Cat Development: Difference between revisions
No edit summary |
mNo edit summary |
||
| (63 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Header}} | ||
==Introduction== | |||
[[File:Max Cat.jpg|right|300px]] | |||
[[File:Cat_6_toes.jpg|thumb|Cat with 6 toes]] | |||
Cats (''Felis catus'') are seasonally polyestrous animals that have multiple estrous cycles only during certain periods of the year. | |||
The cat genome was initially sequenced in 2007{{#pmid:17975172|PMID17975172}} and has been recently annotated in August 2014.{{#pmid:25143822|PMID25143822}} | |||
{{Cat Links}} | |||
<br><br> | |||
{{Animals}} | |||
==Some Recent Findings== | |||
{| | |||
|-bgcolor="F5FAFF" | |||
| | |||
* '''Development of urinary organs in domestic cat during the embryonic and fetal periods'''{{#pmid:30341968|PMID30341968}} "The embryonic origin of the urogenital system came from the intermediate mesoderm. Kidney development involves three successive renal systems with a fast chronological overlap: the pronephro, the mesonephro, and the metanephro. Due to the lack of specific knowledge about this system in cats the present work aimed to describe their urinary organs development, focusing on the structures seen in pronephro, mesonephro, and metanephro during the embryonic and fetal stages of development. The techniques used in this study were: light microscopy, immunohistochemistry, scanning electron microscopy, and transmission electron microscopy. For that, embryos and fetuses from 12 pregnant mixed-breed domestic cats in different gestational stages were used to describe the proposed organs. The pronephro is present at early stages of embryonary development in embryos from 15 to 19 days with the presence of pronephro's corpuscles, ducts and tubules. The mesonephro is found, in general, between days 17 and 37, and contains mesonephric ducts, mesonephric tubules, and glomeruli. The metanephro is seen since 21 days of pregnancy with the presence of glomeruli, proximal and distal contorted tubules and at day 37, the cortex-medullary region is already differentiated. The evaluation of these structures enhances the knowledge about embryology of the urinary system in cats, aiding a better anatomical understanding of the system in the specie allowing the correlation with other species." {{renal}} | |||
* '''Assisted Reproduction in the Female Cat'''{{#pmid:29656770|PMID29656770}} "Assisted reproduction in the queen can range from simple ovulation induction to more advanced techniques such as in vitro fertilization. This article describes techniques available and the success associated with each." {{ART}} | |||
* '''Lipid Droplet Phase Transition in Freezing Cat Embryos and Oocytes Probed by Raman Spectroscopy'''{{#pmid:30099990|PMID30099990}} "Embryo and oocyte cryopreservation is a widely used technology for cryopreservation of genetic resources. One limitation of cryopreservation is the low tolerance to freezing observed for oocytes and embryos rich in lipid droplets. We apply Raman spectroscopy to investigate freezing of lipid droplets inside cumulus-oocyte complexes, mature oocytes, and early embryos of a domestic cat. Raman spectroscopy allows one to characterize the degree of lipid unsaturation, the lipid phase transition from the liquid-like disordered to solid-like ordered state, and the triglyceride polymorphic state. ...Raman spectroscopy is proved to be a promising tool for in situ monitoring of the lipid phase state in a single embryo/oocyte during its freezing." ([https://www.renishaw.com/en/a-basic-overview-of-raman-spectroscopy--25805 Raman spectroscopy] is a vibrational spectroscopic technique used to provide information on molecular vibrations and crystal structures.) | |||
* '''Follicular growth monitoring in the female cat during estrus'''{{#pmid:21798582|PMID21798582}} "This study was designed to describe follicular dynamics by transabdominal ultrasonography. Secondly, the stage of follicular growth was associated to behavioral and vaginal changes. Ovarian ultrasonography was performed during nine anovulatory and 12 ovulatory cycles. Forty-eight follicles were followed during anovulatory cycles: on the first day of estrus behavior, 4.8 ± 0.2 follicles (2 to 7 per female) of 2.3 ± 0.01 mm mean diameter were present. Follicular growth continued at a rate of 0.2 ± 0.04 mm per day. At least one follicle in the cohort reached a diameter greater than 3.0 mm." | |||
|} | |||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! More recent papers | |||
|- | |||
| [[File:Mark_Hill.jpg|90px|left]] {{Most_Recent_Refs}} | |||
Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Cat+Development ''Cat Development''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Cat+Embryology ''Cat Embryology''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Feline+Embryology ''Feline Embryology''] | |||
|} | |||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! Older papers | |||
|- | |||
| {{Older papers}} | |||
* '''Development of external genitalia in fetal and neonatal domestic cats'''{{#pmid:19262023|PMID19262023}} "The female urogenital folds budded from each side of the genital tubercle and, gradually extended to the tip of the genital tubercle by the 6.8 cm stage in crown-rump length. Then, the well-developed urogenital folds ensheathed completely the genital tubercle to form the prepuce of clitoris and the labia, flanking the external opening of vagina as the folds of skin which were equivalent to the labia minora in humans. The genital swellings known to become the labia majora in humans were clearly recognized in the caudolateral region of the genital tubercle during the fetal stage. These swellings became flat and obscure after birth. Thus, in cats the genital swellings did not join to the formation of the labia in the same way as in humans. The sex difference in the external genitalia was first observed at the 3.2-3.3 cm stages. In the male, the anogenital raphe appeared and the caudal portion of the genital swellings moved and fused each other at the caudal region of the genital tubercle. In the female, both features were not easy to observe." {{genital}} | |||
|} | |||
==Developmental Timeline== | |||
[[File:Cat oocyte calcium concentration.jpg|thumb|Cat oocyte calcium concentration{{#pmid:20003339|PMID20003339}}]] | |||
Twenty-two stages have been described for the prenatal development of the domestic cat.{{#pmid:11841356|PMID11841356}} | |||
The following data on early development is based upon the time after copulation{{#pmid:7803616|PMID7803616}} | |||
oviduct embryo development | |||
* 64 hours - 1 to 4 cells (17 of 20; 85.0%) | |||
* 76 hours - 5 to 8 cells (18 of 28; 64.3% ) | |||
* 100 hours - 9 to 16 cells (14 of 24; 58.3%) | |||
* 124 hours - morulae (15 of 21; 71.4% ) | |||
uterine embryo development | |||
* 148 hours - compact morulae or early blastocysts | |||
* days 12-14 - implantation occurs | |||
See also this historic paper on early cat development. | |||
{{Hill1924 TOC}} | |||
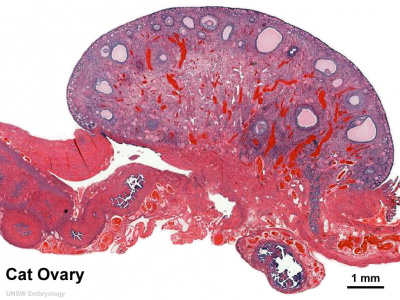
==Cat Ovary== | |||
[[File:Ovary-_histology_overview.jpg|400px]] | |||
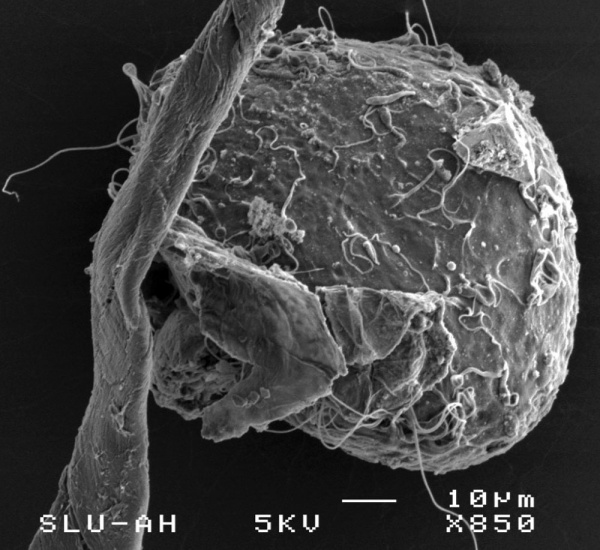
==Oocyte and Spermatozoa== | |||
The following scanning electron micrographs are from a recent paper on fresh and frozen cat oocytes.{{#pmid:17908298|PMID17908298}} Scale bar is 10 microns. | |||
[[File:Cat oocyte zona pellucida 01.jpg|300px]] [[File:Cat oocyte zona pellucida 02.jpg|300px]] | |||
[ | [[File:Cat_spermatozoa_bound_to_oocyte_zona_pellucida.jpg|600px]] | ||
=== | ==Genetics== | ||
'''Lineage:''' Eukaryota; Opisthokonta; Metazoa; Eumetazoa; Bilateria; Coelomata; Deuterostomia; Chordata; Craniata; Vertebrata; Gnathostomata; Teleostomi; Euteleostomi; Sarcopterygii; Tetrapoda; Amniota; Mammalia; Theria; Eutheria; Laurasiatheria; Carnivora; Feliformia; Felidae; Felinae; Felis; Felis catus | |||
The cat genome was initially sequenced in 2007{{#pmid:17975172|PMID17975172}} and has been recently annotated in August 2014.<ref name = PMIDGigaScience>Tamazian, G. etal., '''Annotated features of domestic cat - Felis cats genome.''' [http://www.gigasciencejournal.com/content/3/1/13/abstract# GigaScience] 2014, 3:13</ref> | |||
http://www. | |||
* Mitochondria - entire mitochondrial genome 17,009 bp has been sequenced. | |||
:'''Links:''' [http://www.ncbi.nlm.nih.gov/genome/78 Genome] [http://www.ncbi.nlm.nih.gov/nuccore/NC_001700 Mitochondrial Genome] | |||
==Renal Development== | |||
The time course data below is based on a recent cat study.{{#pmid:30341968|PMID30341968}} | |||
* 15 to 19 days - {{pronephros}} is present at early stages in embryos with the presence of pronephro's corpuscles, ducts and tubules. | |||
* 17 and 37 days - {{mesonephros}} contains mesonephric ducts, mesonephric tubules, and glomeruli. | |||
* 21 days - {{metanephros}} with the presence of glomeruli, proximal and distal contorted tubules | |||
* 37 days - cortex-medullary region is differentiated. | |||
:Links: {{renal}} | |||
==Placenta== | |||
* zonary placenta without cotyledons | |||
* relatively small marginal hematoma | |||
* materno-fetal barrier is endothelial-chorial | |||
* superficially invasive into the endometrium but not into the myometrium | |||
* placental labryrinth has characteristic giant cells | |||
===Placental cord=== | |||
* two pairs of vessels in the cord | |||
** two arteries and two veins | |||
* allantoic duct | |||
* cord average length 2 to 3 cm and 0.3 to 0.5 cm in diameter | |||
* inserts at the margin of the zonary organ | |||
* no spirals, no vitelline duct, and no additional vessels or structures | |||
:'''Links:''' [http://placentation.ucsd.edu/cat.html Comparative Placentation - Cat] | |||
==Additional Images== | |||
===Historic Images=== | |||
{{Historic Disclaimer}} | |||
<gallery> | |||
File:Mall Meyer1921 fig210.jpg|Fig. 210. Normal well-preserved cat fetus | |||
File:Mall_Meyer1921_fig211.jpg|Fig. 211. Normal poorly preserved cat fetus of approximately the same length | |||
File:Wislocki1920_plate_4.jpg|Plate 4. Cat Fetus and Placenta | |||
File:Bailey296 297.jpg|Fig. 297. Transverse section through the thoracic region of a cat embryo of 25 mm | |||
File:Bailey331.jpg|Fig. 331. Sections of a cat's ovary. | |||
File:Gray1112.jpg | |||
File:Gray0903.jpg|Fig. 903. Cat cochlear duct and ganglia | |||
</gallery> | |||
==References== | ==References== | ||
| Line 64: | Line 133: | ||
<references/> | <references/> | ||
===Reviews=== | |||
{{#pmid:28677231}} | |||
===Articles=== | |||
{{#pmid:32847086}} | |||
{{#pmid:30865874}} | |||
{{#pmid:30443927}} | |||
{{#pmid:19151510}} | |||
{{#pmid:19262023}} | |||
{{#pmid:18405438}} | |||
{{#pmid:12606460}} | |||
{{#pmid:11841356}} | |||
{{Ref-Hill1924}} | |||
===Search Pubmed=== | |||
[http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed&cmd=search&term=cat%20development cat development] | [http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed&cmd=search&term=feline%20development feline development] | |||
{{Animals}} | |||
{{ | {{Glossary}} | ||
{{ | {{Footer}} | ||
[[Category:Cat]] | [[Category:Cat]] [[Category:Estrous Cycle]] | ||
Latest revision as of 05:23, 28 August 2020
| Embryology - 30 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Cats (Felis catus) are seasonally polyestrous animals that have multiple estrous cycles only during certain periods of the year.
The cat genome was initially sequenced in 2007[1] and has been recently annotated in August 2014.[2]
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Cat Development | Cat Embryology | Feline Embryology |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Developmental Timeline
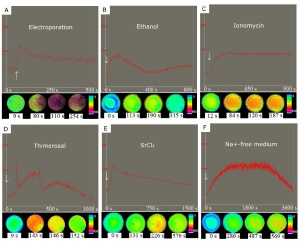
Twenty-two stages have been described for the prenatal development of the domestic cat.[9]
The following data on early development is based upon the time after copulation[10]
oviduct embryo development
- 64 hours - 1 to 4 cells (17 of 20; 85.0%)
- 76 hours - 5 to 8 cells (18 of 28; 64.3% )
- 100 hours - 9 to 16 cells (14 of 24; 58.3%)
- 124 hours - morulae (15 of 21; 71.4% )
uterine embryo development
- 148 hours - compact morulae or early blastocysts
- days 12-14 - implantation occurs
See also this historic paper on early cat development.
- 1924 Cat Development: 1. Ovum of the Cat | 2. Process of Cleavage | 3. Formation of the Blastocyst | 4. Discussion | Plates | cat
Cat Ovary
Oocyte and Spermatozoa
The following scanning electron micrographs are from a recent paper on fresh and frozen cat oocytes.[11] Scale bar is 10 microns.
Genetics
Lineage: Eukaryota; Opisthokonta; Metazoa; Eumetazoa; Bilateria; Coelomata; Deuterostomia; Chordata; Craniata; Vertebrata; Gnathostomata; Teleostomi; Euteleostomi; Sarcopterygii; Tetrapoda; Amniota; Mammalia; Theria; Eutheria; Laurasiatheria; Carnivora; Feliformia; Felidae; Felinae; Felis; Felis catus
The cat genome was initially sequenced in 2007[1] and has been recently annotated in August 2014.[12]
- Mitochondria - entire mitochondrial genome 17,009 bp has been sequenced.
- Links: Genome Mitochondrial Genome
Renal Development
The time course data below is based on a recent cat study.[3]
- 15 to 19 days - pronephros is present at early stages in embryos with the presence of pronephro's corpuscles, ducts and tubules.
- 17 and 37 days - mesonephros contains mesonephric ducts, mesonephric tubules, and glomeruli.
- 21 days - metanephros with the presence of glomeruli, proximal and distal contorted tubules
- 37 days - cortex-medullary region is differentiated.
- Links: renal
Placenta
- zonary placenta without cotyledons
- relatively small marginal hematoma
- materno-fetal barrier is endothelial-chorial
- superficially invasive into the endometrium but not into the myometrium
- placental labryrinth has characteristic giant cells
Placental cord
- two pairs of vessels in the cord
- two arteries and two veins
- allantoic duct
- cord average length 2 to 3 cm and 0.3 to 0.5 cm in diameter
- inserts at the margin of the zonary organ
- no spirals, no vitelline duct, and no additional vessels or structures
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
References
- ↑ 1.0 1.1 Pontius JU, Mullikin JC, Smith DR, Lindblad-Toh K, Gnerre S, Clamp M, Chang J, Stephens R, Neelam B, Volfovsky N, Schäffer AA, Agarwala R, Narfström K, Murphy WJ, Giger U, Roca AL, Antunes A, Menotti-Raymond M, Yuhki N, Pecon-Slattery J, Johnson WE, Bourque G, Tesler G & O'Brien SJ. (2007). Initial sequence and comparative analysis of the cat genome. Genome Res. , 17, 1675-89. PMID: 17975172 DOI.
- ↑ Tamazian G, Simonov S, Dobrynin P, Makunin A, Logachev A, Komissarov A, Shevchenko A, Brukhin V, Cherkasov N, Svitin A, Koepfli KP, Pontius J, Driscoll CA, Blackistone K, Barr C, Goldman D, Antunes A, Quilez J, Lorente-Galdos B, Alkan C, Marques-Bonet T, Menotti-Raymond M, David VA, Narfström K & O'Brien SJ. (2014). Annotated features of domestic cat - Felis catus genome. Gigascience , 3, 13. PMID: 25143822 DOI.
- ↑ 3.0 3.1 Mario LC, Morais MP, Borghesi J, Favaron PO, Oliveira FD, Anunciação ARA, Agopian RG, Gomes SA & Miglino MA. (2018). Development of urinary organs in domestic cat during the embryonic and fetal periods. Microsc. Res. Tech. , , . PMID: 30341968 DOI.
- ↑ Johnson AK. (2018). Assisted Reproduction in the Female Cat. Vet. Clin. North Am. Small Anim. Pract. , 48, 523-531. PMID: 29656770 DOI.
- ↑ Okotrub KA, Mokrousova VI, Amstislavsky SY & Surovtsev NV. (2018). Lipid Droplet Phase Transition in Freezing Cat Embryos and Oocytes Probed by Raman Spectroscopy. Biophys. J. , 115, 577-587. PMID: 30099990 DOI.
- ↑ Malandain E, Rault D, Froment E, Baudon S, Desquilbet L, Begon D & Chastant-Maillard S. (2011). Follicular growth monitoring in the female cat during estrus. Theriogenology , 76, 1337-46. PMID: 21798582 DOI.
- ↑ Inomata T, Ariga M, Sakita K, Kashiwazaki N, Ito J, Yokoh K, Ichikawa M, Ninomiya H & Inoue S. (2009). Development of external genitalia in fetal and neonatal domestic cats. J. Vet. Med. Sci. , 71, 139-45. PMID: 19262023
- ↑ Wang C, Swanson WF, Herrick JR, Lee K & Machaty Z. (2009). Analysis of cat oocyte activation methods for the generation of feline disease models by nuclear transfer. Reprod. Biol. Endocrinol. , 7, 148. PMID: 20003339 DOI.
- ↑ Knospe C. (2002). Periods and stages of the prenatal development of the domestic cat. Anat Histol Embryol , 31, 37-51. PMID: 11841356
- ↑ Swanson WF, Roth TL & Wildt DE. (1994). In vivo embryogenesis, embryo migration, and embryonic mortality in the domestic cat. Biol. Reprod. , 51, 452-64. PMID: 7803616
- ↑ Hermansson U, Axnér E & Holst BS. (2007). Application of a zona pellucida binding assay (ZBA) in the domestic cat benefits from the use of in vitro matured oocytes. Acta Vet. Scand. , 49, 28. PMID: 17908298 DOI.
- ↑ Tamazian, G. etal., Annotated features of domestic cat - Felis cats genome. GigaScience 2014, 3:13
Reviews
Glatzle M, Hoops M, Kauffold J, Seeger J & Fietz SA. (2017). Development of Deep and Upper Neuronal Layers in the Domestic Cat, Sheep and Pig Neocortex. Anat Histol Embryol , 46, 397-404. PMID: 28677231 DOI.
Articles
Piras AR, Ariu F, Zedda MT, Paramio MT & Bogliolo L. (2020). Selection of Immature Cat Oocytes with Brilliant Cresyl Blue Stain Improves In Vitro Embryo Production during Non-Breeding Season. Animals (Basel) , 10, . PMID: 32847086 DOI.
Prozorowska E, Ratajczak M & Jackowiak H. (2019). Ultrastructural study of uterine epithelium in the domestic cat during prenatal development. Theriogenology , 130, 49-61. PMID: 30865874 DOI.
Prozorowska E, Jackowiak H & Skieresz-Szewczyk K. (2018). Morphology and topography of internal reproductive organs in the female cat during prenatal and postnatal development: Scanning electron microscope and three-dimensional reconstruction study. J. Morphol. , 279, 1764-1775. PMID: 30443927 DOI.
Inomata T, Ninomiya H, Sakita K, Kashiwazaki N, Ito J, Ariga M & Inoue S. (2009). Developmental changes of Müllerian and Wolffian ducts in domestic cat fetuses. Exp. Anim. , 58, 41-5. PMID: 19151510
Inomata T, Ariga M, Sakita K, Kashiwazaki N, Ito J, Yokoh K, Ichikawa M, Ninomiya H & Inoue S. (2009). Development of external genitalia in fetal and neonatal domestic cats. J. Vet. Med. Sci. , 71, 139-45. PMID: 19262023
Ciani F, Cocchia N, Rizzo M, Ponzio P, Tortora G, Avallone L & Lorizio R. (2008). Sex determining of cat embryo and some feline species. Zygote , 16, 169-77. PMID: 18405438 DOI.
França LR & Godinho CL. (2003). Testis morphometry, seminiferous epithelium cycle length, and daily sperm production in domestic cats (Felis catus). Biol. Reprod. , 68, 1554-61. PMID: 12606460 DOI.
Knospe C. (2002). Periods and stages of the prenatal development of the domestic cat. Anat Histol Embryol , 31, 37-51. PMID: 11841356
Hill JP. and Tribe M. The early development of the cat (Felis domestica). (1924) Quart. J. Microsc. Sci., 68: 513-602.
Search Pubmed
cat development | feline development
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 30) Embryology Cat Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cat_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G