Cardiovascular - Venous Development
| Embryology - 28 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Development of the heart and vascular system begins very early in mesoderm both within (embryonic) and outside (extra embryonic, yolk sac and placental) the embryo. Vascular development therefore occurs in many places, the most obvious though is the early forming heart, which grows rapidly creating an externally obvious cardiac "bulge" on the early embryo. The cardiovascular system is extensively remodelled throughout development, this current page discusses systemic venous development. Note that placental vessels are discussed in placental notes.
Eph-B4 (EPHB4; Eph receptor gene family; 7q22.1,) has been identified as an early marker of venous endothelium development[1], see also the review.[2] In the retina, angiopoietin-4 (ANGPT4) has also been identified as a factor for venous development.[3]
See also the related pages Ductus Venosus, Arterial Development, Placental Villi Blood Vessels and Coronary Circulation Development.
| Historic Venous | ||
|---|---|---|
| Historic Papers: 1902 Rabbit Inferior Vena Cava | 1909 Cervical Veins | 1925 Human Inferior Vena Cava
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Venous Embryology | Vein Development | Ductus Venosus Development | Cervical Vein Development | Azygos Vein Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Textbooks
- Human Embryology (2nd ed.) Larson Ch7 p151-188 Heart, Ch8 p189-228 Vasculature
- The Developing Human: Clinically Oriented Embryology (6th ed.) Moore and Persaud Ch14: p304-349
- Before we Are Born (5th ed.) Moore and Persaud Ch12; p241-254
- Essentials of Human Embryology Larson Ch7 p97-122 Heart, Ch8 p123-146 Vasculature
- Human Embryology Fitzgerald and Fitzgerald Ch13-17: p77-111
Vena Cava Development
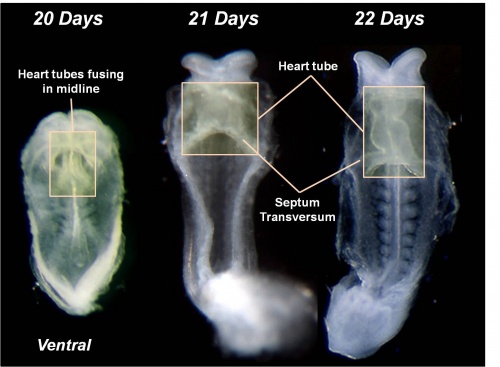
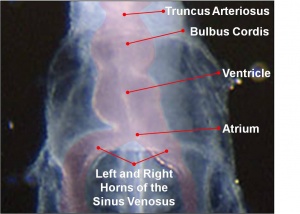
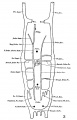
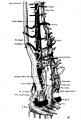
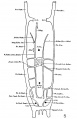
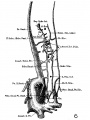
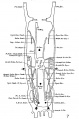
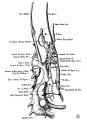
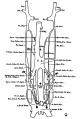
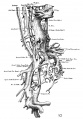
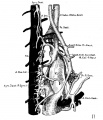
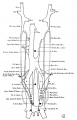
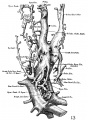
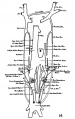
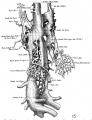
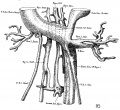
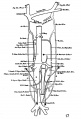
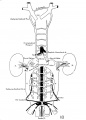
The images below are from a historic 1925 paper by Mcclure and Butler described the development of the human vent cava using embryos from a number of human embryo collections.[6]
Fig. 1 human embryo 4 mm no. 588
Fig. 2 mm human embryo 4 mm no. 588
Fig. 5 human embryo 10.1 mm no. 623 (Carnegie stage 17)
Fig. 6 human embryo 10.1 mm no. 623 (Carnegie stage 17)
Fig. 7 human embryo 15 mm no. 841 (Carnegie stage 18)
Fig. 8 human embryo 15 mm no. 841 (Carnegie stage 18)
Fig. 9 human embryo 15 mm Harvard Collection, no. 2051
Fig. 10 human embryo 15 mm Harvard Collection, no. 2051 Ventrolateral aspect.
Fig. 11 human embryo 15 mm Harvard Collection, no. 2051 venous ring of right side
Fig. 16 human embryo 45 mm Harvard Collection, no. 2128
Fig. 18 embryonic veins of the domestic cat
Infrahepatic Inferior Caval and Azygos Vein
Infrahepatic inferior caval and azygos vein formation in mammals with different degrees of mesonephric development.[7]
- "Controversies regarding the development of the mammalian infrahepatic inferior caval and azygos veins arise from using topography rather than developmental origin as criteria to define venous systems and centre on veins that surround the mesonephros. We compared caudal-vein development in man with that in rodents and pigs (rudimentary and extensive mesonephric development, respectively), and used Amira 3D reconstruction and Cinema 4D-remodelling software for visualisation. The caudal cardinal veins (CCVs) were the only contributors to the inferior caval (IVC) and azygos veins. Development was comparable if temporary vessels that drain the large porcine mesonephros were taken into account. The topography of the CCVs changed concomitant with expansion of adjacent organs (lungs, meso- and metanephroi). The iliac veins arose by gradual extension of the CCVs into the caudal body region. Irrespective of the degree of mesonephric development, the infrarenal part of the IVC developed from the right CCV and the renal part from vascular sprouts of the CCVs in the mesonephros that formed 'subcardinal' veins. The azygos venous system developed from the cranial remnants of the CCVs. Temporary venous collaterals in and around the thoracic sympathetic trunk were interpreted as 'footprints' of the dorsolateral-to-ventromedial change in the local course of the intersegmental and caudal cardinal veins relative to the sympathetic trunk. Interspecies differences in timing of the same events in IVC and azygos-vein development appear to allow for proper joining of conduits for caudal venous return, whereas local changes in topography appear to accommodate efficient venous perfusion. These findings demonstrate that new systems, such as the 'supracardinal' veins, are not necessary to account for changes in the course of the main venous conduits of the embryo."
Renal Venous Development
The renal arterial and venous systems are also reorganised extensively throughout development with changing kidney position.
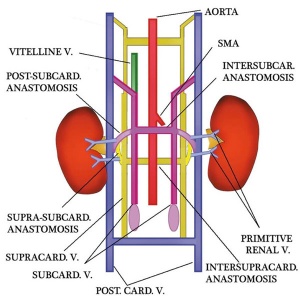
|
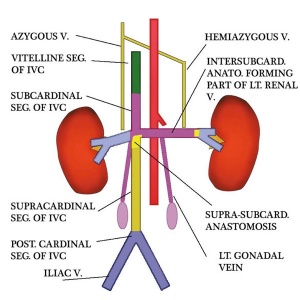
|
| Embryo renal venous | Adult renal venous |
- Links: Renal Development
Fetal Blood Flow
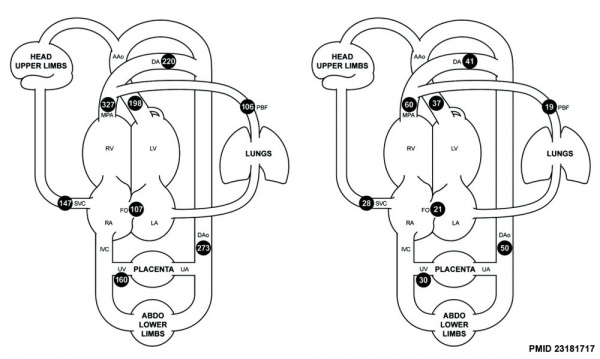
Mean Late Fetal Blood Flows[8]
(8 subjects) in the major vessels of the human fetal circulation by phase contrast MRI. (median gestational age 37 weeks, age range of 30–39 weeks)
| (left) Mean flows in ml/kg/min | (right) Proportions of the combined ventricular output in the major vessels of the human fetal circulation by phase contrast MRI. |
|
|
- Cardiovascular Links: Fetal Blood Flow values | Mean Fetal Blood Flow | Proportions Ventricular Output | Ventricular Output (colour) | heart | blood | cardiovascular
Historic
McClure CFW. and Butler EG. The development of the vena cava inferior in man. (1925) Amer. J Anat. 35(3): 331-383.
Lewis FT. On the cervical veins and lymphatics in four human embryos, with an interpretation of anomalies on the subclavian and jugular veins in the adult. (1909)
Lewis FT. The development of the vena cava inferior. (1902) Amer. J Anat. 1(3): 229-244.
References
- ↑ Takama M & Nosoh Y. (1980). Purification and some properties of 6-phosphoglucose isomerase from Bacillus caldotenax. J. Biochem. , 87, 1821-7. PMID: 7400125 DOI.
- ↑ 2.0 2.1 Wolf K, Hu H, Isaji T & Dardik A. (2019). Molecular identity of arteries, veins, and lymphatics. J. Vasc. Surg. , 69, 253-262. PMID: 30154011 DOI.
- ↑ 3.0 3.1 Elamaa H, Kihlström M, Kapiainen E, Kaakinen M, Miinalainen I, Ragauskas S, Cerrada-Gimenez M, Mering S, Nätynki M & Eklund L. (2018). Angiopoietin-4-dependent venous maturation and fluid drainage in the peripheral retina. Elife , 7, . PMID: 30444491 DOI.
- ↑ Meier A & Alkadhi H. (2019). Venous Collateral Pathways in Superior Thoracic Inlet Obstruction: A Systematic Analysis of Anatomy, Embryology, and Resulting Patterns. AJR Am J Roentgenol , , 1-11. PMID: 31039029 DOI.
- ↑ Krishnan A, Samtani R, Dhanantwari P, Lee E, Yamada S, Shiota K, Donofrio MT, Leatherbury L & Lo CW. (2014). A detailed comparison of mouse and human cardiac development. Pediatr. Res. , 76, 500-7. PMID: 25167202 DOI.
- ↑ McClure CFW. and Butler EG. The development of the vena cava inferior in man. (1925) Amer. J Anat. 35(3): 331-383.
- ↑ Hikspoors JP, Mekonen HK, Mommen GM, Cornillie P, Köhler SE & Lamers WH. (2016). Infrahepatic inferior caval and azygos vein formation in mammals with different degrees of mesonephric development. J. Anat. , 228, 495-510. PMID: 26659476 DOI.
- ↑ Seed M, van Amerom JF, Yoo SJ, Al Nafisi B, Grosse-Wortmann L, Jaeggi E, Jansz MS & Macgowan CK. (2012). Feasibility of quantification of the distribution of blood flow in the normal human fetal circulation using CMR: a cross-sectional study. J Cardiovasc Magn Reson , 14, 79. PMID: 23181717 DOI.
Reviews
Nigro B & Ayarragaray JEF. (2021). Anomalies of Inferior Vena Cava: Implications and Considerations in Retroperitoneal Surgical Procedures. Ann Vasc Surg , , . PMID: 34644626 DOI.
Kelly RG. (2012). The second heart field. Curr. Top. Dev. Biol. , 100, 33-65. PMID: 22449840 DOI.
Carmeliet P & Jain RK. (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature , 473, 298-307. PMID: 21593862 DOI.
Degani S. (2008). Fetal cerebrovascular circulation: a review of prenatal ultrasound assessment. Gynecol. Obstet. Invest. , 66, 184-96. PMID: 18607112 DOI.
Tchirikov M, Schröder HJ & Hecher K. (2006). Ductus venosus shunting in the fetal venous circulation: regulatory mechanisms, diagnostic methods and medical importance. Ultrasound Obstet Gynecol , 27, 452-61. PMID: 16565980 DOI.
Kiserud T. (2005). Physiology of the fetal circulation. Semin Fetal Neonatal Med , 10, 493-503. PMID: 16236564 DOI.
Kiserud T & Acharya G. (2004). The fetal circulation. Prenat. Diagn. , 24, 1049-59. PMID: 15614842 DOI.
Articles
Jiji RS & Kramer CM. (2011). Cardiovascular magnetic resonance: applications in daily practice. Cardiol Rev , 19, 246-54. PMID: 21808168 DOI.
Ribatti D & Djonov V. (2011). Angiogenesis in development and cancer today. Int. J. Dev. Biol. , 55, 343-4. PMID: 21732277 DOI.
Cammarato A, Ahrens CH, Alayari NN, Qeli E, Rucker J, Reedy MC, Zmasek CM, Gucek M, Cole RN, Van Eyk JE, Bodmer R, O'Rourke B, Bernstein SI & Foster DB. (2011). A mighty small heart: the cardiac proteome of adult Drosophila melanogaster. PLoS ONE , 6, e18497. PMID: 21541028 DOI.
Min JK, Park H, Choi HJ, Kim Y, Pyun BJ, Agrawal V, Song BW, Jeon J, Maeng YS, Rho SS, Shim S, Chai JH, Koo BK, Hong HJ, Yun CO, Choi C, Kim YM, Hwang KC & Kwon YG. (2011). The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J. Clin. Invest. , 121, 1882-93. PMID: 21540552 DOI.
Guo C, Sun Y, Zhou B, Adam RM, Li X, Pu WT, Morrow BE, Moon A & Li X. (2011). A Tbx1-Six1/Eya1-Fgf8 genetic pathway controls mammalian cardiovascular and craniofacial morphogenesis. J. Clin. Invest. , 121, 1585-95. PMID: 21364285 DOI.
Arráez-Aybar LA, Turrero-Nogués A & Marantos-Gamarra DG. (2008). Embryonic cardiac morphometry in Carnegie stages 15-23, from the Complutense University of Madrid Institute of Embryology Human Embryo Collection. Cells Tissues Organs (Print) , 187, 211-20. PMID: 18057862 DOI.
Search Pubmed
Search Pubmed: Cardiovascular System Development
Additional Images
Historic
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
| Cardiovascular Terms |
|---|
Cardiovascular System Development See also Heart terms, Immune terms and Blood terms.
|
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 28) Embryology Cardiovascular - Venous Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cardiovascular_-_Venous_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G