Paper - The early development of the meninges of the spinal cord in human embryos (1951)
| Embryology - 22 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Sensenig EC. The early development of the meninges of the spinal cord in human embryos. (1951) Contrib. Embryol., Carnegie Inst. Wash. Publ. 611.
| Online Editor |
|---|
| This 1951 paper is a historic histological study of the development of the meninges of the human embryo spinal cord. Our current understanding of interstitial cell development and their role in male differentiation can be seen in the links below.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Early Development of the Meninges of the Spinal Cord in Human Embryos
Contributions To Embryology, No. 228
E. Carl Sensenig
Medical College of Alabama, Birmingham, Alabmma
With four plates (1951)
Introduction
This study of the embryology of the spinal meninges is a part of a series of investigations concerned with the development of the vertebral canal and its contents.
Most of the embryological investigations on the meninges have dealt primarily with the membranes of the brain and skull, some of them giving secondary attention to those of the spinal cord. A few investigations have dealt solely with the development of the pia mater, arachnoid mater, and dura mater of the spinal cord. Because of differences between the development of the spinal cord and vertebral canal and that of the brain and skull, a separate treatment for each is indicated. Of necessity, the scanty literature dealing with the development of the meninges will be considered in its entirety, regardless of the species or regions discussed.
The author gratefully acknowledges the receipt of two grants from the Joseph Henry Fund of the National Academy of Sciences, which made this study possible. For the many privileges offered by the Department of Embryology, sincere appreciation is expressed to the Carnegie Institution of Washington, and to Dr. G. W. Corner, Director. The author is indebted to Mr. Chester Reather for the photographs herein reproduced.
Historical
The first description known to the present author of the embryological development of the meninges is that of Tiedemann (1816), who states that the pia and dura appear in 7- and 8-week-old human embryos, and the arachnoid becomes identifiable in 5-month and distinct in 6-month embryos.
It was Bischoff (1842), however, who first expressed an opinion as to the origin of the meninges. He describes them all as differentiating from the peripheral layers of the neural tube. He clearly states that the neural tube, besides forming the gray and white matter of the central nervous system, forms the fibrous tissue of the dura, the serous tissue of the arachnoid, and the vascular tissue of the pia. Remak (1850-1855) modified Bischoff's view, regarding only the pia mater and arachnoid as being of neural-tube origin, and the dura as a derivative of the skeleton-forming layer. Reichert (1859, 1861) followed Remak but considered the arachnoid to consist of two parts: a visceral layer associated with the pia mater and having a common origin from the neural tube, and a parietal layer associated with the dura and having a common origin from skeleton-forming tissue.
Others among the early embryologists suggested a quite dififerent origin of the meninges. In 1861 Kollmann reported that the cranial meninges develop from a single mass of embryonal tissue, which he compared to Wharton’s jelly, situated between the cerebral surface and the cranial rudiments. In the same year Kolliker (1861) stated that the spinal meninges are not a product of the medullary plate, but of the primitive vertebra (Urwirbel). His (1865) closely followed Kolliker in considering them derivatives of the somite.
It was at this time that the germ-layer concept was being evolved, as indicated by the introduction of the terms ectoderm and endoderm (Haeckel, 1872; Lankester, 1873) and mesoderm (Huxley, 1871), and by the widespread acceptance of Haeckel's theory of the role of the germ layers in embryonic differentiation. Consequently, the subsequent studies were concerned primarily with establishing the germ-layer derivation of these structures.
The first to attempt this was Hensen (1876), who agreed with Kolliker that the dura and arachnoid are of mesodermal origin (primitive vertebra), but assumed a special origin of doubtful affinity for the pia, which he termed the “membrana prima.” Shortly thereafter, Mihalkovics (I877) published an account which agreed with Kolliker’s mesodermal origin of the meninges, but disagreed as to the time of separation of the dura from the more peripheral osteogenic layer.
The first investigation dealing solely with the development of the meninges was that of Salvi (r898), who studied mammalian forms (guinea pig, rabbit, sheep, and man). Salvi supported a mesodermal origin for these membranes, particularly those of the brain. He presented his material so well that this was the accepted view for many years. Papers by Gronberg (1902), His (1903), and Strasser (1901), though otherwise of interest, added little to our knowledge of the developmental sequence of the meninges. Sterzi‘s (1901, 1902) papers should be mentioned as a vast source of information concerning the comparative anatomy of the meninges.
The researches of Weed (1917) on the pig, Clermont (1922) on the mole, Farrar (1907) and HansenPruss (1923) on birds, and van Gelderen (1925) on teleosts broadened the background in comparative embryology. They all supported Salvi's findings by accepting a mesodermal origin for all three membranes.
Renewed interest in the embryological derivation of these membranes was created in 1924 by the appearance of the first of a series of papers by Harvey and Burr (1924, 1926) and Harvey, Burr, and Van Campenhout (1931, 1933) which indicated, on an experimental basis, an ectodermal origin for the piaarachnoid complex. Sayad and Harvey (1923) had observed that no adhesions of the meninges occurred during the healing of a dura injury when the piaarachnoid was not involved. In contrast, Lear and Harvey (1924) found that in the healing of piaarachnoid injuries, adhesions formed between these membranes and the uninjured dura. From this evidence Harvey and Burr (1924) inferred that the differences in regeneration between the pia-arachnoid and the dura were due to a different embryonic origin. Experimental evidence to support their inferential deduction was obtained by making transplants of neural-tube tissue in amphibian (A1nl:l}~'.~'t0:ncz punctatrmz) embryos. Two series of neural-tube transplants were made, one including neural-crest cells and the other devoid of such cells. On the basis of their results, Harvey and Burr (1924, 1926) concluded that the meninges do not arise from mesoderm. They considered that the pia-arachnoid is predominantly derived from neural—crest cells, whereas the dura is derived from mesoderm.
Flexner (1929), using the same form (z1m[:l_t'.\'t0n1rz) as Harvey and Burr, carefully repeated this experimental procedure. His results failed to support a neural-crest origin for the leptomeninges. Flcxner points out, however, that there is a mixture of neuralcrest and mesodermal cells about the neural tube, forming the meninx primitiva, the greater part coming from the somites. Harvey and Burr (1926) also admitted such a mixture, but considered the neural crests to supply the greater amount.
Harvey, Burr, and Van Campenhout (1931, 1933) returned to the problem and expanded it to include chorioallantoic grafts in chick embryos, Nile blue sulphate cell-distribution studies in Amblystoma, and heteroplastic transplants of neural-tube tissue from the frog (Rana) into the bodies of amphibians (Amblystonm). They regarded their findings from this multiple approach as supporting their earlier conclusions that the leptomeninx develops predominantly from neural-crest cells and the pachymeninx from mesoderm.
The most recent contributor to this discussion, on the basis of evidence from experiments on amphibia, is Spoflord (1945), who acknowledges the presence of neural-crest cells in the meninx primitiva in A n1b1y.ctonzu embryos, but not in amounts that would give rise to a “substantial component of the meninges.”
Hochstetter’s (1934) lengthy work on the development of the spinal meninges was the first careful analysis of the development of these structures it1 human embryos. He treated the development of the meninges from a purely descriptive point of view, and, ignoring the experiments of Harvey and Burr, of Flexner, and of Harvey, Burr, and Van Campenhout, he adhered to a mesodermal origin for all three membranes.
The latest paper directly concerned with the development of the meninges in mammals is Ask’s (1941) study on human embryos. He carefully reviews earlier works, but follows Hoc|1stetter's thesis of a mesodermal origin for these structures. Ask (1941), though also using a purely descriptive method, does take into account the works of Harvey, Burr, and Van Campenhout. He does not consider their evidence as conclusive, because, first, the transplants differ in more than merely the presence or absence of neural crests; and, secondly, these authors failed to supply sullicient comparisons with controlled materials. Ask does not refer to Flexner's investigations. Theiler's (1947) study is chiefly concerned with later development and the formation of the ligaments of the dura mater.
Material
Table 1 - Human Embryos of the Carnegie Collection used in this Study
| Carnegie Embryos - Sensenig (1951) - Spinal Cord Meninges | |||||
|---|---|---|---|---|---|
| Age Group (gestation age) |
Embryo no. |
Crown Rump Length |
Thickness of sections (microns) |
Plane of section |
Illustration |
| X (20-22 days) | 2795 | 2.0 (5) | 6 | Transverse | |
| 8244 | (6) | 8 | Transverse | ||
| 4216 | 2.17 (7) | 15 | Transverse | ||
| XI (22-24 days) | 5074 | 3.0 (10) | 10 | Transverse | |
| 7611 | 2 .4 (16) | 8 | Transverse | ||
| 2053 | 3.1 (20) | 10 | Transverse | Fig. 1, pl. 1 | |
| XII (24-26 days) | 6097 | 3 .4 (25) | 10 | Transverse | |
| 7724 | 3.5 (29) | 8 | Sagittal | ||
| 7999 | 3.2 (29) | 10 | Transverse | ||
| XIII (26-28 days) | 7889 | 4.2 | 6 | Frontal | |
| 8066 | 5.3 | 8 | Transverse | ||
| 8119 | 5.3 | 8 | Transverse | Fig. 2, pl. 1 | |
| XIV (28-30 days) | 5787 | 6.8 | 10 | Sagittal | |
| 7829 | 7.0 | 8 | Transverse | ||
| 8141 | 7.3 | 8 | Frontal | ||
| XV (30-32 days) | 721 | 9.0 | 15 | Transverse | Figs. 3, 4, pl. 1 |
| 3385 | 8.3 | 20 | Transverse | ||
| 6504 | 7.5 | 6 | Sagittal | ||
| 6506 | 7.5 | 10 | Frontal | ||
| XVI (32-34 days) | 792 | 8.0 | 20 | Transverse | |
| 887 | 9.0 | 40 | Transverse | ||
| 6507 | 9 .0 | 8 | Frontal | ||
| 6511 | 8.1 | 10 | Sagittal | ||
| 6512 | 7 .0 | 10 | Transverse | ||
| 6516 | 10.5 | 8 | Sagittal | ||
| 6517 | 10.5 | 8 | Transverse | ||
| 8112 | 10.9 | 8 | Frontal | ||
| XVII (34-36 days) | 6519 | 10.8 | 8 | Sagittal | |
| 6521 | 13.2 | 8 | Transverse | Figs. 5, 6, pl. 1 | |
| 8101 | 13.0 | 10 | Transverse | ||
| 8118 | 12.6 | 10 | Frontal | Fig. 21, pi. 4 | |
| XVIII (36-38 days) | 4430 | 14.0 | 15 | Transverse | |
| 6524 | 11 .7 | 10 | Transverse | ||
| 6525 | 13.8 | 8 | Sagittal | ||
| 6528 | 13 .4 | 8 | Frontal | ||
| 7707 | 14.5 | 10 | Transverse | ||
| 8172 | 16.5 | 20 | Transverse | Figs. 7, 8, pl. 2 | |
| 8235 | 14.0 | 10 | Sagittal | ||
| XIX (38-40 days) | 1390 | 18.0 | 20 | Sagittal | |
| 4405 | 15.8 | 10 | Transverse | ||
| 5609 | 18.0 | 25 | Frontal | ||
| 8092 | 16.3 | 20 | Transverse | ||
| XX (40-42 days) | 2393 | 23 .1 | 20 | Sagittal | |
| 3527 | 22 .0 | 25 | Sagittal | ||
| 4059 | 21.6 | 15 | Frontal | ||
| 6426 | 21.5 | 20 | Transverse | ||
| 7274 | 18.0 | 20 | Transverse | Figs. 9, 10, pl. 2 | |
| 7906 | 19.5 | 20 | Frontal | ||
| XXI (42-44 days) | 7254 | 22.5 | 20 | Transverse | |
| 7392 | 22.7 | 20 | Transverse | Figs. 11, 12, pl. 2: fig. 22, pl. 4 | |
| 7864 | 24.0 | 20 | Frontal | ||
| XXII (44-46 days) | 4339 | 24.5 | 15 | Transverse | |
| 6701 | 24 .0 | 20 | Frontal | ||
| 6832 | 25.8 | 20 | Frontal | Fig. 23, pl. 4 | |
| XXIII (46-48 days) | 4570 | 30.7 | 15 | Transverse | Figs. 13, 14, pl. 3; fig. 24, pl. 4 |
| 6573 | 31.5 | 20 | Transverse | Figs. 15, 16, pl. 3 | |
| 7425 | 27.0 | 20 | Frontal | ||
| Older embryos: | |||||
| 8 weeks | 3669 | 34.0 | 50 | Transverse | |
| 5762 | 34.0 | 25 | Sagittal | ||
| 5712 | 35 .0 | 25 | Frontal | ||
| 1915 | 35.5 | 50 | Transverse | ||
| 4021 | 38 .0 | 15 | Transverse | ||
| 9 weeks | 6658 | 40.0 | 40 | Sagittal | |
| 4985 | 41.0 | 20 | Transverse | ||
| 301 | 44 .0 | 20 | Transverse | ||
| 1686 | 46.0 | 100 | Sagittal | ||
| 4475 | 48.0 | 20 | Transverse | Figs. 19, 20, pl. 4 | |
| 10 weeks | 3990 | 54.0 | 20 | Transverse | |
| 481 | 57.0 | 200 | Frontal | ||
| 11 weeks | 4229 | 58.0 | 20 | Transverse | |
| 1656 | 67 .0 | 200 | Sagittal | ||
| 4291 | 69 .0 | 100 | Transverse | ||
| Week 12 | 1455 | 78.5 | 50 | Sagittal | |
| 172 | 80.0 | 100 | Transverse | ||
| Template:CE72181 | 80.0 | 20 | Transverse | Figs. 17, 18, pl. 3 | |
| |||||
| Reference: Sensenig EC. The early development of the meninges of the spinal cord in human embryos. (1951) Contrib. Embryol., Carnegie Inst. Wash. Publ. 611. | |||||
| Carnegie Fetal - Sensenig (1951) - Spinal Cord Meninges | |||||
|---|---|---|---|---|---|
| Age Group (gestation age) |
Embryo no. |
Crown Rump Length |
Thickness of sections (microns) |
Plane of section |
Illustration |
| 8 weeks | 3669 | 34.0 | 50 | Transverse | |
| 5762 | 34.0 | 25 | Sagittal | ||
| 5712 | 35 .0 | 25 | Frontal | ||
| 1915 | 35.5 | 50 | Transverse | ||
| 4021 | 38 .0 | 15 | Transverse | ||
| 9 weeks | 6658 | 40.0 | 40 | Sagittal | |
| 4985 | 41.0 | 20 | Transverse | ||
| 301 | 44 .0 | 20 | Transverse | ||
| 1686 | 46.0 | 100 | Sagittal | ||
| 4475 | 48.0 | 20 | Transverse | Figs. 19, 20, pl. 4 | |
| 10 weeks | 3990 | 54.0 | 20 | Transverse | |
| 481 | 57.0 | 200 | Frontal | ||
| 11 weeks | 4229 | 58.0 | 20 | Transverse | |
| 1656 | 67 .0 | 200 | Sagittal | ||
| 4291 | 69 .0 | 100 | Transverse | ||
| 12 weeks | 1455 | 78.5 | 50 | Sagittal | |
| 172 | 80.0 | 100 | Transverse | ||
| 72181 | 80.0 | 20 | Transverse | Figs. 17, 18, pl. 3 | |
| |||||
| Reference: Sensenig EC. The early development of the meninges of the spinal cord in human embryos. (1951) Contrib. Embryol., Carnegie Inst. Wash. Publ. 611. | |||||
Description
A developmental study of the meninges usually begins with the condition present in a 15 mm embryo (age group xvii or xviii). At that time the meninx primitiva is well defined and the pia mater is beginning to differentiate. It is advantageous, however, to examine the development of the areas concerned from the period of early somite formation.
In embryos of 4 to 10 somites (age group x), the paraxial mesoderm forms discrete somites, and wherever migration from them has occurred, the cells pass vcntromedially toward the notochord. The notochord is, however, still continuous with the gut and in contact, dorsally, with the neural tube (groove). The spinal ganglia and the meninx primitiva will develop in the cell-free spaces between somites and neural tube.
The migration of cells from the somites has increased in embryos of 10 to 20 somites (age group xi), and the neural crests have undergone rapid development. Extending ventrally from the neural crests, and appearing to be continuous with them, is a single layered strand of cells (fig. 1, pl. 1). This extends along the lateral edge of the neural tube and contains cells which are more flattened and elongated than those migrating from the somite.
In embryos of 20 to 30 somites (age group xii), a great advance has occurred in the migration of cells about the neural tube, as well as a further increase in the size of the neural crests (spinal ganglia). The ventrally migrating neural crest cells are still quite clearly distinct from the somites. This may indicate that the tissues immediately adjacent to the periphery of the neural tube originate from the neural crest. It is in this group that vascularization of the tissue surrounding the neural tube begins.
In the next developmental stages, age groups xiii and xiv (embryos of 4 to 8 mm.), advances are particularly evident in the vertebral rudiments and in the spinal ganglia, with the latter starting their migration ventrad (fig. 2, pl. 1). The vascularization of the tissues directly adjacent to the neural tube has continued, and endothelium-lined channels are beginning to form in the older embryos.
In age group xv (approximately 7 to 9 mm.), the vertebral rudiments now form concentrations that are distinct from the intermediate zone separating them from the neural tube (fig. 3, pl. 1). This intermediary zone, which is less densely cellular and within which lie the ganglia, is the meninx primitiva, from which the meninges have been generally considered to arise. The neural tube is now completely surrounded by endothelium-lined vascular channels (fig. 3). Directly adjacent to the cord, and particularly evident at its ventrolateral surfaces, is a single-layered cell membrane. This is the first indication of the pia mater, and, where it is present, it lies between the vascular channels and the periphery of the spinal cord.
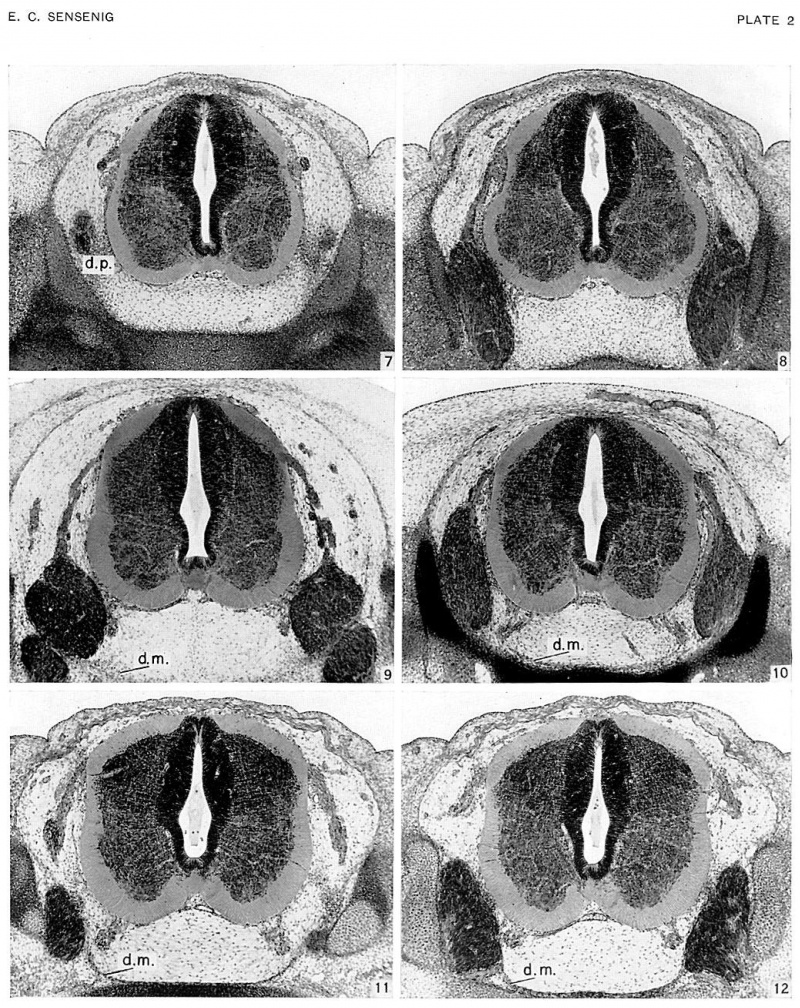
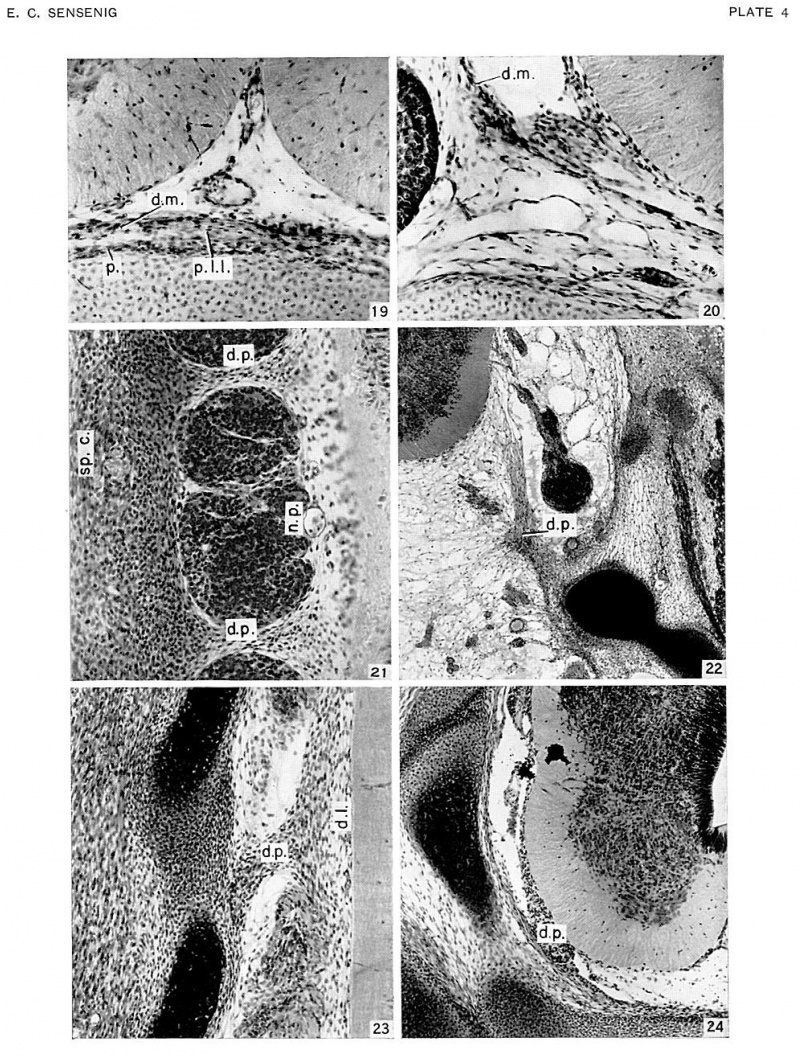
In age groups xvi and xvii (approximately 8 to 13 mm), the vertebral rudiments stand out more and more distinctly from the intermediate zone (fig. 6, pl. 1). Over the dorsal surface of the spinal cord the closure membrane is separating into a peripheral and denser body-wall portion and a deeper and looser portion (fig. 6). The latter becomes part of the meninx primitiva, and this can be identified everywhere about the cord. The meninx primitiva has broadened, and its cells are more scattered than in the previous age group. It is deepest ventral to the neural tube and in the lateral parts between adjacent ganglia. The ganglia have migrated so that their ventral tips lie at the level of origin of the ventral nerve root. The vascular channels now surround the spinal ganglia, and small vessels penetrate into the spinal cord. A frontal section through the ganglia (fig. 21, pl. 4) shows extensions of the dense lateral concentrations of the vertebral canal, passing medially between each two adjacent ganglia to become continuous with the meninx adjacent to the cord. These extensions are the first indications of the denticulate ligaments of the pia mater. These primordia of the dentate processes are also observed in transverse sections (firr. 6), forming a cellular concentration between the ventralateral part of the neural tube and the ventral part of the neural arch rudiments.
In age group xviii, the vertebral canal is clearly distinguishable from its wall, within which chondrification is rapidly advancing (figs. 7, 8, pl. 2). The ventral migration of the ganglia has continued, so that their ventral extremities are entering the intervertebral foramina (fig. 7), and their dorsal extremities lie at about the mid-level of the spinal cord. The tissue of the meninx primitiva is loosening and spaces are becoming evident within it, particularly in the region ventral to the cord. Over the dorsal surfaces of the centra and the intervertebral disks the cells of the meninx primitiva are, for the first time, showing indications of stratification. This stratification is more evident over the intervertebral disks (fig. 7), where it extends laterally and then sweeps dorsad over the medial surface of the ganglia. A similar stratification appears in the pia mater, and this is particularly evident adjacent to the anterior longitudinal sulcus (fig. 8). These conditions are most pronounced in the cervical region and become less distinct in a caudal direction. The rudiments of the dentate processes now form denser and more restricted concentrations and can be identified in the interganglionic transverse sections (fig. 8), where they extend in a dorsoventral direction from the pia, slightly above the emergence of the ventral root, to the mid-point of the rudimentary pedicle.
In age groups xix and xx, developmental advances are apparent in the increased chondrification of the ventral vertebral wall and the greater concentration in the dorsal vertebral wall (the membrana reuniens), in the further ventral migration of the ganglia, and in the formation of large cavities in the meninx primitiva and a reticular arrangement of its cells (figs. 9, 10, pl. 2). The layers of stratified cells overlying the dorsal surfaces of the centra and intervertebral disks, which were beginning to be distinguishable in the previous age group, are now separated from these structures by a narrow network of loose cells (fig. 10). This stratified concentration of cells is the rudiment of the ventral dura mater, and may be traced both laterally and medially to the ganglia. The part lateral to the ganglia passes over into, and becomes indistinguishable from, the dense stratum of the meninx primitiva that forms the more dorsal canal wall. Passing caudad, the ventral dura mater rudiment lies in ever closer proximity to the centra and intervertebral disks, becoming continuous with these structures in the lower cervical segments. It can, however, be identified throughout the thoracic regions.
In age groups xxi and xxii, cavity formation in the meninx primitiva has progressed, and the rudimentary ventral dura is more distinct (figs. 11, 12, pl. 2). Ventral to the medial edge of the ganglia, this dense dural rudiment is separated from the lateral extremities of the centra and intervertebral disks. ‘Within this space a large longitudinal venous channel is developing. The dura can be identified around the spinal ganglia, which are now shifting into the intervertebral foramina, but becomes less distinct as it passes dorsad. The ventral dura can be followed throughout the cervical, thoracic, and lumbar segments, but in the sacral segments it can be identified only on the dorsal surfaces of the intervertebral disks.
The dentate processes are becoming more discrete. The first of these processes (fig. 22, pl. 4), which is attached to the rudiment of the basioccipital bone, is formed of a mass of elongated cells. The base consists of a densely cellular dorsomedial projection which bifurcates into two cell streams. One of these, the denser, extends dorsally to approximate the pia of the spinal cord; the other radiates out into medially and ventromedially directed streams. A frontal section (fig. 23, pl. 4) shows the dentate process projecting from a more medially placed thickened mesenchymal hand which ultimately will form the dentate ligament.
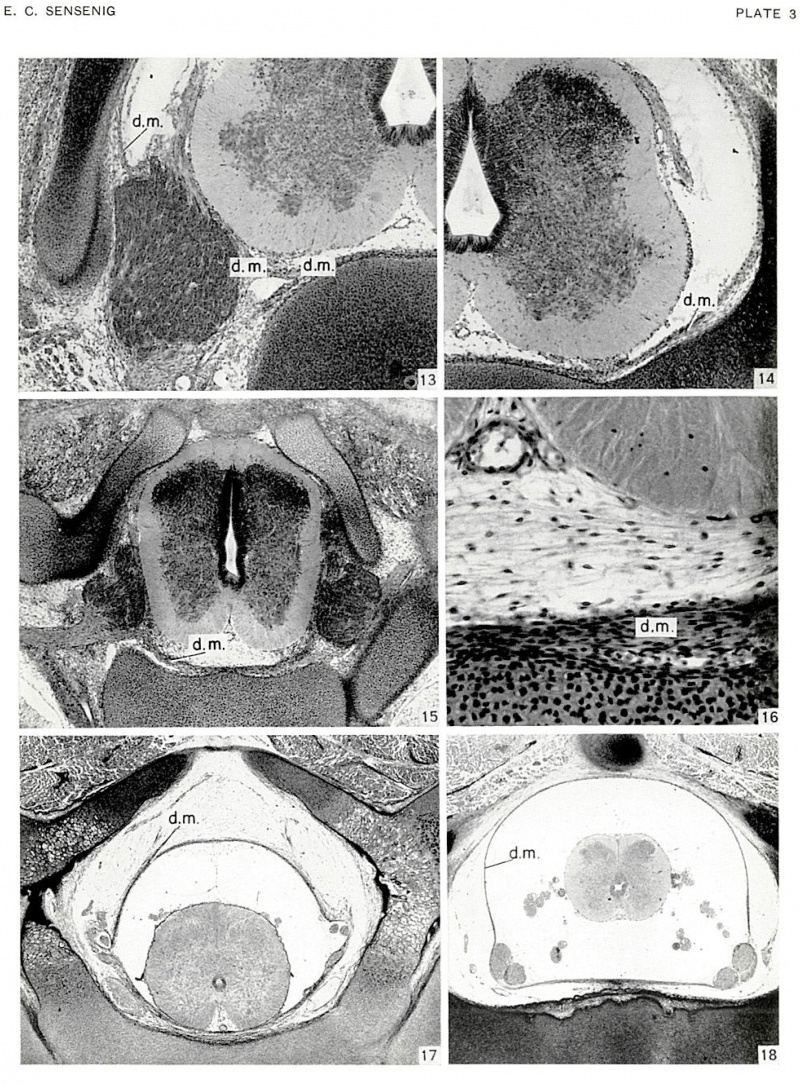
In age group xxiii, the dura mater may be followed completely around the inner wall of the vertebral canal. The ventral dura rudiment is a densely cellular band of tissue with its most medial parts in direct contact with the tissues associated with the dorsal surfaces of the centra and intervertebral disks. The stratified cells lying on the dorsal surface of the centra and intervertebral disks can be separated into three layers: an outer perichondrial layer adjacent to the cartilaginous tissue; an intermediate layer which is more restricted in its transverse extent and ultimately forms the dorsal longitudinal ligament; and an inner layer which is the ventral dura rudiment.
The spinal ganglia are now largely situated within the intervertebral foramina (figs. 13, 15, pl. 3). As the ventral dura mater approaches these ganglia, it splits medially to the ganglia to invest a large, longitudinally directed venous channel (fig. 13), beyond which its two leaves, one directed dorsally and the other ventrally, pass over the medial surface of the ganglia. At interganglionic levels these longitudinal veins have not yet become paired, laterally placed, discrete channels (fig. 16, pl. 3).
The lateral dura mater is separated from the neural arch (fig. 14, pl. 3) or at least is easily distinguished from the underlying perichondrium. As it passes dorsally it fuses with the closure membrane. This membrane is now distinctly separable into a deeper layer, the dura, a thicker and intermediate layer, the muscular layer, and a peripheral or cuticular layer.
In the older embryos of this group the epidural cavity is forming (figs. 13, 14) by the separation of ventrolateral and lateral dura rudiments from the adjacent perichondrium. The subdural (future subarachnoid) space is becoming cell-free through the disappearance of its cavity-filled reticulum (figs. 13, 14).
The pia still consists of a layer one cell thick, adjacent to the spinal cord, and an outer layer of variable thickness within which lies the arterial plexus. The transversely stratified cell layer ventral to the anterior median sulcus is still evident in the younger embryos (figs. 15, 16), but this is breaking up into a subdural space in the older embryos (figs. 13, 14) of the group. In embryos of 30 mm. it is possible to distinguish the paired dorsal and the single ventral spinal arteries (figs. 13-16). As these receive the majority of their blood from the spinal segmental arteries, they do not form continuous longitudinal channels.
From the stage of 30 mm. up, the development of the pia and dura is characterized by progressive changes toward the adult condition. The pia mater of a _j.‘i mm. embryo forms a thin sheath investing the cord (figs. 19, 20, pl. 4). On its outer surface the vascular channels have now formed a discrete plexus of small vessels. From this plexus small vessels penetrate radially into the cord, each carrying with it an extension of pial tissue.
The dura mater, in embryos from 30 to 50 mm. in length, completes its separation from the surrounding wall of the vertebral canal. The last parts to separate from that wall are those adjacent to the centra antl intervertebral disks and to the arches. As this separation progresses, the thick, stratified dural membrane undergoes a condensation of its layers, becoming more closely packed and forming a dense envelope that surrounds the rapidly developing subdural cavity.
The migration of the spinal ganglia into their definitive position in the intervertebral foramina is completed throughout almost the whole length of the cord by the stage of 50 mm. This permits the formation of a complete and uninterrupted dural sheath (figs. 17, 18, pl. 3).
In the embryos studied, of less than 80 mm., no sign of a developing arachnoid membrane was observed.
Resume And Discussion
Participation of the Neural Crest in the Formation of the Meninges
As was mentioned in the descriptive section, in human embryos of ID to 20 somites (age group xi) :1 single layer of cells, apparently continuous with the neural crests (fig. 1, pl. 1), extends ventrad along the lateral edges of the neural tube. In the succeeding stage, 20 to 30 somites (age group xii), neural crest cells and cells of somitic origin are still separately recognizable. In subsequent stages the two kinds of cells become intermingled beyond possibility of being distinguished. These observations are in agreement with those of Holmdahl (1934), who demonstrated that in human embryos neural-crest cells combine with cells of somitic origin to form an undifferentiated mesenchyme which surrounds the neural tube. There is, therefore, no doubt that neural-crest cells mingle with somitic cells in the meninx primitiva, the predominating part of the meninx coming, however, from the somites.
On the evidence at hand, then, Harvey and Burr’s (1926) statement, “Moreover it is definitely proved that the leptomeninx arises from neural crest cells early in embryonic life and the pachymeninx takes origin in mesenchyme at a relatively later period,” is not substantiated so far as the human embryo is concerned. Their further observation (Harvey and Burr, 1926; Harvey, Burr, and Van Campenhout, 1931, 1933) that the leptomeninx is very largely formed by neural-crest cells is likewise not supported. As is pointed out elsewhere in the present discussion, the arachnoid portion of the human leptomeninx forms by delamination from the inner surface of the dura. There is no evidence to indicate that these two meninges, which are so intimately associated in development, are derivatives of separate germ layers. It would appear, then, that the differences in the regeneration of the meninges described by Sayad and Harvey (I923) and Lear and Harvey (1924) cannot be explained on the basis of the origin of the leptomeninx and the pachymeninx from different germ layers. It can better be explained by assuming that in their later development these structures are modified in response to different functional requirements.
A summation of the available evidence thus supports a mesodermal origin for all the meninges, with the reservation that ectodermal cells from the neural crest take a small part in the early formation of the tissue that gives rise to the pia mater.
It should be pointed out that here, as in various other current embryological problems, there is difficulty in rigid adherence to the theory of specificity of the germ layers. Of particular interest in this connection is a series of experimental investigations initiated by Vogt (1926, 1939) on the existence of mesoderm in the more caudal part of the neural plate. Bijtel (1931) added to Vogt’s observations, and Spofford (1945) presented further material which is applicable to the study of the meninges, for it shows that mesoderm derived from the posterior neural plate gives rise to the myotomes and to the meninges in the posterior trunk and tail region in Amblystoma embryos. These observations cast additional doubt on the neural-crest origin of the leptomeninx.
Differentiation of the Meninges from the Menix Primitiva
The term meninx primitiva was introduced by Salvi (1898) to describe the loose intermediate tissue that fills in the region between the periphery of the neural tube and the inner wall of the rudimentary vertebral canal. According to him, all three of the spinal meninges arise within this meninx primitiva: the pia mater from the tissue adjacent to the neural tube, the dura mater from the tissue adjacent to the inner surface of the vertebral canal, and the arachnoid and its trabeculae from the tissue between pia and dura.
Pia Mater
The description given above of the development of the pia mater in age groups xv to xx is sufhcient to indicate that Salvi’s concept of a primitive meningeal layer is correct, and that the pia mater originates by differentiation from it. A discrete pial membrane begins to be differentiated in embryos of about 30 mm., at which age it is evident only along the lateral borders of the spinal cord. In the 50-mm. embryo the pia mater forms a complete sheath about the cord, its outer vascular part consisting of a network of Fine channels. The condensation of pial tissue which is to form the denticulate ligament is already indicated in embryos of 10 to 12 mm. by concentrations extending laterally from the pial rudiment to the canal wall. These are the dentate processes, and they rapidly become more evident as de velopment progresses.
Subarachnoid or Subdural Space
During fetal life there is no separation of the arachnoid from the dura mater, and thus the subdural space does not exist until after birth. In embryos of 12 to 15 mm. the meninx primitiva becomes well defined. Immediately thereafter, the reticular tissue of the meninx primitiva lying between the pia and dura becomes less regularly dispersed, by reason of the development of cavities within it, at first (in embryos of 15 to 16 mm.) in the areas ventral to the spinal cord, and, later, lateral and dorsal to the cord. The subarachnoid space thus begins to form. By the stage of 30 mm. the majority of the cells have disappeared, and a large cell—free space separates the pia from the dura except for the occasional arachnoid trabeculae which project across the space.
Arachnoid Membrane
Most writers on the meninges have accepted the statement, which they quote from Salvi, that early in development the meninx primitiva subdiviclcs into an inner part, the leptomeninx, from which later develops the pia-arachnoid complex, and an outer part, the pachymeninx, which gives rise to the dura. Thereby arose the concept that the pia and arachnoid develop together and form structures developmentally distinct from the dura. Hochstetter (1934) presents as new observations the facts that the arachnoid membrane is associated with the dttra, from which it separates late in development, and that the arachnoid is not developmentally associated with the pia. Clermont (1922) and Flexner (1929) quote Hochstetter's (r934) observations and cite Salvi (1898) as holding the same view. A careful translation of Salvi’s paper shows that he specifically described close association between pia and arachnoid within the cranium, but stated that in the spinal regions the arachnoid is delaminated from the inner surface of the dura, as Hochstetter observed many years later.
The ultimate separation of the arachnoid from the dura mater occurs either very late in fetal life or in early postnatal life. In the mole, Clermont (1922) could not recognize the arachnoid at birth in either cranial or spinal regions. In the oldest human embryo, 2_“O mm., studied by Hochstetter (1934), no evidence of a separation was observed. In human embryos Ask (1941) considered the arachnoid differentiated, but not separated from the dura, in a 120 mm embryo, but could not distinguish it with certainty in one of 138 mm. In an 80-mm. embryo examined in this study, it was possible to identify the arachnoid in restricted areas, yet this membrane was not separated from the dura in the oldest embryo observed, one of 245 mm. In embryos of 180 and 245 mm. the arachnoid was separated from the dura in isolated areas, but such separation was doubtless a result of faults in the fixation and preparation of the tissue. Such artifacts may also account for Tiedemann’s (1816) observation of a distinct arachnoid in 6-month embryos and Ask's (19.11) in a 120 mm embryo. It is evident that in the spinal region the araclmoid is associated with the dura and separates from the latter at a much later developmental stage than was available in the material studied.
Dura Mater
The dura mater is first distinguishable in embryos of I2 to 15 mm. along the dorsal surfaces of the primordial centra and intervertebral disks. It develops from the innermost part of the vertebral canal wall and thus from tissues of sclerotomic origin. This ventral dural rudiment rapidly develops laterally and dorsally, so that by the 30-mm. stage it may be followed about the entire canal wall, although its separation from that wall is not complete until the 50-mm. stage, when it begins to lose its loosely stratified construction and to condense into a firm membrane. By the So-mm. stage it has become a dense, fibrous membrane, approaching the condition seen in the adult.
It is, however, impossible to define a plane of distinct separation between the outermost meninx primitiva and the innermost tissue of the rudimentary vertebral canal. Hochstetter (1934), followed by Ask (1941), was unable to determine with certainty whether the cells forming the dura mater are all derived from the meninx primitiva or whether cells of perichondral origin are also involved. If Fischel’s (1929) observation that the dura mater first forms at some distance from the inner wall of the vertebral canal were correct, there would be no dispute concerning the derivation of the dura from the meninx primitiva. The fact is, on the contrary, that prior to the first appearance of the ventral dura rudiment there is a loosening of the cells of the meninx primitiva everywhere except directly adjacent to the wall of the vertebral canal. Here the cells remain more numerous and form a layer adjacent to the canal wall. It appears that condensation of this layer gives rise to the dura mater. Once condensation has occurred, however, a dura rudiment is formed which is continuous with the vertebral rudiments, the similarity of the tissues and their close association making it exceedingly difiicult to specify a separate origin for each. As development proceeds, the dura and perichondrium become distinct, and separation of the two takes place shortly.
The observations made by Salvi ( 1898), Weed (1917), Hochstetter (1934), and Ask (1941) and in the present study indicate that all three spinal meninges develop from the meninx primitiva. The evidence is not sufficient, however, to support Salvi's (I898) observation that no part of the dura mater has its origin in the tissues of the vertebral rudiments. Salvi specifies that the tissue that gives rise to the meninges is derived from the most medial part of the somite. Sensenig (1943, 1949) pointed out that very shortly after the somites are formed, their ventramedial walls break down and cells migrate in and about the neural tube. The innermost of these cells, or those closest to the neural tube, form the meninx primitiva, and the outer cells form the vertebral rudiments.
Epidural Space
The epidural space is first indicated at about the 20-mm. stage, at the point where the ventral dura rudiment is sweeping dorsolaterally onto the medial surface of the ganglia. Here a large venous channel is forming. The final separation of the dura from the vertebral canal progresses slowly and is not complete until the 50-mm. stage. No true cavity exists here, as the area is filled with a loose tissue containing a plexiform venous bed.
Cranial and Spinal Dura
The development of the spinal meninges differs from that of the cranial meninges in the complete separation of the spinal dura mater from its bony walls. It would appear, however, that it is incorrect to describe, in the definitive condition, a cranial dura mater formed of a double layer, i.e., an outer or periosteal and an inner or membranous layer, in contrast with a spinal dura formed only of a single membranous layer. The membranous layers of the two regions are continuous at the foramen magnum, and such differences as occur between them are adaptations to regional requirements. In the cranium, the size of the cerebral and cerebellar hemispheres necessitates a large bony vault for their protection, and this need, associated with the need for protection of organs of special sense, calls for a strong, nonarticulated bony skull, which in turn must supply a solid base for the movement of the suspended jaw. No articular motion occurs within the skull, except that associated with the occipito-atlanto-axial complex. The membranous dura becomes separated from the bony vault only where venous channels form between the two structures, these channels ultimately forming the definitive dura] sinuses. In contrast, in the vertebral regions, the spinal cord and its membranes lie within a bony canal constructed of a series of articulated segments, of which great mobility as well as strength is demanded. Rotation, flexion, and extension of one segment upon another, and of one series of segments in relation to another series, demand secondary specializations. Thus there is a relatively complete separation of the membranous dura from the surrounding periehondrium of the vertebrae and intervening ligaments. The spinal dura is attached only at intervals to the bony wall, and the central nervous system is suspended within the dural tube by the denticulate ligaments. It is interesting to note that the first separation of the spinal dura from the vertebral canal forms, as in the skull region, through the development of longitudinal vascular channels. These lie at the lateral edges of the vertebral bodies and intervertebral disks, and form the first indication of the epidural space. Furthermore, the adult epidural space contains primarily a system of venous channels, of which the magnitude and significance have been well demonstrated by Pleasants (1911) and Batson (1940, 1942).
Summary
- In human embryos the pia mater, arachnoid, and dura mater develop within the undifferentiated mesenchyme that fills the space between the periphery of the neural tube and the inner wall of the vertebral canal.
- The cells of this mesenchyme adjacent to the spinal cord form (a) the pia mater, investing the spinal cord and containing a vascular plexus; (b) the denticulate ligament, a thickening of the pia, extending the length of the spinal cord.
- The intermediate mass of mesenchymal cells forms (a) the dentate processes of the denticulate ligament; (b) the arachnoid trabeculae.
- The outermost cells of this mesenchyme, which lie adjacent to, and cannot be accurately delimited from, the inner wall of the vertebral rudiments, form (a) the dura mater; (b) the arachnoid mater by subsequent clelamination from the inner surface of the dura mater.
- The tissue from which the meninges arise is predominantly derived from the paraxial somitic mesoderm.
- Ectodermal cells, of neural—crest origin, mingle with the mesodermal cells in the formation of the meninges. These ectodermal cells appear to be primarily involved in the formation of the pia mater.
Literature Cited
Ask, O. 1941. Studien iiber die embryologische Entwieklung cles menschlichen Ruckgrats und seines Inhaltes unter normalen Verhiillnissen und bei gexvissen Formen von Spinal bifida. Uppsala Liikareforen. Fi3rhandl., vol. 46, PP- 243-348
Batson, O. V. 1940. The function of the vertebral veins and their role in the spread of metastases. Ann. Surg., vol. 112, pp. 138-149.
1942. The role of the vertebral veins in metastatic processes. Ann. Intern. Med., vol. 16, pp. 38-45.
Bischoff, Th. J. W. 1842. Entwicklungsgeschichte der Siiugethiere und des Mcnschen. 575 pp. Leipzig.
Bijtel, J. H. 1931. Uber die Entwicklung des Schwanzes bei Amphibien. Arch. f. Entwicklungsmech. (1. Organ., vol. 125, pp. 448-486.
Clermont, 1922. Sur le cléveloppement des meninges chez la taupe (Talpa c-uropm). Arch. de biol., vol. 32, pp. 1—35.
Farrar, C. B. 1907. The embryonic pia. Amer. Iour. Insan., vol. 63, pp. 295-299.
Fischel., A. 1929. Lehrbuch der Entwicklung des Menschen. 812 pp. Vienna.
Flexner, L. B. 1929. The development of the meninges in Amphibia: A study of normal and experimental animals. Carnegie Inst. Wash. Pub. 394, Contrib. to Embryol., vol. 20, pp. 31-49.
Gelderen, C. van. 1925. Uber die Entwicklung der I-Iirn~ hiiute bei Teleostiern. Anat. Anz., vol. 60, pp. 48-57.
Gaoxnake, G. 19oz. Die Ontogenese eines niedern Siiugergehirns nach Untersuchungen an Erimzavcc curopmvrs. Zool. ]ahrb., Abt. 2, Anat. u. Ontog., vol. 15, pp. 261-384.
Haeckel., E. 1872. Die Kalkschwiimmez Iiine Monographie. 3 vols. Berlin.
Hansen-Pruss, O. C. 1923. Meninges of birds, with a consideration of the sinus rhomboidalis. Iour. Comp. Neurol., vol. 36, pp. 193-217.
Harvey SC. and Burr HS. An experimental study of the origin of the meninges. (1924) Proc. Soc. Exper. Biol. and Med. 22: 52-53.
- 1926. The development of the meninges. Arch. Neurol. and Psychiat., vol. 15, pp. 545-567.
- and E. Vax C.\.\lPl-‘.NllOUT. 1931. Development of the meninges in the chick. Proc. Soc. Exper. Biol. and Med., vol. 28, pp. 974-976.
1933. Development of the meninges; further experiments. Arch. Neurol. and Psychiat., vol. 29, pp. 683-690.
Hessen, N, V. 1876. Beobachtungen iiber die Befruchtung und Entwicklung ales Kaninchens und Meersclnveinclietis. Ztschr. f. Anat. u. Entwicltlungsgesclr, vol. 1, pp. 213373: 353-13
His, W. 1865. Die Hiiute und Héihlen des Korpers. 34 pp. Basel.
- 1903. Die Iliiute und I-Iiihlen des Kcirpers. (Wie derabdruck eines akadcmischen Programmes vom Jahre 1865.) Arch. f. Anat. u. Physiol., Anat. Abt., pp. 368-404.
Hochstetter, F. 1934. Uber die Entwicklung und Differenzierung der Hiillen des Riickenmarkcs bcim Menschen. Morphol. ];1hrb., vol. 74, pp. 1-104.
Holmdahl, D. E. 1934. Neurallciste und Ganglicnleistc beim Menschen. Ztschr. f. mikr.-anat. Forsch., vol. 36, pp. 137-178.
Huxley, T. H. 1871. A manual of the anatomy of vertebrated animals. 510 pp. London.
Kolliker, A. von. 1861. Entwicklungsgeschichte des Menschen und dcr hiihcren Thiere. 468 pp. Leipzig.
1880. Grundriss dcr Entwicklungsgcschichte des Mcnschen und dcr héiheren Thiere. 418 pp. Leipzig.
Kollman, I. 1861. Die Entwicklung der Adergeflcchtc. Ein Beitrag zur Entwicltlungsgeschichte des Gehirns. 44 pp. Leipzig.
LANKESTER, E. R. 1873. On the primitive cell-layers of the embryo as the basis of genealogical classification of animals, and on the origin of vascular and lymph systems. Ann. and Mag. Nat. Hist., scr. 4, vol. 11, pp. 321-338.
Lear, M., and S. CI‘L\R\'EY. 1924. The regeneration of the meninges. Ann. Surg., vol. 80, pp. 536-544.
Mihalkovics, V. 1877. Entwicklungsgeschichtc dcs Gchirns. Nach Untersuchungen an hohern Wirbcltliicrcn und dem Menschcn. 195 pp. Leipzig.
Pleasants, I. H. 1911. Obstruction of the inferior vena cava with a report of 18 cases. Johns Hopkins Hosp. Rcpt., vol. 16, pp. 363-548.
Reichert, K. B. 1859, 1861. Der Ban des menschlichen Gchirns durch Abbilclungen mit erliiutcrntlem Texte dargcstellt. 24 pls., 192 pp. Leipzig.
Remak, R. 1850-1855. Untersuchungen iiber die Entwicklung dcr Wirbelthiere. 194 pp. Berlin.
Salvi, G. 1898. L'istogenesi e la struttura dclle mcningi. Mem. Soc. toscana di sci. nat., Pisa (these de Paris, 1898), vol. 16, pp. 187-228.
Sayad, W. Y., and S. C. Harvey. 1923. The regeneration of the meninges. Ann. Surg., vol. 77, pp. 129-141.
Sensenig, E. C. 1943. The origin of the vertebral column in thc deer-mouse, Pcronzyscus maniculams rufirms. Anat. Rcc., vol. 86, pp. 123-141.
- 1949. The early development of the human vertebral column. Carnegie Inst. Wash. Pub. 583, Contrib. to Embryol., vol. 33, pp. 21-41.
Spofford, W. R. 1945. Observations on the posterior part of the neural plate in /Inzblyxtanza. Iour. Exper. Zool., vol- 99. pp- 35-52
S??, G. 1901. Riccrche intorno all’ anatomia comparata ed all ontogcnesi dellc mcningi c considcrazioni sulla filogcnesi. Atti R. lst. veneto di sci., Iett. ed arti, vol. 60, pt. 2, pp. 1101-1372.
- 1902. Recherchcs sur Panatomic comparée et sur Pontogenése des méninges. Arch. ital. de biol., vol. 37, pp- 257-269
Strasser, H. 1901. Ubcr die I-Iiillcn des Gchirns und des Riickenmarks. lhrc Functioncn und ihrc Entwiclclung. Compt. rend. Assoc. des anat., 3d scss., Anat., suppl., pp- 175-184
Theiler, K. 1947. Die Entwicklung dcr konstruktiven Form dcr Riickenmarkshiiute beim Menschcn. Schweizer Arch. f. Neurol. u. Psychiat., vol. 61, pp. 337-370.
Tiedemann, F. 1816. Anatomic und Bildungsgeschichtc des Gehirns im Féitus des Menschcn ncbsl. einer vergleicl1en— den Darstellung des Hirnbaues in den Thiercn. 172 pp. Nuremberg.
Vogt, W. 1926. Uber Wachstum und Gestaltungsbcwegungen am hinteren Kfirpcrende dcr Amphibien. Verhandl. Anat. Gesellsch. Jena, vol. 61, pp. 62-75.
- 1939. Die Rumpfschwanzknospe bei Amphibien und die Thcoric dcr secundiiren Korperentwicklung. Verhandl. Anat. Gcscllsch. Icna, vol. 88, pp. 112-127.
Weed, L. H. 1917. The development of the cerebrospinal spaces in pig and in man. Carnegie Inst. Wash. Pub. 225, Comrib. to E1nbryol., vol. 5. 116 pp.
Plates
Plate 1
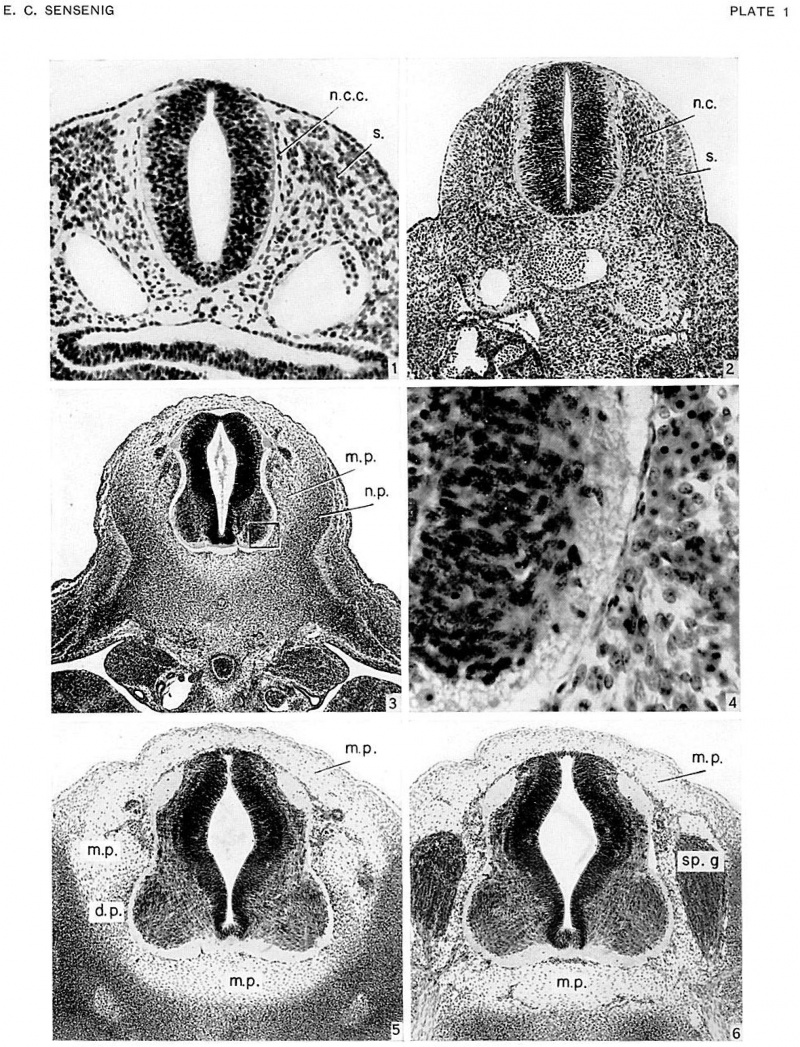
Fig. 1. Transverse section of cervical region of Carnegie Embryo 2053, 3.1 mm., age group 11, cut 10 microns. X:-.50. Shows distribution of paraxial mesodcrm about neural tube, and a single-layered strand of cells (n.c.c.) along periphery of neural tube which originates dorsally from the neural crests.
Fig. 2. Transverse section of upper thoracic region of Carnegie Embryo 8119, 5.3 mm., age group 13, cut 8 X 100. Shows initial vascularization about periphery of neural tube. Differentiation between neural- crest and mesodermal cells still distinct.
Fig. 3. Transverse section of upper thoracic region of Carnegie Embryo 721, 9.0 mm., age group 15, cut 15 microns. X60. Shows intermediate zone (meninx primitiva) between denser vertebral rudiments and periphery of neural tube, and advanced vascularization about neural tube. microns.
Fig. 4. Outlined area in figure 3. X500. Ventrolateral part of neural tube and adjacent tissue of meninx primitiva. Single layer of cells of meninx primitiva lying on periphery of neural tube represents pia mater and can be regarded as of neural-crest origin.
Figs. 5, 6. Transverse sections of 4th thoracic segment of Carnegie Embryo 6521, 13.2 mm., 17, cut 8 microns. X75. Shows distribution of meninx primitiva between neural tube and inner boundary of rudimentary vertebral canal.
Fig. 5, an interganglionic section, demonstrating the rudimentary dentate processes.
Fig. 6, a ganglionic section, the ganglia separated from canal wall by vascular channels. Tissue of meninx primitiva separates ganglia from neural tube.
Plate 2
Plate 3
| Fig. 13. Transverse section through centrum of lower cervical region of embryo, no. 4570, 30.7 mm, age group 23, cut 15 microns x75. The ganglia are entering the intervertebral foramina. Ventral dura passing laterally splits to surround large venous channel and then invests ganglia. Dura rudiment present dorsal to ganglion against lateral wall of vertebral canal. Subdural cavity forming. | Fig. 14. Transverse section through intervertebral disk of embryo, no. 4570, 30.7 mm, age group 23, cut 15 microns x75. Dura rudiment may be identified to tip of neural arches and along ventral part of closure membrane. Note development of subdural space. |
| Fig. 15. Transverse section of mid-thoracic region of embryo, no. 6573 31.3 mm, age group 23, cut 30 microns. x50. Dorsal canal wall compressed. Ventral dura distinct and separating from underlying perichondrium. | Fig. 16. Same section as figure 15. x300. Shows stratification of cells ventral to anterior median sulcus. Dura separating, except in mid-line, from underlying perichondrium of intervertebral disk. |
| Fig. 17. Transverse section of mid-thoracic region of embryo, no. 7218, 80.0 mm cut 20 micron. x30. Dura mater now is a distinct membrane with subdural and epidural cavities formed. Note cell-free subdural cavity, and large venous channels in ventrolateral part of epidural space. | Fig. 18. Transverse section of lumbar region of embryo, no. 7218, 80.0 mm cut 20 micron. x30. Shows complete dural sac of lumbar region, and subdural and epidural spaces. |
Plate 4
| Fig. 19. Transverse section of lower cervical region of embryo no. 4473, 43.0 mm cut 20 microns. X230. Shows single cell layer of pia mater and anterior spinal artery. also dura mater and perichondrium with rudiment of posterior longitudinal ligament between them. | Fig. 20. Transverse section of upper thoracic region of embryo no. 4475, 48.0 mm cut 20 microns x250. Pia mater and durzl mater lying medial to spinal ganglion. |
| Fig. 21. Frontal section of thoracic region of embryo no. Template:CE8113. 12.6 mm age group 17, cut 10 microns. x200. Interganglionic tissue connecting dense laterally situated mesenchyme with lateral border of neural tube representing the earliest indication of a dentate process. | Fig. 22. Transverse section of occipitocervical region of embryo no. 7592. 32.7 mm age group 21 cut 20 microns x50. First dentate process extending dorsomediad from rudiment of occipital bone to neural tube. |
| Fig. 23. Frontal suction of mid-thoracic region of embryo no. 6832; 23.3 mm.. age group 22 cut 20 microns x150. Dentate process joins laterally the cerebral arch rudiment, and medially a thickening of the pia mater. the rudimentary denticulate ligament. | Fig. 24. Transverse section of upper thoracic region of embryo no. 4570, 30.7 mm., age group 23 cut 15 microns x100 Inter-ganglionic section showing dentate process. |
Abbreviations used in Plates: d.1., dentate ligament d.m., dura mater d.p., dentate process m.p., meninx primitiva n.c., neural crest n.c.c., neural-crest cells n.p., neural process p., perichondrium pm., rudimentary posterior longitudinal ligament s., somite :p.c., spinal cord :p.g., spinal ganglion
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
- Links: plate 1 | plate 2 | plate 3 | plate 4 | Sensenig 1951 | Spinal Cord | Meninges
Reference
Sensenig EC. The early development of the meninges of the spinal cord in human embryos. (1951) Contrib. Embryol., Carnegie Inst. Wash. Publ. 611.
Cite this page: Hill, M.A. (2024, May 22) Embryology Paper - The early development of the meninges of the spinal cord in human embryos (1951). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_early_development_of_the_meninges_of_the_spinal_cord_in_human_embryos_(1951)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
- Neural
- Spinal Cord
- Ventricular System
- Historic Embryology
- Carnegie Embryo 1390
- Carnegie Embryo 2053
- Carnegie Embryo 2393
- Carnegie Embryo 2795
- Carnegie Embryo 3385
- Carnegie Embryo 3527
- Carnegie Embryo 4059
- Carnegie Embryo 4216
- Carnegie Embryo 4339
- Carnegie Embryo 4405
- Carnegie Embryo 4430
- Carnegie Embryo 4570
- Carnegie Embryo 5074
- Carnegie Embryo 5609
- Carnegie Embryo 5787
- Carnegie Embryo 6097
- Carnegie Embryo 6426
- Carnegie Embryo 6504
- Carnegie Embryo 6506
- Carnegie Embryo 6507
- Carnegie Embryo 6511
- Carnegie Embryo 6512
- Carnegie Embryo 6516
- Carnegie Embryo 6517
- Carnegie Embryo 6519
- Carnegie Embryo 6521
- Carnegie Embryo 6524
- Carnegie Embryo 6525
- Carnegie Embryo 6528
- Carnegie Embryo 6573
- Carnegie Embryo 6701
- Carnegie Embryo 6832
- Carnegie Embryo 721
- Carnegie Embryo 7254
- Carnegie Embryo 7274
- Carnegie Embryo 7392
- Carnegie Embryo 7425
- Carnegie Embryo 7611
- Carnegie Embryo 7707
- Carnegie Embryo 7724
- Carnegie Embryo 7829
- Carnegie Embryo 7864
- Carnegie Embryo 7889
- Carnegie Embryo 7906
- Carnegie Embryo 792
- Carnegie Embryo 7999
- Carnegie Embryo 8066
- Carnegie Embryo 8092
- Carnegie Embryo 8101
- Carnegie Embryo 8112
- Carnegie Embryo 8118
- Carnegie Embryo 8119
- Carnegie Embryo 8141
- Carnegie Embryo 8172
- Carnegie Embryo 8235
- Carnegie Embryo 8244
- Carnegie Embryo 887
- Draft