Abnormal Development - Thalidomide: Difference between revisions
| Line 87: | Line 87: | ||
==Thalidomide Metabolism in Different Species== | ==Thalidomide Metabolism in Different Species== | ||
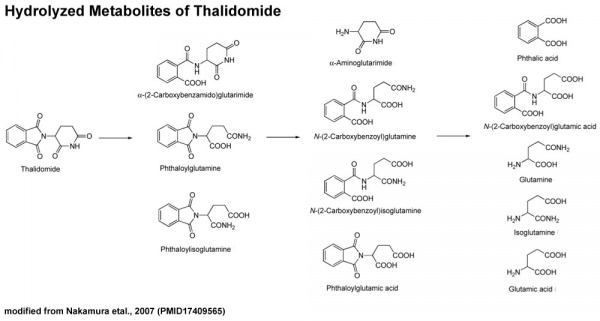
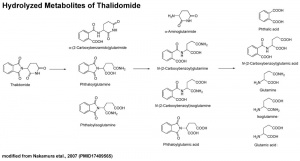
[[File:Thalidomide-_hydrolyzed_metabolites.jpg|thumb|Thalidomide hydrolyzed metabolites]] | |||
It also appears that not all species metabolise thalidomide the same, shown in a study comparing the metabolic breakdown products in mice and man:<ref name="PMID12738721"><pubmed>12738721</pubmed></ref> "Our results show that thalidomide metabolite profiles in multiple myeloma patients differ considerably from those in mice. The lack of measurable hydroxylated metabolites in urine and in 1 case plasma of these patients suggests that such metabolites are not responsible for the therapeutic effects of thalidomide in multiple myeloma." | It also appears that not all species metabolise thalidomide the same, shown in a study comparing the metabolic breakdown products in mice and man:<ref name="PMID12738721"><pubmed>12738721</pubmed></ref> "Our results show that thalidomide metabolite profiles in multiple myeloma patients differ considerably from those in mice. The lack of measurable hydroxylated metabolites in urine and in 1 case plasma of these patients suggests that such metabolites are not responsible for the therapeutic effects of thalidomide in multiple myeloma." | ||
Revision as of 08:33, 10 September 2010
Introduction
Thalidomide is a drug that was introduced on to the market on October 1, 1957 in West Germany. Thalidomide soon became a drug prescribed to pregnant women to combat symptoms associated with morning sickness. When taken during the first trimester of pregnancy, thalidomide prevented the proper growth of the fetus resulting in horrific birth defects in thousands of children around the world.
It was the linking of newborn abnormalities with the taking of thalidomide by an Australian clinician, William McBride, that identified it as a teratogenic agent causing a "thalidomide embryopathy"[1] and also reported independently in Germany by Widukind Lenz.[2] When taken, mainly in first world countries, between day 20 to 36 after fertilisation (34–50 days LMP) children were born with limb and other defects. In the late 1950's and early 1960's these children became known as "Thalidomide babies".
Not all species embryos are affected by the drug in the same way, with human and rabbit being most susceptible to the teratogenic effects. In addition, the effect on human development is also dependent upon the time and dose of the drug exposure, the "critical periods".
More recent research has shown a clinical revival for thalidomide in its use in non-pregnant women during cancer chemotherapy.[3][4]
- Related Links: Limb Development | Abnormalities | Lecture - Limb Development | Lecture - Musculoskeletal Development
Some Recent Findings
|
Thalidomide Physical and Chemical Properties
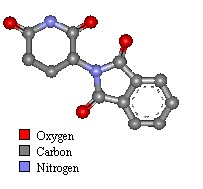
Thalidomide was first synthesised in 1954 by Wilhelm Kunz, a German drug discovery pharmacist for Chemie Grünenthal, searching for new organic compounds. The drug was manufactured as a two enantiomer isomer mix (laevo+ and dextro-). The the teratogenic form was laevo+ thalidomide.
- Molecular Mass: 258.23 Da
- Color: white crystalline
- Odor: Odorless
- Taste: taste-less
- Melting point: 271°C.
- Insoluble in ether and benzene
- Has a low solubility in water, methanol, ethanol and glacial acetic acid
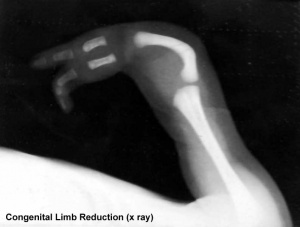
Limb Reduction
Text extract below from [8], note that all timings are using the clinical dates of Last Menstrual Period (LMP), which differ by about 14 days more to the developmental date from fertilization.
Thalidomide does not produce malformations if only taken before the 34th day after the last menstruation (LMP) and usually no malformation if taken only after the 50th day. Within the sensitive period from day 35 to day 49, there is the following sequence:
- 35th - 37th day Absence of the ears and deafness
- 39th - 41st day Absence of arms
- 43rd - 44th day Phocomelia with three fingers
- 46th - 48th day Thumbs with three joints
If thalidomide was taken throughout the sensitive period, the consequence may be severe defects of ears, arms and legs and of internal malformations, which often led to early death. About 40% of thalidomide victims died before their first birthday.
Cereblon Binding
Cereblon (CRBN) was named based on its possible role in cerebral development and the presence of a Lon protease domain. Functionally it had been previously identified as being associated with neural development. Thalidomide specifically binds cereblon, which inhibits the ubiquitin ligase activity of the SCF protein ligase complex, possibly leading to abnormal regulation of the BMP and FGF8 signaling pathways. The SCF complex (Skp, Cullin, F-box containing complex) is a multi-protein E3 ubiquitin ligase complex catalyzing the ubiquitination of proteins destined for proteasomal degradation.
- Links: OMIM - Cereblon | UniProt - CRBN
Signaling Pathway
Two possible mechanisms:[5]
- cereblon dependent process
- cereblon independent process (producing reactive oxygen species)[9]
Thalidomide shown to:
- inhibits production of some cytokines (tumor necrosis factor–alpha and vascular endothelial growth factor)
- inducing apoptosis and producing reactive oxygen species
- fgf8 is a downstream target
Vascular Effect
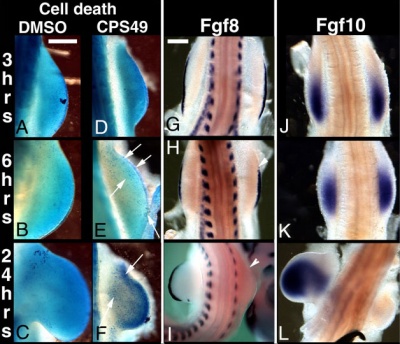
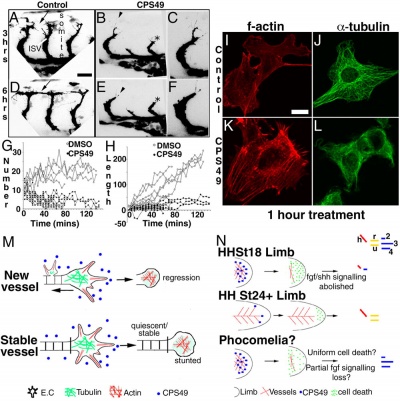
This recent study[7] has used a chicken limb model system and treatment with a chemical, CPS49 a tetrafluorinated analogue of thalidomide that is chemically and structurally related to thalidomide breakdown products.
|
|
| Chicken limb development signaling[7] | Thalidomide - CPS49 vascular effect on chicken model[7] |
The researchers identified an antiangiogenic activity, inhibition of blood vessel growth, of CPS49 within the developing limb and in a number of in vitro tissue culture models of vascular growth. The vessel loss was described as the "primary trigger" leading to an increased cell death and impairment in limb signaling pathways, resulting consequently in both limb outgrowth failure and limb truncations. The possibility that fibroblast growth factor levels are altered, may also fit in with the specific thalidomide binding to cereblon.
Primate Model
Crab-eating Macaque (Macaca fascicularis, Cynomolgus Monkey, Philippine Monkey, Long-tailed Macaque)[6] "Cynomolgus monkeys were orally administered thalidomide at 15 or 20mg/kg-d on days 26-28 of gestation, and fetuses were examined on day 100-102 of gestation. Limb defects such as micromelia/amelia, paw/foot hyperflexion, polydactyly, syndactyly, and brachydactyly were observed in seven of eight fetuses."
Thalidomide Metabolism in Different Species
It also appears that not all species metabolise thalidomide the same, shown in a study comparing the metabolic breakdown products in mice and man:[10] "Our results show that thalidomide metabolite profiles in multiple myeloma patients differ considerably from those in mice. The lack of measurable hydroxylated metabolites in urine and in 1 case plasma of these patients suggests that such metabolites are not responsible for the therapeutic effects of thalidomide in multiple myeloma."
Australia - Advisory Committee on Prescription Medicines
The Australian Drug Evaluation Committee (ADEC) was established in 1963 following the thalidomide experience and in 2010 this committee was replaced by the Advisory Committee on Prescription Medicines (ACPM). The new ACPM advises and makes recommendations to the Therapeutic Goods Administration (TGA) on prescription medicines listed on the Australian Register of Therapeutic Goods (ARTG), established under the Therapeutic Goods Act 1989. There were approximately 54,000 products on the Australian Register of Therapeutic Goods as at 23 May 2008.
Advisory Committee on Prescription Medicines
- inclusion of a prescription medicine on the Australian Register of Therapeutic Goods (the Register)
- changes to an entry of a prescription medicine on the Register
- removal or retention of a prescription medicine on the Register
Teratology
Now consider how different environmental effects during pregnancy may influence developmental outcomes. The terms listed below are often used to describe these environmental effects
- Teratogen (Greek, teraton = monster) any agent that causes a structural abnormality (congenital abnormalities) following fetal exposure during pregnancy. The overall effect depends on dosage and time of exposure. (More? Critical Periods of Development)
- Absolute risk the rate of occurrence of an abnormal phenotype among individuals exposed to the agent. (e.g. fetal alcohol syndrome)
- Relative risk the ratio of the rate of the condition among the exposed and the nonexposed. (e.g. smokers risk of having a low birth weight baby compared to non-smokers) A high relative risk may indicate a low absolute risk if the condition is rare.
- Mutagen a chemical or agent that can cause permanent damage to the deoxyribonucleic acid (DNA) in a cell. DNA damage in the human egg or sperm may lead to reduced fertility, spontaneous abortion (miscarriage), birth defects and heritable diseases.
- Fetotoxicant is a chemical that adversely affects the developing fetus, resulting in low birth weight, symptoms of poisoning at birth or stillbirth (fetus dies before it is born).
- Synergism when the combined effect of exposure to more than one chemical at one time, or to a chemical in combination with other hazards (heat, radiation, infection) results in effects of such exposure to be greater than the sum of the individual effects of each hazard by itself.
- Toxicogenomics the interaction between the genome, chemicals in the environment, and disease. Cells exposed to a stress, drug or toxicant respond by altering the pattern of expression of genes within their chromosomes. Based on new genetic and microarray technologies.
References
- ↑ <pubmed>331548</pubmed>
- ↑ <pubmed>14464040</pubmed>
- ↑ <pubmed>16505120</pubmed>
- ↑ <pubmed>17453375</pubmed>
- ↑ 5.0 5.1 <pubmed>20223979 </pubmed>
- ↑ 6.0 6.1 <pubmed>19751816</pubmed>
- ↑ 7.0 7.1 7.2 7.3 <pubmed>19433787</pubmed>
- ↑ W. Lenz The History of Thalidomide a lecture given at the 1992 UNITH Congress.
- ↑ <pubmed>18418038</pubmed>
- ↑ <pubmed>12738721</pubmed>
Reviews
<pubmed>2228814</pubmed> <pubmed>3067416</pubmed> <pubmed>3533365</pubmed> <pubmed>6991189</pubmed>
Articles
<pubmed>17213509</pubmed>"The Thalidomide Victims Association of Canada (TVAC) was founded in 1988 and is the only organization in North America to work with and for Thalidomide victims. Our mission is to empower our members and to improve their quality of life through various programs and customized services. With the return of Thalidomide on the market, TVAC also took on the mandate of informing the public on the devastating effects of this medication and to promote awareness and caution when using any teratogenic products currently available".
<pubmed>1290446</pubmed>
Books on Thalidomide
A selection of recent general public information books on Thalidomide, available from various internet commercial suppliers (search using the book title). Please note that this listing does not reflect an endorsement of the book or its content and is provided for educational purposes only.
- Thalidomide Kid (Paperback) by Kate, Rigby (Author)
- Thalidomide - A Medical Dictionary, Bibliography, and Annotated Research Guide to Internet References (Paperback) by ICON Health Publications (Author) Internet supplier link: Amazon
Search Pubmed
June 2010 "thalidomide teratogenicity" All (147) Review (57) Free Full Text (11)
Search Pubmed: thalidomide teratogenicity | Thalidomide | McBride WG |
External Links
- OMIM - CEREBLON
- Medline Plus - Thalidomide
- USA FDA - Thalidomide
- Thalidomide Victims Association of Canada
- Thalidomide Isomerism and Optical Isomerism
- United Nations International Drug Control Programme
- Australian Drug Foundation (ADF)
- Centre for Education and Information on Drugs and Alcohol (CEIDA) (Australia)
- Child Health and Safety (Australia)
- NIDA (USA)- Consequences of Prenatal Drug Exposure
- Australian Medicines Handbook (no electronic version yet)
- Australian Congenital Anomalies Monitoring System (ACAMS)
- Australian Institute of Health and Welfare (AIHW)
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, May 7) Embryology Abnormal Development - Thalidomide. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Thalidomide
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G