Gastrointestinal Tract - Liver Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
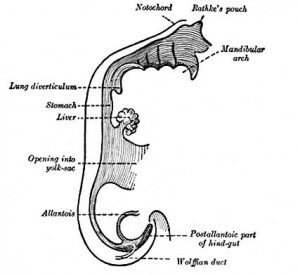
This section of notes gives an overview of how the liver develops. Initially, the transverse septum (septum transversum) arises at an embryonic junctional site. The junctional region externally is where the ectoderm of the amnion meets the endoderm of the yolk sac. The junctional region internally is where the foregut meets the Midgut. The mesenchymal structure of the transverse septum provides a support within which both blood vessels and the liver begin to form. Arises at embryonic junction (septum transversum): externally is where ectoderm of amnion meets endoderm of yolk sac and internally is where the foregut meets the midgut. Mesenchymal structure of transverse septum provides a support within which both blood vessels and liver begin to form in the underlying splanchnic mesoderm.
In the early embryo, the liver and heart grow rapidly forming obvious external swellings on the ventral embryo surface. The liver's initial embryonic function is mainly cardiovascular. Firstly, as a vascular connection between the developing placental vessels to the heart. Secondly, as a haemopoietic tissue where blood stem cells reside before bone marrow development.
The fetal liver also has an endocrine role by 16-hydroxylation, that results in estriol being the major estrogen type produced in late human pregnancy.
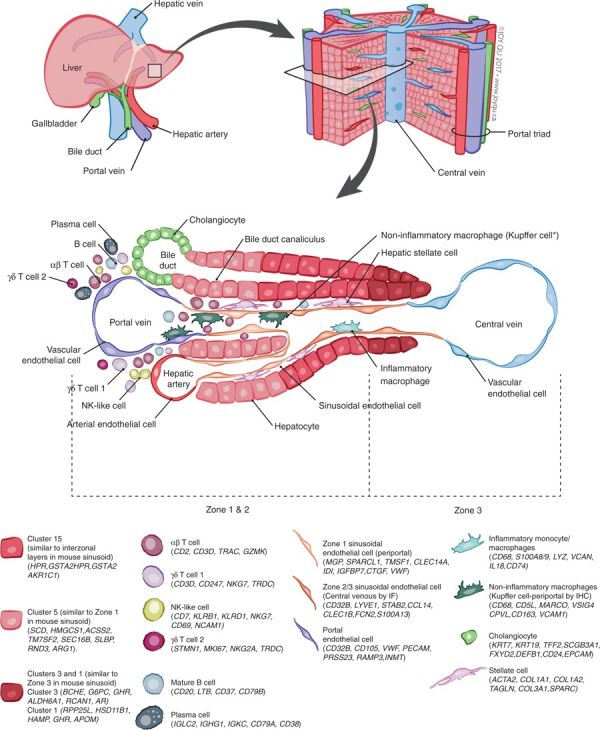
A recent molecular study has shown that within the adult liver at least 20 discrete cell populations exist these include: hepatocytes, endothelial cells, cholangiocytes, hepatic stellate cells, B cells, conventional and non-conventional T cells, NK-like cells, and distinct intrahepatic monocyte/macrophage populations.[1]
See also liver histology showing both developmental and adult histology.
| Historic Embryology |
|
Marcello Malpighi (1628 – 1694) was an Italian biologist and physician who in 1666 first named the liver lobules - "the livers of all vertebrates are conglomerate glands, being composed of lobules which in turn contain acini". He is also known for structures that bear his name in the spleen Malpighian bodies (white pulp) and renal Malpighian corpuscle (renal corpuscle). See also Mall's 1906 Liver Historical Note |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Liver Embryology | Liver Development | Hepatic Embryology | Fetal Hepatocytes | Fetal Liver Function |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Movies
|
|
Liver Development Stages
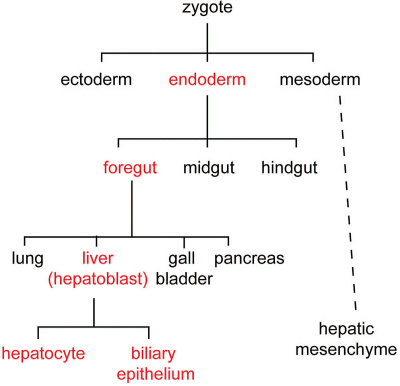
Cell lineage during hepatic development (red) from uncommitted endoderm to functional adult hepatocytes and biliary epithelium.[10]
| Feature | ||
|---|---|---|
| hepatic diverticulum development (ductal plate) | ||
| cell differentiation
septum transversum forming liver stroma hepatic diverticulum forming hepatic trabeculae | ||
| epithelial cord proliferation enmeshing stromal capillaries | ||
| hepatic gland and its vascular channels enlarge
hematopoietic function appeared | ||
| obturation due to epithelial proliferation
bile ducts became reorganized (continuity between liver cells and gut) | ||
| biliary ductules developed in periportal connective tissue
produces ductal plates that receive biliary capillaries | ||
| Human data[11], see also liver development in the rat embryonic period (Carnegie stages 15-23).[12] (More? Detailed Timeline | Timeline human development) | ||
| Carnegie Stage | Age (days) | CRL (mm) | Biliary system | Vascular | Hepatic parenchyma |
|---|---|---|---|---|---|
| 14 | 33 | 7 |
|
|
|
| 18 | 46 | 15 |
|
|
|
| 21 | 53 | 22.5 | Bile duct morphology as earlier stage. Common bile duct empties at the level of the proximal duodenum. |
|
Hepatic parenchyma a large rounded mass. |
| 23 | 58 | 27 | Bile duct morphology as earlier stage. |
|
|
| Data from a recent human study[13] Links: liver | Carnegie stage 14 | 18 | 21 | 23 | simple embryonic timeline | Timeline human development | |||||
- Size - the liver initially occupies the entire anterior body area.
- Hepatoblast - endoderm the bipotential progenitor for both hepatocytes and cholangiocytes.
- Vascular - mesoderm blood vessels enter the liver (3 systems: systemic, placental, vitelline)
- Sinusoids - first blood vessels from vessels in septum transversum mesenchyme. Initially continuous endothelium, become fenestrated in fetal period and reticular development ongoing.
Adult Liver Cells
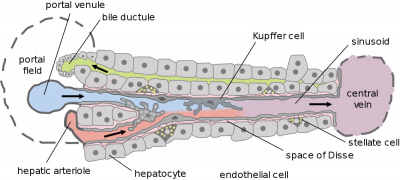
|
|
| Summary Cell Map of the Adult Human Liver | |
|---|---|
| This study[1] has shown in the adult human liver at least 20 discrete cell populations exist. These include: hepatocytes, endothelial cells, cholangiocytes, hepatic stellate cells, B cells, conventional and non-conventional T cells, NK-like cells, and distinct intrahepatic monocyte/macrophage populations.
|
|
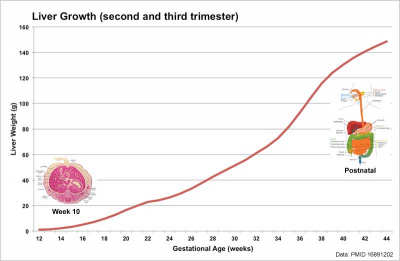
Liver Growth
Human Liver Growth (weight grams)[14]
Liver Buds
- Differentiates to form the hepatic diverticulum and hepatic primordium, generates the gallbladder then divides into right and left hepatic (liver) buds.
- Three connecting stalks (cystic duct, hepatic ducts) which fuse to form bile duct.
Left Hepatic Bud
- left lobe, quadrate, caudate (both q and c anatomically Left)
- caudate lobe of human liver consists of 3 anatomical parts: Spiegel's lobe, caudate process, and paracaval portion.
Right Hepatic Bud
- right lobe
Liver Structural Origins
- Hepatic Buds - form hepatocytes, produce bile from week 13 (forms meconium of newborn)
- Vitelline Veins - form sinusoids
- Mesenchyme - form connective tissue and Kupffer cells
Function - Haemopoiesis
Embryonic liver also involved in blood formation, after the yolk sac and blood islands acting as a primary site.
Components of Liver Formation
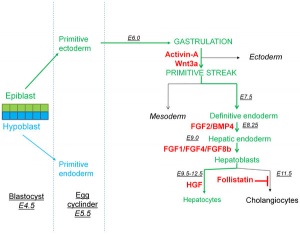
Primitive Endoderm
|
|
|
Data from mouse [16]
Hepatoblasts - endoderm-derived cells can differentiate into either:
- hepatocytes - populate the bulk of the liver parenchyma.
- cholangiocytes - line the intrahepatic bile ducts.
- Links: Endoderm | Mouse Development
Development
Stage 13
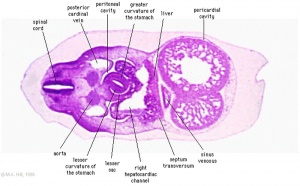
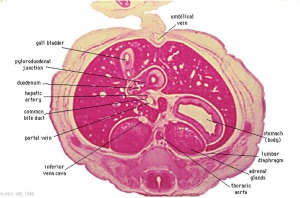
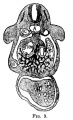
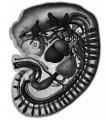
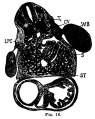
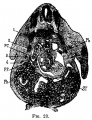
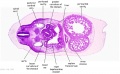
The images below link to larger cross-sections of the mid-embryonic period (end week 4) stage 13 embryo starting just above the level of the liver and then in sequence through the liver to the level of the stomach. Note the relative position of the liver with respect to the abdominal cavity, the gallbladder and the heart.
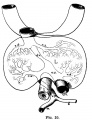
The transverse septum differentiates to form the hepatic diverticulum and the hepatic primordium, these two structures together will go on to form different components of the mature liver and gallbladder. At this stage large vascular channels can be seen coursing through the liver primordium.
|
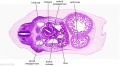
|
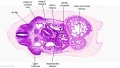
|
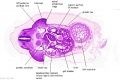
|
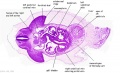
|
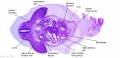
|
| D3L | D4L | D5L | D6L | D7L | E1L |
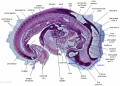
|
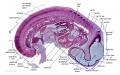
| ||||
| G6L | G7L |
- Links: Carnegie stage 13 - serial sections | Embryo Serial Sections | Embryo Carnegie stage 13 Movies
Stage 22
| Virtual Slide Features - Stage 22 Liver | |||||||
|---|---|---|---|---|---|---|---|
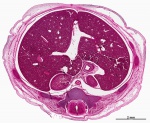
|
Virtual Slide - Stage 22 Liver and Ductus Venosus All Virtual Slides
The links shown in the table below are to specific features shown on the Human embryo (stage 22) Liver and Ductus Venosus virtual slide. See also notes on Liver Development Clicking the text will open the slide at a detailed view with the structure generally located in the centre of the view. The slide then can also be zoomed out from the set magnification using the controls in the upper left or the mouse. Use your browser back button to return to this table. |
You can also make your own selected feature view.
See also Permalink help | |||||
| Cardiovascular | Liver | Endocrine | Musculoskeletal | Neural | Gastrointestinal | ||
| Virtual Slide Features - Stage 22 Liver | |||||||
|---|---|---|---|---|---|---|---|

|
Virtual Slide - Stage 22 Liver and Ductus Venosus All Virtual Slides
The links shown in the table below are to specific features shown on the Human embryo (stage 22) Liver and Ductus Venosus virtual slide. See also notes on Liver Development Clicking the text will open the slide at a detailed view with the structure generally located in the centre of the view. The slide then can also be zoomed out from the set magnification using the controls in the upper left or the mouse. Use your browser back button to return to this table. |
You can also make your own selected feature view.
See also Permalink help | |||||
| Cardiovascular | Liver | Endocrine | Musculoskeletal | Neural | Gastrointestinal | ||
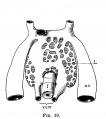
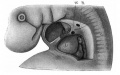
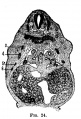
The images below link to larger cross-sections of the end of the embryonic period (week 8) stage 22 embryo starting just above the level of the liver and then in sequence through the entire liver. (Note the sections are viewed from below, LR axis is reversed)
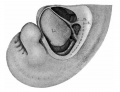
The rapidly developing liver also forms a visible surface bulge on the embryo directly under the heart bulge. The liver now occupies the entire ventral body cavity with parts of the gastrointestinal tract and urinary system "embedded" within its structure. Note in this image the large central ductus venosus.
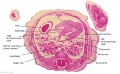
|
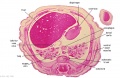
|
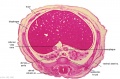
|
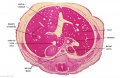
|
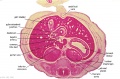
|
| E3L | E4L | E5L | E6L | E7L |
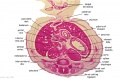
|
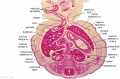
|
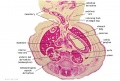
|
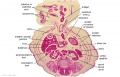
|
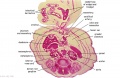
|
| F1L | F2L | F3L | F4L | F5L |
- Links: Carnegie stage 22 - serial sections | Embryo Serial Sections | Embryo Carnegie stage 22 Movies
Selected Stage 22 Images
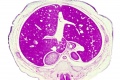
|
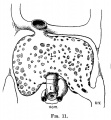
E3 Overview of liver region for selected high power views shown below. Note the position and size of the developing liver spanning the entire abdomen and within the liver the large central ductus venosus. |
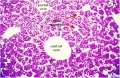
|
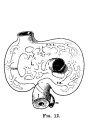
E4 Central veins of liver. Radiating appearance of hepatic sinusoids. unlabeled version |
|
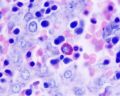
E5 Central vein with endothelial lining, containing nucleated erythrocytes, fetal red blood cells. The fetal liver has an important haemopoietic role. unlabeled version |
Week 9
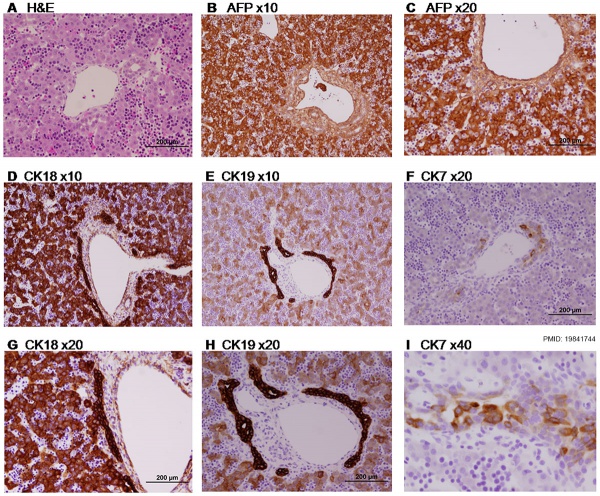
Paraffin-embedded sections of human embryonic liver at 9 weeks (GA 11 weeks).[17] |
|
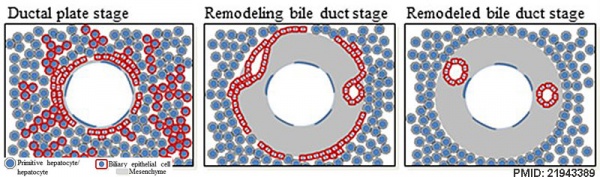
Ductal Plate
The ductal plate is a primitive biliary epithelium which develops in mesenchyme adjacent to portal vein branches (periportal hepatoblasts). During liver development it is extensively reorganised (ductal plate remodelling) within the developing liver to form the intrahepatic bile ducts (IHBD). If remodelling does not occur, leading to excess of embryonic bile duct structures in the portal tract, these developmental abnormalities are described as "ductal plate malformation" (DPM).
Cholangiocyte tubulogenesis: "ductal plate" stages -> "remodeling bile duct" stage -> "remodeled bile duct"
Cartoon model of bile duct formation.[8]
See also Ductal Plate Malformations
Bile Secretion
The epithelial cells that line the bile ducts are called cholangiocytes.
The pathway below describes the production and passage of bile for final excretion into the duodenum:
- hepatocytes produce bile
- secreted into bile canaliculi
- connected to intrahepatic bile ducts
- intrahepatic bile ducts connect to the hepatic duct
- then the cystic duct for storage in the gallbladder
- then the common bile duct into the duodenum
The term extrahepatic bile ducts (EHBDs) is used to describe the hepatic, cystic, and common bile ducts.
The developing bile ducts express VEGF while hepatoblasts express angiopoietin-1, these two signals are thought to regulate arterial vasculogenesis and remodeling of the hepatic artery respectively.[18]
Liver Vascular
Vascular development data below from[19]
Venous
- week 4 - hepatic primordium in contact with vitelline veins and the umbilical veins.
- week 4 to 6 - efferent venous vessels form from the vitelline veins. Afferent venous liver circulation present.
- Week 6 onward - portal vein formed from segments of the vitelline veins
- portal sinus (from subhepatic intervitelline anastomosis) connects umbilical vein to portal system.
- ductus venosus connects portal sinus to vena cava inferior.
- birth - both umbilical vein and ductus venosus collapse.
- portal vein becomes the only afferent vein of the liver.
Arterial
- week 8 - hepatic artery forms.
- week 10 to 15 - intrahepatic arterial branches progressively extend from the central to the peripheral areas of the liver.
Hepatic sinusoids
- week 4 - hepatic cords invade the septum transversum
- progressively acquire structural and functional characters, through a multistage process.
Liver Blood Flow
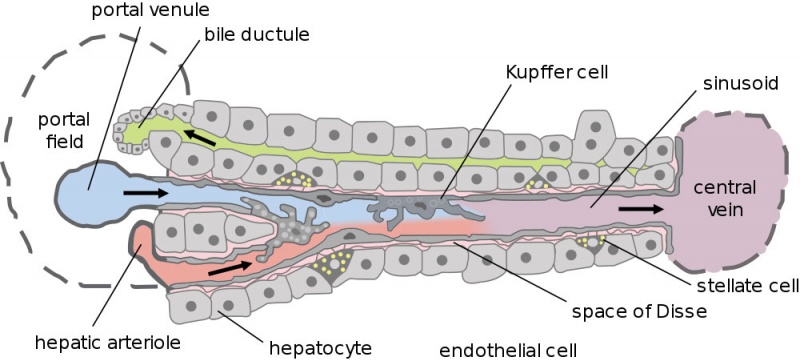
Dual blood supply of the liver merges upon entry into the liver lobule at the portal field. The blood flows along the sinusoid and exits at the central vein.
- branches of the portal vein
- branches of the hepatic artery
Portal Vein - is the sole supplier to the liver until about human 20 mm CRL stage. Portal vein primary branches extend around the periphery of each primitive liver lobule. This branching process continues, from primary to secondary, with each development supplying all newly forming liver lobules. Primary branches also lie parallel to the branches of hepatic veins, that drain the blood from the centre of each early lobule.
Hepatic Artery - from the coeliac axis, initially contact the hepatic duct and gallbladder, later grows into the connective tissue about the larger bile ducts and branches of the portal vein. The hepatic artery will also supply the capsule of the liver.
Hepatocytes
These are the adult functional cells forming the majority of the liver (80% of the cells).
Many different functions including:
- Storage of substances including glucose (as glycogen), vitamin A (possibly in specialized adipocytes), vitamin B12, folic acid and iron.
- Lipid Turnover synthesis of plasmalipoproteins
- Plasma Protein Synthesis albumin, alpha and beta globulins, prothrombin, fibrinogen
- Metabolism fat soluble compounds (drugs, insecticides), steroid hormones turnover
- Secretion bile (about 1 litre/day)
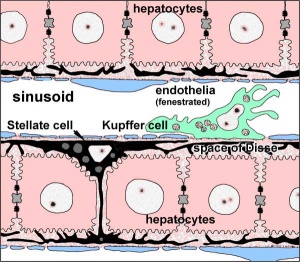
Kupffer Cells
Kupffer Cells are a population of tissue macrophages found in the lumen of hepatic sinusoids, their role is endocytic acting against blood-borne materials entering the liver.
Primordial (primitive) macrophages arise in the yolk sac and then differentiate into fetal macrophages, either of these enter the blood and migrate into the developing liver.[20]
Tissue macrophages are a family of cells found in many organs[21]: liver Kupffer cells, neural microglia, respiratory alveolar macrophages, and integumentary epidermal Langerhans cells. In the embryo, they have a common embryonic origin from yolk sac (YS) erythro-myeloid progenitors (EMPs). In the adult, they are self-maintained in tissues independently of hematopoietic stem cells (HSCs).[22]
- Search PubMed: Kupffer cell development
Liver Associated Vessels
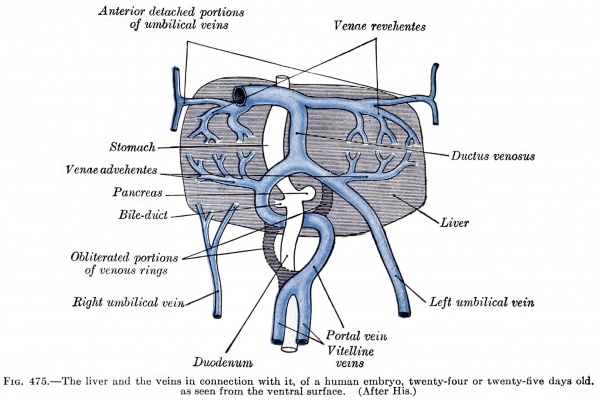
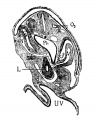
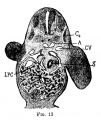
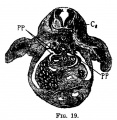
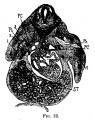
Liver ventral surface and associated veins (human embryo, 24-25 days, after His.) |
|
Adult Liver Transplants
- About 6,000 liver transplant operations are performed in the United States (http://www.liverfoundation.org/education/info/transplant/)
- About 600–700 in the UK every year (http://www.britishlivertrust.org.uk/home/the-liver/liver-transplantation/a-history-of-liver-transplantation-and-current-statistics.aspx).
- The main limitation on numbers are the availability of donor organs.[23]
Histology
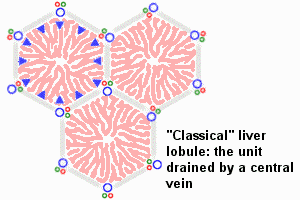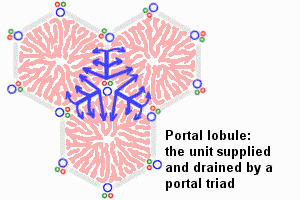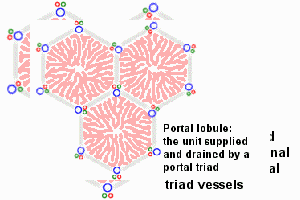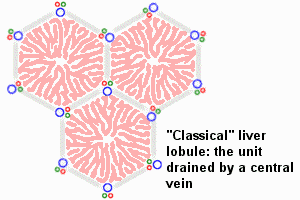
The Liver Lobule
Adult liver Portal Triad
- Links: liver histology
Abnormalities
| ICD-11 LB20 Structural developmental anomalies of gallbladder, bile ducts or liver |
|---|
|
LB20.00 Fibropolycystic liver disease LB20.21 Biliary atresia - Biliary atresia is a rare disease characterised by an inflammatory biliary obstruction of unknown origin that presents in the neonatal period. It is the most frequent surgical cause of cholestatic jaundice in this age group. Untreated, this condition leads to cirrhosis and death within the first years of life. |
Congenital Absence of Portal Vein
Congenital Absence of the Portal Vein (CAPV) is a rare abnormality where the intestinal and splenic venous drainage bypass the liver and drain directly into the systemic veins through various porto-systemic shunts.
Ductal Plate Malformations
- Interlobular bile ducts - autosomal recessive polycystic kidney disease
- Smaller interlobular ducts - von Meyenburg complexes
- Larger intrahepatic bile ducts - Caroli's disease
Hepatobiliary cysts
Fetal hepatic cysts are generally benign, with a low likelihood of associated anomalies of the hepatobiliary tract, abnormal liver function or clinical symptoms.[24]
Maternal Liver
There are several maternal pregnancy-associated direct and indirect liver diseases, see recent review.[25]
- Intrahepatic cholestasis of pregnancy (ICP) - most common liver disease in pregnancy, presenting mainly in the second and third trimesters, with pruritis, elevated serum bile acids, and abnormal liver function tests.[26][27] Many countries have developed guidelines to address the risks, diagnosis and management of ICP. (see Western Australia information)
- Bile Acids levels greater than 10 μmol/L are a common diagnostic marker.
- Liver Function Tests - significant aminotransferase increase, serum bilirubin not usually raised.
- Hyperemesis gravidarum - the clinical term for severe form of nausea and vomiting, which are common symptoms of early pregnancy (4 - 16 weeks). Causal factors include increased human chorionic gonadotropin (hCG) and steroids, multiple pregnancy and vitamin deficiency. The condition can lead to dehydration, ketonuria, catabolism and may require hospitalisation.
- Acute fatty liver disease of pregnancy (AFLP) - fatty infiltration of the liver, occurring usually in the third trimester though can also occur postnatally.
- Pre-eclampsia
- HELLP - haemolysis (with a micoangiopathic blood smear), elevated liver enzymes (LFTs) and low platelet count.
Animal Models
Mouse
See molecular review by Zorn.[10]
- E9.5 to E15 - liver bud undergoes growth and is the major site of haematopoiesis.
- E13 - bi-potential hepatoblasts differentiate into hepatocytes or biliary epithelial cells.
- E16.5 - ductal plate partially becomes bi-layered
- E17.5 - ductal plate remodelling, focal dilations appear between the two cell layers.
References
- ↑ 1.0 1.1 MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, Manuel J, Khuu N, Echeverri J, Linares I, Gupta R, Cheng ML, Liu LY, Camat D, Chung SW, Seliga RK, Shao Z, Lee E, Ogawa S, Ogawa M, Wilson MD, Fish JE, Selzner M, Ghanekar A, Grant D, Greig P, Sapisochin G, Selzner N, Winegarden N, Adeyi O, Keller G, Bader GD & McGilvray ID. (2018). Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun , 9, 4383. PMID: 30348985 DOI.
- ↑ Popescu DM, Botting RA, Stephenson E, Green K, Webb S, Jardine L, Calderbank EF, Polanski K, Goh I, Efremova M, Acres M, Maunder D, Vegh P, Gitton Y, Park JE, Vento-Tormo R, Miao Z, Dixon D, Rowell R, McDonald D, Fletcher J, Poyner E, Reynolds G, Mather M, Moldovan C, Mamanova L, Greig F, Young MD, Meyer KB, Lisgo S, Bacardit J, Fuller A, Millar B, Innes B, Lindsay S, Stubbington MJT, Kowalczyk MS, Li B, Ashenberg O, Tabaka M, Dionne D, Tickle TL, Slyper M, Rozenblatt-Rosen O, Filby A, Carey P, Villani AC, Roy A, Regev A, Chédotal A, Roberts I, Göttgens B, Behjati S, Laurenti E, Teichmann SA & Haniffa M. (2019). Decoding human fetal liver haematopoiesis. Nature , 574, 365-371. PMID: 31597962 DOI.
- ↑ Aizarani N, Saviano A, Sagar L, Mailly S, Durand JS, Herman P, Pessaux TF, Baumert D & Grün. (2019). A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature , , . PMID: 31292543 DOI.
- ↑ Yang L, Li LC, Lamaoqiezhong X, Wang WH, Wang YC, Wang CR & Xu. (2019). The contributions of mesoderm-derived cells in liver development. Semin. Cell Dev. Biol. , 92, 63-76. PMID: 30193996 DOI.
- ↑ Huppert SS & Iwafuchi-Doi M. (2019). Molecular regulation of mammalian hepatic architecture. Curr. Top. Dev. Biol. , 132, 91-136. PMID: 30797519 DOI.
- ↑ Hikspoors JPJM, Peeters MMJP, Mekonen HK, Kruepunga N, Mommen GMC, Cornillie P, Köhler SE & Lamers WH. (2017). The fate of the vitelline and umbilical veins during the development of the human liver. J. Anat. , 231, 718-735. PMID: 28786203 DOI.
- ↑ Yang L, Wang WH, Qiu WL, Guo Z, Bi E & Xu CR. (2017). A single-cell transcriptomic analysis reveals precise pathways and regulatory mechanisms underlying hepatoblast differentiation. Hepatology , 66, 1387-1401. PMID: 28681484 DOI.
- ↑ 8.0 8.1 Vestentoft PS, Jelnes P, Hopkinson BM, Vainer B, Møllgård K, Quistorff B & Bisgaard HC. (2011). Three-dimensional reconstructions of intrahepatic bile duct tubulogenesis in human liver. BMC Dev. Biol. , 11, 56. PMID: 21943389 DOI.
- ↑ Wandzioch E & Zaret KS. (2009). Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science , 324, 1707-10. PMID: 19556507 DOI.
- ↑ 10.0 10.1 Zorn AM. (2008). Liver development. , , . PMID: 20614624 DOI. | online extract | PDF
- ↑ Godlewski G, Gaubert-Cristol R, Rouy S & Prudhomme M. (1997). Liver development in the rat and in man during the embryonic period (Carnegie stages 11-23). Microsc. Res. Tech. , 39, 314-27. PMID: 9407542 <314::AID-JEMT2>3.0.CO;2-H DOI.
- ↑ Godlewski G, Gaubert-Cristol R, Rouy S & Prudhomme M. (1997). Liver development in the rat during the embryonic period (Carnegie stages 15-23). Acta Anat (Basel) , 160, 172-8. PMID: 9718390
- ↑ Lhuaire M, Tonnelet R, Renard Y, Piardi T, Sommacale D, Duparc F, Braun M & Labrousse M. (2015). Developmental anatomy of the liver from computerized three-dimensional reconstructions of four human embryos (from Carnegie stage 14 to 23). Ann. Anat. , 200, 105-13. PMID: 25866917 DOI.
- ↑ Archie JG, Collins JS & Lebel RR. (2006). Quantitative standards for fetal and neonatal autopsy. Am. J. Clin. Pathol. , 126, 256-65. PMID: 16891202 DOI.
- ↑ Pauwelyn K, Roelandt P, Notelaers T, Sancho-Bru P, Fevery J & Verfaillie CM. (2011). Culture of mouse embryonic stem cells with serum but without exogenous growth factors is sufficient to generate functional hepatocyte-like cells. PLoS ONE , 6, e23096. PMID: 21829697 DOI.
- ↑ Kaufman and Bard, The Anatomical Basis of Mouse Development 1999 Academic Press
- ↑ Tzur G, Israel A, Levy A, Benjamin H, Meiri E, Shufaro Y, Meir K, Khvalevsky E, Spector Y, Rojansky N, Bentwich Z, Reubinoff BE & Galun E. (2009). Comprehensive gene and microRNA expression profiling reveals a role for microRNAs in human liver development. PLoS ONE , 4, e7511. PMID: 19841744 DOI.
- ↑ Crawford JM. (2002). Development of the intrahepatic biliary tree. Semin. Liver Dis. , 22, 213-26. PMID: 12360416 DOI.
- ↑ Collardeau-Frachon S & Scoazec JY. (2008). Vascular development and differentiation during human liver organogenesis. Anat Rec (Hoboken) , 291, 614-27. PMID: 18484606 DOI.
- ↑ Naito M, Hasegawa G, Ebe Y & Yamamoto T. (2004). Differentiation and function of Kupffer cells. Med Electron Microsc , 37, 16-28. PMID: 15057601 DOI.
- ↑ Gordon S & Plüddemann A. (2017). Tissue macrophages: heterogeneity and functions. BMC Biol. , 15, 53. PMID: 28662662 DOI.
- ↑ Mass E, Ballesteros I, Farlik M, Halbritter F, Günther P, Crozet L, Jacome-Galarza CE, Händler K, Klughammer J, Kobayashi Y, Gomez-Perdiguero E, Schultze JL, Beyer M, Bock C & Geissmann F. (2016). Specification of tissue-resident macrophages during organogenesis. Science , 353, . PMID: 27492475 DOI.
- ↑ Sharma R, Greenhough S, Medine CN & Hay DC. (2010). Three-dimensional culture of human embryonic stem cell derived hepatic endoderm and its role in bioartificial liver construction. J. Biomed. Biotechnol. , 2010, 236147. PMID: 20169088 DOI.
- ↑ Leombroni M, Buca D, Celentano C, Liberati M, Bascietto F, Gustapane S, Marrone L, Manzoli L, Rizzo G & D'Antonio F. (2017). Outcomes associated with fetal hepatobiliary cysts: systematic review and meta-analysis. Ultrasound Obstet Gynecol , 50, 167-174. PMID: 27553859 DOI.
- ↑ Kelly C & Pericleous M. (2018). Pregnancy-associated liver disease: a curriculum-based review. Frontline Gastroenterol , 9, 170-174. PMID: 30046419 DOI.
- ↑ Bicocca MJ, Sperling JD & Chauhan SP. (2018). Intrahepatic cholestasis of pregnancy: Review of six national and regional guidelines. Eur. J. Obstet. Gynecol. Reprod. Biol. , 231, 180-187. PMID: 30396107 DOI.
- ↑ Wood AM, Livingston EG, Hughes BL & Kuller JA. (2018). Intrahepatic Cholestasis of Pregnancy: A Review of Diagnosis and Management. Obstet Gynecol Surv , 73, 103-109. PMID: 29480924 DOI.
Books
Zorn AM. (2008). Liver development. , , . PMID: 20614624 DOI. | online extract | PDF
Reviews
Schulze RJ, Schott MB, Casey CA, Tuma PL & McNiven MA. (2019). The cell biology of the hepatocyte: A membrane trafficking machine. J. Cell Biol. , 218, 2096-2112. PMID: 31201265 DOI.
Kelly C & Pericleous M. (2018). Pregnancy-associated liver disease: a curriculum-based review. Frontline Gastroenterol , 9, 170-174. PMID: 30046419 DOI.
Zaret KS. (2016). From Endoderm to Liver Bud: Paradigms of Cell Type Specification and Tissue Morphogenesis. Curr. Top. Dev. Biol. , 117, 647-69. PMID: 26970006 DOI.
Gordillo M, Evans T & Gouon-Evans V. (2015). Orchestrating liver development. Development , 142, 2094-108. PMID: 26081571 DOI.
Zong Y & Stanger BZ. (2012). Molecular mechanisms of liver and bile duct development. Wiley Interdiscip Rev Dev Biol , 1, 643-55. PMID: 23799566 DOI.
Dixon LJ, Barnes M, Tang H, Pritchard MT & Nagy LE. (2013). Kupffer cells in the liver. Compr Physiol , 3, 785-97. PMID: 23720329 DOI.
Nakamura T, Sakai K, Nakamura T & Matsumoto K. (2011). Hepatocyte growth factor twenty years on: Much more than a growth factor. J. Gastroenterol. Hepatol. , 26 Suppl 1, 188-202. PMID: 21199531 DOI.
Ando H. (2010). Embryology of the biliary tract. Dig Surg , 27, 87-9. PMID: 20551648 DOI.
Kung JW, Currie IS, Forbes SJ & Ross JA. (2010). Liver development, regeneration, and carcinogenesis. J. Biomed. Biotechnol. , 2010, 984248. PMID: 20169172 DOI.
Si-Tayeb K, Lemaigre FP & Duncan SA. (2010). Organogenesis and development of the liver. Dev. Cell , 18, 175-89. PMID: 20159590 DOI.
Le Lay J & Kaestner KH. (2010). The Fox genes in the liver: from organogenesis to functional integration. Physiol. Rev. , 90, 1-22. PMID: 20086072 DOI.
Roskams T & Desmet V. (2008). Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) , 291, 628-35. PMID: 18484608 DOI.
Articles
Szpinda M, Paruszewska-Achtel M, Woźniak A, Mila-Kierzenkowska C, Elminowska-Wenda G, Dombek M, Szpinda A & Badura M. (2015). Volumetric Growth of the Liver in the Human Fetus: An Anatomical, Hydrostatic, and Statistical Study. Biomed Res Int , 2015, 858162. PMID: 26413551 DOI.
Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, Reid LM & Alvaro D. (2012). The biliary tree--a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol , 9, 231-40. PMID: 22371217 DOI.
Friedman JR & Kaestner KH. (2011). On the origin of the liver. J. Clin. Invest. , 121, 4630-3. PMID: 22105167 DOI.
Carpentier R, Suñer RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M & Lemaigre FP. (2011). Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology , 141, 1432-8, 1438.e1-4. PMID: 21708104 DOI.
Collardeau-Frachon S & Scoazec JY. (2008). Vascular development and differentiation during human liver organogenesis. Anat Rec (Hoboken) , 291, 614-27. PMID: 18484606 DOI.
Rudolph AM. (1983). Hepatic and ductus venosus blood flows during fetal life. Hepatology , 3, 254-8. PMID: 6832717
Historic
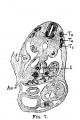
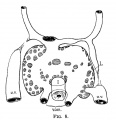
Mall FP. A study of the structural unit of the liver. (1906) Amer. J Anat. 5:227-308.
Severn CB. A morphological study of the development of the human liver. I. Development of the hepatic diverticulum. (1971) Amer. J Anat. 131: 133-158. PMID 5575887
Severn CB. A morphological study of the development of the human liver. II. Establishment of liver parenchyma, extrahepatic ducts and associated venous channels. (1972) Amer. J Anat. 133: 85-107. PMID 5008885
Search Pubmed
Search Bookshelf Liver Development
Search Pubmed Now: Liver Development | Embryonic Liver Development
Additional Images
Historic
Mall FP. A study of the structural unit of the liver. (1906) Amer. J Anat. 5:227-308.
Terms
| Gastrointestinal Tract Terms | ||
|---|---|---|
| ||
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Gastrointestinal Tract - Liver Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Gastrointestinal_Tract_-_Liver_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G