Chicken Development
| Embryology - 26 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
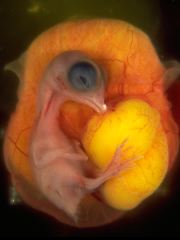
The chicken (taxon -Gallus gallus) embryo develops and hatches in 20 to 21 days and has been extensively used in embryology studies. Historically, the chicken embryo was one of the first embryos studied, readily available and easy to incubate, embryo development can be directly observed by cutting a small window in the egg shell. A key to this model organism study was the establishment of a staging atlas by Hamburger & Hamilton in 1951[1], which allowed specifc developmental landmarks to be seen and correlated with experimental manipulations of development. This much cited paper included images of all key stages and was more recently republished in the journal Developmental Dynamics (1993), for a new generation of avian researchers. Probably just as important has been the recent chicken genome sequencing, providing a resource to extend our knowledge of this excellent developmental model.
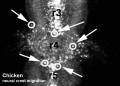
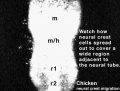
Fertilized eggs can be easily maintained in humidified incubators and during early stages of development the embryo floats on to of the egg yolk that it is using for nutrition. As the embryo grows it sinks into, or below the, yolk. The regular appearance of somites allowed early experimenters to acurately stage the embryo. The embryo was accessible and easy to manipulate (limb grafts/removal etc) that were informative about developmental processes. Chicken cells and tissues (neural ganglia/fragments) are also easy to grow in tissue culture. The discovery that quail cells have a different nuclear appearance meant that transplanted cells (chick/quail chimeras) could be tracked during development. For example, LeDourian's studies showed how neural crest cells migrate widely throughout the embryo.
| This collapsible and sortable table compares the chicken incubation period with other bird species. | ||||||||||||||||||||||||||||
|
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 | ||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Chicken Embryology | Chicken Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Gallus gallus
Taxonomy Id: 9031
Preferred common name: chicken
Rank: species
Genetic code: Translation table 1 (Standard) Mitochondrial genetic code: Translation table 2
Other names: dwarf Leghorn chickens (includes), red jungle fowl (includes), chickens (common name), Gallus domestics (misnomer), Gallus galls domesticus (misnomer)
Lineage (abbreviated ): Eukaryota; Metazoa; Chordata; Craniata; Vertebrata; Archosauria; Aves; Neognathae; Galliformes; Phasianidae; Phasianinae; Gallus
Chicken Stages
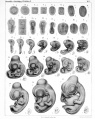
Chicken stages - Hamburger & Hamilton staged the chicken embryo in 1951. The Hamburger Hamilton Stages are most commonly used series for chicken staging. The original paper had approx 25 citations between 1955 - 59, while in the year 1991 alone there were over 300 citations. Series of Embryonic Chicken Growth. J. Morphology, 88 49 - 92 (1951). Atlas recently republished by J.R. Sanes in Developmental Dynamics 195 229-275 (1993).
| Hamburger Hamilton Stages (1951) | ||
|---|---|---|
| 3.5-4.5 hr | Shell membrane of egg formed in isthmus of oviduct | |
| Germ wall formed from marginal periblast | ||
| 4.5-24.0 hr | Shell of egg formed in uterus | |
| Preprimitive streak (embryonic shield) | ||
| 6-7 hr | Initial primitive streak, 0.3-0.5 mm long | |
| 12-13 hr | Intermediate primitive streak | |
| 18-19 hr | Definitive primitive streak, ±1.88 mm long | |
| 19-22 hr | Head process (notochord) | |
| 23-25 hr | Head fold | |
| 23-26 hr | 1 somite; neural folds | |
| ca. 23-26 hr | 1-3 somites; coelom | |
| 26-29 hr | 4 somites; blood islands | |
| 29-33 hr | 7 somites; primary optic vesicles | |
| ca. 33 hr | 8-9 somites; anterior amniotic fold | |
| 33-38 hr | 10 somites; 3 primary brain vesicles | |
| 40-45 hr | 13 somites; 5 neuromeres of hindbrain | |
| 45-49 hr | 16 somites; telencephalon | |
| 48-52 hr | 19 somites; atrioventricular canal | |
| ca. 50-52 hr | 20-21 somites; tail bud | |
| 50-53 hr | 22 somites; trunk flexure; visceral arches I and II, clefts 1 and 2 | |
| ca. 50-54 hr | 23 somites; premandibular head cavities | |
| 50-55 hr | 24-27 somites; visceral arch III, cleft 3 | |
| 51-56 hr | 26-28 somites; wing bud; posterior amniotic fold | |
| 52-64 hr | 29-32 somites; leg bud; epiphysis | |
| 3 da | 30-36 somites extending beyond level of leg bud; allantois | |
| 3.0-3.5 da | 37- 40 somites extending into tail; maxillary process | |
| 3.0-3.5 da | 40-43 somites; rotation completed; eye pigment | |
| 3.5 da | 43-44 somites; visceral arch IV, cleft 4 | |
| 3.5-4.0 da | Somites extend to tip of tail | |
| 4 da | Dorsal contour from hindbrain to tail is a curved line | |
| 4.5 da | Toe plate | |
| 4.5-5.0 da | Elbow and knee joints | |
| 5 da | 1st 3 toes | |
| 5.0-5.5 da | Beak | |
| 5.5-6.0 da | 3 digits, 4 toes | |
| 6.0-6.5 da | Rudiment of 5th toe | |
| 6.5-7.0 da | Feather germs; scleral papillae; egg tooth | |
| 7.0-7.5 da | Web between 1st and 2nd digits | |
| 7.5 da | Anterior tip of mandible has reached beak | |
| 7.5-8.0 da | Web on radial margin of wing and 1st digit | |
| 8 da | Nictitating membrane | |
| 8.5-9.0 da | Phalanges in toes | |
| 10 da | Length of 3rd toe from tip to middle of metatarsal joint = 5.4 ±0.3 mm; length of beak from anterior angle of nostril to tip of bill = 2.5mm; primordium of comb; labial groove; uropygial gland | |
| 11 da | Length of 3rd toe = 7.4 ±0.3mm; length of beak = 3.0 mm | |
| 12 da | Length of 3rd toe = 8.4 ± 0.3 mm; length of beak = 3.1 mm | |
| 13 da | Length of 3rd toe = 9.8 ± 0.3 mm; length of beak = 3.5 mm | |
| 14 da | Length of beak = 4.0 mm; length of 3rd toe = 12.7 ± 0.5 mm | |
| 15 da | Length of beak from anterior angle of nostril to tip of upper bill = 4.5 mm; length of 3rd toe = 14.9 ± 0.8 mm | |
| 16 da | Length of beak = 4.8 mm; length of 3rd toe = 16.7 ± 0.8 mm | |
| 17 da | Length of beak = 5.0 mm; length of 3rd toe = 18.6 ± 0.8 mm | |
| 18 da | Length of beak = 5.7 mm; length of 3rd toe = 20.4 ± 0.8 mm | |
| 19-20 da | Yolk sac half enclosed in body cavity; chorio-allantoic membrane contains less blood and is "sticky" in living embryo | |
| 20-21 da | Newly-hatched chick | |
|
Hamburger V. and Hamilton HL. A series of normal stages in the development of the chick embryo. (1951) J Morphol. 88(1): 49-92. PMID 24539719 PDF Original 1951 paper (and all data) was republished in 1992. <pubmed>1304821</pubmed> PDF | ||
Note that there was also an earlier Witschi staging, and a 1900 staging series by Franz Keibel and Karl Abraham[8], and an earlier (1883) series by Foster, Balfour, Sedgwick, and Heape.[9]
Normal Plates of the Development of the Chicken Embryo (1900)
- Links: Chicken Stages | Hamburger Hamilton | Witschi | 1900 | 1883 | PDF Poster- Hamburger Hamilton Stages | 2006 reproduction of the original paper
Chicken Movies
|
|
|
|
|
|
|
| Neural crest migration Chicken Head (movies overview) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
| |||||||||||||||||||||
| Chicken Placode | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
| |||||||||||||||
|
|
|
|
- Links: Movies
Other Chicken Atlases
Vertebrate and Invertebrate Embryos (7th Edition) G.C. Schoenwolf, Prentice Hall, New Jersey
An Atlas of Embryology (1975) W.H. Freeman and B. Bracegirdle, Heinemann Educational Books, UK.
This is an ATLAS (no description of development) , basically reprinted from the original 1963 edition.
Photos with labelled diagrams covering Amphioxus (worm) Frog, Chicken.
An Atlas for Staging Mammalian and Chick Embryos (1987) H. Bultler and B.H. Juurlink, CRC Press Inc., Florida
This ATLAS is not a complete series of development but has interesting comparisons of species.
Mostly photos of embryos with a few drawn diagrams and a series of staging correlation graphs.
Bird Evolution
Birds and Dinosaurs? as quoted in a Curent Biology review "...abundant and ever increasing evidence places birds as one surviving lineage of the diverse clade Dinosauria"[10][11]
Chicken Genomics
The first draft of the chicken genome was publicly released in March, 2004. There are a number of sites that have begun looking into establishing chicken genomics partly due to its powerful history as a model of vertebrate development that is easy to observe, manipulate and is also cheap. (see also NIH Proposal for Chicken Genomics | NCBI Chicken Genome Resources)
A summary of chicken genome resources has recently been identified in a review in Developmental Dynamics by Antin PB and Konieczka JH.[12]
Chicken Sex Determination
In chicken development sex determination depends on a ZZ male/ZW female mechanism.
This differs from mammalian sex determination which is based upon testis expression of an Sry gene in somatic supporting Sertoli cells.
In the gonad, the coelomic epithelium contributes only to non-steroidogenic interstitial cells and nephrogenous mesenchyme contributes both Sertoli cells and steroidogenic cells.
Genital
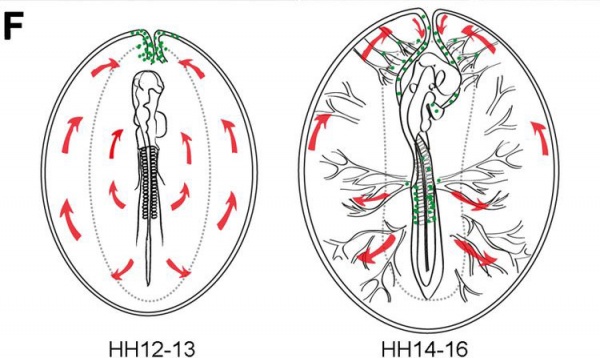
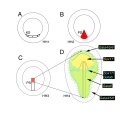
Primordial Germ Cell Migration Model[13]
| HH12–13 - yolk sac circulation courses in loop (red arrows) to enter the embryo via the heart. The majority of PGCs (green dots) localized axially at the border between the area opaca and pellucida, where the sinus terminalis converged in the anterior vitelline veins. | HH14–16 - PGCs (green dots) circulated effectively towards the embryo via the sinus terminalis and the anterior vitelline veins towards the heart. Then PGCs traffic via the aorta to the caudal part of the embryo and become lodged in the genital ridges. |
Chicken Heart
Note these are Hamburger Hamilton Stages of chicken development, see also Heart 3D reconstruction.
Chicken Cardiac Stages
From review[14]
- HH 8 (26–29 hours, 4–6 somites)
- HH 9 (29–33 hours, 7–9 somites) - Cardiac neural crest cells begin the process of EMT and emigrate from the neural tube.
- HH 10–11 (33–45 hours, 10–15 somites) - Primary heart tube
- HH 12-13- (45–49 hours, 16–19 somites) - dextral-looping phase of looping completed at stage 12.
- HH 13+ (50–52 hours, 20–21 somites) - c-shaped heart loop transformed into the s-shaped heart loop. Cardiac neural crest has stopped producing cells.
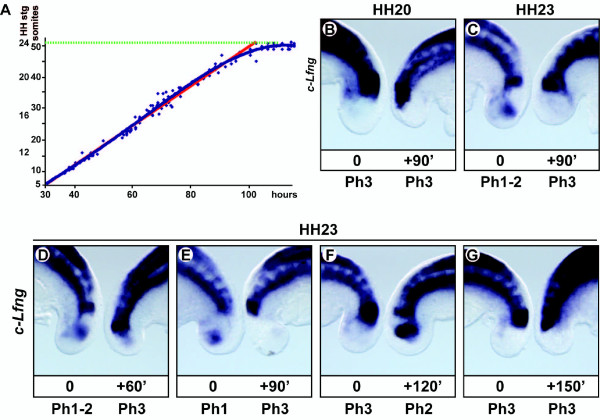
Chicken Somitogenesis
|
| ||||||
| Gene expression | Somite timing |
Chick somitogenesis oscillator[15]
Chicken body elongation model[16]
Chicken Limb
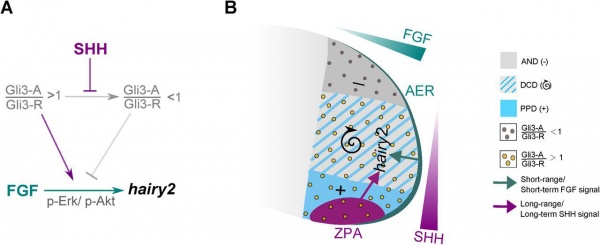
Limb Hairy2 Expression Model[17]
Hairy2 is a "molecular oscillator" involved in both somite and limb development.
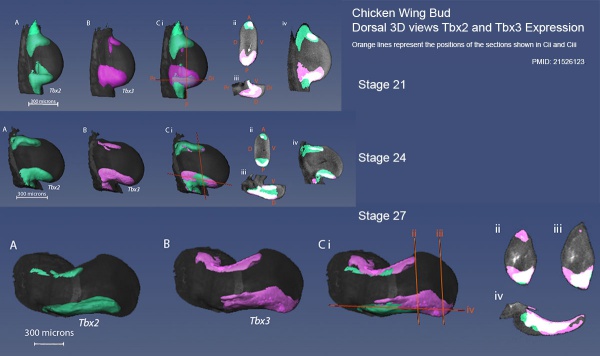
Chicken stage 21 to 27 wing bud Tbx2 and Tbx3 expression[18]
Chicken Head
A recent study of chicken mandible development[5] has shown MORN5 (MORN repeat containing 5, on chromosome 17, was expressed in chick craniofacial structures from stage HH17-18 (E2.5). At stage HH20 (E3) expression was localized in the mandibular prominences lateral to the midline. and from stage HH20 up to HH29 (E6), there was strong expression in restricted regions of the maxillary and mandibular prominences. DOI: 10.3389/fphys.2016.00378
The following gene expression data is from a study of different head regions during development.[19]
- Frontonasal Prominence - CASH1/ASCL1, POSTN, OGN, CYP26A1, NR2E1, and SCARA5.
- Olfactory epithelium SP8, EYA2, and SIX3
- Maxillary/Trigeminal Ganglion - SOX10, TAGLN3.
- Mandibular - DLX1, HAND2 (highest), LHX8, MSX2, PITX2, and TWIST2.
- Mandibular/maxillary prominences differentially expressed - BETA3, HAND2, and MSX2.
Chicken Skin
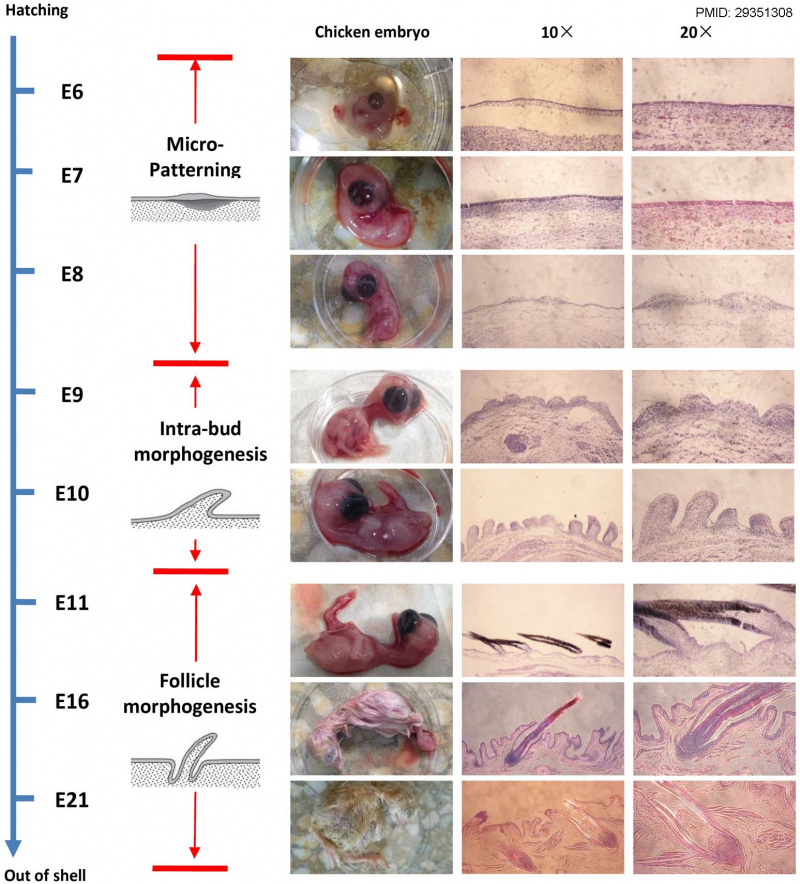
Processes in chicken embryo skin development.[4]
Three different processes in chicken embryo skin development were analyzed: Micro-patterning (E6–E8), intra-bud morphogenesis (E9–E10) and follicle morphogenesis (After E11). Histological sections of three stages of chicken skin during embryonic development.
Historic Studies
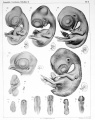
The Elements of Embryology - Volume 1 by Foster, M., Balfour, F. M., Sedgwick, A., & Heape, W. (1883)
The History of the Chick: Egg structure and incubation beginning | Summary whole incubation | First day | Second day - first half | Second day - second half | Third day | Fourth day | Fifth day | Sixth day to incubation end
Keibel F. and Abraham K. Normal Plates of the Development of the Chicken Embryo (Gallus domesticus). (1900) Vol. 2 in series by Keibel F. Normal plates of the development of vertebrates (Normentafeln zur Entwicklungsgeschichte der Wirbelthiere) Fisher, Jena., Germany.
Lillie FR. The development of the chick. (1908) New York.
Elements of Embryology - Volume 1 - Figures
Other Avian Models
Pigeon
The domestic pigeon (Columba livia f. domestica) has an average egg development 18 days. A recent paper describes pigeon development and staging.[20]
Quail
Quail average egg development 15-16 days. The quail historically was used extensively by Nicole Le Douarin in chimera studies with chicken, particularly for neural crest development. See also the quail anatomy portal (For review username: demo, password: quail123).[21]
- Japanese Quail (Coturnix japonica).
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 | ||
|
References
- ↑ Hamburger V & Hamilton HL. (1992). A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. , 195, 231-72. PMID: 1304821 DOI.
- ↑ Davey MG, Towers M, Vargesson N & Tickle C. (2018). The chick limb: embryology, genetics and teratology. Int. J. Dev. Biol. , 62, 85-95. PMID: 29616743 DOI.
- ↑ Kremnyov S, Henningfeld K, Viebahn C & Tsikolia N. (2018). Divergent axial morphogenesis and earlyshhexpression in vertebrate prospective floor plate. Evodevo , 9, 4. PMID: 29423139 DOI.
- ↑ 4.0 4.1 Gong H, Wang H, Wang Y, Bai X, Liu B, He J, Wu J, Qi W & Zhang W. (2018). Skin transcriptome reveals the dynamic changes in the Wnt pathway during integument morphogenesis of chick embryos. PLoS ONE , 13, e0190933. PMID: 29351308 DOI.
- ↑ 5.0 5.1 Cela P, Hampl M, Fu KK, Kunova Bosakova M, Krejci P, Richman JM & Buchtova M. (2016). MORN5 Expression during Craniofacial Development and Its Interaction with the BMP and TGFβ Pathways. Front Physiol , 7, 378. PMID: 27630576 DOI.
- ↑ Atsuta Y & Takahashi Y. (2015). FGF8 coordinates tissue elongation and cell epithelialization during early kidney tubulogenesis. Development , 142, 2329-37. PMID: 26130757 DOI.
- ↑ Canaria CA & Lansford R. (2011). 4D fluorescent imaging of embryonic quail development. Cold Spring Harb Protoc , 2011, 1291-4. PMID: 22046043 DOI.
- ↑ Keibel F. and Abraham K. Normal Plates of the Development of the Chicken Embryo (Gallus domesticus). (1900) Vol. 2 in series by Keibel F. Normal plates of the development of vertebrates (Normentafeln zur Entwicklungsgeschichte der Wirbelthiere) Fisher, Jena., Germany.
- ↑ Foster M. Balfour FM. Sedgwick A. and Heape W. The Elements of Embryology (1883) Vol. 1. (2nd ed.). London: Macmillan and Co.
- ↑ Clarke J & Middleton K. (2006). Bird evolution. Curr. Biol. , 16, R350-4. PMID: 16713939 DOI.
- ↑ Lindow BE & Dyke GJ. (2006). Bird evolution in the Eocene: climate change in Europe and a Danish fossil fauna. Biol Rev Camb Philos Soc , 81, 483-99. PMID: 16893476 DOI.
- ↑ Antin PB & Konieczka JH. (2005). Genomic resources for chicken. Dev. Dyn. , 232, 877-82. PMID: 15739221 DOI.
- ↑ De Melo Bernardo A, Sprenkels K, Rodrigues G, Noce T & Chuva De Sousa Lopes SM. (2012). Chicken primordial germ cells use the anterior vitelline veins to enter the embryonic circulation. Biol Open , 1, 1146-52. PMID: 23213395 DOI.
- ↑ Martinsen BJ. (2005). Reference guide to the stages of chick heart embryology. Dev. Dyn. , 233, 1217-37. PMID: 15986452 DOI.
- ↑ Tenin G, Wright D, Ferjentsik Z, Bone R, McGrew MJ & Maroto M. (2010). The chick somitogenesis oscillator is arrested before all paraxial mesoderm is segmented into somites. BMC Dev. Biol. , 10, 24. PMID: 20184730 DOI.
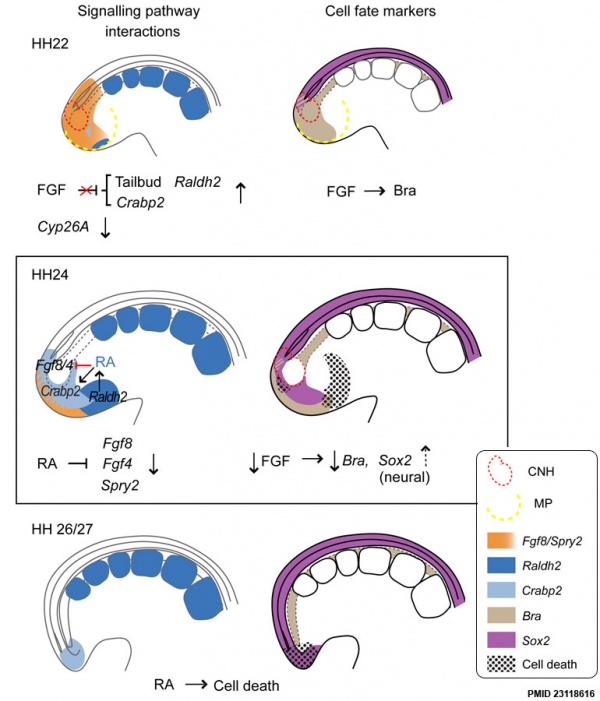
- ↑ Olivera-Martinez I, Harada H, Halley PA & Storey KG. (2012). Loss of FGF-dependent mesoderm identity and rise of endogenous retinoid signalling determine cessation of body axis elongation. PLoS Biol. , 10, e1001415. PMID: 23118616 DOI.
- ↑ Sheeba CJ, Andrade RP & Palmeirim I. (2012). Joint interpretation of AER/FGF and ZPA/SHH over time and space underlies hairy2 expression in the chick limb. Biol Open , 1, 1102-10. PMID: 23213390 DOI.
- ↑ Fisher M, Downie H, Welten MC, Delgado I, Bain A, Planzer T, Sherman A, Sang H & Tickle C. (2011). Comparative analysis of 3D expression patterns of transcription factor genes and digit fate maps in the developing chick wing. PLoS ONE , 6, e18661. PMID: 21526123 DOI.
- ↑ Buchtová M, Kuo WP, Nimmagadda S, Benson SL, Geetha-Loganathan P, Logan C, Au-Yeung T, Chiang E, Fu K & Richman JM. (2010). Whole genome microarray analysis of chicken embryo facial prominences. Dev. Dyn. , 239, 574-91. PMID: 19941351 DOI.
- ↑ Rodrigues T, Brodier L & Matter JM. (2020). Investigating Neurogenesis in Birds. Methods Mol. Biol. , 2092, 1-18. PMID: 31786777 DOI.
- ↑ Ruparelia AA, Simkin JE, Salgado D, Newgreen DF, Martins GG & Bryson-Richardson RJ. (2014). The quail anatomy portal. Database (Oxford) , 2014, bau028. PMID: 24715219 DOI.
Reviews
Articles
Korn MJ & Cramer KS. (2007). Windowing chicken eggs for developmental studies. J Vis Exp , , 306. PMID: 18989413 DOI.
Search Pubmed
Search Pubmed: chicken development
Additional Images
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- e-Chick Atlas
The e-Chick Atlas of Anatomy Explore the 3-D anatomical atlas of Chick embryo development.
- The e-Chick Atlas of Gene Expression A database of Chick gene expression where, uniquely, the gene expression is mapped into the ECA 3-D space and can be queried spatially.
- Developmental Dynamics - Chicken Special Issue (2004) | Poster- Hamburger Hamilton Stages | Republished Hamburger Hamilton Stages Paper
- Developmental Biology - Quail-Chick Chimeras
- Nicole Le Douarin pioneered the use of quail-chick chimeras to study the developmental fate of cells in the bird embryo. The videotape Nicole Le Douarin gave us permission to digitize is titled, "Quail-Chick Chimeras in Development of the Nervous System and Immune System" and it was made in 1987. These digital video sequences and still images come from the first part of her videotape. These chimeras were a key to our understanding cell migration (eg neural crest) in the embryo.
- Quicktime movie sequence 1 (477k) showing newly hatched quail-chick chimeras; white feathers are chick and dark, pigmented feathers are quail.
- Quicktime movie sequence 2 (1.3 MB) Sequence showing the preparation of the chick host; removing a portion of host's neural tube and neural crest.
- Quicktime movie sequence 3 (1.4 MB) Sequence showing the removal and "cleaning off" of donor quail neural tube and neural crest.
- Quicktime movie sequence 4 (1.5 MB) Sequence showing transplantation and grafting of donor quail neural tube and neural crest into the chick host; at the end of this sequence, you see the host chick embryo 5 hours later with its healed in graft.
- Developmental Biology- Laurie Iten's Serially Sectioned Frog and Chick Embryos
- JoVE article chicken egg preparation
- Chicken genomic websites
- AvianNet http://www.ri.bbsrc.ac.uk/chickmap
- NCBI Chicken Genome Resources
- Genome browser - Washington University Genome Sequencing Center (WUGSC)
- Genome browser -  Ensembl
- http://www.ri.bbsrc.ac.uk/chickmap
- http://poultry.mph.msu.edu/mapsnew.html
- http://ag.ansc.purdue.edu/poultry/
- http://www.zod.wau.nl/vf/chickensite/chicken.html
- http://tetra.gig.usda.gov:8400/chickgbase/manager.html
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 26) Embryology Chicken Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Chicken_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G