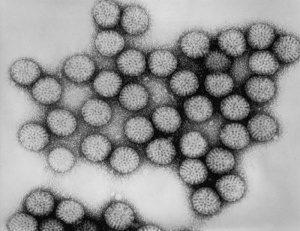
Abnormal Development - Rotavirus
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
Introduction
The rotavirus is a common cause of diarrhoea and vomiting (viral gastroenteritis) in infants and young children. The virus was first identified in 1973 from epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis.[1]
The live attenuated rotavirus vaccine is contraindicated in pregnancy, but can be safely administered to household contacts of pregnant women. There is only a very small risk of transmission of the rotavirus vaccine virus to a susceptible pregnant woman and there is no evidence of risk to the fetus if pregnant women are in contact with recently vaccinated individuals.
(data based on: The Australian Immunisation Handbook 9th Edition 2008 2.3.2 Vaccination of women planning pregnancy, pregnant or breastfeeding women, and preterm infants - updated July 2009 )
- Links: Abnormal Development - Rotavirus | Postnatal - Vaccination | The Australian Immunisation Handbook 9th Edition 2008 | Australian Immunisation Handbook - Rotavirus | 2.3.2 Vaccination of women planning pregnancy, pregnant or breastfeeding women, and preterm infants - updated July 2009 | Medical Microbiology - Rotaviruses
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Abnormal Development Rotavirus <pubmed limit=5>Abnormal Development Rotavirus</pubmed> Search term: Rotavirus |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page. |
Discovery of Rotavirus
The virus was identified in 1973[1] from epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis, and has been summarised in a 2009 paper by Ruth Bishop, who was one of original discoverers of the virus.[5]
- "For centuries, acute diarrhea has been a major worldwide cause of death in young children, and until 1973, no infectious agents could be identified in about 80% of patients admitted to hospital with severe dehydrating diarrhea. In 1973 Ruth Bishop, Geoffrey Davidson, Ian Holmes, and Brian Ruck identified abundant particles of a 'new' virus (rotavirus) in the cytoplasm of mature epithelial cells lining duodenal villi and in feces, from such children admitted to the Royal Children's Hospital, Melbourne. Rotaviruses have now been shown to cause 40-50% of severe acute diarrhea in young children worldwide in both developing and developed countries, and > 600 000 young children die annually from rotavirus disease, predominantly in South-East Asia and sub-Saharan Africa. Longitudinal surveillance studies following primary infection in young children have shown that rotavirus reinfections are common. However the immune response that develops after primary infection is protective against severe symptoms on reinfection. This observation became the basis for development of live oral rotavirus vaccines"
Virus Structure
- Non-enveloped, icosahedral virus of the Reoviridae family containing a genome of 11 segments of double stranded RNA (dsRNA).
- Divided into seven serotypes (Rotavirus A–G).
| Environmental Links: Introduction | low folic acid | iodine deficiency | Nutrition | Drugs | Australian Drug Categories | USA Drug Categories | thalidomide | herbal drugs | Illegal Drugs | smoking | Fetal Alcohol Syndrome | TORCH | viral infection | bacterial infection | fungal infection | zoonotic infection | toxoplasmosis | Malaria | maternal diabetes | maternal hypertension | maternal hyperthermia | Maternal Inflammation | Maternal Obesity | hypoxia | biological toxins | chemicals | heavy metals | air pollution | radiation | Prenatal Diagnosis | Neonatal Diagnosis | International Classification of Diseases | Fetal Origins Hypothesis |
Vaccine
Two oral rotavirus vaccines are available in Australia, and their efficacy and safety in the prevention of rotavirus gastroenteritis have been extensively evaluated.[6][7] Both rotavirus vaccines have been shown to have similar efficacy against rotavirus gastroenteritis (of any severity) of around 70%.
- Rotarix (GlaxoSmithKline) - 2 oral doses (1.5 mL/dose)
- RotaTeq (bioCSL /Merck & Co Inc) - 3 oral doses (2 mL/dose)
References
- ↑ 1.0 1.1 Bishop RF, Davidson GP, Holmes IH & Ruck BJ. (1973). Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet , 2, 1281-3. PMID: 4127639
- ↑ Fathima P, Snelling TL, de Klerk N, Lehmann D, Blyth CC, Waddington CS & Moore HC. (2019). Perinatal Risk Factors Associated With Gastroenteritis Hospitalizations in Aboriginal and Non-Aboriginal Children in Western Australia (2000-2012): A Record Linkage Cohort Study. Pediatr. Infect. Dis. J. , 38, 169-175. PMID: 29620723 DOI.
- ↑ Bines JE, At Thobari J, Satria CD, Handley A, Watts E, Cowley D, Nirwati H, Ackland J, Standish J, Justice F, Byars G, Lee KJ, Barnes GL, Bachtiar NS, Viska Icanervilia A, Boniface K, Bogdanovic-Sakran N, Pavlic D, Bishop RF, Kirkwood CD, Buttery JP & Soenarto Y. (2018). Human Neonatal Rotavirus Vaccine (RV3-BB) to Target Rotavirus from Birth. N. Engl. J. Med. , 378, 719-730. PMID: 29466164 DOI.
- ↑ Coldiron ME, Guindo O, Makarimi R, Soumana I, Matar Seck A, Garba S, Macher E, Isanaka S & Grais RF. (2018). Safety of a heat-stable rotavirus vaccine among children in Niger: Data from a phase 3, randomized, double-blind, placebo-controlled trial. Vaccine , , . PMID: 29752026 DOI.
- ↑ Bishop R. (2009). Discovery of rotavirus: Implications for child health. J. Gastroenterol. Hepatol. , 24 Suppl 3, S81-5. PMID: 19799704 DOI.
- ↑ Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O'Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA & Heaton PM. (2006). Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N. Engl. J. Med. , 354, 23-33. PMID: 16394299 DOI.
- ↑ Soares-Weiser K, Maclehose H, Ben-Aharon I, Goldberg E, Pitan F & Cunliffe N. (2010). Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev , , CD008521. PMID: 20464766 DOI.
Reviews
Desselberger U. (2017). Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors. Pathogens , 6, . PMID: 29231855 DOI.
Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O'Ryan M, Kang G, Desselberger U & Estes MK. (2017). Rotavirus infection. Nat Rev Dis Primers , 3, 17083. PMID: 29119972 DOI.
Kuate Defo Z & Lee B. (2016). New approaches in oral rotavirus vaccines. Crit. Rev. Microbiol. , 42, 495-505. PMID: 25268934 DOI.
Articles
Mwenda JM, Burke RM, Shaba K, Mihigo R, Tevi-Benissan MC, Mumba M, Biey JN, Cheikh D, Poy MSc A, Zawaira FR, Aliabadi N, Tate JE, Hyde T, Cohen AL & Parashar UD. (2017). Implementation of Rotavirus Surveillance and Vaccine Introduction - World Health Organization African Region, 2007-2016. MMWR Morb. Mortal. Wkly. Rep. , 66, 1192-1196. PMID: 29095805 DOI.
. (2017). Progress with the implementation of rotavirus surveillance and vaccines in countries of the WHO African Region, 2007–2016. Wkly. Epidemiol. Rec. , 92, 673-80. PMID: 29106113
Search PubMed
Search term: Rotavirus
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Abnormal Development - Rotavirus. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Rotavirus
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G