Fetal ECHO Meeting 2012: Difference between revisions
| Line 232: | Line 232: | ||
!Description | !Description | ||
|- | |- | ||
|[[Image:Ventricular Septal Defect.jpg|thumb| | |[[Image:Ventricular Septal Defect.jpg|thumb|200px|VSD]] | ||
|'''Ventricular Septal Defect''' | |'''Ventricular Septal Defect''' | ||
|25% of CHD; more frequent in males | |25% of CHD; more frequent in males | ||
| Line 242: | Line 242: | ||
|Comprises a classic group of four defects: pulmonary stenosis, VSD, dextroposition of the aorta and right ventricle hypertrophy. An additional ASD creates a ‘pentalogy’ of Fallot. Results in cyanosis. | |Comprises a classic group of four defects: pulmonary stenosis, VSD, dextroposition of the aorta and right ventricle hypertrophy. An additional ASD creates a ‘pentalogy’ of Fallot. Results in cyanosis. | ||
|- | |- | ||
|[[Image:Transposition of the Great Vessels.jpg|thumb| | |[[Image:Transposition of the Great Vessels.jpg|thumb|200px|Transposition of the Great Vessels]] | ||
|'''Transposition of the Great Vessels''' | |'''Transposition of the Great Vessels''' | ||
|10-11% of CHD | |10-11% of CHD | ||
|The most common form of transposition is where the aorta arises from the right ventricle while the pulmonary trunk arises from the left. This occurs either via abnormal rotation of the arterial pole or abnormal development of the outflow septum. In order for a newborn to survive prior to surgery there must be mixing of systemic and pulmonary circulations through a VSD, ASD or patent ductus arteriosus. It is the most common cause of cyanotic heart disease in newborns and is surgically corrected. | |The most common form of transposition is where the aorta arises from the right ventricle while the pulmonary trunk arises from the left. This occurs either via abnormal rotation of the arterial pole or abnormal development of the outflow septum. In order for a newborn to survive prior to surgery there must be mixing of systemic and pulmonary circulations through a VSD, ASD or patent ductus arteriosus. It is the most common cause of cyanotic heart disease in newborns and is surgically corrected. | ||
|-style="background:lightsteelblue" | |-style="background:lightsteelblue" | ||
|[[Image:Atrial Septal Defect.jpg|thumb| | |[[Image:Atrial Septal Defect.jpg|thumb|200px|ASD]] | ||
|'''Atrial Septal Defect''' | |'''Atrial Septal Defect''' | ||
|6-10% of CHD; more common in females | |6-10% of CHD; more common in females | ||
|The atrial septal myocardium is derived from multiple sources making various places susceptible to defects. The most common is a patent foramen ovale. Others include an ostium secundum defect and ostium primum defect (where there is usually some defect with the AV cushions as well). Defects in the sinus venosus act as ASDs yet are really defects in the wall separating the right pulmonary veins and SVC. A common atrium results from a complete lack of atrial septation. ASDs results in cyanosis due to a right-to-left shunt. | |The atrial septal myocardium is derived from multiple sources making various places susceptible to defects. The most common is a patent foramen ovale. Others include an ostium secundum defect and ostium primum defect (where there is usually some defect with the AV cushions as well). Defects in the sinus venosus act as ASDs yet are really defects in the wall separating the right pulmonary veins and SVC. A common atrium results from a complete lack of atrial septation. ASDs results in cyanosis due to a right-to-left shunt. | ||
|- | |- | ||
|[[Image:Pulmonary Atresia.jpg|thumb| | |[[Image:Pulmonary Atresia.jpg|thumb|200px|Pulmonary Atresia]][[Image:Pulmonary Stenosis.jpg|thumb|x100px|Pulmonary Stenosis]] | ||
|'''Pulmonary Atresia & Pulmonary Stenosis''' | |'''Pulmonary Atresia & Pulmonary Stenosis''' | ||
|10% of CHD | |10% of CHD | ||
|Unequal division of trunks causes cusps to fuse to form a dome with a narrow/non-existent lumen and hence obstruction to blood flow from the right ventricle to the pulmonary artery. The most common side of stenosis/obstruction is at the pulmonary valve itself (rather than distal or proximal to the valve). Heart-lung transplantation may be the only therapy. | |Unequal division of trunks causes cusps to fuse to form a dome with a narrow/non-existent lumen and hence obstruction to blood flow from the right ventricle to the pulmonary artery. The most common side of stenosis/obstruction is at the pulmonary valve itself (rather than distal or proximal to the valve). Heart-lung transplantation may be the only therapy. | ||
|-style="background:lightsteelblue" | |-style="background:lightsteelblue" | ||
|[[Image:Patent Ductus Arteriosus.jpg|thumb| | |[[Image:Patent Ductus Arteriosus.jpg|thumb|200px|Patent Ductus Arteriosus]] | ||
|'''Patent Ductus Arteriosus''' | |'''Patent Ductus Arteriosus''' | ||
|6-8% of CHD; 2-3 times more common in females; common in preterm newborns | |6-8% of CHD; 2-3 times more common in females; common in preterm newborns | ||
|Failure of contraction of the muscular wall of the DA. This imposes greater pressure on the pulmonary circulation. Many will close spontaneously, however if this does not occur, surgical closure is advised to prevent the development of congestive heart failure. | |Failure of contraction of the muscular wall of the DA. This imposes greater pressure on the pulmonary circulation. Many will close spontaneously, however if this does not occur, surgical closure is advised to prevent the development of congestive heart failure. | ||
|- | |- | ||
|[[Image:Aortic Stenosis.jpg|thumb| | |[[Image:Aortic Stenosis.jpg|thumb|200px|Aortic Stenosis]] | ||
|'''Aortic Stenosis''' | |'''Aortic Stenosis''' | ||
|7% of CHD | |7% of CHD | ||
|Stenosis is caused either by a muscular obstruction below the aortic valve, obstruction at the valve itself or aortic narrowing above the valve. The most common site is at the valve itself which results from a persistence of endocardial tissue that normally degenerates. Results in LV hypertrophy and heart murmurs. | |Stenosis is caused either by a muscular obstruction below the aortic valve, obstruction at the valve itself or aortic narrowing above the valve. The most common site is at the valve itself which results from a persistence of endocardial tissue that normally degenerates. Results in LV hypertrophy and heart murmurs. | ||
|-style="background:lightsteelblue" | |-style="background:lightsteelblue" | ||
|[[Image:Hypoplastic Left Heart.jpg|thumb| | |[[Image:Hypoplastic Left Heart.jpg|thumb|200px|Hypoplastic Left Heart]] | ||
[[Image:Functional Hypoplastic Left Heart.jpg|thumb|x100px|Functional Hypoplastic Left Heart]] | [[Image:Functional Hypoplastic Left Heart.jpg|thumb|x100px|Functional Hypoplastic Left Heart]] | ||
|'''Hypoplastic Left Heart Syndrome''' | |'''Hypoplastic Left Heart Syndrome''' | ||
| Line 273: | Line 273: | ||
|The left ventricle is incapable of supporting the systemic circulation, hence the right ventricle maintains both pulmonary and systemic circulations aided by an ASD. Other defects such as stenosis or atresia of the mitral and aortic valves, anomalous pulmonary venous connections and hypoplasia of the aortic arch can be responsible for hypoplastic left heart syndrome. Infants usually die within weeks. | |The left ventricle is incapable of supporting the systemic circulation, hence the right ventricle maintains both pulmonary and systemic circulations aided by an ASD. Other defects such as stenosis or atresia of the mitral and aortic valves, anomalous pulmonary venous connections and hypoplasia of the aortic arch can be responsible for hypoplastic left heart syndrome. Infants usually die within weeks. | ||
|- | |- | ||
|[[Image:Coarctation of the Aorta.jpg|thumb| | |[[Image:Coarctation of the Aorta.jpg|thumb|200px|Coarctation of the Aorta]] | ||
|'''Coarctation of the Aorta''' | |'''Coarctation of the Aorta''' | ||
|5-7% of CHD | |5-7% of CHD | ||
| Line 283: | Line 283: | ||
|In partial anomalous pulmonary venous drainage (PAPVD) a portion of the pulmonary venous blood flow returns to the right atrium. When over 50% of the veins return to the right atrium the condition is clinically significant. All types of total anomalous pulmonary venous connection (TAPVC) (those compatible with survival) are accompanied by an atrial septal defect. Pulmonary veins in TAPVC tend to open into one of the systemic veins before returning to the right atrium. The overloaded pulmonary circuit leads to cyanosis, tachypnoea and dyspnoea. Treatment is via surgical redirection. | |In partial anomalous pulmonary venous drainage (PAPVD) a portion of the pulmonary venous blood flow returns to the right atrium. When over 50% of the veins return to the right atrium the condition is clinically significant. All types of total anomalous pulmonary venous connection (TAPVC) (those compatible with survival) are accompanied by an atrial septal defect. Pulmonary veins in TAPVC tend to open into one of the systemic veins before returning to the right atrium. The overloaded pulmonary circuit leads to cyanosis, tachypnoea and dyspnoea. Treatment is via surgical redirection. | ||
|- | |- | ||
|[[Image:Tricuspid Atresia.jpg|thumb| | |[[Image:Tricuspid Atresia.jpg|thumb|200px|Tricuspid Atresia]] | ||
|'''Tricuspid Atresia''' | |'''Tricuspid Atresia''' | ||
|1-3% of CHD | |1-3% of CHD | ||
|Complete lack of formation of the tricuspid valve which results in an hypoplastic right ventricle. The pulmonary circulation can be maintained via a VSD, and an ASD is necessary for survival. Results in cyanosis and tachypnoea. Treatment is initially via administration of prostaglandins followed by surgery to place a shunt to maintain the pulmonary circulation. | |Complete lack of formation of the tricuspid valve which results in an hypoplastic right ventricle. The pulmonary circulation can be maintained via a VSD, and an ASD is necessary for survival. Results in cyanosis and tachypnoea. Treatment is initially via administration of prostaglandins followed by surgery to place a shunt to maintain the pulmonary circulation. | ||
|-style="background:lightsteelblue" | |-style="background:lightsteelblue" | ||
|[[Image:Double Outlet Right Ventricle.jpg|thumb| | |[[Image:Double Outlet Right Ventricle.jpg|thumb|200px|DORV]] | ||
|'''Double Outlet Right Ventricle''' | |'''Double Outlet Right Ventricle''' | ||
|1-1.5% of CHD | |1-1.5% of CHD | ||
|Both large arteries arise wholly or mainly from the right ventricle. This defect is considered to be present if the aorta obtains 50% of its blood from the right ventricle. The defect arises due to absence of the secondary heart field such that myocardium is not added to the outflow tract during looping. Arrangement of the atrioventricular valves, coronary arteries and the ventriculoarterial connections as well as clinical manifestations are variable. | |Both large arteries arise wholly or mainly from the right ventricle. This defect is considered to be present if the aorta obtains 50% of its blood from the right ventricle. The defect arises due to absence of the secondary heart field such that myocardium is not added to the outflow tract during looping. Arrangement of the atrioventricular valves, coronary arteries and the ventriculoarterial connections as well as clinical manifestations are variable. | ||
|- | |- | ||
|[[Image:Interrupted Aortic Arch.jpg|thumb| | |[[Image:Interrupted Aortic Arch.jpg|thumb|200px|Interrupted Aortic Arch]] | ||
|'''Interrupted Aortic Arch''' | |'''Interrupted Aortic Arch''' | ||
|Very rare | |Very rare | ||
Revision as of 21:57, 4 October 2012
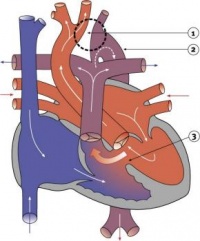
Fetal ECHO Meeting 5th-8th October 2012 ROYAL PRINCE ALFRED HOSPITAL, SYDNEY, AUSTRALIA
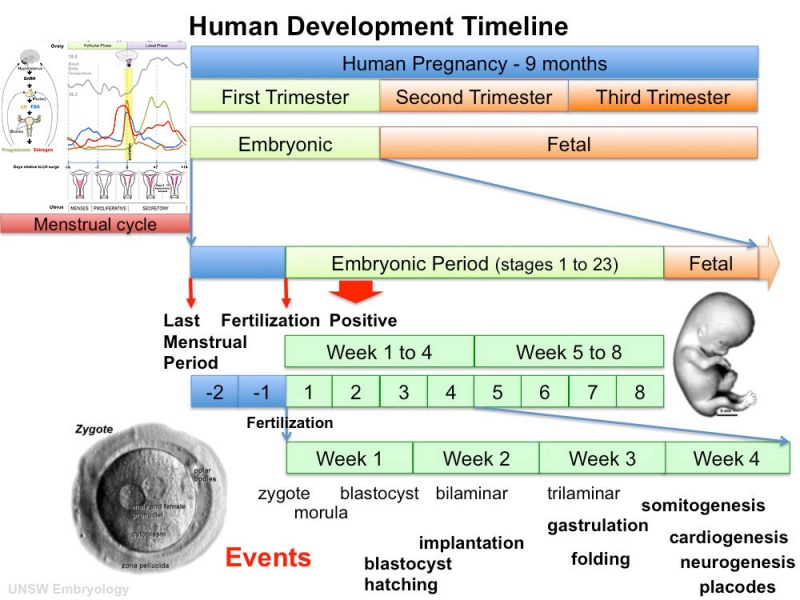
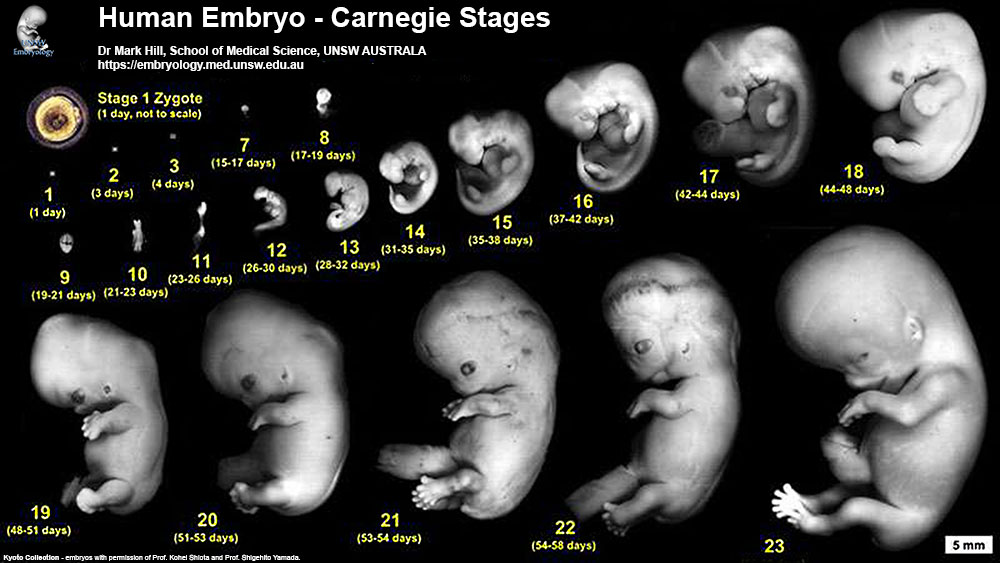
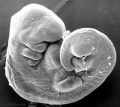
Human Embryo Development
Weeks shown are ovulation age (OA) for gestational age (GA) add 2 weeks.
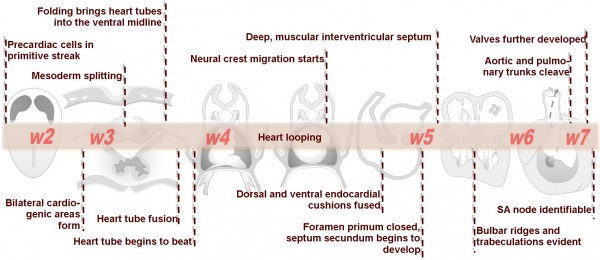
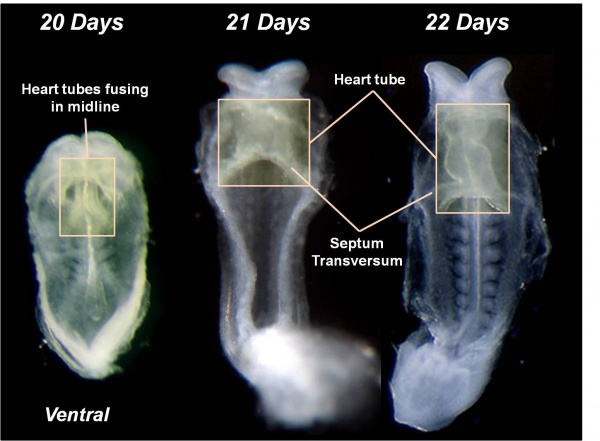
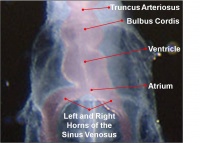
Heart Development
Weeks shown are ovulation age (OA) for clinical Gestational Age (GA) add 2 weeks.
- Links: PO timeline | GA timeline
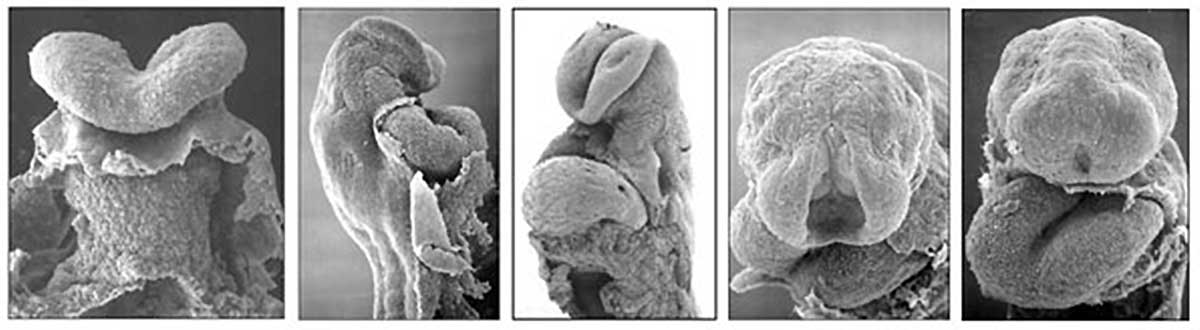
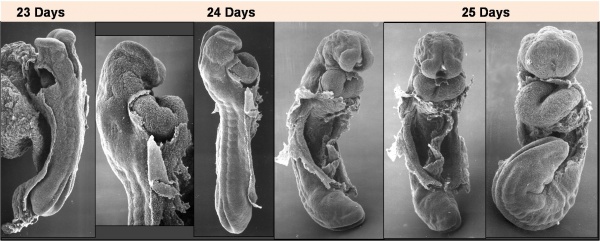
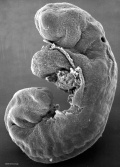
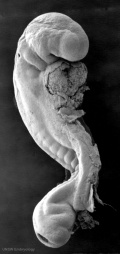
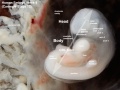
Image day 21 to 25 (GA Week 5)
|
|
|
| Stage 11 | Stage 12 | Stage 13 |
Heart Week 7 and 10
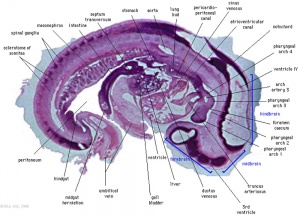
|
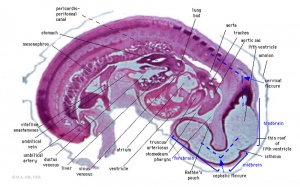
|
| G6L | G7L |
| Stage 13 Embryo (GA Week 7) | ||||||
|---|---|---|---|---|---|---|
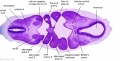
|
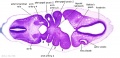
|
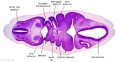
|
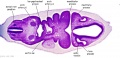
|
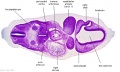
|
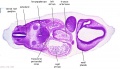
|
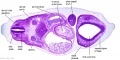
|
| B1L | B2L | B3L | B4L | B5L | B6L | B7L |
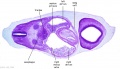
|
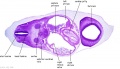
|
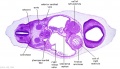
|
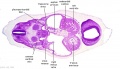
|
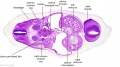
|
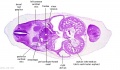
|
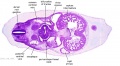
|
| C1L | C2L | C3L | C4L | C5L | C6L | C7L |
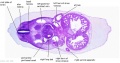
|
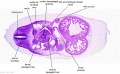
|
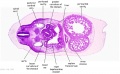
|
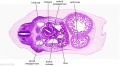
|
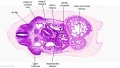
|
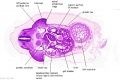
|
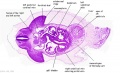
|
| D1L | D2L | D3L | D4L | D5L | D6L | D7L |
| Stage 22 Embryo (GA Week 10) | ||
|---|---|---|
| Section | Name | Description |
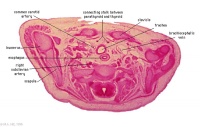
|
C6L | L and R common carotid arteries. L and R internal jugular veins. |
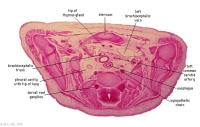
|
C7L | L brachiocephalic vein (transverse anastomosis of L, R jugular veins). L common carotid artery. Brachiocephalic trunk. |
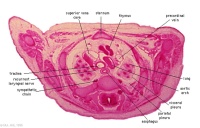
|
D1L | Aortic arch. Thymus (retrosternal). Trachea. Oesophagus. Large L and R jugular veins. |
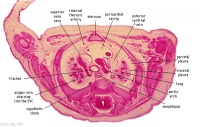
|
D2L | Aortic arch. L jugular lymph sac lying laterally with hemiazygos joining it. Trachea. Oesophagus. Draining of azygos vein into R jugular vein. |
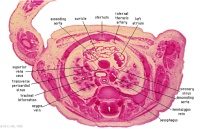
|
D3L | Ascending aorta attached cranial to transverse pericardial sinus. Superior vena cava expanding into R jugular vein. Trachea (bifurcation). Oesophagus. Azygos (R) and hemiazygos (L) veins. |
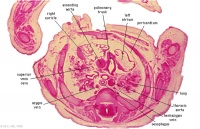
|
D4L | Ascending aorta. Pulmonary trunk and arteries. Thoracic aorta. Oesophagus. Bronchi. Note azygos vein on right, and precursor of hemiazygos vein on left side.
(Image excerpt different scale from previous images) |
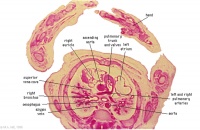
|
D5L | Superior vena cava. Ascending arch of aorta. Pulmonary trunk with the other two semilunar valves.
Sinus above one valve and branching of pulmonary trunk into L and R pulmonary arteries. |
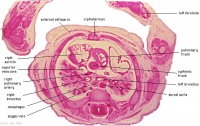
|
D6L | Superior vena cava. Ascending aorta with transverse pericardial sinus behind. Semilunar valve at origin of pulmonary trunk. L. atrium and auricle. Thoracic aorta. |
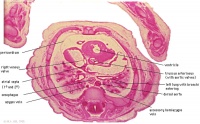
|
D7L | R atrium with R venous valve. R ventricle close to origin of pulmonary trunk. L.ventricular wall and three semilunar valves of aortic ostium. |
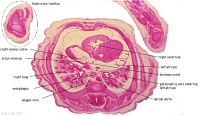
|
E1L | Section through all four chambers of heart.
left ventricle- outflow tract close to origin of ascending aorta. Deep coronary sulcus and transverse pericardial sinus. right atrium- R venous valve of the inferior vena cava, the small septum secundum, the aperture in the dorsal part of septum primum (ostium secundum). (The foramen ovale arises later with the elongation of the septum secundum). left atrium- entrance of the L and R pulmonary veins and the auricle. |
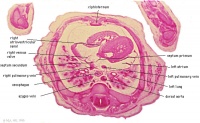
|
E2L | Section through all four chambers of heart.
Ventricles. Right venous valve. Between the tiny ridge of the septum secundum of the R atrium and the R venous valve. right atrium- drainage of the inferior vena cava (cf. E3). R atrioventricular canal. Septum primum. left atrium- drainage sites of R and L pulmonary veins. |
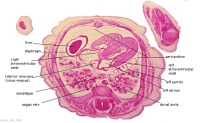
|
E3L | Section through all four chambers of heart.
Ventricles. Diaphragm and liver. Pericardium and cavity. R atrium and two cusps of the atrioventricular valve. L atrium and its auricle. Thoracic aorta. Inferior vena cava. Coronary sinus. |
Cardiovascular Movies
|
|
| Heart Fields |
| Page | Play |
Embryonic Heart Rate
In a 1996 study normal successful human gestations were defined by EHR criteria at different early embryonic (34-56 days GA, from last menstrual period) developmental stages (at the earliest stages when embryo length is difficult to measure gestational sac diameters are included). [1]
- Stage 9-10 2 mm embryo (gestational sac diameter of 20 mm) EHR at least 75 beats / minute
- Stage 11-12 5 mm embryo (gestational sac diameter of 30 mm) EHR at least 100 beats / minute
- Stage 16 10 mm embryo EHR at least 120 beats / minute
- Stage 18 15 mm embryo EHR at least 130 beats / minute
Abnormalities
| Abnormalities | |||
|---|---|---|---|
| Diagram | Abnormality | Epidemiology | Description |
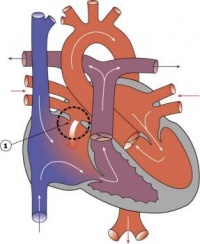
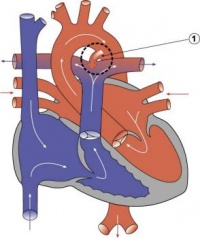
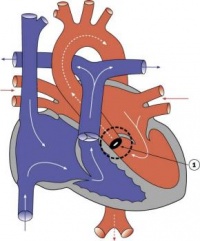
| Ventricular Septal Defect | 25% of CHD; more frequent in males | Result from growth failure of the membranous IV septum or endocardial cushions leading to a lack of closure of the IV foramen. Many VSDs are actually from defects in the outflow tract septum. 30-50% close spontaneously; large VSDs result in dyspnoea and cardiac failure in infancy. | |
| Tetralogy of Fallot | 9-14% of CHD | Comprises a classic group of four defects: pulmonary stenosis, VSD, dextroposition of the aorta and right ventricle hypertrophy. An additional ASD creates a ‘pentalogy’ of Fallot. Results in cyanosis. | |
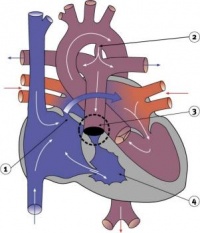
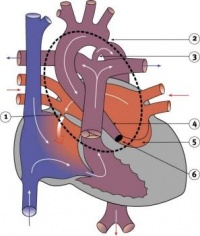
| Transposition of the Great Vessels | 10-11% of CHD | The most common form of transposition is where the aorta arises from the right ventricle while the pulmonary trunk arises from the left. This occurs either via abnormal rotation of the arterial pole or abnormal development of the outflow septum. In order for a newborn to survive prior to surgery there must be mixing of systemic and pulmonary circulations through a VSD, ASD or patent ductus arteriosus. It is the most common cause of cyanotic heart disease in newborns and is surgically corrected. | |
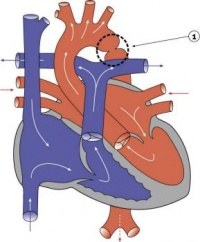
| Atrial Septal Defect | 6-10% of CHD; more common in females | The atrial septal myocardium is derived from multiple sources making various places susceptible to defects. The most common is a patent foramen ovale. Others include an ostium secundum defect and ostium primum defect (where there is usually some defect with the AV cushions as well). Defects in the sinus venosus act as ASDs yet are really defects in the wall separating the right pulmonary veins and SVC. A common atrium results from a complete lack of atrial septation. ASDs results in cyanosis due to a right-to-left shunt. | |
| Pulmonary Atresia & Pulmonary Stenosis | 10% of CHD | Unequal division of trunks causes cusps to fuse to form a dome with a narrow/non-existent lumen and hence obstruction to blood flow from the right ventricle to the pulmonary artery. The most common side of stenosis/obstruction is at the pulmonary valve itself (rather than distal or proximal to the valve). Heart-lung transplantation may be the only therapy. | |
| Patent Ductus Arteriosus | 6-8% of CHD; 2-3 times more common in females; common in preterm newborns | Failure of contraction of the muscular wall of the DA. This imposes greater pressure on the pulmonary circulation. Many will close spontaneously, however if this does not occur, surgical closure is advised to prevent the development of congestive heart failure. | |
| Aortic Stenosis | 7% of CHD | Stenosis is caused either by a muscular obstruction below the aortic valve, obstruction at the valve itself or aortic narrowing above the valve. The most common site is at the valve itself which results from a persistence of endocardial tissue that normally degenerates. Results in LV hypertrophy and heart murmurs. | |
| Hypoplastic Left Heart Syndrome | 4-8% of CHD | The left ventricle is incapable of supporting the systemic circulation, hence the right ventricle maintains both pulmonary and systemic circulations aided by an ASD. Other defects such as stenosis or atresia of the mitral and aortic valves, anomalous pulmonary venous connections and hypoplasia of the aortic arch can be responsible for hypoplastic left heart syndrome. Infants usually die within weeks. | |
| Coarctation of the Aorta | 5-7% of CHD | Aortic constriction in the region of the ductus arteriosus. It is associated with patent ductus arteriosus, VSD and aortic stenosis. Closure of the ductus arteriosus in neonates with a coarctation imposes a greater afterload on the left ventricle which can lead to congestive heart failure. Treatment aims at maintaining the ductus arteriosus via prostaglandins. | |
| Partial/Total Anomalous Pulmonary Venous Connection | <4% of CHD; more common in females | In partial anomalous pulmonary venous drainage (PAPVD) a portion of the pulmonary venous blood flow returns to the right atrium. When over 50% of the veins return to the right atrium the condition is clinically significant. All types of total anomalous pulmonary venous connection (TAPVC) (those compatible with survival) are accompanied by an atrial septal defect. Pulmonary veins in TAPVC tend to open into one of the systemic veins before returning to the right atrium. The overloaded pulmonary circuit leads to cyanosis, tachypnoea and dyspnoea. Treatment is via surgical redirection. | |
| Tricuspid Atresia | 1-3% of CHD | Complete lack of formation of the tricuspid valve which results in an hypoplastic right ventricle. The pulmonary circulation can be maintained via a VSD, and an ASD is necessary for survival. Results in cyanosis and tachypnoea. Treatment is initially via administration of prostaglandins followed by surgery to place a shunt to maintain the pulmonary circulation. | |
| Double Outlet Right Ventricle | 1-1.5% of CHD | Both large arteries arise wholly or mainly from the right ventricle. This defect is considered to be present if the aorta obtains 50% of its blood from the right ventricle. The defect arises due to absence of the secondary heart field such that myocardium is not added to the outflow tract during looping. Arrangement of the atrioventricular valves, coronary arteries and the ventriculoarterial connections as well as clinical manifestations are variable. | |
| Interrupted Aortic Arch | Very rare | An extreme form of coarctation of the aorta involving a gap in the ascending or descending thoracic aorta that results from abnormal proliferation/migration/function of neural crest cells. Different types of interrupted aortic arch are defined based on their relation to the arterial branches of the aortic arch. It is greatly associated with other defects such as a patent ductus arteriosus or VSD. It is treated with prostaglandin to maintain ductus arteriosus followed by surgery. |
References
- ↑ <pubmed>8921130</pubmed>
Online Textbooks
- Developmental Biology (6th ed.) Gilbert, Scott F. Sunderland (MA): Sinauer Associates, Inc.; c2000. Figure 15.6 Cascade of heart development
Search Bookshelf heart development
Reviews
<pubmed>21940548</pubmed> <pubmed>17224285</pubmed>| PMC1858673 <pubmed></pubmed>
Articles
<pubmed>17384040</pubmed>| MC2190734 J Ultrasound Med.
Search Pubmed
Search Pubmed heart development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Cardiovascular Ultrasound is an open access, peer-reviewed, online journal covering clinical, technological, experimental, biological, and molecular aspects of ultrasound applications in cardiovascular physiology and disease. Search - Fetal