USA FDA Fetal Risk Categories
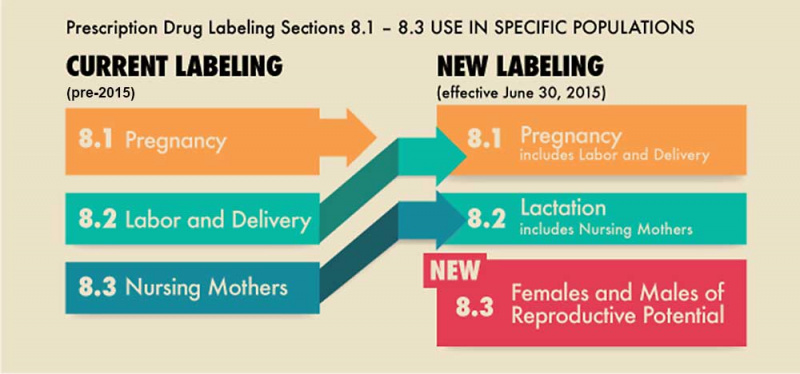
In the United States the government Food and Drug Administration (FDA) historically (before 2015) established the drug labelling classes (A, B, C, D, and X) to define their safety. Since 2015 this drug classification has been replaced with the "FDA Pregnancy and Lactation Labeling Rule" (PLLR).[1] As well as removing the previous letter classes it has added a
new category of #Females and Males of Reproductive Potential.
A 2011 study of USA data from 1976-2008 has shown:[2]
- 6 million pregnancies every year
- 50% of pregnant women reported taking at least one medication
- Pregnant women take an average of 2.6 medications at any time during pregnancy
- First trimester use of prescription medications has increased by more than 60%
- Use of 4 or more medications in the first trimester has tripled (9.9% to 27.6%)
- Small percentage of drugs contraindicated for use in pregnancy or while breast feeding
- contraindicated example include - isotretinoin, mycophenolates
- Drugs Links: Australian Drug Categories | USA Drug Categories | Human Abnormal Development | BGD Tutorial
Some Recent Findings
- Recent changes in pregnancy and lactation labeling: retirement of risk categories[3] "The rapid development and increased availability of novel pharmacologic therapies and pharmaceutical products has amplified the potential for drug exposure during pregnancy. Many drugs are beneficial for disease state management during pregnancy and provide significant fetal and maternal health benefits. However, a paucity of safety data combined with the imprecision of the current risk category system renders risk versus benefit assessment difficult. In response to decades of criticism, the U.S. Food and Drug Administration (FDA) is implementing a new pregnancy and lactation labeling rule designed to improve risk versus benefit assessment of drugs used in pregnant and nursing mothers. These recommendations will provide clear and detailed information for both patients and health care providers, and they will include three main categories: risk summary, clinical considerations, and data. The new labeling rules remove the previous letter risk categorization system (A, B, C, D, X). In this review, we summarize the upcoming FDA labeling changes and discuss their potential consequences on clinical practice."
- Obstetric toxicology: teratogens[4] "The emergency physician frequently encounters women who seek care because of pregnancy- and nonpregnancy-related complaints. Many medications are safe for use during pregnancy, including several that are listed as potential teratogens based on the Food and Drug Administration's (FDA) pregnancy classification; but it is important that the emergency physician know and recognize which drugs can be given in pregnancy and which drugs are absolutely contraindicated. Expert resources should be identified and used because the FDA's classification of drugs based on pregnancy risk does not represent the most up-to-date or accurate assessment of a drug's safety."
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: tFDA Fetal Risk Category
|
FDA History
| US FDA Historic Background
|
| Date
|
Events
|
| 1906
|
Food and Drugs Act is passed by Congress and prohibits interstate transportation of misbranded or adulterated foods, drinks, and drugs.
|
| 1927
|
Food and Drug Administration Agency is formed and given authority to enforce the Food and Drugs Act.
|
| 1938
|
Federal Food, Drug, and Cosmetic (FDC) Act is passed, requiring all drugs to have demonstrated safety prior to marketing.
|
| 1951
|
Durham-Humphrey Amendment established the category of prescription drugs and gave the FDA the power to determine if a drug should be prescription-only or over-the-counter.
|
| 1962
|
Kefauver-Harris Amendment passed after thalidomide was found to cause birth defects. It requires drug manufacturers to provide stronger evidence of safety and effectiveness prior to FDA approval for marketing.
|
| 1966
|
FDA issued requirement for animal studies referred to as “3-Segment Studies” to evaluate safety of drugs. The studies should be designed to address 1) fertility, 2) in utero development, and 3) perinatal and postnatal effects.
|
| 1970
|
FDA requires first package insert for women using oral contraceptives.
|
| 1979
|
As published in 1980, FDA establishes the ABCDX pregnancy risk categories and requires the packaging label to include one of these categories.
|
| 1993
|
FDA revises a policy that excluded women of childbearing potential from being participants in drug trials and requires improved assessments of medication responses specific to gender.
|
| 2007
|
New regulations are passed that require all studies of drugs that involve human participants to register with ClinicalTrials.gov.; some drugs to conduct postapproval studies or have a risk evaluation and mitigation strategy to monitor the drug once it is marketed; and package inserts to include contact information for reporting adverse reactions.
|
| 2008
|
FDA proposes changing the pregnancy labeling categories.
|
| 2015
|
Content and Format of Labeling for Human Prescription Drug and Biologic Products; Requirements for Pregnancy and Lactation Labeling (Final rule 79, FR 72064) is passed and requires the removal of the ABCDX labels from all prescription and biologic product labels and new requirements to include narrative descriptions.
|
| Table historic data[5]
|
| US FDA - Historic Drug Categories (pre-2015)
|
| US FDA - Historic Drug Categories (pre-2015)
|
| Category
|
Description
|
| Category A
|
Controlled studies in women fail to demonstrate a risk to the fetus in the first trimester, there is no evidence of a risk in later trimesters, and the possibility of fetal harm appears remote.
|
| Category B
|
Either animal reproduction studies have not demonstrated a fetal risk but there are no controlled studies in pregnant women, or animal reproduction studies have shown on adverse effect (other than a decrease in fertility) that was not confirmed in controlled studies in women in the first trimester (and there is no evidence of risk in later trimesters).
|
| Category C
|
Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal or other) and there are no controlled studies in women, or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus.
|
| Category D
|
There is positive evidence of human fetal risk, but the benefits from use in pregnant women may be acceptable despite the risk (eg, if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective).
|
| Category X
|
Studies in animals or human beings have demonstrated fetal abnormalities or there is evidence of fetal risk based on human experience or both, and the risk of use of the drug in pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant.
|
|
FDA Pregnancy and Lactation Labeling Rule
- "The PLLR requires changes to the content and format for information presented in prescription drug labeling in the Physician Labeling Rule (PLR) format to assist health care providers in assessing benefit versus risk and in subsequent counseling of pregnant women and nursing mothers who need to take medication, thus allowing them to make informed and educated decisions for themselves and their children. The PLLR removes pregnancy letter categories – A, B, C, D and X. The PLLR also requires the label to be updated when information becomes outdated."

- Links: Final Rule
Pregnancy Exposure Registry
The USA has is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to drugs (by trade name) during pregnancy. The information below is extracted from, and not a complete summary of, PLLR requirements.
Pregnancy Risk Summary
Drugs with systemic absorption
- When use of a drug is contraindicated during pregnancy, that information must be stated first in the Risk Summary
- Risk statement based on human data
- Risk statement based on animal data
- Risk statement based on pharmacology
- Background risk information in general population
- Background risk information in disease population
Risk Based on Animal Data
Systemic drug absorption
There are no adequate and well-controlled studies of [TRADENAME] in pregnant women. The limited available information on [TRADENAME] use during pregnancy is not sufficient to inform a drug-associated risk of major birth defects or miscarriage. In animal reproduction studies, oral administration of [drug name] to pregnant rats and rabbits during the period of organogenesis at doses up to 40 and 20 times the maximum recommended human dose (MRHD), respectively, resulted in decreased fetal body weight gain and delayed skeletal ossification but no teratogenic effects were observed. Decreased fetal body weight and delayed skeletal ossification were not observed at doses up to 10 and 5 times the MRHD in rats and rabbits, respectively [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15- 20%, respectively.
Pregnancy Clinical Considerations
Clinical Considerations (five optional subheadings)
- Disease-Associated Maternal and/or Embryo/Fetal Risk
- Dose Adjustments During Pregnancy and the Post- Partum Period
- Maternal Adverse Reactions
- Fetal/Neonatal Adverse Reactions
- Labor or Delivery
Lactation Risk Summary
Systemic drug absorption
- Presence of drug in milk (if unknown, must state so)
- Concentration in milk
- Actual or estimated infant daily dose
- Effects of drug on the breastfed infant (if unknown, must state so)
- Effects of the drug on milk production (if unknown, must state so)
- Risk/Benefit Statement
Females and Males of Reproductive Potential
Include when there are requirements or recommendations for pregnancy testing and/or contraception and/or when human and/or animal data suggest drug effects on fertility.
Three headings
- Pregnancy Testing
- Contraception
- Infertility
Example of Labelling
Based on its mechanism of action, TRADENAME can cause fetal harm when administered to a pregnant
woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Female patients of reproductive potential should have a negative pregnancy test ...
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment and for at least 2 weeks after the last dose of TRADENAME. Advise patients that TRADENAME can reduce the effectiveness of oral contraceptives and to use alternative effective contraception during treatment with TRADENAME [see Warnings and Precautions (5.x), Drug Interactions (7.x), Clinical Pharmacology (12.x)].
Infertility
Females
Decreased fertility and ovarian toxicity were observed in female rats treated with DRUGNAME. Advise female patients of reproductive potential ...
Males
Effects on spermatogenesis have been observed in animals treated with DRUGNAME. Advise male patients of the potential risk...
Anesthetics
FDA September 2014 - Discussion - anesthetic drugs to infants and young children
References
- ↑ Pernia S & DeMaagd G. (2016). The New Pregnancy and Lactation Labeling Rule. P T , 41, 713-715. PMID: 27904304
- ↑ Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C & Hernández-Díaz S. (2011). Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am. J. Obstet. Gynecol. , 205, 51.e1-8. PMID: 21514558 DOI.
- ↑ <pubmed>24390829</pubmed>
- ↑ <pubmed>23137407</pubmed>
- ↑ Brucker MC & King TL. (2017). The 2015 US Food and Drug Administration Pregnancy and Lactation Labeling Rule. J Midwifery Womens Health , 62, 308-316. PMID: 28556499 DOI.
Search PubMed: US Drug Categories | Drug Categories | teratogenic drugs
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Terms
| Drug Terms
|
- adverse reaction - (adverse event) An unwanted effect caused by the administration of drugs. Onset may be sudden or develop over time (See Side Effects).
- approved drugs - In the U.S., the Food and Drug Administration (FDA) must approve a substance as a drug before it can be marketed. The approval process involves several steps including pre-clinical laboratory and animal studies, clinical trials for safety and efficacy, filing of a New Drug Application by the manufacturer of the drug, FDA review of the application, and FDA approval/rejection of application (See Food and Drug Administration).
- AUC - acronym for Area Under the plasma concentration versus time Curve is an important parameter when determining drug effects, both therapeutic and teratogenic.
- clinical trial - A research study to answer specific questions about vaccines or new therapies or new ways of using known treatments. Clinical trials (also called medical research and research studies) are used to determine whether new drugs or treatments are both safe and effective. Carefully conducted clinical trials are the fastest and safest way to find treatments that work in people. Trials are in four phases: Phase I tests a new drug or treatment in a small group; Phase II expands the study to a larger group of people; Phase III expands the study to an even larger group of people; and Phase IV takes place after the drug or treatment has been licensed and marketed. (See Phase I, II, III, and IV Trials).
- cohort - In epidemiology, a group of individuals with some characteristics in common.
- contraindication - A specific circumstance when the use of certain treatments could be harmful.
- double-blind study - A clinical trial design in which neither the participating individuals nor the study staff knows which participants are receiving the experimental drug and which are receiving a placebo (or another therapy). Double-blind trials are thought to produce objective results, since the expectations of the doctor and the participant about the experimental drug do not affect the outcome; also called double-masked study. See Blinded Study, Single-Blind Study, and Placebo.
- drug clearance - measured as the volume of blood or plasma from which a compound is irreversibly removed per unit time.
- drug distribution - movement of a drug from one location in the body to another, generally by passive diffusion down the concentration gradient.
- drug-drug interaction - A modification of the effect of a drug when administered with another drug. The effect may be an increase or a decrease in the action of either substance, or it may be an adverse effect that is not normally associated with either drug.
- drug metabolism - (drug biotransformation)
- efficacy - Referring to a drug or treatment, the maximum ability of a drug or treatment to produce a result regardless of dosage. A drug passes efficacy trials if it is effective at the dose tested and against the illness for which it is prescribed. In the procedure mandated by the FDA, Phase II clinical trials gauge efficacy, and Phase III trials confirm it (See Food and Drug Administration (FDA), Phase II and III Trials).
- Food and Drug Administration - (FDA) The U.S. Department of Health and Human Services agency responsible for ensuring the safety and effectiveness of all drugs, biologics, vaccines, and medical devices, including those used in the diagnosis, treatment, and prevention of HIV infection, AIDS, and AIDS-related opportunistic infections. The FDA also works with the blood banking industry to safeguard the nation's blood supply. Internet address: http://www.fda.gov/.
- informed consent - The process of learning the key facts about a clinical trial before deciding whether or not to participate. It is also a continuing process throughout the study to provide information for participants. To help someone decide whether or not to participate, the doctors and nurses involved in the trial explain the details of the study.
- oral bioavailability - refers to the percentage of the oral dose of that drug that ends up in the systemic circulation, few drugs have complete oral bioavailability.
- orphan drugs - An FDA category that refers to medications used to treat diseases and conditions that occur rarely. There is little financial incentive for the pharmaceutical industry to develop medications for these diseases or conditions. Orphan drug status, however, gives a manufacturer specific financial incentives to develop and provide such medications.
- pharmacokinetics - The processes (in a living organism) of absorption, distribution, metabolism, and excretion of a drug or vaccine.
- Phase I trials - Initial studies to determine the metabolism and pharmacologic actions of drugs in humans, the side effects associated with increasing doses, and to gain early evidence of effectiveness; may include healthy participants and/or patients.
- Phase II trials - Controlled clinical studies conducted to evaluate the effectiveness of the drug for a particular indication or indications in patients with the disease or condition under study and to determine the common short-term side effects and risks.
- Phase III trials - Expanded controlled and uncontrolled trials after preliminary evidence suggesting effectiveness of the drug has been obtained, and are intended to gather additional information to evaluate the overall benefit-risk relationship of the drug and provide and adequate basis for physician labeling.
- Phase IV trials - Post-marketing studies to delineate additional information including the drug's risks, benefits, and optimal use.
- placebo - A placebo is an inactive pill, liquid, or powder that has no treatment value. In clinical trials, experimental treatments are often compared with placebos to assess the treatment's effectiveness. (See Placebo Controlled Study).
- placebo controlled study - A method of investigation of drugs in which an inactive substance (the placebo) is given to one group of participants, while the drug being tested is given to another group. The results obtained in the two groups are then compared to see if the investigational treatment is more effective in treating the condition.
- placebo effect - A physical or emotional change, occurring after a substance is taken or administered, that is not the result of any special property of the substance. The change may be beneficial, reflecting the expectations of the participant and, often, the expectations of the person giving the substance.
- plasma proteins - can bind drugs, acidic drugs bind to albumin, basic drugs bind to lipoproteins or to alpha-1 acid glycoprotein.
- Preclinical - Refers to the testing of experimental drugs in the test tube or in animals - the testing that occurs before trials in humans may be carried out.
- side effects - Any undesired actions or effects of a drug or treatment. Negative or adverse effects may include headache, nausea, hair loss, skin irritation, or other physical problems. Experimental drugs must be evaluated for both immediate and long-term side effects (See Adverse Reaction).
- statistical significance - The probability that an event or difference occurred by chance alone. In clinical trials, the level of statistical significance depends on the number of participants studied and the observations made, as well as the magnitude of differences observed.
- toxicity - An adverse effect produced by a drug that is detrimental to the participant's health. The level of toxicity associated with a drug will vary depending on the condition which the drug is used to treat.
- treatment IND - Investigational New Drug (IND) application, which is part of the process to get approval from the FDA for marketing a new prescription drug in the U.S. It makes promising new drugs available to desperately ill participants as early in the drug development process as possible. Treatment INDs are made available to participants before general marketing begins, typically during Phase III studies. To be considered for a treatment IND a participant cannot be eligible to be in the definitive clinical trial.
|
|
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 30) Embryology USA Drug Categories. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/USA_Drug_Categories
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G


