Abnormal Development - Folic Acid and Neural Tube Defects: Difference between revisions
| Line 81: | Line 81: | ||
* Genetic, dysmorphic or metabolic disorders or a family history of serious genetic disorder | * Genetic, dysmorphic or metabolic disorders or a family history of serious genetic disorder | ||
* Perinatal or serious neonatal infection including children of mothers who are HIV positive | * Perinatal or serious neonatal infection including children of mothers who are HIV positive | ||
* Psychosocial problems eg., infants of drug-addicted or alcoholic mothers. | * Psychosocial problems eg., infants of drug-addicted or alcoholic mothers. | ||
== References == | == References == | ||
Revision as of 15:08, 2 November 2010
Introduction
In 2001, the Australian estimated birth prevalence of neural tube defects was 0.5 per 1,000 births (National Perinatal Statistics Unit). Low maternal dietary folic acid (folate) has been shown to be associated with the development of neural tube defects.
Research over the last 20 years has suggested a relationship between maternal diet and the birth of an affected infant, and recent evidence has confirmed that folic acid, a water soluble vitamin, found in many fruits (particularly oranges, berries and bananas), leafy green vegetables, cereals and legumes, may prevent the majority of neural tube defects.
 |
 |
In the U.S.A. the Food and Drug Administration in 1996 authorized that all enriched cereal grain products be fortified with folic acid, with optional fortification beginning in March 1996 and mandatory fortification in January 1998. (More? [#USAdata USA Data])
The March of Dimes Folic Acid Campaign (a major US charity group) has as one of its major objectives to reduce neural tube defects by 30% by 2001 using community programs, professional education, and mass media information.
- Links: Neural System - Abnormalities | [spinabifida.htm Spina Bifida]
Some Recent Findings
- Prevalence of neural tube defects in Australia prior to mandatory fortification of bread-making flour with folic acid.[1]"The birth prevalence of NTDs from 1998-2005, was 5/10,000 births. The total prevalence including terminations of pregnancy was 13/10,000 births. A 26% declining trend in total prevalence was seen from 1992-2005, but the main decline occurred prior to 1998. Women who were Indigenous, socially disadvantaged, young, living in remote areas and had multiple gestations were more likely to give birth to babies with NTDs."
- Low folic acid supplement intake rate among women in northern China with a high-prevalence of neural tube defects, 2008.[2]"In 2008, we conducted a cross-sectional survey to investigate awareness and intake rates of folic acid supplementation among women of childbearing age from 293 counties (all national-level poverty counties) in northern China that had a high prevalence of NTDs ( about 16.0/10,000 births of at least 28 weeks gestation in 2008)."
- Association of folate receptor (folr1, folr2, folr3) and reduced folate carrier (slc19a1) genes with meningomyelocele.[3] "This study involved the analyses of selected single nucleotide polymorphisms across the folate receptor genes and the folate carrier gene in a large population sample. It provided evidence that the rare alleles of specific single nucleotide polymorphisms within these genes appear to be statistically significant for association to meningomyelocele in the patient population that was tested."
Australian Statistics
- Women who have one infant with a neural tube defect have a significantly increased risk of recurrence
- 40-50 per thousand compared with 2 per thousand for all births.
- A randomised controlled trial conducted by the Medical Research Council of the United Kingdom demonstrated a 72% reduction in risk of recurrence by periconceptional (ie before and after conception) folic acid supplementation (4mg daily).
- Other epidemiological research, including work done in Australia, suggests that primary occurrences of neural tube defects may also be prevented by folic acid either as a supplement or in the diet.
- This has been confirmed in a randomised controlled trial from Hungary, which found that a multivitamin supplement containing 0.8mg folic acid was effective in reducing the occurrence of neural tube defects in first births.
USA Statistics
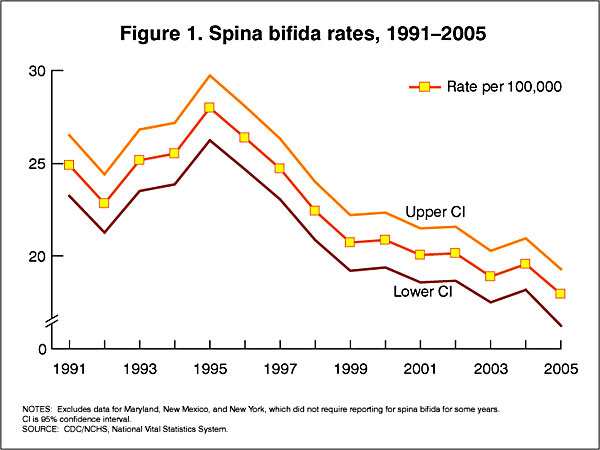
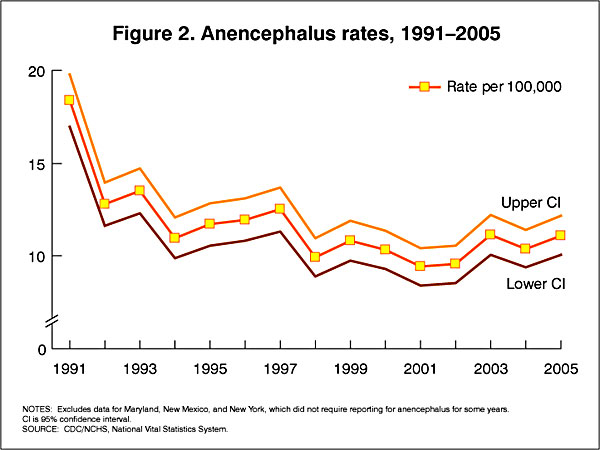
In the U.S.A. the Food and Drug Administration in 1996 authorized that all enriched cereal grain products be fortified with folic acid, with optional fortification beginning in March 1996 and mandatory fortification in January 1998. The data below shows the subsequent changes in anencephaly and spina bifida rate over that period.
Data CDC Report[4]
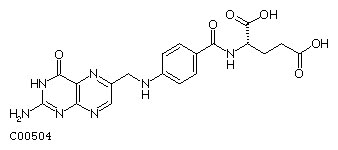
Folic Acid
Formula: C19H19N7O6
Alternate Names: Folic acid, Folate, Pteroylglutamic acid
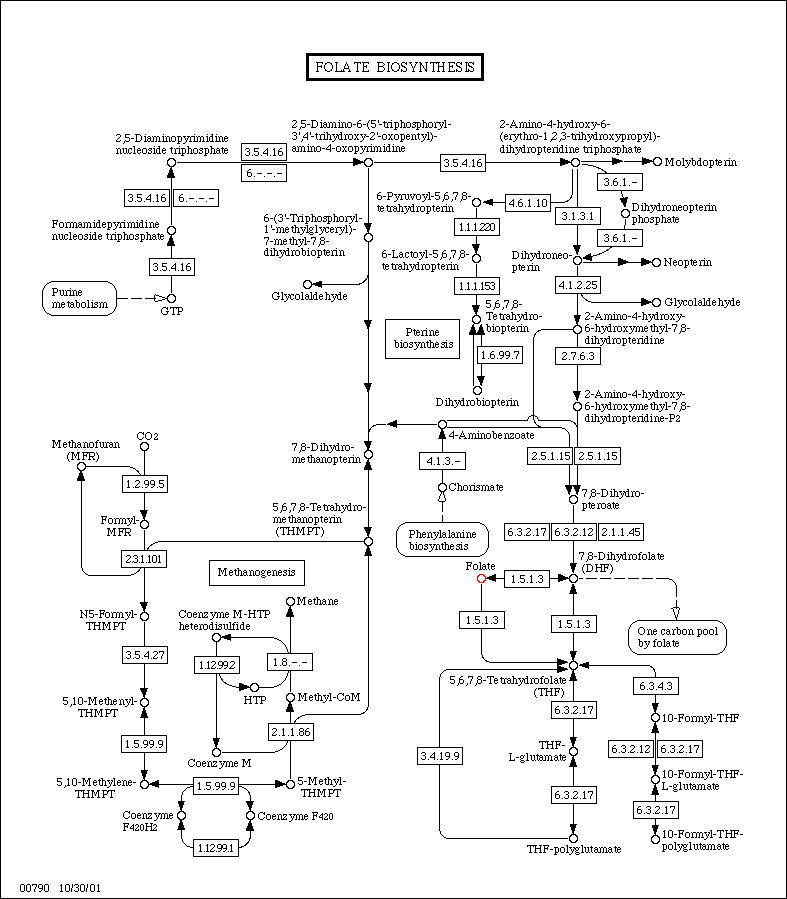
(Data from KEGG)
Folate Supplementation and Other Abnormalities
A recent study of periconceptional folate supplementation using the Cochrane Pregnancy and Childbirth Group's Trials Register (July 2010) identified no statistically significant evidence of any effects on prevention of cleft palate, cleft lip, congenital cardiovascular defects, miscarriages or any other birth defects.[5]
Australian NHMRC
The Australian NHMRC (1988) recommends neonates be assessed for follow-up care under the following conditions.
- Birthweight less than 1500g or gestational age less than 32 weeks
- Small-for-gestational-age neonates
- Perinatal asphyxia
- Apgar score less than 3 at 5 minutes
- clinical evidence of neurological dysfunction
- delay in onset of spontaneous respiration for more than 5 minutes and requiring mechanical ventilation
- Clinical evidence of central nervous system abnormalities ie., seizures, hypotonia
- Hyperbilirubinaemia of greater than 350umol/l in full term neonates
- Genetic, dysmorphic or metabolic disorders or a family history of serious genetic disorder
- Perinatal or serious neonatal infection including children of mothers who are HIV positive
- Psychosocial problems eg., infants of drug-addicted or alcoholic mothers.
References
Reviews
<pubmed>16631914</pubmed> <pubmed>16685040</pubmed> <pubmed>15937577</pubmed>
Articles
Search PubMed
Search Bookshelf: Folic Acid and Neural Tube Defects | Folic Acid
- Folic Acid Supplementation for the Prevention of Neural Tube Defects An Update of the Evidence for the U.S. Preventive Services Task Force
- Screening for Neural Tube Defects— Including Folic Acid/Folate Prophylaxis Guide to Clinical Preventive Services, 2nd Edition: 1996
Search Pubmed: Folic Acid and Neural Tube Defects | Folic Acid
External Links
- Australia Department of Health Nutrition and Healthy Eating Trends in Neural Tube Defects in Australia
- See also the NHMRC Publication Dietary Folic Acid and Neural Tube Defects
- National Perinatal Statistics Unit
- Australian Journal of Nutrition and Dietetics
- Australian Nutrition Foundation
- CSIRO Human Nutrition
- Food, Nutrition & Health Information - Centre for Advanced Food Research
- Office of Dietary Supplements, National Institutes of Health (USA)Dietary Supplement Fact Sheet: Folate
- Foodwatch
- Healthy Eating, Healthy Living
- AIS Sport Nutrition - information from the Australian Institute of Sport. Supplies nutrition research, a question-and-answer section, publications, and other resources.
- America's Eating Habits - a report from the U.S. Department of Agriculture's economic research service. Covers dietary recommendations, health claims in food advertising, reality about cholesterol, and various other topics.
- British Nutrition Foundation - an organization that works with academic, private, and public groups on nutrition. Includes nutrition education, facts, news, and publications.
- Center for Food Safety & Applied Nutrition - a repository of links relevant to all manner of food issues, including food safety. Emphasizes FDA regulations and related research programs.
- Nutrition.gov - a collection of information and links to other sites on food safety, food programs, and federally funded research centers.
- Nutrition.org - site of the American Society for Nutritional Sciences. Features an encyclopedia that summarizes food sources, diet recommendations, and deficiencies for a number of macro- and micronutrients.
- Nutrition Navigator - a guide to nutrition websites reviewed and rated by Tufts University nutritionists. Supplies news and sections arranged by topic.
- U.S. Department of Agriculture
- Children's Nutrition Research Center
- Facts about the DASH Diet
- Healthfinder Kids
- Interactive Healthy Eating Index
- National Dairy Council
- Nutrition Explorations
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, May 2) Embryology Abnormal Development - Folic Acid and Neural Tube Defects. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Folic_Acid_and_Neural_Tube_Defects
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G