Abnormal Development - Hepatitis Virus
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose. |
Introduction
Hepatitis (inflammation of the liver) is caused in humans by one of 7 viruses (A, B, C, D, E) with the 2 additional F has not been confirmed as a distinct genotype; and G is a newly described flavivirus. CDC states that all of these viruses can cause an acute disease with symptoms lasting several weeks including yellowing of the skin and eyes (jaundice); dark urine; extreme fatigue; nausea; vomiting and abdominal pain. It can take several months to a year to feel fit again.
- An estimated 400 million people worldwide are living with chronic hepatitis B infection.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Teratogen Hepatitis Virus |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
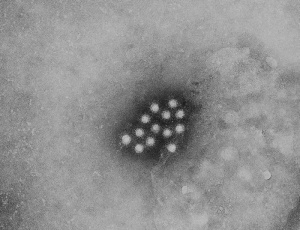
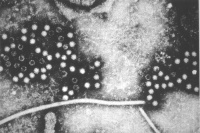
Hepatitis Virus
Virus particles measure 42nm in overall diameter and contain a 27nm diameter DNA-based core.
Hepatitis Transmission Risk to the Fetus
Hepatitis A
- Fetal transmission of virus occurs with extreme rarity.
Hepatitis B
- Can occur as a consequence of intrapartum exposure, transplacental transmission, and breastfeeding.
- 20% - 30% of HBsAg-positive/HbeAg-negative women will transmit virus to their infants.
- 90% of HBsAg- and HBeAg-positive women will transmit virus to their infants.
- Immunoprophylaxis at birth with both HBIG and Hepatitis B vaccine within 12 hours of birth decreases the risk of transmission.
- Passive (HBIG) and active immunization is 85-95% effective in preventing neonatal HBV infection.
- Chronic infection occurs in almost all children who are infected with hepatitis B during the perinatal period and in up to 50% of children who become infected between 1 and 5 years of age.
Hepatitis C
- The overall risk of transmission is approximately 2-6% with unknown maternal viral titers.
- All pregnant women with HCV should have viral titers performed.
- The placenta appears to act as an immunological organ providing antiviral protection against hepatitis C viral transmission in the majority of cases.[4]
- Children and adolescents with chronic infection generally have no symptoms.
- Breast-feeding does not appear to transmit virus.
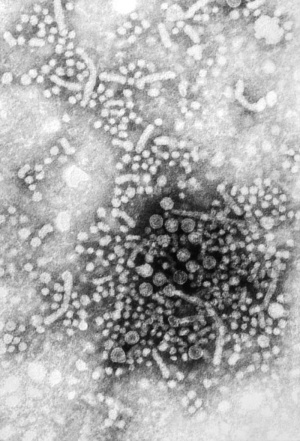
Hepatitis E virions (CDC)
Adult Hepatitis B Treatment
Women who are HBsAg-positive have a very high risk for vertical transmission to their infants. NIH currently recommends that infants of HBsAg-positive women receive hepatitis B immunoglobulin and hepatitis B vaccination within 12 hours of birth and receive a complete set of 3 vaccinations and long-term follow-up. This has been shown to substantially reduce the risk for perinatal transmission.
U.S. Food and Drug Administration have approved 7 agents, used as monotherapy or in combination, for use in the treatment of adults with Hepatitis B.
- interferons (interferon-α2b and peginterferon-α2a)
- Interferon use has a defined self-limited course.
- nucleoside or nucleotide analogues (lamivudine, adefovir, entecavir, tenofovir, and telbivudine)
- therapy can be long-term or indefinite treatment.
Ribavirin
Ribavirin (tribavirin) an antiviral drug used to treat hepatitis C, RSV infection, and viral hemorrhagic fever. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Formula: C8H12N4O5.
Teratogenic effects have been demonstrated in all animal species exposed to ribavirin. Ribavirin is prescribed for chronic hepatitis C and is contraindicated in women who are pregnant and in the male sexual partners of women who are pregnant. In the USA a voluntary registry has been established for pregnant women (272 pregnant women, with 180 live births) with prenatal exposure to ribavirin.
Exposure is classified as
- direct-women taking ribavirin during pregnancy
- 6 months prior to conception
- indirect-women exposed through sexual contact, 6 months prior to or during pregnancy, with a man who is taking or has taken ribavirin in the past 6 months.
A preliminary report from this study[2] states: "Patterns suggesting a common etiology or relationship with ribavirin exposure are not seen. Based on the patterns of birth defects reported, preliminary findings do not suggest a clear signal of human teratogenicity for ribavirin. However, the current sample size is insufficient for definitive conclusions, and ribavirin exposure should be avoided during pregnancy and during the 6 months prior to pregnancy, in accordance with prescribing information." ClinicalTrials.gov identifier: NCT00114712.
Liver Damage
Sequelae of chronic Hepatitis C infection are progressive liver fibrosis leading to cirrhosis, end-stage liver disease, and Hepatocellular Carcinoma (HCC).
Cirrhosis
Hepatocellular Carcinoma
References
- ↑ Nimgaonkar I, Ding Q, Schwartz RE & Ploss A. (2018). Hepatitis E virus: advances and challenges. Nat Rev Gastroenterol Hepatol , 15, 96-110. PMID: 29162935 DOI.
- ↑ 2.0 2.1 Sinclair SM, Jones JK, Miller RK, Greene MF, Kwo PY & Maddrey WC. (2017). The Ribavirin Pregnancy Registry: An Interim Analysis of Potential Teratogenicity at the Mid-Point of Enrollment. Drug Saf , 40, 1205-1218. PMID: 28689333 DOI.
- ↑ Lai J, Fay KE & Bocchini JA. (2011). Update on childhood and adolescent immunizations: selected review of US recommendations and literature: part 2. Curr. Opin. Pediatr. , 23, 470-81. PMID: 21743328 DOI.
- ↑ <pubmed>20814429</pubmed>| PLoS One.
- ↑ Practice Committee of American Society for Reproductive Medicine. (2008). Hepatitis and reproduction. Fertil. Steril. , 90, S226-35. PMID: 19007636 DOI.
- ↑ Ornoy A & Tenenbaum A. (2006). Pregnancy outcome following infections by coxsackie, echo, measles, mumps, hepatitis, polio and encephalitis viruses. Reprod. Toxicol. , 21, 446-57. PMID: 16480851 DOI.
- ↑ Lee C, Gong Y, Brok J, Boxall EH & Gluud C. (2006). Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ , 332, 328-36. PMID: 16443611 DOI.
Books
- Medical Microbiology. 4th edition. Baron S, editor. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
- Molecular Biology of the Cell. 4th edition. Alberts B, Johnson A, Lewis J, et al. New York: Garland Science; 2002.
- Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection. Geneva: World Health Organization; 2014 Apr. Available from: http://www.ncbi.nlm.nih.gov/books/NBK263483/
Reviews
Yogeswaran K & Fung SK. (2011). Chronic hepatitis B in pregnancy: unique challenges and opportunities. Korean J Hepatol , 17, 1-8. PMID: 21494071 DOI.
Articles
Giles ML, Visvanathan K, Lewin SR & Sasadeusz J. (2012). Chronic hepatitis B infection and pregnancy. Obstet Gynecol Surv , 67, 37-44. PMID: 22278077 DOI.
Kumar A. (2012). Hepatitis B virus infection and pregnancy: a practical approach. Indian J Gastroenterol , 31, 43-54. PMID: 22528342 DOI.
Connell LE, Salihu HM, Salemi JL, August EM, Weldeselasse H & Mbah AK. (2011). Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. , 31, 1163-70. PMID: 21745298 DOI.
Search Pubmed
Search PubMed: term = Hepatitis Virus teratology | embryo infection | fetal infection | neonatal infection
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- International Committee on Taxonomy of Viruses
- Australian Government The Australian Immunisation Handbook 9th Edition 2008
- NIH (USA) Consensus Development Conference Management of Hepatitis B October 20–22, 2008 Bethesda, Maryland | Management of Hepatitis C: 2002 June 10-12, 2002
| Environmental Links: Introduction | low folic acid | iodine deficiency | Nutrition | Drugs | Australian Drug Categories | USA Drug Categories | thalidomide | herbal drugs | Illegal Drugs | smoking | Fetal Alcohol Syndrome | TORCH | viral infection | bacterial infection | fungal infection | zoonotic infection | toxoplasmosis | Malaria | maternal diabetes | maternal hypertension | maternal hyperthermia | Maternal Inflammation | Maternal Obesity | hypoxia | biological toxins | chemicals | heavy metals | air pollution | radiation | Prenatal Diagnosis | Neonatal Diagnosis | International Classification of Diseases | Fetal Origins Hypothesis |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Abnormal Development - Hepatitis Virus. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Hepatitis_Virus
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G