Vision - Cornea Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
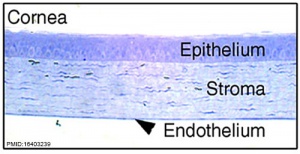
These notes introduce the development of the cornea of the eye. The adult cornea has three layers: an outer epithelium layer (ectoderm), a middle stromal layer of collagen-rich extracellular matrix between stromal keratocytes (neural crest) and an inner layer of endothelial cells (neural crest).
The cornea is a vision-specific specialised sensory epithelia that in humans differentiates mainly in the postnatal period. It arises initially from cranial ectoderm adjacent to the lens placode and forms a presumptive corneal epithelium. Later neural crest cells migrate between the lens and presumptive structure to form both the corneal endothelium and the stromal fibroblasts (keratocytes). Neural crest development in humans, reptiles and birds differs from that seen in rodents, cats, rabbits, and cattle.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Cornea Development <pubmed limit=5>Cornea Development</pubmed> Search term: Cornea Embryology <pubmed limit=5>Cornea Embryology</pubmed> |
Carnegie Stages - Eye
| Carnegie Stage | Event | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | optic primordia appear. | ||||||||||||||||||
| 11 | Right and left optic primordia meet at the optic chiasma forming a U-shaped rim. | ||||||||||||||||||
| 12 | optic neural crest reaches its maximum extent and the optic vesicle becomes covered by a complete sheath, | ||||||||||||||||||
| 13 | By the end of the fourth week the optic vesicle lies close to the surface ectoderm. Optic evagination differentiation allows identification of optic part of retina, future pigmented layer of retina, and optic stalk. The surface ectoderm overlying the optic vesicle, in response to this contact, has thickened to form the lense placode. | ||||||||||||||||||
| 14 | (about 32 days) Lens placode is indented by the lens pit, cup-shaped and still communicates with the surface by a narrowing pore. | ||||||||||||||||||
| 15 | (about 33 days) Lens pit is closed. The lens vesicle and optic cup lie close to the surface ectoderm and appear to press against the surface. | ||||||||||||||||||
| 16 | (37 days) Growth of the lens body results in a D-shaped lens cavity. Perilental blood vessels (tunica vasculosa lentis) are visible. Prior to the development of the eyelids, one small sulcus or groove forms above the eye (eyelid groove) and another below it. | ||||||||||||||||||
| 17 - 19 | Retinal pigment is visible and the retinal fissure is largely closed. Eyelids grooves deepen, eyelid folds develop, first below, and then above, the eye. | ||||||||||||||||||
| 18 | Mesenchyme invades the region between the lens epithelium and the surface ectoderm. | ||||||||||||||||||
| 19 - 22 | Eyelid folds develop into the eyelids and cover more of the eye as the palpebral fissure takes shape. The upper and the lower eyelids meet at the outer canthus in Stage 19. | ||||||||||||||||||
| 20 | Lens cavity is lost and a lens suture begins to form. The inner canthus is established. | ||||||||||||||||||
| 23 | retina comprises the pigmented layer, external limiting membrane, proliferative zone, external neuroblastic layer, transient fiber layer, internal neuroblastic layer, nerve fiber layer, and internal limiting membrane. Eyelids closure is complete (Note - shown as still open in the Kyoto embryo). | ||||||||||||||||||
Data from a study of human embryonic carnegie stages[3] and other sources.
| |||||||||||||||||||
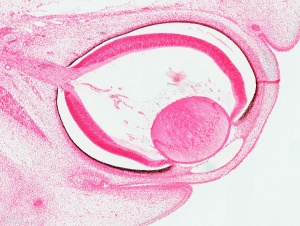
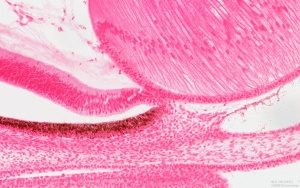
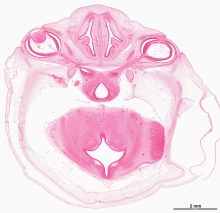
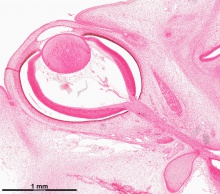
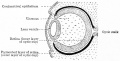
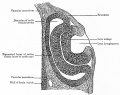
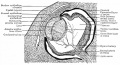
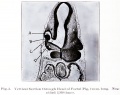
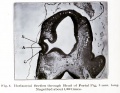
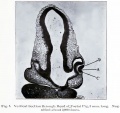
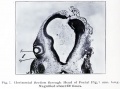
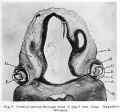
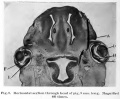
Human Cornea
Week 8 Stage 22
The images below link to virtual slides of the human developing eye at Carnegie stage 22. Click on the image to open or select specific regions from the regions of interest links.
|
|
Virtual Slide - Regions of Interest |
Links: Embryo Virtual Slides
Cornea Epithelia
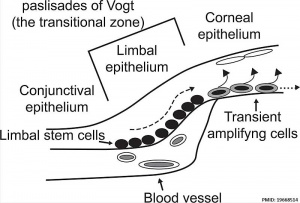
The cornea ocular surface is composed of three epithelia, conjunctival, limbal and corneal.
|
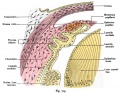
Corneal epithelial cells cartoon[4] |
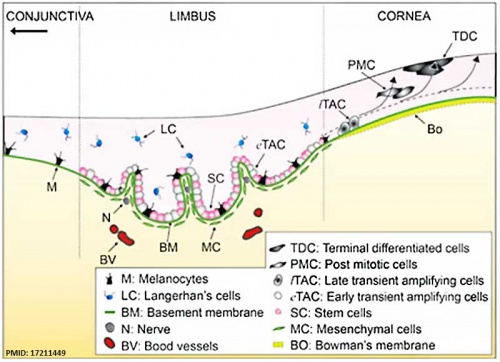
The Adult Human Limbal Palisades of Vogt
Bar represents 500 μm in A and B, 200 μm in C and E, and 50 μm in D
|
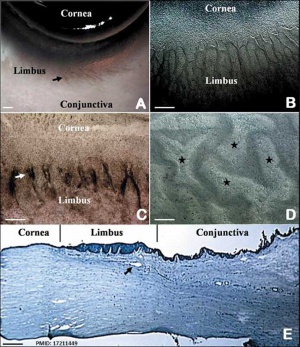
Adult human limbal palisades of Vogt[5] |
Limbal Stem Cells
Cartoon showing the location of limbal stem cells at the limbal basal layer.[5]
- Links: Stem Cells
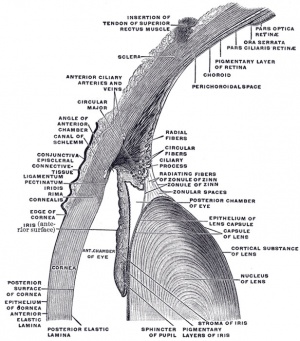
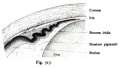
Descemet Membrane
Corneal endothelium basement membrane beginning in children at 3 μm thick and increases in adults to 10 μm. Consists of collagen type IV and VIII fibrils.
Composed of two layers:
- anterior banded layer - commencing in week 10 (GA week 12) as collagen lamellae and proteoglycans.
- posterior non-banded layer - deposited by endothelial cells over time and thickens postnatally over decades.
Descemet membrane was historically named after Jean Descemet (1732–1810) a French physician.
Palisades of Vogt
The palisades of Vogt are a series of radially oriented fibrovascular ridges concentrated along the upper and lower corneoscleral limbus, the vasculature component consists of radially oriented hairpin loops of narrow arterial and venous vessels. Named by Vogt in 1921. (for review see[6])
Aggregate into distinct crescentic zones and lie peripheral to the terminal capillary loops of the limbus and central to Schlemm’s canal. Lying between the connective tissue palisades are intervening radial zones of thickened conjunctival epithelium, the so-called inter-palisades or epithelial rete ridges.
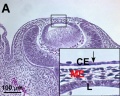
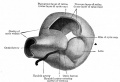
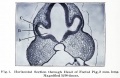
Mouse Cornea
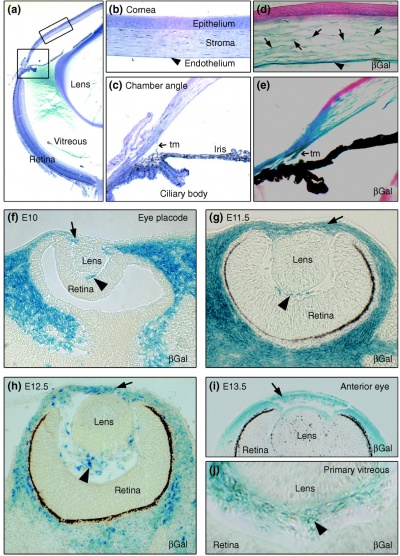
Neural crest-derived cells contribute to mouse cornea development.[7] |
|
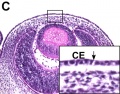
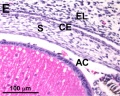
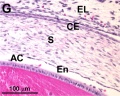
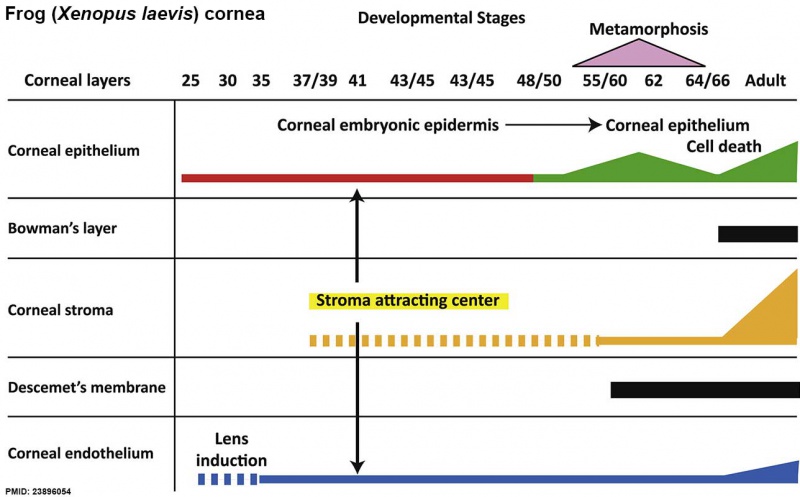
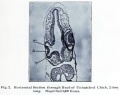
Frog Cornea
This developmental timeline is from a recent frog (Xenopus laevis) cornea study[8]
- stage 25 - cornea starts from a simple embryonic epidermis overlying the developing optic vesicle.
- stage 30 - detachment of the lens placode, cranial neural crest cells start to invade the space between the lens and the embryonic epidermis to construct the corneal endothelium.
- stage 41 - a second wave of migratory cells containing presumptive keratocytes invades the matrix leading to the formation of inner cornea and outer cornea. A unique cell mass (stroma attracting center) connects the two layers like the center pole of a tent.
- stage 48 - many secondary stromal keratocytes individually migrate to the center and form the stroma layer.
- stage 60 - the stroma space is filled by collagen lamellae and keratocytes, and the stroma attracting center disappears. At early metamorphosis, the embryonic epithelium gradually changes to the adult corneal epithelium, which is covered by microvilli.
- stage 62 - the embryonic epithelium thickens and cell death is observed in the epithelium, coinciding with eyelid opening.
- After metamorphosis - cornea has attained the adult structure of three cellular layers, epithelium, stroma, and endothelium, and between the cellular layers lie two acellular layers (Bowman's layer and Descemet's membrane)
- Links: Frog Development
Molecular
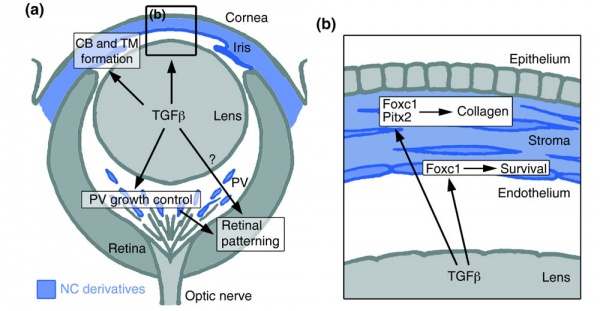
Mouse Eye TGF-beta Model - Summary of the TGFβ-dependent development of anterior and posterior ocular structures.[7]
a Neural crest-derived cells (NC, blue) contribute to structures of the anterior eye segment and the primary vitreous (PV).
|
b In the cornea, prospective stromal keratocytes and endothelial cells are of neural crest origin.
|
Additional Images
Historic Images
References
- ↑ Lwigale PY. (2015). Corneal Development: Different Cells from a Common Progenitor. Prog Mol Biol Transl Sci , 134, 43-59. PMID: 26310148 DOI.
- ↑ Ho LT, Harris AM, Tanioka H, Yagi N, Kinoshita S, Caterson B, Quantock AJ, Young RD & Meek KM. (2014). A comparison of glycosaminoglycan distributions, keratan sulphate sulphation patterns and collagen fibril architecture from central to peripheral regions of the bovine cornea. Matrix Biol. , 38, 59-68. PMID: 25019467 DOI.
- ↑ Pearson AA. (1980). The development of the eyelids. Part I. External features. J. Anat. , 130, 33-42. PMID: 7364662
- ↑ Kayama M, Kurokawa MS, Ueno H & Suzuki N. (2007). Recent advances in corneal regeneration and possible application of embryonic stem cell-derived corneal epithelial cells. Clin Ophthalmol , 1, 373-82. PMID: 19668514
- ↑ 5.0 5.1 Li W, Hayashida Y, Chen YT & Tseng SC. (2007). Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. , 17, 26-36. PMID: 17211449 DOI.
- ↑ Goldberg MF & Bron AJ. (1982). Limbal palisades of Vogt. Trans Am Ophthalmol Soc , 80, 155-71. PMID: 7182957
- ↑ 7.0 7.1 Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, Hjalt TA, Suter U, Karlsson S, Hafezi F, Born W & Sommer L. (2005). Compound developmental eye disorders following inactivation of TGFbeta signaling in neural-crest stem cells. J. Biol. , 4, 11. PMID: 16403239 DOI.
- ↑ Hu W, Haamedi N, Lee J, Kinoshita T & Ohnuma S. (2013). The structure and development of Xenopus laevis cornea. Exp. Eye Res. , 116, 109-28. PMID: 23896054 DOI.
Journals
- Cornea "For corneal specialists and for all general ophthalmologists with an interest in this exciting subspecialty, Cornea brings together the latest clinical and basic research on the cornea and the anterior segment of the eye." [jour PuMed Listing]
Reviews
Lwigale PY. (2015). Corneal Development: Different Cells from a Common Progenitor. Prog Mol Biol Transl Sci , 134, 43-59. PMID: 26310148 DOI.
Maycock NJ & Marshall J. (2014). Genomics of corneal wound healing: a review of the literature. Acta Ophthalmol , 92, e170-84. PMID: 23819758 DOI.
Hassell JR & Birk DE. (2010). The molecular basis of corneal transparency. Exp. Eye Res. , 91, 326-35. PMID: 20599432 DOI.
Masters BR. (2009). Correlation of histology and linear and nonlinear microscopy of the living human cornea. J Biophotonics , 2, 127-39. PMID: 19343693 DOI.
The International Journal of Developmental Biology Vol. 48 Nos. 8/9 (2004) Eye Development
Articles
MAURICE DM. (1957). The structure and transparency of the cornea. J. Physiol. (Lond.) , 136, 263-86. PMID: 13429485
Bookshelf cornea development
Search Pubmed
Search Pubmed: cornea development
Search Entrez: cornea development
Terms
- Limbal epithelial stem cells - cells located at the limbal basal layer.
- palisades of Vogt - series of radially oriented fibrovascular ridges concentrated along the upper and lower corneoscleral limbus, the vasculature component consists of radially oriented hairpin loops of narrow arterial and venous vessels. Named by Vogt in 1921. PMID 7182957
.
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- UNSW SoMS research - MDTR
- UNSW Virtual Slides Eye Development Histology (requires login)
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Vision - Cornea Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Vision_-_Cornea_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G