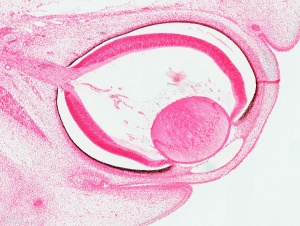
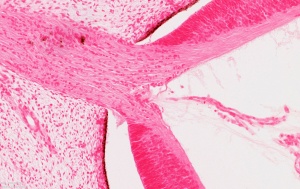
Vision - Retina Development
| Embryology - 6 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
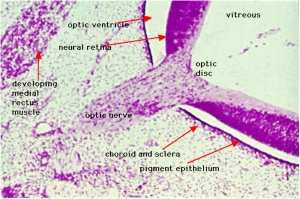
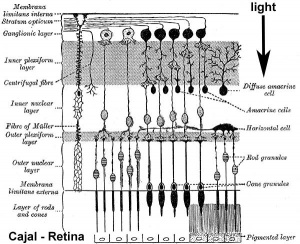
The retina and its development has been the subject of research from the early histology studies of Santiago Ramón y Cajal (1852 - 1934) and Camillo Golgi (1843 - 1926). It has a complex differentiation involving both a neural retina and a pigmented retina, in the embryo separated by a space and in the adult closely apposed to each other.
The retina is also a key focus of clinical research, as genetic or environmental damage and vision loss is generally a result of damage to the retinal cells or their processes. Retinitis pigments is a degenerative retinal disease. Retinoblastoma is retinal cancer that allowed researchers to identify retinoblastoma protein (Rb), the first identified tumour suppressor protein acting as a G1 cell cycle checkpoint.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Retina Embryology <pubmed limit=5>Retina Embryology</pubmed> |
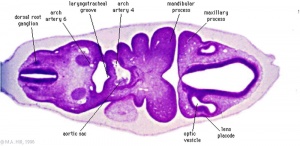
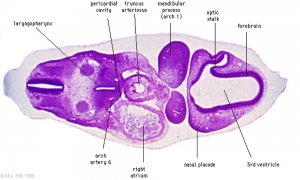
Timeline
| Carnegie Stage | Event | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | optic primordia appear. | ||||||||||||||||||
| 11 | Right and left optic primordia meet at the optic chiasma forming a U-shaped rim. | ||||||||||||||||||
| 12 | optic neural crest reaches its maximum extent and the optic vesicle becomes covered by a complete sheath, | ||||||||||||||||||
| 13 | By the end of the fourth week the optic vesicle lies close to the surface ectoderm. Optic evagination differentiation allows identification of optic part of retina, future pigmented layer of retina, and optic stalk. The surface ectoderm overlying the optic vesicle, in response to this contact, has thickened to form the lense placode. | ||||||||||||||||||
| 14 | (about 32 days) Lens placode is indented by the lens pit, cup-shaped and still communicates with the surface by a narrowing pore. | ||||||||||||||||||
| 15 | (about 33 days) Lens pit is closed. The lens vesicle and optic cup lie close to the surface ectoderm and appear to press against the surface. | ||||||||||||||||||
| 16 | (37 days) Growth of the lens body results in a D-shaped lens cavity. Perilental blood vessels (tunica vasculosa lentis) are visible. Prior to the development of the eyelids, one small sulcus or groove forms above the eye (eyelid groove) and another below it. | ||||||||||||||||||
| 17 - 19 | Retinal pigment is visible and the retinal fissure is largely closed. Eyelids grooves deepen, eyelid folds develop, first below, and then above, the eye. | ||||||||||||||||||
| 18 | Mesenchyme invades the region between the lens epithelium and the surface ectoderm. | ||||||||||||||||||
| 19 - 22 | Eyelid folds develop into the eyelids and cover more of the eye as the palpebral fissure takes shape. The upper and the lower eyelids meet at the outer canthus in Stage 19. | ||||||||||||||||||
| 20 | Lens cavity is lost and a lens suture begins to form. The inner canthus is established. | ||||||||||||||||||
| 23 | retina comprises the pigmented layer, external limiting membrane, proliferative zone, external neuroblastic layer, transient fiber layer, internal neuroblastic layer, nerve fiber layer, and internal limiting membrane. Eyelids closure is complete (Note - shown as still open in the Kyoto embryo). | ||||||||||||||||||
Data from a study of human embryonic carnegie stages[5] and other sources.
| |||||||||||||||||||
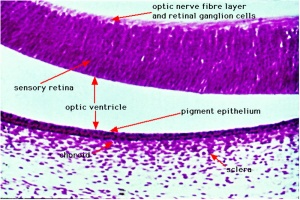
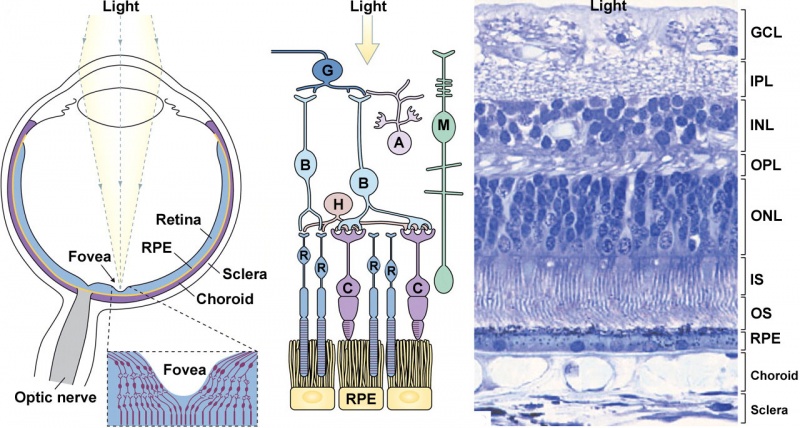
Adult Retina
Vertebrates have ten identifiable layers formed from neurons, their processes (nerve fibers), membranes, photoreceptors and pigmented cells. Light must pass through nearly all these layers to the photoreceptors.
- Inner limiting membrane - Müller cell footplates.
- Nerve fiber layer - retinal ganglion axons eventually the optic nerve.
- Ganglion cell layer - neuronal cell bodies of retinal ganglion cells, their axons form the nerve fiber layer and eventually the optic nerve.
- Inner plexiform layer - another layer of neuronal processes.
- Inner nuclear layer - neuronal cell bodies
- Outer plexiform layer - another layer of neuronal processes.
- Outer nuclear layer - neuronal cell bodies
- External limiting membrane - layer separating inner segment portions of photoreceptors from their cell nuclei.
- Photoreceptor layer - rods and cones that convert light into signals.
- Retinal pigment epithelium.
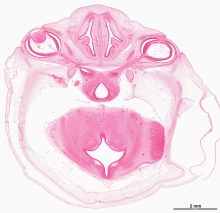
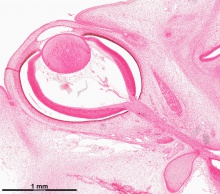
Week 5
Week 8
The images below link to virtual slides of the human developing eye at Carnegie stage 22. Click on the image to open or select specific regions from the regions of interest links.
|
|
Virtual Slide - Regions of Interest
|
Links: Stage 22 | Embryo Virtual Slides
Retinal Pigment Epithelium
Retinal pigment epithelium (RPE) cells are generated directly from the optic neuroepithelium. The choroidal melanocytes, the other pigmented cells, are derived from neural crest cells that have migrated towards the eye.
Retinal pigment epithelium cells:
- cuboidal cells
- apical side form multiple villi
- these villi are in direct contact with the outer segments of the photoreceptor cells
- lateral sides are joined together by tight, adherens and gap junctions
- basal side contacts the underlying basal membrane (Bruch’s membrane)
Ciliary Body
A proposed model of ciliary body development begins with specification at the optic vesicle stage, when the neural retina and pigmented epithelium are also specified. The molecular signals could involve overlapping BMP and FGF signals. The lens has recently been shown in the chicken model to not be required for specification of the iris and ciliary body.[6]
References
- ↑ Chen J, Ma L, Wang S, Wang X, Sun Y, Gao L, Li J & Zhou G. (2017). Analysis of expression of transcription factors in early human retina. Int. J. Dev. Neurosci. , 60, 94-102. PMID: 28377129 DOI.
- ↑ Hendrickson A. (2016). Development of Retinal Layers in Prenatal Human Retina. Am. J. Ophthalmol. , 161, 29-35.e1. PMID: 26410132 DOI.
- ↑ Kwan KM, Otsuna H, Kidokoro H, Carney KR, Saijoh Y & Chien CB. (2012). A complex choreography of cell movements shapes the vertebrate eye. Development , 139, 359-72. PMID: 22186726 DOI.
- ↑ Zhang Q & Eisenstat DD. (2012). Roles of homeobox genes in retinal ganglion cell differentiation and axonal guidance. Adv. Exp. Med. Biol. , 723, 685-91. PMID: 22183394 DOI.
- ↑ Pearson AA. (1980). The development of the eyelids. Part I. External features. J. Anat. , 130, 33-42. PMID: 7364662
- ↑ Dias da Silva MR, Tiffin N, Mima T, Mikawa T & Hyer J. (2007). FGF-mediated induction of ciliary body tissue in the chick eye. Dev. Biol. , 304, 272-85. PMID: 17275804 DOI.
Online Textbooks
- Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System [Internet]. Salt Lake City (UT): University of Utah Health Sciences Center; 1995-. Retinal Neurogenesis: Early stages in the Development of Neurons and Pathways. Available from: https://www.ncbi.nlm.nih.gov/books/NBK52777/
Reviews
- The International Journal of Developmental Biology (2004) Eye Development
{{#pmid: Andreazzoli M. (2009). Molecular regulation of vertebrate retina cell fate. Birth Defects Res. C Embryo Today , 87, 284-95. PMID: 19750521 DOI.
Cvekl A & Wang WL. (2009). Retinoic acid signaling in mammalian eye development. Exp. Eye Res. , 89, 280-91. PMID: 19427305 DOI.
Galli-Resta L, Leone P, Bottari D, Ensini M, Rigosi E & Novelli E. (2008). The genesis of retinal architecture: an emerging role for mechanical interactions?. Prog Retin Eye Res , 27, 260-83. PMID: 18374618 DOI.
Taylor D. (2007). Developmental abnormalities of the optic nerve and chiasm. Eye (Lond) , 21, 1271-84. PMID: 17914430 DOI.
Finlay BL. (2008). The developing and evolving retina: using time to organize form. Brain Res. , 1192, 5-16. PMID: 17692298 DOI.
Cayouette M, Poggi L & Harris WA. (2006). Lineage in the vertebrate retina. Trends Neurosci. , 29, 563-70. PMID: 16920202 DOI.
Bookshelf retina development
Articles
Yao J, Zhou J, Liu Q, Lu D, Wang L, Qiao X & Jia W. (2010). Atoh8, a bHLH transcription factor, is required for the development of retina and skeletal muscle in zebrafish. PLoS ONE , 5, e10945. PMID: 20532172 DOI.
Boije H, Ring H, López-Gallardo M, Prada C & Hallböök F. (2010). Pax2 is expressed in a subpopulation of Müller cells in the central chick retina. Dev. Dyn. , 239, 1858-66. PMID: 20503381 DOI.
Georgi SA & Reh TA. (2010). Dicer is required for the transition from early to late progenitor state in the developing mouse retina. J. Neurosci. , 30, 4048-61. PMID: 20237275 DOI.
Yue F, Johkura K, Shirasawa S, Yokoyama T, Inoue Y, Tomotsune D & Sasaki K. (2010). Differentiation of primate ES cells into retinal cells induced by ES cell-derived pigmented cells. Biochem. Biophys. Res. Commun. , 394, 877-83. PMID: 20206598 DOI.
Levin N, Dumoulin SO, Winawer J, Dougherty RF & Wandell BA. (2010). Cortical maps and white matter tracts following long period of visual deprivation and retinal image restoration. Neuron , 65, 21-31. PMID: 20152110 DOI.
Ghosh F & Arnér K. (2010). Cell type differentiation dynamics in the developing porcine retina. Dev. Neurosci. , 32, 47-58. PMID: 20150723 DOI.
Keeley PW & Reese BE. (2010). Role of afferents in the differentiation of bipolar cells in the mouse retina. J. Neurosci. , 30, 1677-85. PMID: 20130177 DOI.
Sánchez-Sánchez AV, Camp E, Leal-Tassias A & Mullor JL. (2010). Wnt signaling has different temporal roles during retinal development. Dev. Dyn. , 239, 297-310. PMID: 20014102 DOI.
Hackler L, Wan J, Swaroop A, Qian J & Zack DJ. (2010). MicroRNA profile of the developing mouse retina. Invest. Ophthalmol. Vis. Sci. , 51, 1823-31. PMID: 19933188 DOI.
Search Pubmed
Search Pubmed: retina embryology
Search Entrez: retina embryology
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 6) Embryology Vision - Retina Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Vision_-_Retina_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G