Neural - Cerebrum Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
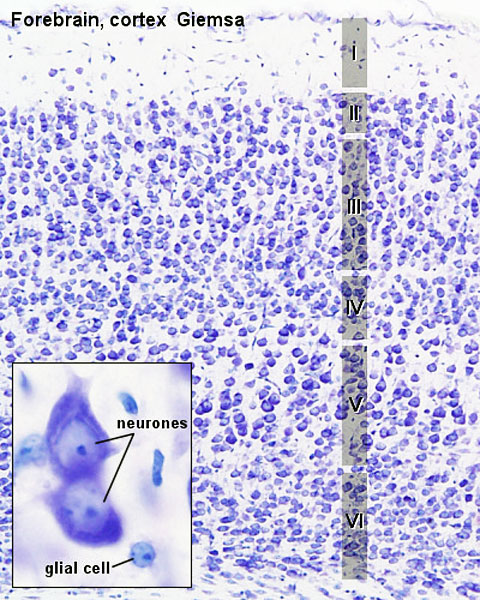
The brain (cerebral cortex, cerebrum, cortex) as it is generally recognised. Though the cerebrum includes the cerebral cortex and the subcortical structures (hippocampus, basal ganglia, and olfactory bulb). The adult cerebral cortex like other neural structures has a laminar organisation, the mammalian neocortex consists of six layers, while the reptilian and avian cortices have only three layers (equivalent to mammalian layers I, V and VI). The adult human cerebrum contains about 16,340,000,000 ± 2,170,000,000 (sixteen billion three hundred forty million) neurons and 60,840,000,000 ± 7,020,000,000 other cell types.[2]
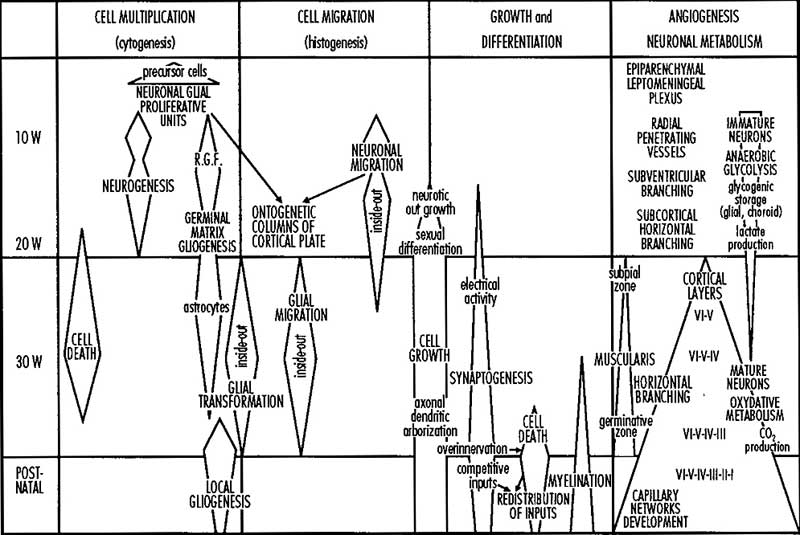
A simplified developmental sequence can be described as cell proliferation, cell migration, and finally cortical organization. In development, lamination occurs in an "inside-out" sequence earlier inside and later born neurons outside. The cortex is divided into areas which serve distinct functions including motor, sensory and cognitive processing. The lamination process requires a range of different signals including; Reelin (Reln, an extracellular protein), Disabled-1 (Dab1, an intracellular signaling molecule), and Cullin-5 (Cul5, an E3 ubiquitin ligase).
The cortex progenitor cell types are either neuron-restricted or bipotent (neuron-glial) progenitors that generate glial-restricted progenitors at mid- and late neurogenesis.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Cerebral Cortex Development | Cerebral Cortex Embryology | Cerebrum Development | Cortex Development | |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Development Overview
Neuralation begins at the trilaminar embryo with formation of the notochord and somites, both of which underly the ectoderm and do not contribute to the nervous system, but are involved with patterning its initial formation. The central portion of the ectoderm then forms the neural plate that folds to form the neural tube, that will eventually form the entire central nervous system.
- Early developmental sequence: Epiblast - Ectoderm - Neural Plate - Neural groove and Neural Crest - Neural Tube and Neural Crest
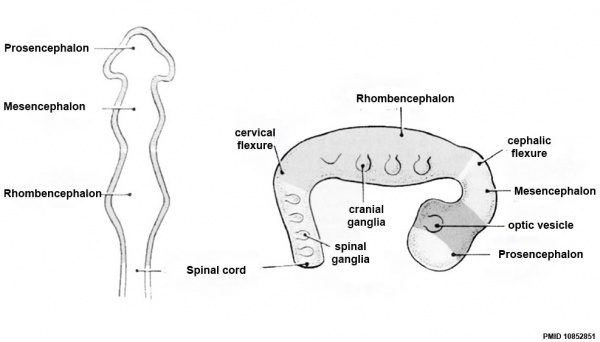
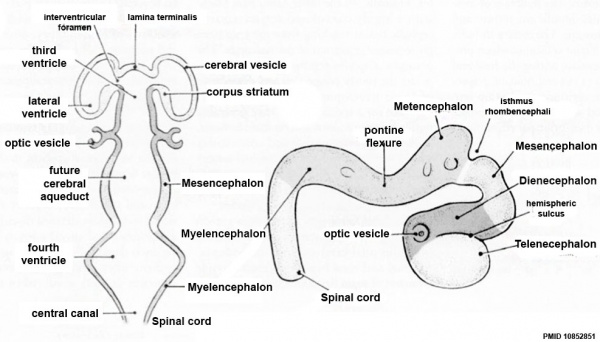
| Neural Tube | Primary Vesicles | Secondary Vesicles | Adult Structures |
|---|---|---|---|
| week 3 | week 4 | week 5 | adult |
| prosencephalon (forebrain) | telencephalon | Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles | |
| diencephalon | epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle | ||
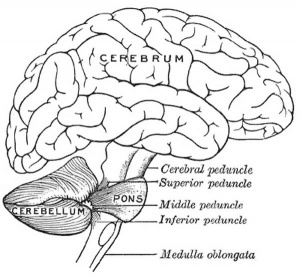
| mesencephalon (midbrain) | mesencephalon | tectum, Cerebral peduncle, cerebral aqueduct, pons | |
| rhombencephalon (hindbrain) | metencephalon | cerebellum | |
| myelencephalon | medulla oblongata, isthmus | ||
| spinal cord, pyramidal decussation, central canal | |||
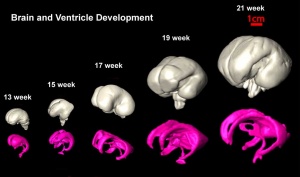
Early Brain Vesicles
Primary Vesicles
Secondary Vesicles
Late Embryonic Brain
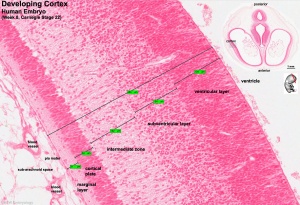
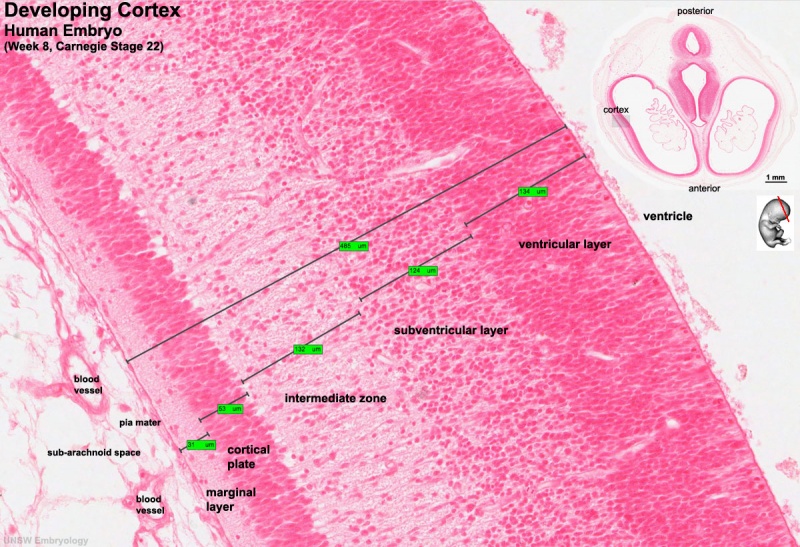
Human embryo developing cortex (Week 8, Carnegie stage 22)
- small embryo shows approximate level of section.
- insert top right shows whole head section.
- small shaded box on whole section shows region of large image.
- layer thicknesses are shown in microns.
Early Fetal Brain
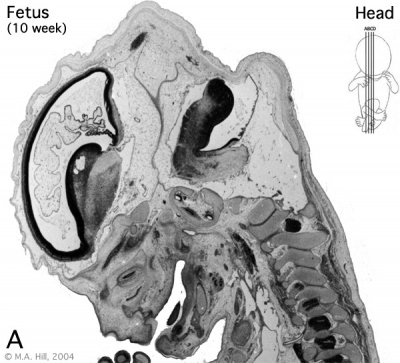
|
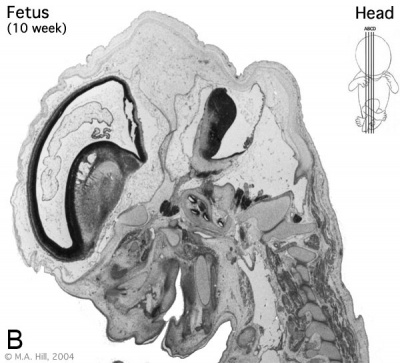
|
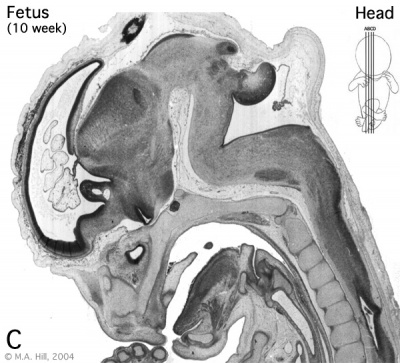
|
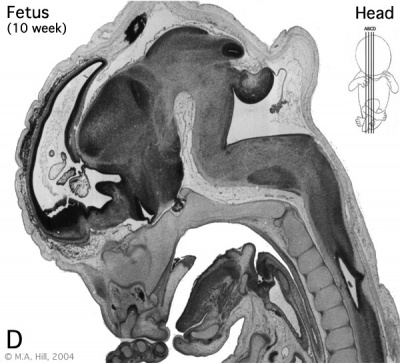
|
The above images are from a week 10 human fetus.
Fetal Brain
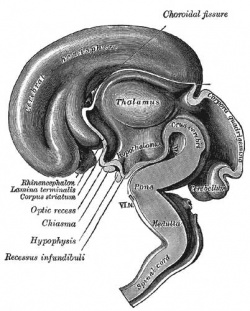
|
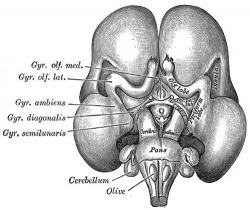
|
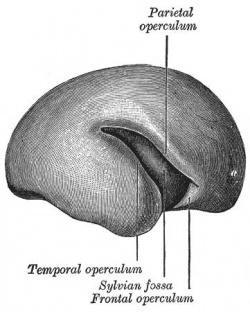
|
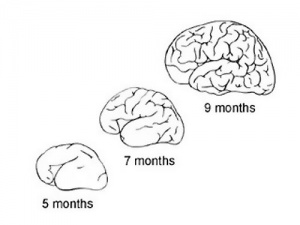
| Fetal brain (3 months) | Fetal brain (4 months) | Fetal brain (5 months) |
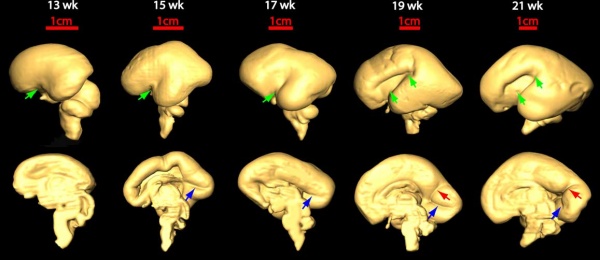
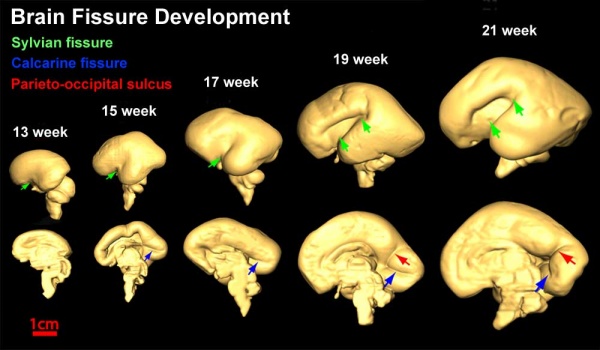
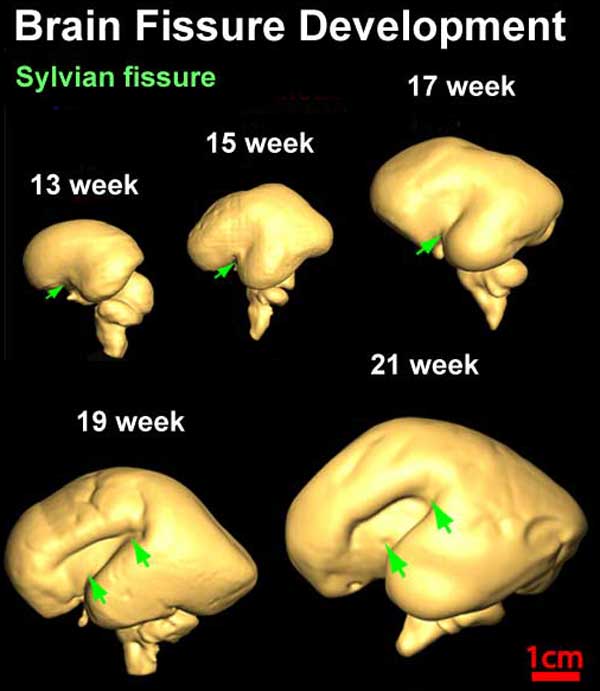
Fissures are the major indentations, sulci (singular sulcus), that divide the brain surface into lobes and appear during fetal development as the brain grows. The images below show MRI analysis of the developing human fetal brain.
- Links: Magnetic Resonance Imaging
Developmental Overview
Cortical Neurons
[[File:Telencephalon development signals.jpg|thumb|Telencephalon development signals[11]
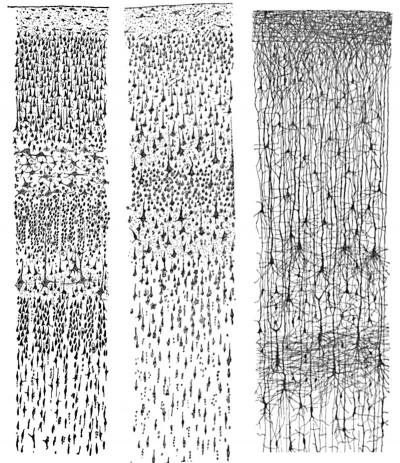
Cortical layers in a historic drawing by Cajal.
Cajal-Retzius Neurons
Cajal-Retzius (CR) cells are some of the earliest generated cortical neurons arising from restricted domains of the pallial ventricular zone, and then migrate from the borders of the developing pallium to cover the cortical primordium. These early forming neurons then control the radial migration of neurons and the formation of cortical layers. In mice, this has been shown by these cells secreting the extracellular glycoprotein Reelin (Reln) and it has been suggested that these cells also fine tune multiple signaling pathways underlying the regulation of cortical regionalization.[11]
Molecular
- Fgfr1 and Fgfr2 - control excitatory cortical neuron development within the entire cerebral cortex.[13]
- Fgfr2 - proper formation of the medial prefrontal cortex (mPFC).[13]
- MARCKS - (myristoylated alanine-rich C-kinase substrate protein) a cellular substrate for PKC modulates radial glial placement and expansion.[14]
- MicroRNA - noncoding RNAs that regulate mRNA expression, highly expressed during development.[15][16]
Sensorimotor Cortex
The sensorimotor cortex is the region comprising the precentral and postcentral gyri and covers the primary sensory and motor areas of the brain. This region is involved with central integration and processing of the sensory input and motor output of the brain.
A study of this region of the human fetal brain from the end of the first trimester GA 13 weeks (11 weeks) and the to end of the second trimester GA 28 weeks (26 weeks) has identified four stages of development:[7]
| Sensorimotor Cortex Timeline | ||
|---|---|---|
| Gestational Age GA weeks | Fertilization Age FA weeks | Event |
| 18-19 | 16-17 | appearance of the inferior part of the central cerebral sulcus |
| 20-22 | 18-20 | development of the pericentral lateral regions and the beginning of opercularization |
| 24-26 | 22-24 | development of parietal and temporal cortices and the covering of the postcentral insular region |
| 27-28 | 25-26 | maturation of the central cerebral regions |
| Table data.[7] Links: sensorimotor cortex | cerebral cortex | second trimester | Sensory System Development | ||
- central cerebral sulcus - (central fissure, fissure of Rolando, Rolandic fissure) fold in the cerebral cortex.
- opercularization - during fetal development the insula begins to invaginate from the surface of the immature cerebrum of the brain, until at full term, the opercula completely cover the insula.
- posterior insula - is composed of the anterior and posterior long insular gyri and the postcentral insular sulcus, which separates them.
- Links: Sensory System Development
Corpus Callosum
The corpus callosum is the area of the brain which connects the two cerebral hemispheres. Maximum increase in thickness and width of the corpus callosum occurred between 19 and 21 weeks' gestation.[17]
Human Timeline:
- 74 days - callosal axons appear.
- 84 days - subdivisions of the genu and splenium can be identified.
- 115 days - adult morphology is seen.
Agenesis of Corpus Callosum
Agenesis of the corpus callosum (ACC) is a partial or complete absence of the corpus callosum, a rare cerebral malformation.
- Links: NINDS Information
Cerebral Vascular Development
| Overview cartoon | Early vascular changes |
|---|---|
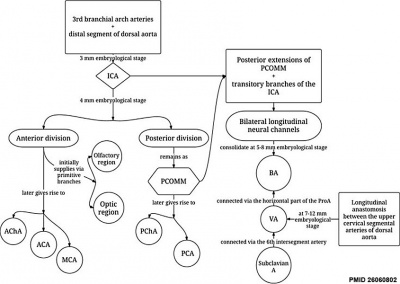
|

|
Chronological development of cerebral brain arteries initially with the rise of the internal carotid artery and subsequently with the development of the posterior circulation.[18]
- Links: blood vessel | Head Development
Animal Models
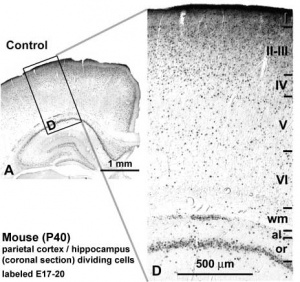
Mouse Cortex
| Timeline comparison of the migration and layer arrangement in neocortex and hippocampal CA1 during cortical development[19] | |
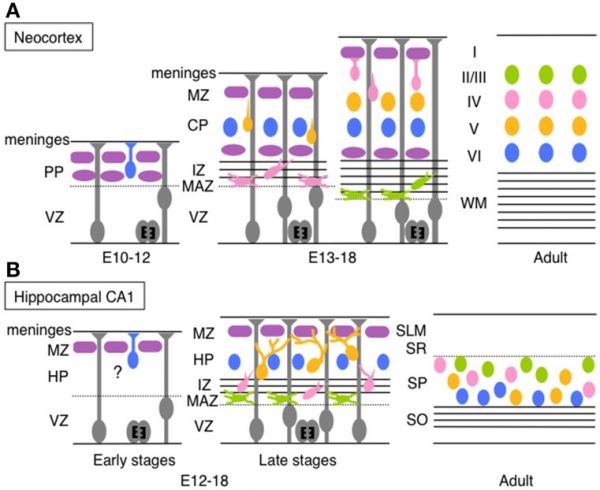
|
(A) Neocortical neurons born between E10 and E12 radially migrate using the somal translocation mode. In contrast, late-born neurons transform their migration mode sequentially to multipolar migration, locomotion mode, and terminal translocation mode during their radial migration. These neurons form neocortical layers in a birthdate-dependent inside-out manner.
PP, preplate; VZ, ventricular zone; MZ, marginal zone; CP, cortical plate; IZ, intermediated zone; MAZ, multipolar cell accumulation zone; WM, white matter; HP, hippocampal plate; SLM, stratum lacunosum-moleculare; SR, stratum radiatum; SP, stratum pyramidale; SO, stratum oriens. (text modified from original figure legend) |
Abnormalities
Agenesis of Corpus Callosum
Agenesis of the corpus callosum (ACC) is a partial or complete absence of the corpus callosum, a rare cerebral malformation.
- Links: NINDS Information
Hemimegalencephaly
Increased proliferation
Heterotopia
Ectopic migration
Lissencephaly
A malformations derived from abnormal neuronal migration leading to agyria (convolutions of the cerebral cortex are not fully formed) and pachygyria (convolutions of the cerebral cortex unusually thick ).
Pachygyria (Greek, pachy = "thick")
Microlissencephaly
Decreased proliferation
Polymicrogyria
Abnormal organization, numerous small gyri and a thick disorganized cortical plate lacking normal lamination, disruption of microtubule-based processes underlies a large spectrum of neuronal migration.[20]
Schizencephaly
Abnormal organization
References
- ↑ Habas PA, Kim K, Rousseau F, Glenn OA, Barkovich AJ & Studholme C. (2010). Atlas-based segmentation of developing tissues in the human brain with quantitative validation in young fetuses. Hum Brain Mapp , 31, 1348-58. PMID: 20108226 DOI.
- ↑ Herculano-Houzel S, Catania K, Manger PR & Kaas JH. (2015). Mammalian Brains Are Made of These: A Dataset of the Numbers and Densities of Neuronal and Nonneuronal Cells in the Brain of Glires, Primates, Scandentia, Eulipotyphlans, Afrotherians and Artiodactyls, and Their Relationship with Body Mass. Brain Behav. Evol. , 86, 145-63. PMID: 26418466 DOI.
- ↑ Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, Lind PA, Pizzagalli F, Ching CRK, McMahon MAB, Shatokhina N, Zsembik LCP, Thomopoulos SI, Zhu AH, Strike LT, Agartz I, Alhusaini S, Almeida MAA, Alnæs D, Amlien IK, Andersson M, Ard T, Armstrong NJ, Ashley-Koch A, Atkins JR, Bernard M, Brouwer RM, Buimer EEL, Bülow R, Bürger C, Cannon DM, Chakravarty M, Chen Q, Cheung JW, Couvy-Duchesne B, Dale AM, Dalvie S, de Araujo TK, de Zubicaray GI, de Zwarte SMC, den Braber A, Doan NT, Dohm K, Ehrlich S, Engelbrecht HR, Erk S, Fan CC, Fedko IO, Foley SF, Ford JM, Fukunaga M, Garrett ME, Ge T, Giddaluru S, Goldman AL, Green MJ, Groenewold NA, Grotegerd D, Gurholt TP, Gutman BA, Hansell NK, Harris MA, Harrison MB, Haswell CC, Hauser M, Herms S, Heslenfeld DJ, Ho NF, Hoehn D, Hoffmann P, Holleran L, Hoogman M, Hottenga JJ, Ikeda M, Janowitz D, Jansen IE, Jia T, Jockwitz C, Kanai R, Karama S, Kasperaviciute D, Kaufmann T, Kelly S, Kikuchi M, Klein M, Knapp M, Knodt AR, Krämer B, Lam M, Lancaster TM, Lee PH, Lett TA, Lewis LB, Lopes-Cendes I, Luciano M, Macciardi F, Marquand AF, Mathias SR, Melzer TR, Milaneschi Y, Mirza-Schreiber N, Moreira JCV, Mühleisen TW, Müller-Myhsok B, Najt P, Nakahara S, Nho K, Olde Loohuis LM, Orfanos DP, Pearson JF, Pitcher TL, Pütz B, Quidé Y, Ragothaman A, Rashid FM, Reay WR, Redlich R, Reinbold CS, Repple J, Richard G, Riedel BC, Risacher SL, Rocha CS, Mota NR, Salminen L, Saremi A, Saykin AJ, Schlag F, Schmaal L, Schofield PR, Secolin R, Shapland CY, Shen L, Shin J, Shumskaya E, Sønderby IE, Sprooten E, Tansey KE, Teumer A, Thalamuthu A, Tordesillas-Gutiérrez D, Turner JA, Uhlmann A, Vallerga CL, van der Meer D, van Donkelaar MMJ, van Eijk L, van Erp TGM, van Haren NEM, van Rooij D, van Tol MJ, Veldink JH, Verhoef E, Walton E, Wang M, Wang Y, Wardlaw JM, Wen W, Westlye LT, Whelan CD, Witt SH, Wittfeld K, Wolf C, Wolfers T, Wu JQ, Yasuda CL, Zaremba D, Zhang Z, Zwiers MP, Artiges E, Assareh AA, Ayesa-Arriola R, Belger A, Brandt CL, Brown GG, Cichon S, Curran JE, Davies GE, Degenhardt F, Dennis MF, Dietsche B, Djurovic S, Doherty CP, Espiritu R, Garijo D, Gil Y, Gowland PA, Green RC, Häusler AN, Heindel W, Ho BC, Hoffmann WU, Holsboer F, Homuth G, Hosten N, Jack CR, Jang M, Jansen A, Kimbrel NA, Kolskår K, Koops S, Krug A, Lim KO, Luykx JJ, Mathalon DH, Mather KA, Mattay VS, Matthews S, Mayoral Van Son J, McEwen SC, Melle I, Morris DW, Mueller BA, Nauck M, Nordvik JE, Nöthen MM, O'Leary DS, Opel N, Martinot MP, Pike GB, Preda A, Quinlan EB, Rasser PE, Ratnakar V, Reppermund S, Steen VM, Tooney PA, Torres FR, Veltman DJ, Voyvodic JT, Whelan R, White T, Yamamori H, Adams HHH, Bis JC, Debette S, Decarli C, Fornage M, Gudnason V, Hofer E, Ikram MA, Launer L, Longstreth WT, Lopez OL, Mazoyer B, Mosley TH, Roshchupkin GV, Satizabal CL, Schmidt R, Seshadri S, Yang Q, Alvim MKM, Ames D, Anderson TJ, Andreassen OA, Arias-Vasquez A, Bastin ME, Baune BT, Beckham JC, Blangero J, Boomsma DI, Brodaty H, Brunner HG, Buckner RL, Buitelaar JK, Bustillo JR, Cahn W, Cairns MJ, Calhoun V, Carr VJ, Caseras X, Caspers S, Cavalleri GL, Cendes F, Corvin A, Crespo-Facorro B, Dalrymple-Alford JC, Dannlowski U, de Geus EJC, Deary IJ, Delanty N, Depondt C, Desrivières S, Donohoe G, Espeseth T, Fernández G, Fisher SE, Flor H, Forstner AJ, Francks C, Franke B, Glahn DC, Gollub RL, Grabe HJ, Gruber O, Håberg AK, Hariri AR, Hartman CA, Hashimoto R, Heinz A, Henskens FA, Hillegers MHJ, Hoekstra PJ, Holmes AJ, Hong LE, Hopkins WD, Hulshoff Pol HE, Jernigan TL, Jönsson EG, Kahn RS, Kennedy MA, Kircher TTJ, Kochunov P, Kwok JBJ, Le Hellard S, Loughland CM, Martin NG, Martinot JL, McDonald C, McMahon KL, Meyer-Lindenberg A, Michie PT, Morey RA, Mowry B, Nyberg L, Oosterlaan J, Ophoff RA, Pantelis C, Paus T, Pausova Z, Penninx BWJH, Polderman TJC, Posthuma D, Rietschel M, Roffman JL, Rowland LM, Sachdev PS, Sämann PG, Schall U, Schumann G, Scott RJ, Sim K, Sisodiya SM, Smoller JW, Sommer IE, St Pourcain B, Stein DJ, Toga AW, Trollor JN, Van der Wee NJA, van 't Ent D, Völzke H, Walter H, Weber B, Weinberger DR, Wright MJ, Zhou J, Stein JL, Thompson PM & Medland SE. (2020). The genetic architecture of the human cerebral cortex. Science , 367, . PMID: 32193296 DOI.
- ↑ Wang XL, Ma YX, Xu RJ, Ma JJ, Zhang HC, Qi SB, Xu JH, Qin XZ, Zhang HN, Liu CM, Chen JQ, Li B, Yang HL & Saijilafu. (2020). c-Myc controls the fate of neural progenitor cells during cerebral cortex development. J. Cell. Physiol. , 235, 4011-4021. PMID: 31625158 DOI.
- ↑ Ohira K. (2020). Dopamine as a growth differentiation factor in the mammalian brain. Neural Regen Res , 15, 390-393. PMID: 31571646 DOI.
- ↑ Kurabayashi N, Tanaka A, Nguyen MD & Sanada K. (2018). The LPA-LPA4 axis is required for establishment of bipolar morphology and radial migration of newborn cortical neurons. Development , 145, . PMID: 30217809 DOI.
- ↑ 7.0 7.1 7.2 Afif A, Trouillas J & Mertens P. (2015). Development of the sensorimotor cortex in the human fetus: a morphological description. Surg Radiol Anat , 37, 153-60. PMID: 24972575 DOI.
- ↑ Rajagopalan V, Scott J, Habas PA, Kim K, Corbett-Detig J, Rousseau F, Barkovich AJ, Glenn OA & Studholme C. (2011). Local tissue growth patterns underlying normal fetal human brain gyrification quantified in utero. J. Neurosci. , 31, 2878-87. PMID: 21414909 DOI.
- ↑ Zhang Z, Liu S, Lin X, Teng G, Yu T, Fang F & Zang F. (2011). Development of laminar organization of the fetal cerebrum at 3.0T and 7.0T: a postmortem MRI study. Neuroradiology , 53, 177-84. PMID: 20981415 DOI.
- ↑ Trivedi R, Husain N, Rathore RK, Saksena S, Srivastava S, Malik GK, Das V, Pradhan M, Pandey CM & Gupta RK. (2009). Correlation of diffusion tensor imaging with histology in the developing human frontal cerebrum. Dev. Neurosci. , 31, 487-96. PMID: 19622880 DOI.
- ↑ 11.0 11.1 Griveau A, Borello U, Causeret F, Tissir F, Boggetto N, Karaz S & Pierani A. (2010). A novel role for Dbx1-derived Cajal-Retzius cells in early regionalization of the cerebral cortical neuroepithelium. PLoS Biol. , 8, e1000440. PMID: 20668538 DOI.
- ↑ Berbel P, Navarro D, Ausó E, Varea E, Rodríguez AE, Ballesta JJ, Salinas M, Flores E, Faura CC & de Escobar GM. (2010). Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity. Cereb. Cortex , 20, 1462-75. PMID: 19812240 DOI.
- ↑ 13.0 13.1 Stevens HE, Smith KM, Maragnoli ME, Fagel D, Borok E, Shanabrough M, Horvath TL & Vaccarino FM. (2010). Fgfr2 is required for the development of the medial prefrontal cortex and its connections with limbic circuits. J. Neurosci. , 30, 5590-602. PMID: 20410112 DOI.
- ↑ Weimer JM, Yokota Y, Stanco A, Stumpo DJ, Blackshear PJ & Anton ES. (2009). MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex. Development , 136, 2965-75. PMID: 19666823 DOI.
- ↑ Fineberg SK, Kosik KS & Davidson BL. (2009). MicroRNAs potentiate neural development. Neuron , 64, 303-9. PMID: 19914179 DOI.
- ↑ Kuwahara A, Hirabayashi Y, Knoepfler PS, Taketo MM, Sakai J, Kodama T & Gotoh Y. (2010). Wnt signaling and its downstream target N-myc regulate basal progenitors in the developing neocortex. Development , 137, 1035-44. PMID: 20215343 DOI.
- ↑ Achiron R & Achiron A. (2001). Development of the human fetal corpus callosum: a high-resolution, cross-sectional sonographic study. Ultrasound Obstet Gynecol , 18, 343-7. PMID: 11778993 DOI.
- ↑ Menshawi K, Mohr JP & Gutierrez J. (2015). A Functional Perspective on the Embryology and Anatomy of the Cerebral Blood Supply. J Stroke , 17, 144-58. PMID: 26060802 DOI.
- ↑ Hayashi K, Kubo K, Kitazawa A & Nakajima K. (2015). Cellular dynamics of neuronal migration in the hippocampus. Front Neurosci , 9, 135. PMID: 25964735 DOI.
- ↑ Jaglin XH, Poirier K, Saillour Y, Buhler E, Tian G, Bahi-Buisson N, Fallet-Bianco C, Phan-Dinh-Tuy F, Kong XP, Bomont P, Castelnau-Ptakhine L, Odent S, Loget P, Kossorotoff M, Snoeck I, Plessis G, Parent P, Beldjord C, Cardoso C, Represa A, Flint J, Keays DA, Cowan NJ & Chelly J. (2009). Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat. Genet. , 41, 746-52. PMID: 19465910 DOI.
Reviews
Blaauw J & Meiners LC. (2020). The splenium of the corpus callosum: embryology, anatomy, function and imaging with pathophysiological hypothesis. Neuroradiology , , . PMID: 32062761 DOI.
Martínez-Cerdeño V & Noctor SC. (2018). Neural Progenitor Cell Terminology. Front Neuroanat , 12, 104. PMID: 30574073 DOI.
Molnár Z & Clowry G. (2019). Human cerebral cortex development. J. Anat. , 235, 431. PMID: 31435944 DOI.
Fernández V, Llinares-Benadero C & Borrell V. (2016). Cerebral cortex expansion and folding: what have we learned?. EMBO J. , 35, 1021-44. PMID: 27056680 DOI.
Diaz AL & Gleeson JG. (2009). The molecular and genetic mechanisms of neocortex development. Clin Perinatol , 36, 503-12. PMID: 19732610 DOI.
Rakic P. (2009). Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. , 10, 724-35. PMID: 19763105 DOI.
Articles
Zhan J, Dinov ID, Li J, Zhang Z, Hobel S, Shi Y, Lin X, Zamanyan A, Feng L, Teng G, Fang F, Tang Y, Zang F, Toga AW & Liu S. (2013). Spatial-temporal atlas of human fetal brain development during the early second trimester. Neuroimage , 82, 115-26. PMID: 23727529 DOI.
Shoemaker LD, Orozco NM, Geschwind DH, Whitelegge JP, Faull KF & Kornblum HI. (2010). Identification of differentially expressed proteins in murine embryonic and postnatal cortical neural progenitors. PLoS ONE , 5, e9121. PMID: 20161753 DOI.
Zimmer C, Lee J, Griveau A, Arber S, Pierani A, Garel S & Guillemot F. (2010). Role of Fgf8 signalling in the specification of rostral Cajal-Retzius cells. Development , 137, 293-302. PMID: 20040495 DOI.
Simó S, Jossin Y & Cooper JA. (2010). Cullin 5 regulates cortical layering by modulating the speed and duration of Dab1-dependent neuronal migration. J. Neurosci. , 30, 5668-76. PMID: 20410119 DOI.
Stevens HE, Smith KM, Maragnoli ME, Fagel D, Borok E, Shanabrough M, Horvath TL & Vaccarino FM. (2010). Fgfr2 is required for the development of the medial prefrontal cortex and its connections with limbic circuits. J. Neurosci. , 30, 5590-602. PMID: 20410112 DOI.
Kuwahara A, Hirabayashi Y, Knoepfler PS, Taketo MM, Sakai J, Kodama T & Gotoh Y. (2010). Wnt signaling and its downstream target N-myc regulate basal progenitors in the developing neocortex. Development , 137, 1035-44. PMID: 20215343 DOI.
Search PubMed
Search Pubmed: Cerebrum Embryology | Cerebrum Development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Zagreb Neuroembryological Collection - contains more than 500 prenatal human brains stained with various classical neurohistological, as well as modern histochemical and immunohistochemical methods. The bank is located at the Croatian Institute for Brain Research. http://www.hiim.hr/nova
| Neural Terms |
|---|
Neural Development
|
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Neural - Cerebrum Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Cerebrum_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G