Abnormal Development - Measles Virus
| Embryology - 27 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
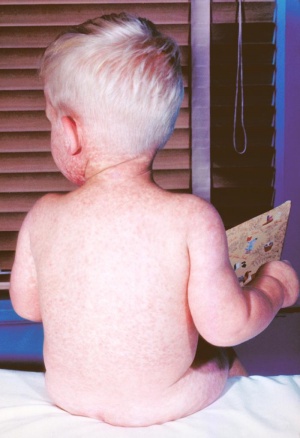
Measles (rubeola, German Measles, Three-Day Measles) is a paramyxovirus appearing mainly as a respiratory viral infection, clinically different from rubella. A single-stranded RNA virus which is highly contagious. Before measles vaccination (USA 1963) more than 90% of children had an infection before puberty and in developing countries it is still a common and often fatal childhood disease. Childhood immunisation and immunity persists in only about 80% of adults.
Pregnancy effects of measles results in a higher risk of premature labor, spontaneous abortion, low-birth-weight, and possibly rare cases of birth defects with no definable pattern of malformation.[1]
20 March 2014 - Measles Elimination Achieved in Australia
The World Health Organization (WHO) announced that measles elimination had been achieved by Australia, Macao (China), Mongolia and the Republic of Korea.
Some Recent Findings
|
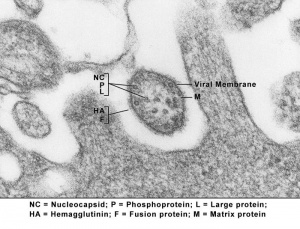
Virus Structure
Lineage: Viruses; ssRNA viruses; ssRNA negative-strand viruses; Mononegavirales; Paramyxoviridae; Paramyxovirinae; Morbillivirus; Measles virus
- ssRNA; linear; Length: 15,894 nt Measles virus, complete genome
- virus replication involves a viral RNA-dependent RNA polymerase (vRdRp), using as a template a nucleocapsid (NC) made of a single strand of RNA in tight complex with the nucleoprotein (N).[8]
- negative-strand genome contains six transcription units encoding the N, phospho (P), matrix (M), fusion (F), hemagglutinin (H), and large (L) or polymerase protein.
- each N protein binds to 6 nucleotides.
- the N polymer entirely covers the 15,894-nucleotide genome.
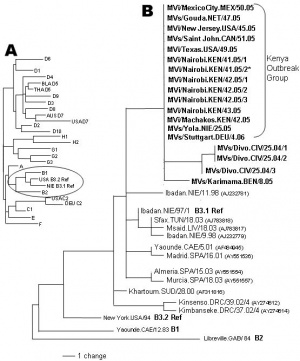
- 23 known measles genotypes.
Model of cell virus RNA accumulation
The following 5 -step model has been described for cell virus accumulation following hours post-infection (hpi)[8]
- 0 to ~5 hpi - incoming viral RNA-dependent RNA polymerase (vRdRp) initiated primary transcription from every gene with no detectable lag phase.
- ~5 to ~12 hpi - mRNA accumulates exponentially.
- ~12 to ~24 hpi - mRNAs, genomes, and antigenomes accumulate exponentially because of the increase of both newly available template and vRdRp.
- ~24 to ~30 hpi - genomes and antigenomes continue to accumulate exponentially at the same rate, whereas the accumulation of the transcripts slows down.
- 30+ hpi - genome and antigenome accumulation slows down, and the cell content in viral transcripts tends to decrease.
Vaccination
Japan - first introduced to Japan in 1966 and adopted in the national regular immunization program from 1978.
References
- ↑ <pubmed>12850161</pubmed>
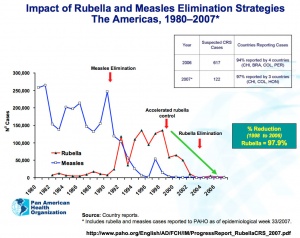
- ↑ Centers for Disease Control and Prevention (CDC). (2013). Rubella and congenital rubella syndrome control and elimination - global progress, 2000-2012. MMWR Morb. Mortal. Wkly. Rep. , 62, 983-6. PMID: 24304830
- ↑ Lin WH, Kouyos RD, Adams RJ, Grenfell BT & Griffin DE. (2012). Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. Proc. Natl. Acad. Sci. U.S.A. , 109, 14989-94. PMID: 22872860 DOI.
- ↑ Grigorov B, Rabilloud J, Lawrence P & Gerlier D. (2011). Rapid titration of measles and other viruses: optimization with determination of replication cycle length. PLoS ONE , 6, e24135. PMID: 21915289 DOI.
- ↑ Stanescu A, Janta D, Lupulescu E, Necula G, Lazar M, Molnar G & Pistol A. (2011). Ongoing measles outbreak in Romania, 2011. Euro Surveill. , 16, . PMID: 21871218
- ↑ Rima BK & Duprex WP. (2009). The measles virus replication cycle. Curr. Top. Microbiol. Immunol. , 329, 77-102. PMID: 19198563
- ↑ Rota J, Lowe L, Rota P, Bellini W, Redd S, Dayan G, van Binnendijk R, Hahné S, Tipples G, Macey J, Espinoza R, Posey D, Plummer A, Bateman J, Gudiño J, Cruz-Ramirez E, Lopez-Martinez I, Anaya-Lopez L, Holy Akwar T, Giffin S, Carrión V, de Filippis AM, Vicari A, Tan C, Wolf B, Wytovich K, Borus P, Mbugua F, Chege P, Kombich J, Akoua-Koffi C, Smit S, Bukenya H, Bwogi J, Baliraine FN, Kremer J, Muller C & Santibanez S. (2006). Identical genotype B3 sequences from measles patients in 4 countries, 2005. Emerging Infect. Dis. , 12, 1779-81. PMID: 17283637 DOI.
- ↑ 8.0 8.1 Plumet S, Duprex WP & Gerlier D. (2005). Dynamics of viral RNA synthesis during measles virus infection. J. Virol. , 79, 6900-8. PMID: 15890929 DOI.
Textbooks
- Medical Microbiology. 4th edition. Baron S, editor. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Medical Microbiology- Measles
- Molecular Biology of the Cell. 4th edition. Alberts B, Johnson A, Lewis J, et al. New York: Garland Science; 2002. Viruses Exploit Host Cell Machinery for All Aspects of Their Multiplication
- Disease Control Priorities in Developing Countries. 2nd edition. Jamison DT, Breman JG, Measham AR, et al., editors. Washington (DC): World Bank; 2006. Chapter 20Vaccine-preventable Diseases
Reviews
Manikkavasagan G & Ramsay M. (2009). The rationale for the use of measles post-exposure prophylaxis in pregnant women: a review. J Obstet Gynaecol , 29, 572-5. PMID: 19757257 DOI.
Stein SJ & Greenspoon JS. (1991). Rubeola during pregnancy. Obstet Gynecol , 78, 925-9. PMID: 1923230
Enders M, Biber M & Exler S. (2007). [Measles, mumps and rubella virus infection in pregnancy. Possible adverse effects on pregnant women, pregnancy outcome and the fetus]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz , 50, 1393-8. PMID: 17999132 DOI.
Ornoy A & Tenenbaum A. (2006). Pregnancy outcome following infections by coxsackie, echo, measles, mumps, hepatitis, polio and encephalitis viruses. Reprod. Toxicol. , 21, 446-57. PMID: 16480851 DOI.
Articles
Chiba ME, Saito M, Suzuki N, Honda Y & Yaegashi N. (2003). Measles infection in pregnancy. J. Infect. , 47, 40-4. PMID: 12850161
Search Pubmed
Search Pubmed: Measles Virus | rubeola | Congenital rubeola Infection
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Eurosurveillance is a European peer-reviewed scientific journal devoted to the epidemiology, surveillance, prevention and control of communicable diseases, with a focus on such topics that are of relevance to Europe.
- CDC Rubella (German Measles, Three-Day Measles) | About Rubella
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 27) Embryology Abnormal Development - Measles Virus. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Measles_Virus
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G