Maternal Immune: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
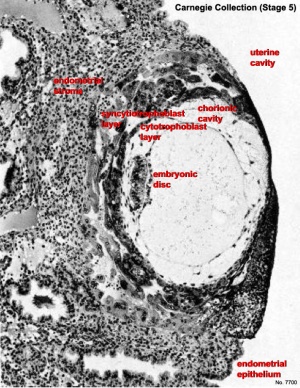
[[File:Stage5_bf11L.jpg|thumb|300px|alt=Image of human conceptus fully implanted|Human conceptus fully implanted ([[Carnegie_stage_5|Stage 5]]).]] | [[File:Stage5_bf11L.jpg|thumb|300px|alt=Image of human conceptus fully implanted|Human conceptus fully implanted ([[Carnegie_stage_5|Stage 5]]).]] | ||
[[File:Lymphocyte 02.jpg|thumb|300px|alt=Circulating large granular lymphocyte|Circulating large granular lymphocyte]] | [[File:Lymphocyte 02.jpg|thumb|300px|alt=Circulating large granular lymphocyte|Circulating large granular lymphocyte]] | ||
[[File:Implantation LIF.jpg|thumb|300px|alt=Uterine Leukemia Inhibitory Factor (LIF) Expression|Uterine Leukemia Inhibitory Factor (LIF) Expression | [[File:Implantation LIF.jpg|thumb|300px|alt=Uterine Leukemia Inhibitory Factor (LIF) Expression|Uterine Leukemia Inhibitory Factor (LIF) Expression{{#pmid:18046411|PMID18046411}}]] | ||
How does the implanting conceptus avoid immune rejection by the maternal immune system? There are a number of maternal and embryonic mechanisms that are thought to act to prevent immune rejection of the implanting conceptus, though the complete mechanism(s) are unknown. This is particularly relevant to [[Assisted_Reproductive_Technology|Assisted Reproductive Technologies]] involving donor eggs. | How does the implanting conceptus avoid immune rejection by the maternal immune system? There are a number of maternal and embryonic mechanisms that are thought to act to prevent immune rejection of the implanting conceptus, though the complete mechanism(s) are unknown. This is particularly relevant to [[Assisted_Reproductive_Technology|Assisted Reproductive Technologies]] involving donor eggs. | ||
Recent studies have also identified innate lymphoid cells (ILCs) are present in the uterus during the first trimester. | Recent studies have also identified innate lymphoid cells (ILCs) are present in the uterus during the first trimester.{{#pmid:25052762|PMID25052762}} Innate lymphoid cells form three main groups: ILC1, ILC2, and ILC3. The ILC1 group include natural killer (NK) cells and other interferon (IFN)γ-producing cells. | ||
developmentally related hematopoietic cells, which contribute to host immune defenses. | developmentally related hematopoietic cells, which contribute to host immune defenses. | ||
| Line 16: | Line 16: | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| | | | ||
* '''Where and when should natural killer cells be tested in women with repeated implantation failure?''' | |||
* '''Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface''' | * '''Review - An insight into the role of the death receptor CD95 throughout pregnancy: Guardian, facilitator, or foe'''{{#pmid:30702213|PMID30702213}} "The prototype death receptor CD95 (Fas) and its ligand, CD95L (FasL), have been thoroughly studied due to their role in immune homeostasis and elimination of infected and transformed cells. The fact that CD95 is present in female reproductive cells and modulated during embryogenesis and pregnancy has raised interest in its role in immune tolerance to the fetoplacental unit. CD95 has been shown to be critical for proper embryonic formation and survival. Moreover, altered expression of CD95 or its ligand causes autoimmunity and has also been directly involved in recurrent pregnancy losses and pregnancy disorders. The objective of this review is to summarize studies that evaluate the mechanisms involved in the activation of CD95 to provide an updated global view of its effect on the regulation of the maternal immune system. Modulation of the CD95 system components may be the immune basis of several common pregnancy disorders." | ||
* '''Where and when should natural killer cells be tested in women with repeated implantation failure?'''{{#pmid:25708533|PMID25708533}} "Thirty-two of the 73 patients were considered to have idiopathic repeated implantation failure (RIF), and 17 fertile women with children were taken as controls. Immunohistochemical staining for endometrial CD56+ and blood CD56+ or CD16+ NK cells measured using flow cytometry were compared during the mid-luteal phase in both patients and controls. Seventeen out of the 32 patients with idiopathic RIF and only one of the controls had >250 CD56 cells per high power field 400× in endometrial biopsy (p<0.001). The percentage of blood NK cells out of the total lymphocyte population was higher in women with idiopathic RIF than in controls. There was a positive correlation between blood and endometrial CD56 cells (ρ=0.707; p<0.001). No significant differences were found between patients with other types of RIF and controls. This study suggested that testing for NK cells might be useful in women with idiopathic RIF during the mid-luteal phase." | |||
* '''Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface'''{{#pmid:22679098|PMID22679098}} "The chemokine-mediated recruitment of effector T cells to sites of inflammation is a central feature of the immune response. The extent to which chemokine expression levels are limited by the intrinsic developmental characteristics of a tissue has remained unexplored. We show in mice that effector T cells cannot accumulate within the decidua, the specialized stromal tissue encapsulating the fetus and placenta. Impaired accumulation was in part attributable to the epigenetic silencing of key T cell-attracting inflammatory chemokine genes in decidual stromal cells, as evidenced by promoter accrual of repressive histone marks. These findings give insight into mechanisms of fetomaternal immune tolerance, as well as reveal the epigenetic modification of tissue stromal cells as a modality for limiting effector T cell trafficking." | |||
|} | |} | ||
{| class="wikitable mw-collapsible mw-collapsed" | {| class="wikitable mw-collapsible mw-collapsed" | ||
| Line 26: | Line 29: | ||
| [[File:Mark_Hill.jpg|90px|left]] {{Most_Recent_Refs}} | | [[File:Mark_Hill.jpg|90px|left]] {{Most_Recent_Refs}} | ||
Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Maternal+Immune ''Maternal Immune''] | Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Maternal+Immune ''Maternal Immune''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Uterine+natural+killer ''Uterine natural killer''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=decidual+natural+killer+cells ''decidual natural killer cells''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=CD95+pregnancy ''CD95 pregnancy''] | ||
|} | |||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! Older papers | |||
|- | |||
| {{Older papers}} | |||
* '''Human Endometrial CD98 Is Essential for Blastocyst Adhesion'''{{#pmid:20976164|PMID20976164}} "These results indicate that CD98, a component of tetraspanin-enriched microdomains, appears to be an important determinant of human endometrial receptivity during the implantation window." [http://omim.org/entry/158070 OMIM CD98] | |||
* '''Implantation failure: molecular mechanisms and clinical treatment.'''{{#pmid:20729534|PMID20729534}} "Implantation is a complex initial step in the establishment of a successful pregnancy. Although embryo quality is an important determinant of implantation, temporally coordinated differentiation of endometrial cells to attain uterine receptivity and a synchronized dialog between maternal and embryonic tissues are crucial. The exact mechanism of implantation failure is still poorly understood." | |||
|} | |} | ||
| Line 56: | Line 59: | ||
* Killer Inhibitory Receptor (KIR) activation by fetal HLA-C (expressed on extravillous trophoblast cells) | * Killer Inhibitory Receptor (KIR) activation by fetal HLA-C (expressed on extravillous trophoblast cells) | ||
|} | |} | ||
==Neutrophils== | |||
* second trimester decidual leukocytes{{#pmid:25135830|PMID25135830}} | |||
** expressed neutrophil activation markers and angiogenesis-related proteins - vascular endothelial growth factor-A, Arginase-1, and CCL2, similarly shown in tumor-associated neutrophils. | |||
==Chemokine Gene Silencing== | ==Chemokine Gene Silencing== | ||
:''Remove the attraction of maternal immune cells.'' | :''Remove the attraction of maternal immune cells.'' | ||
A mouse study | A mouse study{{#pmid:22679098|PMID22679098}} has shown that the normal immune response to inflammation, accumulation of effector T cells in response to chemokine secretion does not occur during implantation. This is prevented locally by epigenetic silencing of chemokine expression in the decidual stromal cells. | ||
==Corticotropin-Releasing Hormone== | ==Corticotropin-Releasing Hormone== | ||
:''Kill the maternal immune cells.'' | :''Kill the maternal immune cells.'' | ||
Both maternal and implanting conceptus release CRH at the embryo implantation site. This hormone then binds to receptors on the surface of trophoblast (extravillous trophoblast) cells leading to expression of a protein (Fas ligand, FasL) that activates the extrinsic cell death pathway on any local maternal immune cells ( T and B lymphocytes, natural killer cells, monocytes and macrophages). | Both maternal and implanting conceptus release CRH at the embryo implantation site. This hormone then binds to receptors on the surface of trophoblast (extravillous trophoblast) cells leading to expression of a protein (Fas ligand, FasL) that activates the extrinsic cell death pathway on any local maternal immune cells ( T and B lymphocytes, natural killer cells, monocytes and macrophages).{{#pmid:11590404|PMID11590404}} This cannot be the only mechanism, as mice with dysfunctional FasL proteins are still fertile. | ||
| Line 71: | Line 78: | ||
== Uterine Natural Killer Cells== | == Uterine Natural Killer Cells== | ||
Uterine Natural Killer or decidual NK (dNK) function to establishment of an immunosuppressive environment in comparison to circulating Natural Killer cells. | Uterine Natural Killer (uNK) or decidual NK (dNK) function to establishment of an immunosuppressive environment in comparison to circulating Natural Killer cells. There is a concentration of uNK cells in decidual tissue close to the invading conceptus trophoblast calls. | ||
Circulating Natural Killer (NK) cells form part of the normal immune defence: | Circulating Natural Killer (NK) cells form part of the normal immune defence: | ||
| Line 79: | Line 86: | ||
## inhibitory receptors - killer Ig-like receptors (KIRs) and CD94/NKG2A). | ## inhibitory receptors - killer Ig-like receptors (KIRs) and CD94/NKG2A). | ||
## activating receptors - (natural cytotoxicity receptors) NKp46, NKp30, and NKp44. | ## activating receptors - (natural cytotoxicity receptors) NKp46, NKp30, and NKp44. | ||
* express large amounts of CD56 (different from peripheral blood NK cells) CD56<sup>bright</sup> | |||
* secrete angiogenic factors vascular endothelial growth factor (VEGF), placental growth factor, angiopoietin-2, matrix metalloproteinases and interferon γ | |||
===CD56=== | |||
* Neural cell adhesion molecule (NCAM) | |||
* adhesion glycoprotein with five extracellular immunoglobulin-like domains followed by two fibronectin type III repeats | |||
* some cells with the T cell markers CD3 and CD4 also express CD56. | |||
Antibodies | |||
* mouse monoclonal primary anti-CD56 antibody (NCL-CD56-504; Novacastra Laboratories, Milton Keynes, UK) | |||
* rabbit polyclonal primary anti-CD56 antibody ([http://www.cellsignal.com/product/productDetail.jsp?productId=3606 Antibody #3606]; Cell Signaling Technology) | |||
* [http://www.antibodies-online.com/search.php#vred antibodies-online] | |||
==Implantation Factors== | ==Implantation Factors== | ||
[[File:Implantation cartoon 01.jpg|400px]] [[File:Implantation cartoon 02.jpg|400px]] | [[File:Implantation cartoon 01.jpg|400px]] [[File:Implantation cartoon 02.jpg|400px]] | ||
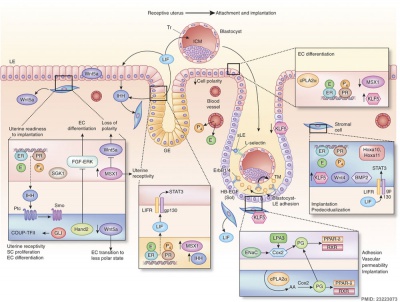
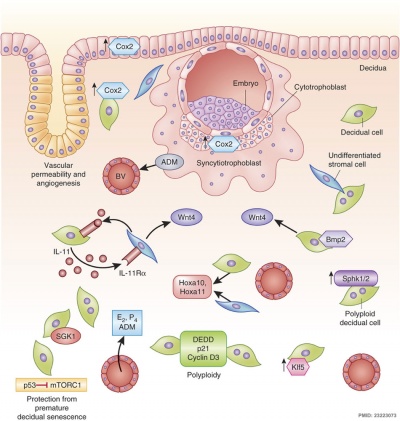
Molecular Implantation and Decidualization | Molecular Implantation and Decidualization{{#pmid:23223073|PMID23223073}} | ||
===Cytokines=== | ===Cytokines=== | ||
In mice, endometrial secretion of two IL-6 family cytokines, leukemia inhibitory factor (LIF) and Interleukin-11 (IL-11), are key requirements for implantation. A recent human study suggests that there is a similar requirement for human conceptus implantation. | In mice, endometrial secretion of two IL-6 family cytokines, leukemia inhibitory factor (LIF) and Interleukin-11 (IL-11), are key requirements for implantation. A recent human study suggests that there is a similar requirement for human conceptus implantation.{{#pmid:19213836|PMID19213836}} | ||
Uterine Leukemia Inhibitory Factor (LIF) Expression | Uterine Leukemia Inhibitory Factor (LIF) Expression{{#pmid:18046411|PMID18046411}} | ||
[[File:Implantation LIF.jpg|700px]] | [[File:Implantation LIF.jpg|700px]] | ||
| Line 102: | Line 124: | ||
===Reviews=== | ===Reviews=== | ||
{{#pmid:25713505}} | |||
{{#pmid:25342175}} | |||
===Articles=== | ===Articles=== | ||
{{#pmid:25310696}} | |||
{{#pmid:25163504}} | |||
{{#pmid:25135830}} | |||
{{#pmid:25066422}} | |||
{{#pmid:24954221}} | |||
{{#pmid:24954224}} | |||
===Search PubMed=== | ===Search PubMed=== | ||
Latest revision as of 11:24, 2 February 2020
| Embryology - 5 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
How does the implanting conceptus avoid immune rejection by the maternal immune system? There are a number of maternal and embryonic mechanisms that are thought to act to prevent immune rejection of the implanting conceptus, though the complete mechanism(s) are unknown. This is particularly relevant to Assisted Reproductive Technologies involving donor eggs.
Recent studies have also identified innate lymphoid cells (ILCs) are present in the uterus during the first trimester.[2] Innate lymphoid cells form three main groups: ILC1, ILC2, and ILC3. The ILC1 group include natural killer (NK) cells and other interferon (IFN)γ-producing cells.
developmentally related hematopoietic cells, which contribute to host immune defenses.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Maternal Immune | Uterine natural killer | decidual natural killer cells | CD95 pregnancy |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Decidual Immune Cells
| Decidual Macrophages (Mϕ) | Decidual T cells | Uterine Natural Killer cells |
|---|---|---|
|
|
|
Neutrophils
- second trimester decidual leukocytes[8]
- expressed neutrophil activation markers and angiogenesis-related proteins - vascular endothelial growth factor-A, Arginase-1, and CCL2, similarly shown in tumor-associated neutrophils.
Chemokine Gene Silencing
- Remove the attraction of maternal immune cells.
A mouse study[5] has shown that the normal immune response to inflammation, accumulation of effector T cells in response to chemokine secretion does not occur during implantation. This is prevented locally by epigenetic silencing of chemokine expression in the decidual stromal cells.
Corticotropin-Releasing Hormone
- Kill the maternal immune cells.
Both maternal and implanting conceptus release CRH at the embryo implantation site. This hormone then binds to receptors on the surface of trophoblast (extravillous trophoblast) cells leading to expression of a protein (Fas ligand, FasL) that activates the extrinsic cell death pathway on any local maternal immune cells ( T and B lymphocytes, natural killer cells, monocytes and macrophages).[9] This cannot be the only mechanism, as mice with dysfunctional FasL proteins are still fertile.
- Links: Implantation
Uterine Natural Killer Cells
Uterine Natural Killer (uNK) or decidual NK (dNK) function to establishment of an immunosuppressive environment in comparison to circulating Natural Killer cells. There is a concentration of uNK cells in decidual tissue close to the invading conceptus trophoblast calls.
Circulating Natural Killer (NK) cells form part of the normal immune defence:
- have the ability to kill virus-infected cells and tumor cells
- release cytokines and chemokines involved in early inflammatory responses.
- regulated by inhibitory receptors and activating receptors
- inhibitory receptors - killer Ig-like receptors (KIRs) and CD94/NKG2A).
- activating receptors - (natural cytotoxicity receptors) NKp46, NKp30, and NKp44.
- express large amounts of CD56 (different from peripheral blood NK cells) CD56bright
- secrete angiogenic factors vascular endothelial growth factor (VEGF), placental growth factor, angiopoietin-2, matrix metalloproteinases and interferon γ
CD56
- Neural cell adhesion molecule (NCAM)
- adhesion glycoprotein with five extracellular immunoglobulin-like domains followed by two fibronectin type III repeats
- some cells with the T cell markers CD3 and CD4 also express CD56.
Antibodies
- mouse monoclonal primary anti-CD56 antibody (NCL-CD56-504; Novacastra Laboratories, Milton Keynes, UK)
- rabbit polyclonal primary anti-CD56 antibody (Antibody #3606; Cell Signaling Technology)
- antibodies-online
Implantation Factors
Molecular Implantation and Decidualization[10]
Cytokines
In mice, endometrial secretion of two IL-6 family cytokines, leukemia inhibitory factor (LIF) and Interleukin-11 (IL-11), are key requirements for implantation. A recent human study suggests that there is a similar requirement for human conceptus implantation.[11]
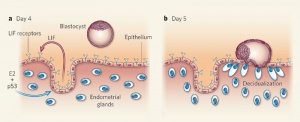
Uterine Leukemia Inhibitory Factor (LIF) Expression[1]
| a - At day 4 of pregnancy, oestrogen E2 induces LIF expression in the endometrial glands, leading to LIF secretion into the uterine lumen. There, LIF binds to its receptors on the surface of epithelial cells. | b - This makes the uterus receptive to the blastocyst, which implants by day 5 of pregnancy. Hu et al. find that LIF expression in the endometrial glands also depends on the regulatory activity of p53. In the absence of p53, insufficient LIF is produced, the uterus does not become adequately receptive, and fewer blastocysts implant. |
References
- ↑ 1.0 1.1 Hu W, Feng Z, Teresky AK & Levine AJ. (2007). p53 regulates maternal reproduction through LIF. Nature , 450, 721-4. PMID: 18046411 DOI.
- ↑ Vacca P, Montaldo E, Croxatto D, Loiacono F, Canegallo F, Venturini PL, Moretta L & Mingari MC. (2015). Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol , 8, 254-64. PMID: 25052762 DOI.
- ↑ Sagrillo-Fagundes L, Bienvenue-Pariseault J, Legembre P & Vaillancourt C. (2019). An insight into the role of the death receptor CD95 throughout pregnancy: Guardian, facilitator, or foe. Birth Defects Res , 111, 197-211. PMID: 30702213 DOI.
- ↑ Santillán I, Lozano I, Illán J, Verdú V, Coca S, Bajo-Arenas JM & Martinez F. (2015). Where and when should natural killer cells be tested in women with repeated implantation failure?. J. Reprod. Immunol. , 108, 142-8. PMID: 25708533 DOI.
- ↑ 5.0 5.1 Nancy P, Tagliani E, Tay CS, Asp P, Levy DE & Erlebacher A. (2012). Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science , 336, 1317-21. PMID: 22679098 DOI.
- ↑ Domínguez F, Simón C, Quiñonero A, Ramírez MÁ, González-Muñoz E, Burghardt H, Cervero A, Martínez S, Pellicer A, Palacín M, Sánchez-Madrid F & Yáñez-Mó M. (2010). Human endometrial CD98 is essential for blastocyst adhesion. PLoS ONE , 5, e13380. PMID: 20976164 DOI.
- ↑ Cakmak H & Taylor HS. (2011). Implantation failure: molecular mechanisms and clinical treatment. Hum. Reprod. Update , 17, 242-53. PMID: 20729534 DOI.
- ↑ Amsalem H, Kwan M, Hazan A, Zhang J, Jones RL, Whittle W, Kingdom JC, Croy BA, Lye SJ & Dunk CE. (2014). Identification of a novel neutrophil population: proangiogenic granulocytes in second-trimester human decidua. J. Immunol. , 193, 3070-9. PMID: 25135830 DOI.
- ↑ Makrigiannakis A, Zoumakis E, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G, Rice KC, Gravanis A & Chrousos GP. (2001). Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat. Immunol. , 2, 1018-24. PMID: 11590404 DOI.
- ↑ Cha J, Sun X & Dey SK. (2012). Mechanisms of implantation: strategies for successful pregnancy. Nat. Med. , 18, 1754-67. PMID: 23223073 DOI.
- ↑ Marwood M, Visser K, Salamonsen LA & Dimitriadis E. (2009). Interleukin-11 and leukemia inhibitory factor regulate the adhesion of endometrial epithelial cells: implications in fertility regulation. Endocrinology , 150, 2915-23. PMID: 19213836 DOI.
Reviews
Lee SK, Kim CJ, Kim DJ & Kang JH. (2015). Immune cells in the female reproductive tract. Immune Netw , 15, 16-26. PMID: 25713505 DOI.
Rätsep MT, Felker AM, Kay VR, Tolusso L, Hofmann AP & Croy BA. (2015). Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction , 149, R91-102. PMID: 25342175 DOI.
Articles
Gong X, Liu Y, Chen Z, Xu C, Lu Q & Jin Z. (2014). Insights into the paracrine effects of uterine natural killer cells. Mol Med Rep , 10, 2851-60. PMID: 25310696 DOI.
Le Bouteiller P. (2015). HLA-G in human early pregnancy: control of uterine immune cell activation and likely vascular remodeling. Biomed J , 38, 32-8. PMID: 25163504 DOI.
Amsalem H, Kwan M, Hazan A, Zhang J, Jones RL, Whittle W, Kingdom JC, Croy BA, Lye SJ & Dunk CE. (2014). Identification of a novel neutrophil population: proangiogenic granulocytes in second-trimester human decidua. J. Immunol. , 193, 3070-9. PMID: 25135830 DOI.
Lima PD, Zhang J, Dunk C, Lye SJ & Croy BA. (2014). Leukocyte driven-decidual angiogenesis in early pregnancy. Cell. Mol. Immunol. , 11, 522-37. PMID: 25066422 DOI.
Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN & Arenas-Hernandez M. (2014). Immune cells in term and preterm labor. Cell. Mol. Immunol. , 11, 571-81. PMID: 24954221 DOI.
Leno-Durán E, Muñoz-Fernández R, Olivares EG & Tirado-González I. (2014). Liaison between natural killer cells and dendritic cells in human gestation. Cell. Mol. Immunol. , 11, 449-55. PMID: 24954224 DOI.
Search PubMed
Search Pubmed: Embryo Adplantation | Embryo Implantation | tubal pregnancy | Endometrial Receptivity | Placenta Abnormalities | Pinopods | decidualization
Embryo Week: Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9
- Carnegie Stages: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | About Stages | Timeline
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, May 5) Embryology Maternal Immune. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Maternal_Immune
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G