Developmental Signals - Hippo
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The Hippo pathway, first identified in Drosophila, controls organ size by regulating cell proliferation and apoptosis.
| Factor Links: AMH | hCG | BMP | sonic hedgehog | bHLH | HOX | FGF | FOX | Hippo | LIM | Nanog | NGF | Nodal | Notch | PAX | retinoic acid | SIX | Slit2/Robo1 | SOX | TBX | TGF-beta | VEGF | WNT | Category:Molecular |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Embryo Retinoic acid | Images <pubmed limit=5>Embryo Retinoic acid</pubmed> |
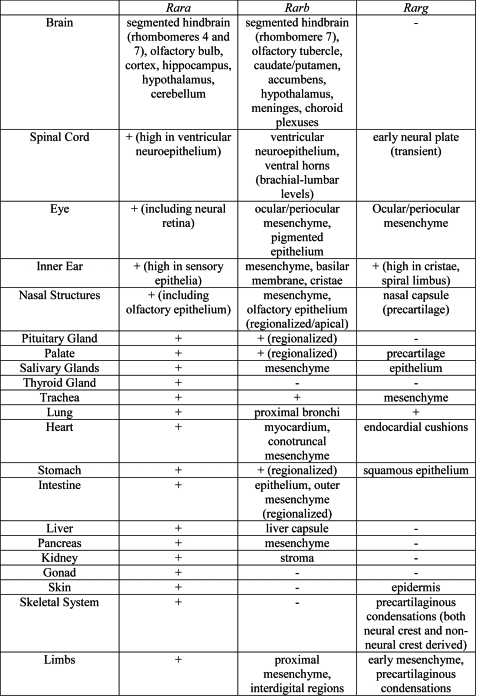
Organ Expression
Summary of Rar gene expression patterns in mouse developing organ systems.[2]
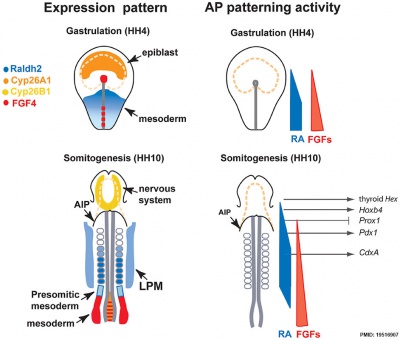
Endoderm
Chicken antero-posterior endoderm patterning[3]
- Links: Endoderm | Chicken Development
Fetal Gonad
Immunohistochemical localisation of retinoid receptor expression in the human fetal gonad[4]
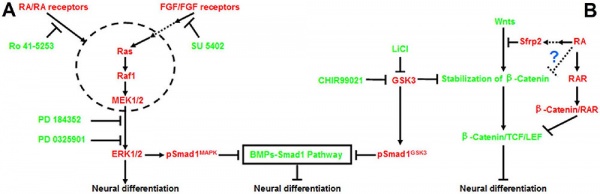
Neural
Model retinoic acid extracellular signal-regulated kinase and Wnt pathway interactions[5]
RA Is Not Required for Radial Expansion of the Embryonic Cortex
References
- ↑ <pubmed>25558812</pubmed>
- ↑ <pubmed>19471585</pubmed>
- ↑ 19516907</pubmed>| PLoS One.
- ↑ Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PTK (2011) Retinoic Acid Signalling and the Control of Meiotic Entry in the Human Fetal Gonad. PLoS ONE 6(6): e20249. PMID 21674038 | PLoS One
- ↑ <pubmed>19642999</pubmed>
Articles
<pubmed>21673209</pubmed>| Can Fam Physician
Search Pubmed
Search Bookshelf Retinoic acid
Search Pubmed Now: Retinoic acid
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Developmental Signals - Hippo. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Developmental_Signals_-_Hippo
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G