532 DOI.
- ↑ Muguruma K, Nishiyama A, Ono Y, Miyawaki H, Mizuhara E, Hori S, Kakizuka A, Obata K, Yanagawa Y, Hirano T & Sasai Y. (2010). Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat. Neurosci. , 13, 1171-80. PMID: 20835252 DOI.
- ↑ Cho KH, Rodríguez-Vázquez JF, Kim JH, Abe H, Murakami G & Cho BH. (2011). Early fetal development of the human cerebellum. Surg Radiol Anat , 33, 523-30. PMID: 21380713 DOI.
- ↑ HERNDON RM. (1964). THE FINE STRUCTURE OF THE RAT CEREBELLUM. II. THE STELLATE NEURONS, GRANULE CELLS, AND GLIA. J. Cell Biol. , 23, 277-93. PMID: 14222815
- ↑ Voogd J & Koehler PJ. (2018). Historic notes on anatomic, physiologic, and clinical research on the cerebellum. Handb Clin Neurol , 154, 3-26. PMID: 29903448 DOI.
- ↑ Bloch-Gallego E, Causeret F, Ezan F, Backer S & Hidalgo-Sánchez M. (2005). Development of precerebellar nuclei: instructive factors and intracellular mediators in neuronal migration, survival and axon pathfinding. Brain Res. Brain Res. Rev. , 49, 253-66. PMID: 16111554 DOI.
- ↑ Tsai LH & Gleeson JG. (2005). Nucleokinesis in neuronal migration. Neuron , 46, 383-8. PMID: 15882636 DOI.
- ↑ Killeen MT & Sybingco SS. (2008). Netrin, Slit and Wnt receptors allow axons to choose the axis of migration. Dev. Biol. , 323, 143-51. PMID: 18801355 DOI.
- ↑ Reeber SL, Otis TS & Sillitoe RV. (2013). New roles for the cerebellum in health and disease. Front Syst Neurosci , 7, 83. PMID: 24294192 DOI.
- ↑ Toledo A, Lang F, Doengi M, Morrison H, Stein V & Baader SL. (2019). Merlin modulates process outgrowth and synaptogenesis in the cerebellum. Brain Struct Funct , , . PMID: 31165301 DOI.
- ↑ Malek R, Matta J, Taylor N, Perry ME & Mendrysa SM. (2011). The p53 inhibitor MDM2 facilitates Sonic Hedgehog-mediated tumorigenesis and influences cerebellar foliation. PLoS ONE , 6, e17884. PMID: 21437245 DOI.
- ↑ Shi X, Garcia G, Van De Weghe JC, McGorty R, Pazour GJ, Doherty D, Huang B & Reiter JF. (2017). Super-resolution microscopy reveals that disruption of ciliary transition-zone architecture causes Joubert syndrome. Nat. Cell Biol. , 19, 1178-1188. PMID: 28846093 DOI.
- ↑ 32.0 32.1 Namavar Y, Barth PG, Poll-The BT & Baas F. (2011). Classification, diagnosis and potential mechanisms in pontocerebellar hypoplasia. Orphanet J Rare Dis , 6, 50. PMID: 21749694 DOI.
- ↑ Aldinger KA, Dempsey JC, Tully HM, Grout ME, Mehaffey MG, Dobyns WB & Doherty D. (2018). Rhombencephalosynapsis: Fused cerebellum, confused geneticists. Am J Med Genet C Semin Med Genet , 178, 432-439. PMID: 30580482 DOI.
- ↑ Miterko LN, Baker KB, Beckinghausen J, Bradnam LV, Cheng MY, Cooperrider J, DeLong MR, Gornati SV, Hallett M, Heck DH, Hoebeek FE, Kouzani AZ, Kuo SH, Louis ED, Machado A, Manto M, McCambridge AB, Nitsche MA, Taib NOB, Popa T, Tanaka M, Timmann D, Steinberg GK, Wang EH, Wichmann T, Xie T & Sillitoe RV. (2019). Consensus Paper: Experimental Neurostimulation of the Cerebellum. Cerebellum , , . PMID: 31165428 DOI.
Journals
- Cerebellum Springer Publishers
Reviews
Lehman VT, Black DF, DeLone DR, Blezek DJ, Kaufmann TJ, Brinjikji W & Welker KM. (2020). Current concepts of cross-sectional and functional anatomy of the cerebellum: a pictorial review and atlas. Br J Radiol , 93, 20190467. PMID: 31899660 DOI.
Wang L & Liu Y. (2019). Signaling pathways in cerebellar granule cells development. Am J Stem Cells , 8, 1-6. PMID: 31139492
Shoja MM, Jensen CJ, Ramdhan R, Chern J, Oakes WJ & Tubbs RS. (2018). Embryology of the Craniocervical Junction and Posterior Cranial Fossa Part II: Embryogenesis of the hindbrain. Clin Anat , , . PMID: 29344994 DOI.
Aldinger KA & Doherty D. (2016). The genetics of cerebellar malformations. Semin Fetal Neonatal Med , 21, 321-32. PMID: 27160001 DOI.
Butts T, Green MJ & Wingate RJ. (2014). Development of the cerebellum: simple steps to make a 'little brain'. Development , 141, 4031-41. PMID: 25336734 DOI.
Voogd J. (2012). A note on the definition and the development of cerebellar Purkinje cell zones. Cerebellum , 11, 422-5. PMID: 22396330 DOI.
Roussel MF & Hatten ME. (2011). Cerebellum development and medulloblastoma. Curr. Top. Dev. Biol. , 94, 235-82. PMID: 21295689 DOI.
Herculano-Houzel S. (2010). Coordinated scaling of cortical and cerebellar numbers of neurons. Front Neuroanat , 4, 12. PMID: 20300467 DOI.
Ten Donkelaar HJ & Lammens M. (2009). Development of the human cerebellum and its disorders. Clin Perinatol , 36, 513-30. PMID: 19732611 DOI.
Moldrich RX, Dauphinot L, Laffaire J, Rossier J & Potier MC. (2007). Down syndrome gene dosage imbalance on cerebellum development. Prog. Neurobiol. , 82, 87-94. PMID: 17408845 DOI.
Zervas M, Blaess S & Joyner AL. (2005). Classical embryological studies and modern genetic analysis of midbrain and cerebellum development. Curr. Top. Dev. Biol. , 69, 101-38. PMID: 16243598 DOI.
Sato T, Joyner AL & Nakamura H. (2004). How does Fgf signaling from the isthmic organizer induce midbrain and cerebellum development?. Dev. Growth Differ. , 46, 487-94. PMID: 15610138 DOI.
Adamsbaum C, Merzoug V, André C, Ferey S & Kalifa G. (2003). [Imaging of the pediatric cerebellum]. J Neuroradiol , 30, 158-71. PMID: 12843872
Articles
Cho KH, Rodríguez-Vázquez JF, Kim JH, Abe H, Murakami G & Cho BH. (2011). Early fetal development of the human cerebellum. Surg Radiol Anat , 33, 523-30. PMID: 21380713 DOI.
Lee EY, Ji H, Ouyang Z, Zhou B, Ma W, Vokes SA, McMahon AP, Wong WH & Scott MP. (2010). Hedgehog pathway-regulated gene networks in cerebellum development and tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. , 107, 9736-41. PMID: 20460306 DOI.
Corrales JD, Rocco GL, Blaess S, Guo Q & Joyner AL. (2004). Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development , 131, 5581-90. PMID: 15496441 DOI.
Search PubMed
Search Pubmed: Cerebellum Embryology | Cerebellum Development | Medulloblastoma
Historic
Herrick CL. The histogenesis of the cerebellum. (1895) J Comp. Neurol. 5: 66-70.
Stroud BB. The mammalian cerebellum, part 1: The development of the cerebellum in man and the cat. (1895) J Comp. Neurol. 5: 71-118.
Bradley OC. The mammalian cerebellum: its lobes and fissures. (1904) J Anat Physiol. 38(4): 448-475. PMID17232617
Bradley OC. The mammalian cerebellum: its lobes and fissures. (1904) J Anat Physiol. 39(1): 99–117. PMID 17232628
Myers BD. A study of the development of certain features of the cerebellum. (1920) Contrib. Embryol., Carnegie Inst. Wash. 41:
Palmgren A. Embryological and morphological studies on the mid-brain and cerebellum of vertebrates. (1921) Acta Zoologica. 64(5): 2-94.
Terms
- Bergmann glia - a cerebellar radial glial population that in development act as scaffolds for the radial migration of granule cell precursors from the external germinal layer (EGL or outer granular layer) to the inner granular cell layer (IGL).
- cerebellar nuclei - clusters of glutamatergic and GABAergic neurons located in the cerebellar white matter that are the synaptic targets of the majority of Purkinje cells. Projection neurons within nuclei account for the output of the cerebellum. Cerebellar nuclei are often termed ‘deep’ although the designation is superfluous.
- External Germinal Layer - (EGL or outer granular layer) a developmental transient layer that is lost following granule cell migration.
- Granule cell - neurons in the internal granule layer that receive excitatory inputs from mossy fibres, that originate in the pons, medulla and spinal cord. These glutamatergic excitatory neutrons receive local inhibitory inputs from Golgi neurons. Granule cells axons are T-shaped and extend into the molecular layer synapsing on Purkinje cell dendrites.
- Inner Granular Layer - (IGL) granule neurons (granule cell) lies deep to the Purkinje cell layer.
- Purkinje cell - neurons located in the cerebellar cortex that receive excitatory inputs from granule cell parallel fibres and inhibitory input from climbing fibres of the inferior olive. These GABAergic inhibitory neurons axons mainly extend to the deep cerebellar nuclei, some axons also directly innervate hindbrain vestibular targets.
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Keith A. Human Embryology and Morphology. (1902) London: Edward Arnold.
Bradley OC. The mammalian cerebellum: its lobes and fissures. (1904) J Anat Physiol. 38(4): 448-475. PMID17232617
Gray H. Anatomy of the human body. (1918) Philadelphia: Lea & Febiger.
Bailey FR. and Miller AM. Text-Book of Embryology (1921) New York: William Wood and Co. The Cerebellum
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 2) Embryology Neural - Cerebellum Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Cerebellum_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G