Paper - The development of the mammalian spleen, with special reference to its hematopoietic activity (1921)
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Thiel GA. and Downey H. The development of the mammalian spleen, with special reference to its hematopoietic activity. (1921) Amer. J Anat. 28(2): 279 - 339.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Mammalian Spleen with Special Reference to its Hematopoietic Activity
Geo. A. Thiel and Hal Downey
Hematological Laboratory, Department of Animal Biology, University of Minnesota
Three Text figures and Three Plates (Eight figures)
Introduction
To anyone looking over the literature on the development of the mammalian spleen the necessity for more work on the finer details of the process is evident. In spite of an extensive literature, one finds few recent papers dealing with the subject from the standpoint of the many interesting hematological problems involved. The most extensive work has been done on the lower vertebrates and on human embryos. But, owing to the lack of some of the most important stages and due to the poor preservation of much of the material, the work on the human fetus has contributed little toward the solution of the many hematological problems.
For the following study the writers have selected the pig and other mammalian embryos, because the material could be obtained easily in all the desired stages. Special consideration was given to the following problems: 1) the origin of the splenic rudiment, especially its relation to the peritoneal epithelium; 2) the origin of the first free cells of the organ; 3) hematopoiesis in the early spleen and the origin of the first cells belonging to the ‘myeloid’ series; 4) influence of enviromental factors on the hematopoietic process; 5) development of the arteries and their surrounding lymphoid sheaths; 6) development of the follicular tissue (white pulp) and the origin of its lymphoid cells; 7) relationship between follicular tissue and spleen pulp (red pulp); 8) comparison of the hematopoietic process of the early spleen with myeloid metaplasia in the spleen of the adult; 9) development of the splenic follicles in postnatal animals.
The investigators who have studied the problem of splenic development can be placed in two distinct groups. The first maintains that the spleen is of mesodermal origin; the second that the organ owes its development to cells that are given off from the endoderm. The first class of workers may be further divided into those who hold that the rudiment of the organ is limited to a differentiation of the mesenchyme, and into those who maintain that the peritoneal epithelium also plays a part in the first stages of development.
The chief exponents of the endodermal theory are Schenk, Gotte, Maurcr, v. Kupffer, Woit, and Glas. These men, with the exception of Maurer and Gotte, believed there was a definite relation between the embryonic spleen and pancreas, and consequently considered ‘the spleen as being dependent upon the endodermal cells which are given off from the primitive diverticulum of the pancreas. The work of Maurer stands alone in maintaining a relation between the endodermal epithelium of the intestinal tract and the splenic rudiment. According to Gotte, the splenic rudiment of the toad is composed of a mass of free "Dotterbildungszellen’- primitive blood-cells derived from the yolk endoderm and forced into the mesenchyme by the heart beat.
Choronshitzky believed that free cells from the intestinal epithelium may enter the mesenchyme and become transformed into mesenchymal tissue, and that therefore the endodermal elements are concerned only to the extent that they are functional in the production of the accumulation of mesenchyrne for the splenic rudiment.
Radford has made a careful study with special reference to Maurer’s conclusions, but failed to find sufficient evidence to corroborate his views. Kraatz, Tonkoff, and Nicolas also report the absence of cells from the digestive tract.
The group of investigators that maintains a mesodermal splenic rudiment is represented by Miiller, Koelliker, Laguesse, Minot, Pinto, Toldt, Janosik, Tonkoff, Choronshitzky, Mietens, and Danchakoff. Of this group Choronshitzky, Tonkoff, Muller, and Mietens find free cells given off from the coelomic epithelium which make their way into the underlying mesenchyme where they proliferate and join the local mesenchyme in the formation of the first rudiment of the organ. Toldt and Janosik derive the rudiment exclusively from coelomic epithelium, while Laguesse states that in Acanthias and tel.eosts the epithelium plays no direct part in the formation of the rudiment, although he admits that in earlier stages, before the rudiment has appeared, it contributes cells to the underlying mesenchyme.
Materials and Methods
Embryos of the pig (Sus domesticus) were used as the chief object of study in making this investigation. A series of embryos beginning with those 7 mm. in length to stages near the end of fetal life were collected and prepared for study. Also embryos of the striped gopher (Citellus tridecemlineatus), guinea-pig, white rat, and sheep were employed for comparative purposes.
On beginning the study of the origin of the spleen, it was found to be expedient to begin with embryos of about 15 or 20 mm. in length. The advantages of such a procedure are obvious; most of the organs of the viscera have begun their development in an embryo of that size, and, furthermore, the presence of the spleen has been reported and figured by Minot, Tonkoff, and others. Thus, in first making a superficial study of such embryos, one could familiarize himself with the general features of the embryonic structures and also develop a technique that would bring out the best details of the organ in question.
Various methods of fixation were employed, but Helly’s Zenker-formol fixative proved to be the most satisfactory. Embryos up to those 12 mm. in length were left whole and placed directly in the fixative. In those from 12 to 30 mm. slits were made in the body wall, or the anterior and posterior portion of the embryo was removed so as to allow the fixative to penetrate more rapidly. In all later stages, the embryos were dissected and the embryonic stomach and spleen removed and placed directly in the fixing fluid to insure rapid and thorough fixation. The material was embedded in paraffin and the sections mounted in serial order. After examining a few embryos it was found that the early splenic tissue was extremely dense and that thin sections were highly essential for a cytological study of the organ. For this reason all of the younger stages were cut at a thickness of 3,1. Various combinations of stains were employed, depending upon the nature of the splenic tissue at its various stages of differentiation. For a general stain Dominici’s eosinorange G-toluidin blue method proved to be the most satisfactory. Benda’s haematoxylin, counterstained with Van Gieson’s picrofuchsin, was of special value in demonstrating the cytoplasmic processes of the early mesenchyme of the splenic rudiment. The May-Giemsa mixture was used most extensively for all the later embryonic stages. Ehrlich’striacid followed with toluidin blue, and Ehrlich’s staining mixture of indulin-aurantiaeosin gave excellent material for a study of the granulocytes of the organ. Weigert-’s elastic tissue stain proved very satisfactory for the demonstration of the distribution of the elastic fibers surrounding the early arteries, and Mallory’s ‘Phosphowolframsatire’ stain together with Krause’s gold chloride, counterstained with .May-Giemsa, gave excellent material for a study of the fibers Within the cells of the early reticulum. Among the many other stains were aqueous and alcoholic mixtures of haematoxylin, Mallory’s connective-tissue stain, and Pappenheim’s methylgreen pyronin.
Observations
Early splenic rudiment In embryos of white rat, gopher, and pig, the animals used in this study, the spleen is seen to develop in the dorsal mesogastrium at a level with the fundus portion of the stomach. When first discernible as a distinct structure it appears as a dense mass of tissue in the left dorsolateral portion or the mesentery, occupying approximately one-half of the dorsolateral extent of that structure.
Owing to the controversy regarding the derivation of the cells of the rudiment, the study of the mesogastrium of younger embryos which have not yet developed a splenic rudiment is of special importance and interest. A 3-mm. gopher embryo seemed to present favorable conditions for such a study.
In this embryo the stomach is already formed and possesses a short but distinctly developed mesentery. The latter is composed of a loose mesenchymatous network covered on both surfaces by the visceral peritoneum consisting of several layers of somewhat elongated cells rather loosely arranged. Within the mesenchyme there are a few isolated free cells and capillaries containing primitive erythrocytes.
The structure of the coelomic epithelium and its relation to the underlying mesenchyme is of special interest, because many authors have claimed that the mesenchyme in the region of the future splenic rudiment is increased‘ by the addition of cells from the epithelium. Toldt and Janosik claim that all of the cells of the rudiment are derived from this source.
In the embryo under discussion the coelomic epithelium appears more as a condensed region of the mesenchyme, rather than as a distinct epithelium. The cells are closely approximated and they are elongated somewhat in the direction vertical to the surface of the mesentery. This condensation is two or three cells deep, but the cells are irregularly placed and not arranged in very distinct layers. There is no boundary line between them and the mesenchyme, and the lower cells are directly continuous with those of the mesenchyme, so that the whole structure appears as a continuous syncytium. This is a general condition throughout the mesentery and is not confined to the region of the future spleen. With such an arrangement cells from the coelomic epithelium could very readily pass into the mesenchyme.
This close relationship between coelomic epithelium and mesenchyme was also described by Choronshitzky, Tonkoff, Laguesse, and Mietens. Choronshitzky believed that both the endoderm and the coelomic epithelium give off cells to the mesenchyme. The coelomic epithelium is designated as a ‘Keimepithel’ for the mesenchyme. Tonkoff noted continuity of epithelium and mesenchyme just before the appearance of -the splenic-rudiment. In later stages he found that the two were-separated by a distinct boundary. Similar conditions were described by Laguesse, and Mietens noted that cells from the epithelium of the mesentery (Bufo) join the mesenchynie in the early stages.
These findings are in accordance with the observations of Mollier, who found peritoneal epithelium and mesenchyme forming a distinct and continuous network over the early embryonic liver. He states that “Das Peritoneum ist fast iiberall keine glatte, scharf begrenste Zellhaut, sondern nimmt im Verein mit tiefer gelegenen Stellen an der Bildung eines Netzwerkes Anteil, in dem alle diese Zellen mit protoplasmatischen Auslaufern zusammenhfingen und grossere und kleinere Maschenriiume abgrenzen.” The process of cell division is very active in embryos of this size, but the proliferative activity is not confined to any particular region of the visceral mesoderm; it is rather uniformly distributed throughout the entire peritoneum. J anoéik held that the mesothelium was very active in the formation of free cells in the region of the early spleen. However, at no stage of embryonic life, has the writer found cell division more active in the mesothelium of the mesentery than in any other part of the peritoneal epithelium. But the mesothelium in the region of the splenic rudiment does give off some cells to the underlying mesen~ chyine, as many mitotic figures are seen in the epithelium wit-h their long axes perpendicular to its free surface. One of the daughter cells of such divisions is consequently crowded beneath the epithelium and becomes part of the mesenchyinatous network. However, equally as many mitotic figures are seen with their long axes parallel with the surface of the epithelium, thus merely adding more cells to the peritoneal tissue.
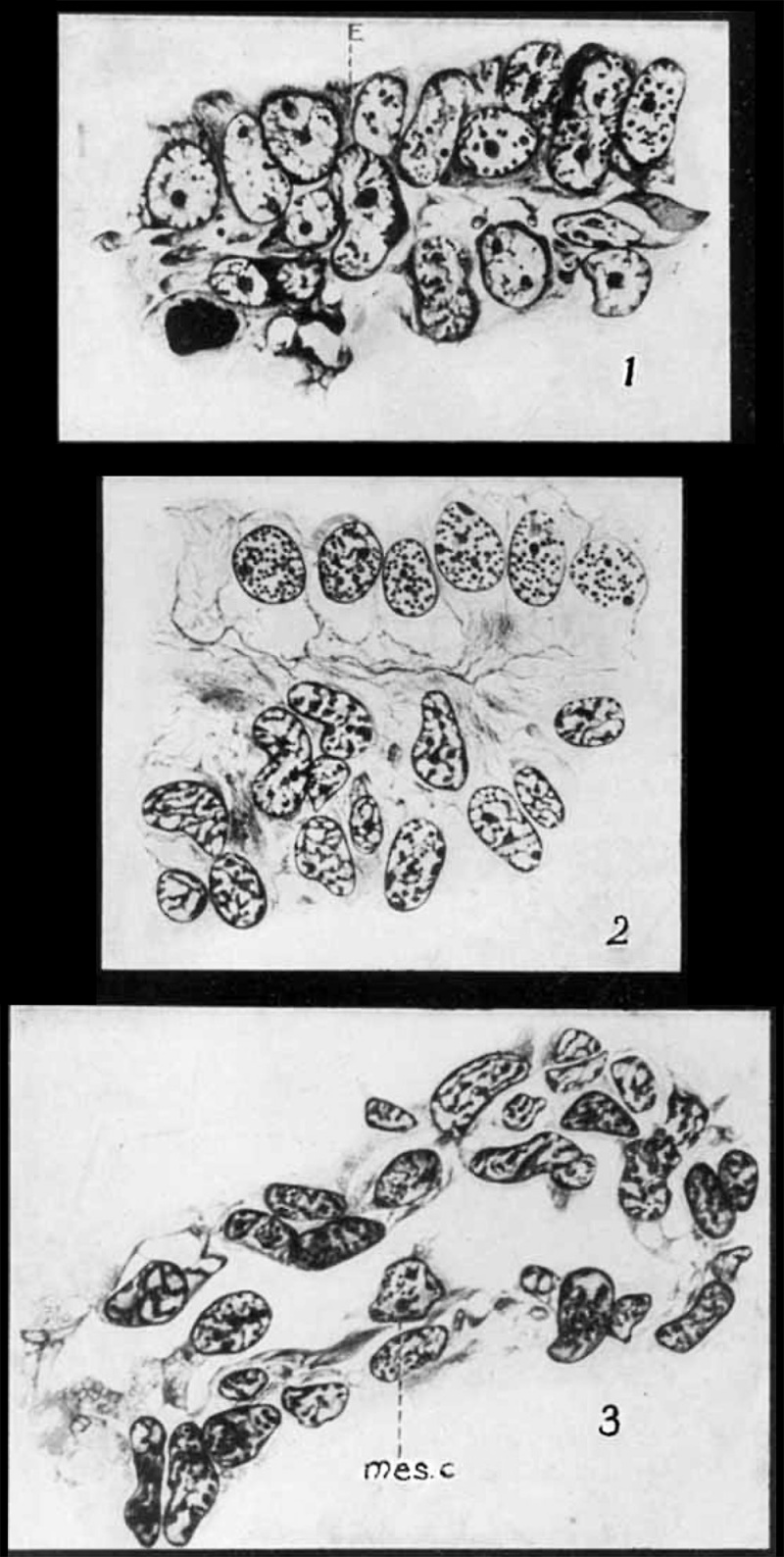
In a 7.5—mm. pig the peritoneum gives evidence of becoming a more distinct structure, but still many areas can be found where cells are being displaced from the peritoneum and are crowded into the mesenchyrne. figure 1 is taken from the peritoneum over the region of the early splenic rudiment. There is very little difference between the cells of the coeloniic epithelium and those of the mesenchyme. The cytoplasm of the former is slightly more basophilic, but the nuclei of the two tissues are identical. In both the epithelium and the mesenchyme, the nuclei are large and oval in shape, possessing a distinct nuclear membrane and, in most cases, two well-marked nucleoli. The remaining chromatic material is distributed in the form of a few small, irregular granules. The cells shown at E are undoubtedly epithelial cells, but from their position one would conclude that they are being crowded into the mesenchyme. To the right and left of the figure the epithelium is more clearly separated from the mesenchyme, and one can readily distinguish the location of the limiting membrane which separates the two tissues during later embryonic life.
The marked changes that take place in the structure of the peritoneal epithelium can be seen by comparing figures 1 and 2. In figure 2, which is taken from a 15-mm. pig, the peritoneal cells are not only sharply separated from the mesenchyme, but they have undergone a marked differentiation that distinguishes them from the characteristic mesenchyme cells. The greatest variation is seen in the nuclear structure. In figure 2 the epithelial nuclei are almost uniformly round structures. The nuclear membrane is still quite distinct, but the chromatin is distributed in finely granular form throughout the whole nucleus. The nucleoli, when present, are not as sharply defined as in the mesenchyme cells. The cytoplasm of the two types of cells shows the same staining reaction, but cell walls become quite evident between adjoining epithelial cells, and at" their base a distinct limiting membrane, which is a direct modification of the basal portion of their cytoplasm, is developed as further evidence of differentiation. The membrane can be distinctly demonstrated with either aqueous or alcoholic mixtures of hematoxylin and can also be clearly recognized in material that has been stained with Dominici’s eosin-orange G and toluidin blue.
The above findings, together with those of other workers, indicate that in the early stages, before the splenic rudiment is formed, it is impossible to make a sharp distinction between the coelomic epithelium and the mesenchyme of the mesogastrium. The cells of both are quite similar in structure (7.5-mm. pig) and continuity of their processes is well established. Proliferative activity on the part of the epithelial cells results in the crowding of some of the daughter cells into the underlying tissue where they become a part of the general mesenchyme. After the splenic rudiment has been established (15—mm. pig) the cells of the epithelium have differentiated to such an extent that they no longer resemble those of the mesenchyme. At this stage the mesenchyme is separated from the epithelium by a sharp line and epithelial cells are no longer added to it. This corresponds exactly to what was observed by Laguesse and Tonkoff. The coelomic epithelium is, therefore, concerned only indirectly with the splenic rudiment through the mesenchyme which by growth and differentiation produces the rudiment.
The first sign of a distinct differentiation into splenie tissue was seen in an 8~mm. gopher embryo. In embryos of this size, the rudiment is more easily distinguished by its position than by the character of its cellular constituents. Its cells appear the same as those of the surrounding mesenchyme, but the tissue is much denser, being composed of mesenchymal cells with short anastomosing processes which form a dense and compact syncytial mass. This early condensation does not extend across the entire mesentery, but is confined to its dorsal portion, near the attachment or junction of the mesentery with the stomach wall. There is no loose mesenchyme between the rudiment and the coelomic epithelium.
Some authors (Tonkoff, Choronshitzky, Laguesse, Kraatz, Kollman, Minot) noted this early condensation of the mesen~ chyme, but others (Gray, Gétte, Mietens, Toldt, Maurer, V. Kupffer, Janosik) did not recognize the rudiment until it was composed of many free cells which were considered to have been derived from the visceral coelomic epithelium, from endoderm, or from primitive wandering cells, the mesenchyme servin g merely as a supporting tissue for these free cells.
Danchakoff found the same conditions in the first stage of splenic development in the chick. She, however, denies the possibility of coelomic epithelium playing a part in the formation of the rudiment of the organ. She states that the “development of the spleen at the expense of the mesenchymal cells without any relation to the endoderm nor the coelomic epithelium may be regarded as a well founded fact.” Such an interpretation may be ‘Well—founded’ for lower vertebrate forms, but in mammals, one cannot disregard the small but strikingly evident role that the peritoneum plays in early embryonic life in adding its proliferative products to the underlying tissue.
In neither of the types of embryos under observation Was there anything seen that would indicate the possibility of endodermal cells from the intestinal epithelium or from the early pancreatic tubules making their way to the splenic rudiment. The immediate proximity of spleen and pancreas in the embryo Was, no doubt, a factor in inducing the earlier Workers (Schenk, l/Voit, Glas, and others) to seek for a genetic relationship between the two organs.
As stated above, the early spleen is characterized by its dense mesenchymal syncytium. Due to intense cell proliferation, it continues to develop until it acquires a length of 2 to 3 mm. in a 15-mm. pig embryo. In embryos of this stage there is but little differentiation Within the splenic tissue.
The Vascularization of the Spleen
I. General
The question of the vascularization of the primitive spleen has received very little "attention, as most of the earlier Workers confined their observations to the fundamental problems concerning the differentiation of the various germ layers and the part they played in the formation of the different organs of the viscera. It was not until hematologists undertook an explanation of the relation of the spleen to the blood and lymphatic systems that a more definite conception of the splenic circulation was established.
Before attempting a discussion of the splenic circulation of the embryo, a survey of the different opinions in regard to the vascularization of the adult organ may be permitted. This question, which has been given much attention, centers upon the relation of the so-called splenic sinuses or ‘Venose Kapillaren’ to the rest of the circulatory bed. The presence of the cellular elements of the blood in the splenic pulp has attracted the attention of many investigators, and various methods have been devised in attempting to ascertain the course of the channels that the cells pursue in passing from the arterial to the Venous circulation.
In comparing the results of previous investigations one finds four different interpretations of the circulatory system through the splenic pulp.
- An open or intermediate circulation through the pulp. Upon leaving the arterial capillaries the blood passes into the meshes of the network of the reticulum. These spaces connect with larger ones that are differentiated into the Venous sinuses which in turn empty into the Veins (Henle, Peremeschko, Hoyer, Bannwarth, Laguesse, Kultschitzky).
- A completely closed circulatory system in which the blood passes through the pulp in a completely closed capillary system (Billroth, Schweiger-Seidel, Kolliker, Kyber, Toldt, Thoma, and at a later period V. Ebner, Helly, Mall (later modified)).
- A partially open and partially closed system (Rindfleisch, Weidenreich, and Mall). Some of the blood is confined to distinct channels, but part of it may circulate through the reticulum of the pulp before reaching the Veins.
- A partially open system due to the porosity of the walls of the Venous sinuses (Mollier, Jolly, Chevalier).
Billroth was the first to describe the splenic sinuses or Venous capillaries, as they were then termed. In View of the fact that many splenic arteries and veins are confined to the trabeculae, Billroth undertook a comparative study of a group of mammals and found that in ox, sheep, and pig the trabecular material is far more abundant than in rabbit, cat, and dog. In the first group the veins retain a rather uniform caliber, and then suddenly break up into short tapering branches that seldom anastomose as they do in rabbit and cat. In the pig the lining of the Veins is a smooth uniform endothelium and does not bulge into the lumen as in the case of the human spleen. These tiny trabecular veins connect directly with the arterial capillaries, thus forming a closed system. Among the more recent workers, we find Helly still an ardent supporter of the closed circulatory system. Helly admits the presence of blood—ce1ls in the splenic pulp, but maintains that the walls of the vessels are very permeable and that the endothelial cells rest upon a structureless membrane through which the bloodcells can readily pass. The presence of such a membrane is denied by Billroth, Schweigger—Seidel, and Maugubi—KudrjaVtzewa, but its presence is maintained by v. Ebner, Schumaeher, and Weidenreich.
Mall studied the spleen of cat and dog and found the following: “from numerous specimens its (the artery’s) communication with the vein is not large but is cut up by bridges of tissue across its lumen before it connects with the vein. This cutting up is so extensive that in uninjected specimens it has been impossible for me to find a single ampulla connected with a vein. In other Words it may be better to say that the ampulla rarely reaches the vein but is separated from it by a small band of splenic pulp.” Weidenreich concludes that two distinct routes are open between the arterial and venous portion of the splenic circulatory system. A branch of the splenic artery, upon leaving a trabecula, forms branches that do not anastomose. These branches soon acquire a lymphoid sheath which at certain intervals forms splenic follicles. Beyond the follicle the artery is resolved into numerous ‘Pilsenarterien’ each of which shows a distinct capsule near its extremity. It is beyond the capsule that the true arterial capillaries are found. The capillary may empty directly into a splenic sinus, or its contents may be poured into the meshes or spaces of the reticulum of the pulp and -collect in larger channels which lead into the venous sinuses of the organ.
Mollier gives a detailed account of the capillary Veins in the spleen of dog, cat, ox, ape, and man. In the spleen of dog, cat, and pig, the sinuses possess no distinct endothelium, but are merely lined with cells of the reticulum of the pulp. These cells retain their original reticular structure and consequently many of the spaces between neighboring cells serve as channels leading from the pulp into the sinuses. From these gleanings of the literature we see that most investigators report an open circulation through the splenic pulp. The work of Helly stands out alone as a last defense of a closed system. The conclusions of some of the earlier workers are far from convincing, for, as has been pointed out by Von W. Schulte, “while the results of injection demonstrate the permeability of the vessel wall, as do in life the passage of cells and fluids, they do not afford us the opportunity of ascertaining the structure of the wall or the nature of the orifices.”
2. Circulation of the Early Embryonic Spleen and the Development of the Venous Sinuses
The Vascularization of the early embryonic spleen has been given but slight attention. It has been superficially investigated by Rathke (in amphibians), Remak (in birds), Arnold, Bischoff and Kolliker (in mammals), and merely mentioned by many others. Peremeschko and Muller held that the spleen was supplied with vessels from the Very earliest stages, and Radford also adds that it soon becomes Very Vascular. Kolliker, however, holds that the vessels do not appear until toward the end of embryonic life. The presence of spaces in the splenic mesenchyme is mentioned by Choronshitzky in his work on the chick and on Various mammalian forms. He states that the Vena lienalis is present before the splenic rudiment is established. Its branches penetrate the rudiment and lose their endothelium. In the rudiment the Veins are merely clefts in the mesenchyme which are in communication with the intercellular spaces of the latter. The embryonic cells can therefore enter the lumina where they become transformed into blood-cells. The splenic artery has no relation to the early rudiment," but grows in later. Toldt and Tonkoff also find blood-Vessels with indistinct walls in the early rudiment. According to Laguesse, the first vessels of the spleen are merely lacunae in the mesenchyme which connect with branches of the sub-intestinal vein (in fishes). Daiber’s studies on the salamander also reveal the presence of lacunae in the very young spleen, but she sees no distinct endothelium at their borders. These lacunae may contain two or three early blood cells which are derivatives of the tissue bordering them. In two— to six—day larvae, the lacunae are bounded by cells that begin to assume the flattened and elongated structure of a definite endothelium.
Mollier is of the opinion that a capillary system is present from the very beginning. His interpretation of its development is embodied in the following quotation: ich bin der Ansiclit dass es sich hier inn ein allgemeines gestaltendes Prinzip handelt, dass uberall die erste Entwicklung von Gefassen auf diesen Weg aus mesenchymatosen Material (Reticulum) erfolgt. Wie ein grosseres Gefass durch die starkere Betonung und Entwicklung bestimmter Bahnen eines capillaren Netzes ausgestattet wird so nieine ich, ist der gleichc Vorgang schon ontogenetisch fruher teltig um durch Betonung und Entwicklung bestimmtcr Reihen von Maschenraumen im Mesenchym-reticulum die ersten Capillarnetze zu schaffen.
He does not deny the possibility of blood-vessels growing by sprouting, but he interprets the sprouting process as a production of mesenchyme cells which at the same time are converted into ‘Gefasszellen.’ Sabin, working with pig embryos, comes to conclusions very similar to those reported by Mollier. By an injection of the splenic artery it was found that in a pig 3 cm. long the entire splenic circulation consists of a capillary network which extends throughout the organ. This condition is maintained until the fetus is 7.5 cm. in length. “Thus the spleen confirms the general principle that the primitive circulation of any organ is in the form of a capillary network out of which the arteries and veins are formed.” The spleen is characterized by persistence of the primitive capillary network until the embryo is 10 cm. long when the type of circulation characteristic of the adult spleen is established.
Sabin’s results are quite contrary to the observations of Danchakoff on normal spleen development in the chick. The latter worker found that the earliest vascular elements of the spleen are exclusively venous. Slits later appear in the mesenchyrne which connect with the early vessels. These slits or sinuses connect together and form a network, thus giving the early spleen a spongy structure. At a much later stage (twelve to thirteen days) “the arteries and their branches seem to grow into the spleen from the outside and here ramify by budding.” The veins and sinuses are local formations, whereas the arteries, which develop as regular narrow tubules, grow into the pulplike tissue of the organ and there continue to divide and subdivide into numerous ramifications.
A comparison of these findings with observations on serial sections of early mammalian embryos, together with the details of the differentiation that accompanies the early vascular transformations within the organ, will be discussed in the following section of this paper.
In order to determine the structure and distribution of the early vessels of the spleen, a study was made of serial sections of pig embryos from 1 to 6 cm. in length. In a 6-cm. embryo, branches of the splenic artery exhibit sufiicient differentiation to facilitate their identification. In all embryos under that size, the only vascular elements in evidence are narrow, irregular, and branching capillaries through which nucleated erythrocytes make their way through the splenic rudiment. These primitive vessels present a distinct endothelial lining, the cytoplasm of which can be easily recognized because of its staining reaction which is more intense than that of the splenic mesenchyme. There are no instances of large unlined sinuses in the organ during the first five weeks of embryonic life.
As early as a 12—mm. embryo it is possible to trace the mesenteric artery along the mid-ventral margin of the dorsal mesogastrium. At various points along its course tiny capillary branches are given off that penetrate the mesenchyme of the early splenic rudiment. These continue to give off branches that form a network which supplies the entire mesenteric tissue. This primitive capillary system persists until the embryo has reached a length of 4 to 6 cm.
In the chick Danchakoff found none of these early capillaries. The venous sinuses, which soon communicated with the intestinal veins, but not with the arteries, were the first vessels to appear. In the pig, however, the first capillaries communicate with both the arteries and veins. By making injections into the dorsal aorta, Sabin revealed the presence of both arterial and venous capillaries in the spleen of a 3-cm. pig. After various but partially successful attempts at injection into the umbilical vein of smaller embryos, the writers resorted to a comprehensive study of serial sections and found the latter far more satisfactory. While the injection method is worthy of support, when successfully carried out, still the difficulty in its manipulation more than outweighs its merits, especially when applied to embryos from 7 to 10 mm. in length. In embryos of that size, due to the non-resistant character of the tissue, it is almost impossible to dissect out the aorta in order to make a direct injection into the splenic artery, and if the cannula is inserted into the umbilical vein, the injection mass fails to get beyond the larger branches of the aorta.
Sabin reports the presence of both arterial and Venous capillaries in the spleen of a 3-cm. pig, but makes no comments concerning the circulation prior to that time. A study of serial sections shows that the network is present in the early mesenchyme of the mesentery even before any marked differentiation in the splenic rudiment has taken place.
When the embryo reaches 4 to 6 cm. in length, a new relation is established between the capillary vessels and the splenic mesenchyme. This change is preceded by the appearance of clear and somewhat open areas in the mesenchymal tissue, in which distinct slits later become evident (fig. 3). At the margins of the slits, the mesenchyme cells still retain their irregular processes, some of which extend well into the lumen of the sinus-like openings.
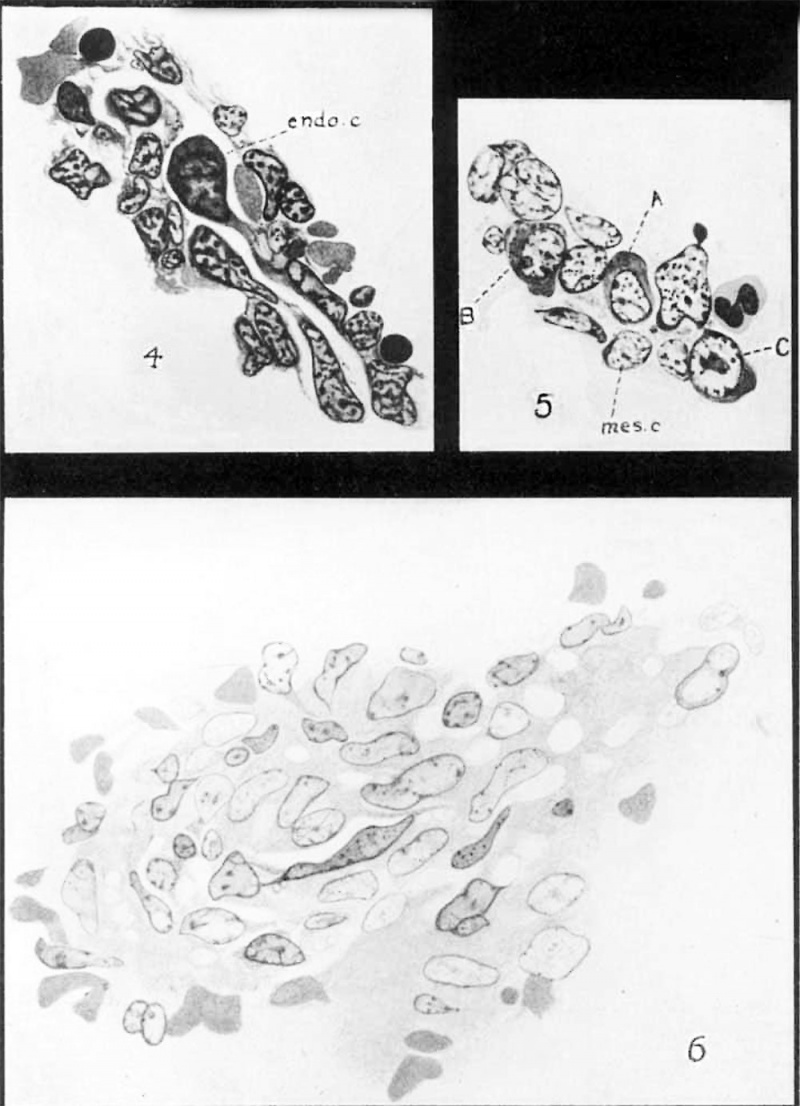
Many of the cells bordering these sinuses have nearly severed their connection with the surrounding mesenchyme. The cytoplasm of some of them has become rounded and very basophilic, while the chromatin of the nucleus has become rearranged, and one or two prominent nucleoli have appeared. One such cell is shown in figure 4. It lies Within the sinus, but it is still connected with the mesenchyme at the margin of the sinus by a broad cytoplasmic strand. Excepting for its connection with the mesenchyme, this cell shows all of the characteristics of the large, lymphocytes (hemocytoblasts of Danchakoff) which become so numerous in the later stages. Numerous examples of this cutting—off process in this and later stages make it quite evident that the mesenchyme bordering these primitive sinuses is very active in the process of furnishing free cells to the sinuses. At the same time these cells assume the characters of primitive lymphocytes or ‘hemocytoblasts’ which later function as the parent cells of the erythroblasts which differentiate within the sinuses. The process is similar to that described by Danchakoff in the developing chick spleen, and by Downey and Weidenreich and many others for the lymph sinuses of lymph nodes of adult mammals. , ‘ In 6- to 7-cm. embryos a connection between the vascular system and the primitive splenic sinuses is established, as is shown by the sudden appearance of the fully differentiated erythrocytes within the sinuses. This vascular transformation begins in the central part of the organ and is at first distinctly localized. In an embryo of 7.5 cm. large areas of the splenic tissue are infiltrated with erythrocytes, but the regions are sharply cut off from the remaining reticulum of the organ. They soon spread toward the periphery, so that in an 8 cm. embryo only a narrow rim of dense mesenchyme remains.
When intense erythropoiesis is established ( 10 to 12 cm.), the sinuses become greatly enlarged and are filled with differentiating erythroblasts, many of the parent cells of which are derived from the cells that border the lumen. Some of the border cells become elongated and assume the characteristics of a flattened endothelium. The walls of the early sinuses are extremely delicate, being composed of a single layer of flattened reticular cells (text fig. A), between which a direct communication with the early splenic pulp is retained. Through these meshes numerous bloodcells migrate and are conveye_d from the sinuses to the veins of the organ.
Text figures A and B are sections of sinuses from a 30-cm. embryo. In figure A, two large cells are migrating from the splenic pulp into an enlarged sinus. The foremost of the cells is a normeblast that already possess considerable hemoglobin and shows a normal accompanying increase in the amount of chromatin material in its nucleus. The cell to its left is a typical lymphoid hemocytoblast with a large open nucleus, possessing a distinct nucleolus and but a small amount of chromatin. Its cytoplasm is strongly basophilic. figure B shows a cell of the same type.
Figs. A and B Sections of splenic six1u.~‘<ss in the splmm of :1 5§}—exn. pig on1bI'y(>. 4 (}0In]I1L 111 :1 ion WU 1e S’) en'c .)11 ) is 1” :1.i11e( ,1rm_ 1 we ea ic; own 9 X 1 c L 1’rh tl l 1 l (T 111 1gl tl dl at ll Llml, me c0111p0se<l of fi:lt.t(>11e1l 1'eticul::L1' cells.
Fig. C Part of it Venous sinus and smmunding pulp in the S})lE‘,(?1'10F‘d.nt1Lll.1lt pig. The sinus w::l| is c0111pnsed of flattouetl 1'0tic11lz1r (cells co11tinu<)us with tl1(».~:e of the-. pulp. Numerous ;~:pa(*.eS between Kheso cells and their ])1‘l)(’,t’/:‘.SPS provide for f1'e<>, c0n1InL111i1::1,ti0n betwetm the pulp zmd the sinus.
By comparing these two figures, a clearer conception of the structure of the wall of the sinuses can be obtained. In text figure A the wall is seen to be continuous with the processes of the neighboring reticular cells, so that an open path with the meshes of the pulp is established. In text figure B a tiny cytoplasmic thread, one of the torn ends of which extends over the cytoplasm of the migrating cell, marks the presence of a continuous Wall at a plane slightly above the free cell, showing that the cell is either penetrating the wall directly or is passing through a mesh in a plane that is slightly below the section here figured.
During the development of the organ the structure of the Venous sinuses remains unchanged. Text figure C was drawn from a preparation of the spleen of adult pig and it represents a portion of a sinus with some of the surrounding pulp. It is evident that the wall of the sinus is composed of a close network of reticulum which is continuous with that of the surrounding pulp. The sinuses are never lined with endothelium; they are merely irregular channels through the reticulum, in the adult as well as in the embryo. Cells from the pulp can readily make their way into the sinus through the numerous openings which occur in the reticular wall, one of which is shown in the figure.
The terminations of the arterial capillaries in the pulp were not studied, but the structure of the arterial capsules in 12 to 17 cm. pig embryos shows that blood—cells from the encapsulated arterioles can readily make their way into the pulp. At this stage the arterial capsules are already established and a striking path of communication between artery and pulp becomes evident. This path lies through the unlined spaces of the tissue of the early capsules, from which numerous channels radiate into the meshes of the pulp. And, as has been shown above, many connections between these meshes and the splenic sinuses are maintained. Thus an open circulation is established before the middle of embryonic life.
3. Arterial Capsules
A study of the differentiation that accompanies the development of the arteries leads one to the questions concerning the splenic capsules or ‘Hulscnartericn’ as first described by Schweiggerfieidel. This observer found the capsules present in both the human and pig spleens. in the human spleen no sharp line of demarcation was seen between the capsule and the reticulum, but in pig the two were distinctly separated from each other. The adventitia was reported as being continuous with the capsule, and the whole structure was thought to serve as a filter.
Muller saw a thickening in the adventitia of the arteries of the human spleen which he thought was similar to the capsules of other forms, He considered them as endings for nerve fibers.
Kyber doubted the communication of the spaces in the capsule with the lumen of the artery, believing that they connect with the lymph system and are filled with lymphoid cells.
Bannwarth, in studying the spleen of cat, found that the spaces of the capsule that are not lined by endothelium serve as channels for the passage of cellular elements from the capsule to the parcnchyme of the organ. He adds that the capsules and the endothelium are derived from a common ‘Keim— oder Grundgewebe’ during their development, and that the later ditlercntiation of this tissue is dependent upon the amount available. If the amount of this tissue is small, an ordinary capillary is developed, but if there is an abundance present a capsule is formed. During embryonic life the latter serve as forerunners of the follicles, but in later life they are buds of growth for tissue that enters the splenic pulp.
Kultschitzky is of the opinion that the cells of the capsules are leucocytes.
Whiting more of the details of the structures and reports that blood cells pass between the muscle cells into the tissue of the capsule and from there into the sinus that surrounds it.
Weidenreich gives considerable attention to the ‘Hulsen’ of the human spleen. Here they are characterized by a gradual increase in the size of the wall of the artery which disappears gradually on the opposite end, thus giving the capsule the shape of a spindle. On the inner surface is an endothelium, the cells of which possess but scanty amounts of protoplasm and large nuclei that extend into the lumen of the capsule. In some cases this condition is so pronounced that the lumen is almost completely obliterated. The capsule itself consists of compact tissue With many irregular nuclei and no distinct cell walls. Many very fine fibers, so arranged that they are often mistaken for cell Walls, lie parallel with the vessel, but no elastic fibers are present. The capsule is cut off from the reticulum by a rather distinct band of fibrous tissue, some of the fibers of which extend into the reticulum. In the outer zone are numerous leucocytes, and throughout the entire capsule spaces unlined by endothelium are filled with deformed erythrocytes and a few scattered leucocytes.
Weidenreich is not positive concerning the nature of the cells which make up the capsule. He finds that the structure of the capsules is similar to that of the inner fibrous membrane of the larger arteries described by Henle, and believed by him to be derived from the endothelium. The capsule is not a thickening of the adventitia nor a continuation of the media, for the muscle cells of the latter are of very different character. Weidenreich cannot agree with Carlier that the capsules are made up of reticulum, for the tissue does not resemble reticulum in appearance and the nuclei are much more irregular than those of the reticulum. He is inclined to agree with Kolliker’s view that the endothelial and capsular cells are derived from a common cell-form.
Sabin agrees with Bannwarth in maintaining that the ellipsoids or capsules appear before the follicles and disappear in later embryonic life when the follicles‘ make their appearance. “Thus the ellipsoid is the primitive lymphoid structure of the spleen.” In the spleen of pig, the development of the capsules is initiated in 15 to 17-cm. embryos. They are recognized as slightly condensed areas of the mesenchyme, penetrated by distinct arterial capillaries or arterioles. The only relation they bear to the early splenic follicles lies in the continuity of a common mesenchymal reticulum from which both are differentiated, but at sharply separated regions along the arterial wall. In a 20-cm. embryo, the structure of the capsule has become more distinct. Weidenreich is inclined to think of the cells that make up the capsule as transformed endothelial cells. However, by making a study of their development in a closely graded series of embryos, the capsule cells are found to be cells of the general mesenchyme that have drawn in their processes and become more compact. The fact that they are not endothelium can be readily demonstrated by applying Mallory’s phosphotungstic-acid stain together with Krause’s gold chloride for reticular fibers. This method brings out the cytoplasmic processes of the reticular cells and also the reticular fibers that extend for long distances from cells of the capsules through the reticular cells of the pulp. These fibersvare not found in the endothelium that lines the vessel of the capsule, nor is the same intimate connection between cytoplasmic processes in evidence.
A great many erythrocytes are found in the walls of the capsules. These red blood-cells pass through the porous wall of the arterial capillary into the unlined spaces between the capsule cells, and from there make their way into the meshes of the reticulum of the pulp.
The presence of a great many contorted nuclei within the capsules of adult spleens has led many workers to believe that they represent the presence of numerous leucocytes. A histogenic study, however, indicates that by far the greater number are the nuclei of the capsular cells.
In the spleen of adult pig a band of fibrous tissue separates the capsule from the splenic pulp, but in the embryo this sharp line of demarcation is not present.
The Origin and Differentiation of the First Blood-Cells
In the pig the blood-forming activity of the spleen begins in embryos of from 3 to 4 cm. in length, but the process of differentiation along this li.ne is subject to considerable variation in different embryos of this size. In 3- to 3.5-cm. embryos a few isolated fields are usually encountered where cells of the mesenchyme are becoming more basophilic and are beginning to lose their connection with the mesenchymal syncytium. When free, these cells undergo further changes in structure and staining reaction until they become the large lymphocytes which are typical of all hematopoietic organs.
From the explanation that has been given of the early system of vascularization it could perhaps be said that ideal conditions are present in the splenic tissue for the development of a new hematopoietic organ by metastasis of a preformed blood—forming organ, the cellular products of which might be held in the meshes of the splenic syneytium where they could find favorable conditions for multiplication and differentiation. This interpretation was held by most of the early heinatologists, and is still being maintained by some members of the dualistic school. Stockard is of the opinion that the blood—forming organs are obliged to shift to different positions in the embryo in order that spaces unlined by an endothelium may be utilized for blood—cell proliferation. He states that “There is evidence to indicate that definite environmental conditions are necessary for blood—cell proliferation and multiplication. Blood-cells do not normally divide when completely enclosed by vascular endothelium. This is the key to the shifting series of so—called hematopoietic organs found during embryonic development.” He further emphasizes the fact that the tells in the yolk sac blo0d—islands “continue to divide until they become surrounded by endothelium.” They then “lose their hematopoietic function and become a vascular net through which the blood circulates. The liver now takes up the role of harboring dividing blood—cells Within its tissue spaces, when these spaces become vascularized by endothelium, here again the blood-cells no longer multiply but merely circulate.” Such an interpretation denies the possibility of local formation of blood elements from the mesencliyrnal cells of the bloodforming organs, and also from the endothelial walls of the early blood-vessels. Many authors, however, regard the endothelium as a definite source of blood-cells of the various classes. According to Schridde, cells of the myeloid series are derived exclusively from the endothelium of the blood-vessels, while Maxiinow, Danchakoff, and Jordan derive all types of blood—cells from the endothclium of the earliest vessels, as Well as from the local mesenchyme of the blood—for1ning organs. Lobenhoifer and H. fischer also claim that the endothelium may assume a hematopoietic function.
In 3 to 4—cm. pigs the spleen shows its first signs of blood cell formation, and is therefore a good object for study. In embryos of this size it is easy to trace all the intermediate stages between the free cells present and the fixed cells of the -mesenchyme. The differentiation of free lymphoid cells the expense of the mesenchyme does not begin in any definite location within the organ, nor is there any evidence that a change in the vascularization of the organ initiates the process, as cells in no relation with vessels and sinuses show signs of differentiation. Cytoplasmic and nuclear changes do not always proceed at a uniform rate, but, in most cases, the first evidence of differentiation is a slight increase in the basophilic reaction of the cytoplasm of the cell-body (fig. 5). This may begin to appear while the cell is still a part of the general syncytium, or the cell may become entirely free and then assume its more pronounced basophilia. In some cases the nuclear modification precedes that of the cytoplasm and a typical large lymphocyte nucleus is seen in a cell that is still connected with the mesenchyme. This condition, however, is rare and is usually confined to mesenchymal cells at the margins of the sinuses.
In making a study of the nuclear structure of the first ameboid cells, one soon finds many intermediate stages between the nuclei of mesenchyme cells and those of the typical large lymphocytes. figure 5 includes a mesenchyme cell (mes.c.) which shows a nucleus that is typical of this tissue. It is a clear, open structure that is round or oval in shape and possesses a rather distinct nuclear membrane. The small amount of chromatin that it contains is distributed in the form of a fine irregular network. Two nucleoli are generally present, but the number is Variable. The cell at A shows considerable change in its cytoplasm, but by comparing its nucleus with that of the mesenchyme cell, their identity is at once apparent. In the cell at B the cytoplasmic change is more pronounced and a slight nuclear modification has taken place. The nucleus has begun to enlarge, and the chromatin which was quite uniformly distributed, is collected in larger knots. Part of the fine chromatic network still remains. In C the nucleus has become very characteristic; its nucleolus is a distinct structure, located in or near the center of the nucleus and surrounded by a clear zone that contains but little chromatin. The chromatin knots that were beginning to appear in B are more numerous and are for the most part confined to the periphery of the nucleus. The cell body is strongly basophilic. A cell of this type continues to differentiate until it becomes the large lymphocyte that is found in all the lymphoid tissue.
These large lymphocytes show a marked proliferative activity in a 4-cm. pig, their products being added to the groups of large free cells that are found in the meshes of the splenic mesenchyme. In a 6-cm. pig the process is more pronounced, many of the cells having already differentiated into erythrocytes.
The details of the modifications that take place in the stem cells as they develop into erythrocytes need not be reviewed here, as many detailed accounts are found in the hematological literature.
It should, however, be noted that the differentiating cells may show considerable Variation in size. The large lymphocytes may undergo direct differentiation, or Various cell divisions may intervene, producing smaller cells which then take on the erythroblastic characters. In the first case the large cells still retain the nuclear structure of the large lymphocyte when they already show the presence of hemoglobin in their cytoplasm. The nuclcus later becomes dense and pycnotic, as seen in the typical normoblast. In the second case, differentiation begins in a smaller cell and the nuclear changes precede those of the cytoplasm.
We are now confronted with the following problem: Is the erythropoietic activity confined entirely to cells derived from the local mesenchyme, or do erythroblasts brought in by the bloodstream continue their multiplication and differentiation within the meshes of the splenic tissue‘? After making a survey of a number of 3- to 4—cm. embryos it was found that groups of blood cells in approximately the same stage of differentiation were located in the larger vessels at the periphery of the organ. If the vessels containing these groups of cells were followed through a series of sections, they were seen to communicate with vessels in the mesentery of the spleen. The most striking feature was the absence of the large parent cells that are seen in the regions of differentiation in the central portion of the organ. By far the greatest number of the cells that compose such intravascular groups are normoblasts or erythroblasts with the typical ‘Radkern’ nucleus. Cell division is very pronounced, many mitotic figures being seen in cells that are strongly acidophilic. Such observations might be interpreted, at first glance, as indicating the presence of erythropoiesis without the participation of the local tissue. However, by tracing the vessels through a series of sections, they Were found to connect with the enlarged sinuses within the organ and with the larger veins in the mesentery. It is probable, therefore, that the cells had begun their differentiation within the central portion of the organ, Where erythropoiesis was very active, and that they had made their way through the mesenchymal tissue into the sinuses, and from here to the larger vessels that leave the organ.
Although the entire process of local differentiation of mesenchymal cells into hemocytoblasts and these in turn into erythrocytes, can be readily observed within the splenic tissue, yet one cannot entirely disregard the possibility of an accompanying differentiation of elements brought in by the blood-stream. In a 6—cm. pig an occasional erythroblast is observed in the peripheral circulation. To deny the possibility of their proliferation and differentiation in the retarded blood currents would be contrary to well—established observations. Their scarcity in number, however, would not make it possible for them to play an appreciable role in the hemogenic process with.in the organ.
According to Danchakoff, the spleen of the chick embryo is not an active erythropoietic organ. The sinuses of a normal spleen contain a few young cells undergoing erythroblastic differentiation, but the process never becomes very marked. In mammals, on the other hand, numerous authors have reported that the spleen of the embryo is a very active erythropoietic organ. The beginning of this activity is seen in pigs of from 4 to 6 cm., although at this stage the process is limited to a few sharply defined areas. The process gradually spreads in the older embryos until in the 17.5-cm. stage it involves practically the entire organ, excepting the region of condensed mesenchyme surrounding the developing arteries (fig. 8, 7.5 cm,).
When the first erythroblasts can be recognized (4 to 6 cm.) there are a few venous sinuses present, and some of them contain erythroblasts. But all of the erythroblasts are not within sinuses, as is claimed by Danehakoff for the chick. They occur in small groups, many of which are at a considerable distance from the nearest sinus. They are differentiated from similar groups of hemocytoblasts located within lacunae in the dense mesenchyme. Since the hemocytoblasts are derived from the mesenehyme by the isolation of some of its cells from the general syncytium, it is to be expected that the free cells will be contained in cavities of the mesenchyme. Neighboring lacunae containing free cells run together and produce the larger spaces containing the first erythroblasts. These spaces are not sinuses, for the latter are produced as splits in the mesenchyme indepenent of the process of hemocytoblast formation, although a few hemocytoblasts may be formed from the margins of the sinuses after the latter have become established (fig. 4). The larger spaces containing nests of erythroblasts may later connect with sinuses, and hemocytoblasts entering the sinuses usually differentiate into erythroblasts. For these reasons numerous sinuses are encountered which contain erythroblasts in Various stages of differentiation. In many cases, however, the erythroblast nests remain separated from the sinuses for some time. Frequently mesenchyme cells are seen to pass between the individual cells of a group, which is further proof that these cells are not located within sinuses. It is quite possible that many of the larger lacunae later function as sinuses.
In the later stages ( 17 cm.) the erythroblasts do not show the distinct grouping which is so characteristic of the earlier stages of erythropoiesis. They are uniformly distributed throughout the entire pulp and are located within the sinuses as well as in the pulp strands between the sinuses. The majority of the hemocytoblasts and younger erythroblasts are outside of the sinuses.
Similar conditions were seen by H. Fischer in the spleens of human embryos, Where hematopoiesis was very active in the veins and venous sinuses of the pulp. According to M. B. Schmidt, on the other hand, intravascular erythropoiesis in the venous sinuses of the pulp from cells derived from the endothelium is of greatest importance.
According to Danchakoff, the lines of differentiation pursued by hemocytoblasts depend entirely on extrinsic environmental conditions. The relations of the primitive blood—cells to the venous sinuses are of particular importance in this connection. In the yolk—sac and bone—marrow of birds and reptiles and in the spleen of birds those hemocytoblasts which pass into the larger blood—channels are differentiated into erythrocytes, while those outside_ of the vessels either differentiate into granuloeytes or remain as lymphoid wandering cells. Close proximity to the sinuses favors granulocyte differentiation. Venzlaff also claims that in the bone—marrow of birds erythrocyte development is largely intravascular.
Intravascular erythropoiesis in mammalian embryos, particularly in the liver, is also claimed by Neumann, Schmidt, Kuborn, Kostanecki, and Nageli, while Van der Stricht, l\=IaXim0W_ Schridde, Lobenhoffer, H. Fischer, and Mollier maintain that erythropoiesis in the mammalian embryonic liver is chiefly extravascular. According to Maximow and Wleidenreich, the process is also largely extravascular in the mammalian bonc—marrow.
For the embryonic marrow Maximow states that the developing cells lie in groups in close contact with the endothelium of the vessels. In such places the endothelial membrane later loosens up and acquires numerous openings. Through these openings blood-plasma enters the tissue, loosens up the groups of developing erythrocytes and forces them into the vessels. Besides the young denucleated erythrocytes a few of the riper normoblasts and megaloblasts are carried into the circulation where they complete their development in a normal manner. Lymphocytes are also carried into the blood—vessels in this manner and many of them migrate through the endothelial membrane. In the early stages, while the marrow is still of the primary, lymphoid type, these lymphocytes differentiate into erythrocytes within the vessels. A similar mode of entrance of erythrocytes from the blood forming tissue to the vessels was described by Lobenhoffer and H. Fischer.
According to Weidenreich, the ‘cell—nests’ of the bone—marrow constitute the blood-forming tissue. These cell—nests are appendages to the venous capillaries, and the endothelium of the latter is deficient in the region of the cell-nests.
Venzlafi maintains that erythrocyte differentiation takes place Within the Venous sinuses of the marrow of birds from lymphocytes which have passed out of the ‘Leukoblastenhaufen’ (cell nests of Weidenreich). The endothelium of the sinuses is deficient in the region of the latter.
For the spleen of the chick Danchakoff states: “The spleen during the second stage of development is characterized by the development of a net of wide venous capillaries developed in the mesenchymatous syncytium, by an intense granulopoiesis outside the vessels and by a potential erythropoiesis within the vessels.” “The granulo- or leukopoiesis develops around the large venous sinuses under conditions identical to those under which they develop in the yolk—sac and in the bone marrow.” For the spleen of the pig it has already been shown that early erythropoiesis is largely extravascular. The first groups of erythroblasts are usually at some distance from the sinuses in lacunae of the dense mesenchyme. As the erythropoietic process becomes more active the mesenchyme acquires a looser texture, owing to the fact that many of its cells are cut oil" from the general syncytium. This finally results in the formation of a loose network of‘ mesenchyme through which the erythroblasts are carried into the sinuses. This is the structure of the pulp in embryos of 15 to 17 cm., and in these spleens the sinuses contain numerous erythroblasts. Some hemocytoblasts and nucleated red cells are also seen in the sinuses of younger embryos, but intravascular erythropoiesis is not very active until communication has been established between the pulp lacunae and the sinuses. According to Danchakoff, the presence of the blood-plasma in the Venous sinuses is responsible for the exclusive intravascular erythropoiesis in the bird’s spleen. This may be the determining factor even in the mammalian spleen, where the process is largely extravascular, because the ‘open’ circulation is established in limited regions of the spleen soon after the first sinuses are formed. This is seen even in a 6—cm. embryo in which small areas of the spleen pulp are infiltrated with fully differentiated erythrocytes. The mesenchyme of these areas forms a loose network, and it is clear that the erythrocytes are not located within vessels. Since the erythropoietic function of the organ is still in its earliest stages, it is clear that the erythrocytes of these areas are not of local origin. Plasma may diffuse throughout the mesenchyme from these areas of infiltration, and this may influence the differentiation of the hemoeytoblasts. Under such conditions, the distinction between intra- and extra-vascular erythropoiesis seems to be of doubtful value.
Few granulocytes are formed in the pigs spleen at any stage of its development. However, a few mononuclear granulocytes were seen in the spleens of 7.5-cm. and 13—cm. embryos. The great majority of them are located in the marginal regions of the organ where the mesenchyme is still very dense and where venous sinuses and the ‘open’ circulation have not yet been established. They are not grouped in ‘nests’ as are the erythroblasts and each cell is located within a lacuna of the dense mesenchyme. The cytoplasm of many of these cells is still Very basophilic and the nuclear structures indicate that the granules have differentiated in cells which have but recently been cut off from the mesenchyme. fine processes are sometimes seen to connect with the surrounding mesenchyme, indicating that the granules may appear before the cells are completely isolated. There is little dense mesenchyme left in the spleen of the 13-cm. embryo, hence the granulocytes are located immediately under the capsule.
In the pig’s spleen there is no evidence whatever for Schridde’s and T1'irk’s claim that the granulocytes are derived from ‘myeloblasts’ which have their origin in the endothelium of the bloodvessels and sinuses. Erythroblasts may be derived from the walls of the sinuses at a time when the latter are merely splits in the mesenchyme, but there is no evidence to indicate that cells from this source might differentiate granules in their cytoplasm. The free mesenchyme cells which differentiate into granulocytes are far removed from vessels or sinuses, and hence it is not likely that they bear any direct relationship to endothelial cells.
The differentiation of a few erythroblasts from lymphoid cells cut off from the marginal mesenchyme of the venous sinuses does not confirm the claims of Schridde and Turk that erythroblasts, as well as myelololasts, are always derived from cells of the vessel walls. The early sinuses have no walls, for they are merely splits in the mesenchyme.
At the 17—cm. stage the greater part of the spleen is composed of free cells; the fixed mesenchymatous tissue has become greatly reduced in amount, and it is evident that the majority of the lymphoid cells which are cut off from the mesenchyme differentiate into erythrocytes. The greater part of the mesenchyme of the pulp is thus used up in the process of erythrocyte production. The remaining fixed tissue later differentiates into the loose network of reticulum which forms the supporting framework of the organ in the adult.
Much of the condensed mesenchyme surrounding the developing arteries is also used up in the production of free cells during the development of the lymphoid arterial sheaths. But in this region the lymphoid cells remain lymphoid and none of them differentiate into erythrocytes or granulocytes.
Development of the Arteries and Lymphoid Arterial Sheaths
The further differentiation of the vascular system leads to the formation of distinct arteries, the development of which initiates the formation of the ‘White pulp’ of the organ. The White pulp is composed of the lymphoid sheaths which soon develop around the arteries, and later distinct nodules are also added to it. The arterial sheaths are at first composed of very dense mesenchyme, as described by Danchakoff for the chick spleen. They are surrounded everywhere by the loose pulp which, at this stage, consists largely of free cells. Later this dense mesenchyme is resolved into a Very loose network, the meshes of which are packed with lymphocytes. Most of these free lymphoid cells are ‘small lymphocytes,’ and in these lymphoid sheaths and in the early follicles it is not difficult to work out their origin.
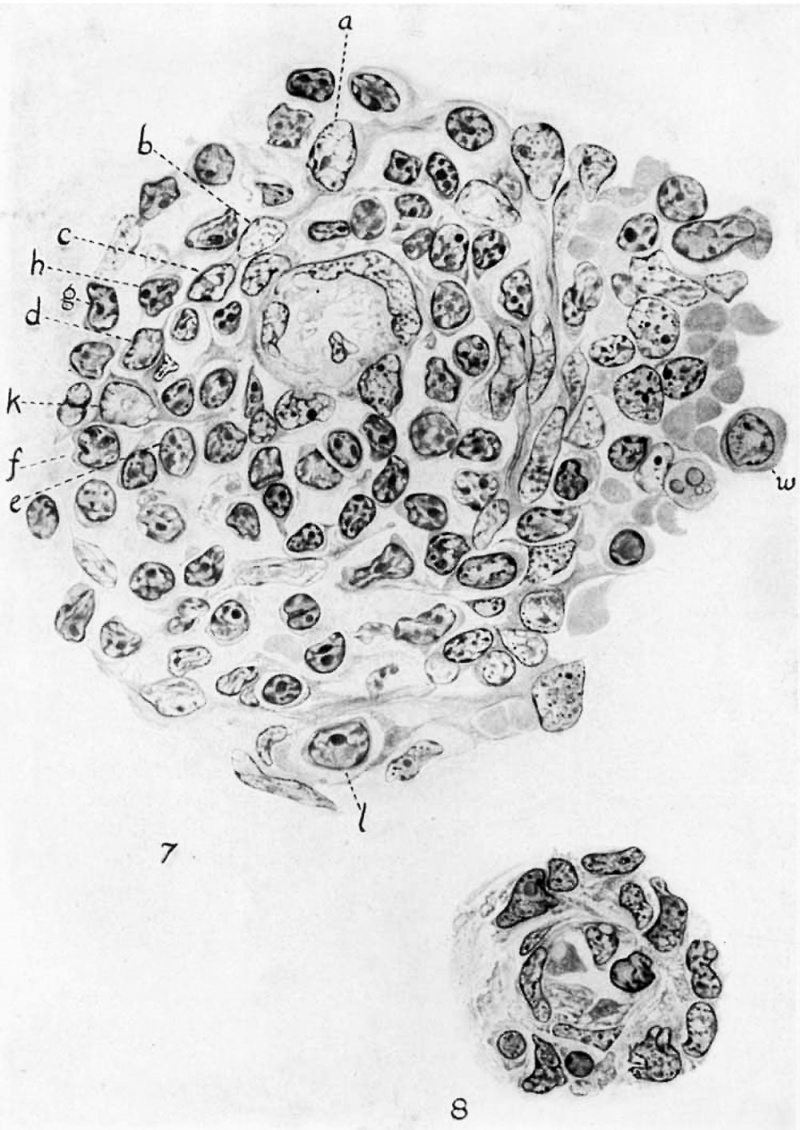
figure 7 is a cross—section of a typical artery and its lymphoid sheath in a 30—cm. embryo. The vessel is surrounded by a single layer of smooth muscle fibers which lie directly underneath the endothelium of the Vessel. When Weigert’s elastic tissue strain is employed, it is found that elastic tissue fibers, which extend parallel with the artery, lie between the endothelial and muscle cells, forming the beginning of the inner elastic membrane of the arterial wall. In this figure but one endothelial nucleus is shown, which extends well into the lumen of the Vessel. The cytoplasmic processes that fringe the remaining portion of the lumen rep resent sections of the cell bodies of neighboring endothelial cells, the nuclei of which were in a different plane than the section here figured.
The reticulum surrounding; the muscular coat of the artery is of particular interest; its cells possess long processes which form a network, in the meshes of which numerous small lymphocytes are located. At the outer border of this early lymphoid sheath, the reticular cells are crowded into parallel bands that lie in concentric order about the artery.
A great Variation is seen in the structure of the reticular nuclei. The cell at in the upper part of the figure possesses a nucleus identical with those of the reticulum of the pulp portion of the organ. It is a large oval structure with a distinct eccentrically placed nucleolus and a Very small amount of chromatin distributed in the form of a fine network. The nuclear membrane is very pronounced in contrast to those of the smaller reticular cells. The cell at 1), although connected by cytoplasmic processes with the reticular cell already described, shows Very marked differences. Its nucleus is scarcely half as large as those of the reticular cells of the pulp. No distinct nucleolus is present, and the small amount of chromatin is uniformly distributed throughout the nucleus, but the nuclear membrane is quite indistinct. Many intermediate stages can be found between these two types of cells. Further study of the same follicle shows that many of the small lymphocytes are formed in situ from reticular cells similar in structure to the cells shown at c and d. A reticular cell identical and continuous with a, excepting that its nucleus has a few larger chromatin granules, is shown at b. The cell c has a nucleus similar to that of b with a slight addition of chromatin, but its cytoplasm is more basophilic and its processes have become very delicate. At d both nucleus and cytoplasm are darker and the cell is almost completely isolated from the general mesenchymatous network. The free cells e, f, g show further advance in differentiation which is completed in h, a typical small lymphocyte.
The cell It shows clearly that large lymphocytes may also be cut off from the mesenchyme. The cytoplasm of this cell is still continuous with the mesenchyme, but the structure of its nucleus approaches that of the large lymphocytes l and m. As indicated in the figure, large lymphocytes are extremely rare in the territory of the early follicles. They are far more numerous in-the pulp. The great majority of the free cells of the early follicles are small and medium-sized lymphocytes. Among the former are many cells whose nuclei have not developed the full amount of chromatin characteristic of the small lymphocyte. With Danchakoff, these cells might be classified as ‘dwarf hemocytoblasts,’ although it is evident that in the pig they are not products of the proliferative activity of large lymphocytes (hemocytoblasts) similar to those in the pulp at l and m. Their nuclei are clear, because the cells have only recently been cut off from the reticulum and have not yet completed their differentiation.
Figure 8 is a section of an early artery in the spleen of a 6-cm. pig. The arterial wall is just beginning its differentiation'—a few elongated nuclei marking the initial stages of muscular development in the media of the vessel. The vascular endothelium is Very distinct, its nuclei bulging into the lumen so that it is nearly obliterated. In the mesenchyme surrounding the artery no discernible differentiation has taken place, but in other regions throughout the mesenchymal tissue of the organ isolated groups of hemocytoblasts are being differentiated into erythroblasts. Figure 8 shows two darkly staining ‘nuclei near the arterial Wall, but cells of the same type are encountered at various distances from the vessel, so that no particular significance can be placed upon their differentiation at this stage.
However, in a 7.5—cm. embryo a marked differentiation has taken place. figure 6 is a section of an early arterial capillary that is cut somewhat obliquely. The endothelium lining the vessel possesses large clear nuclei, oval in outline, that extend so far into the lumen of the vessel that only a narrow slit-like open— ing is discernible. Surrounding the endothelium is a group of small reticular cells that closely resemble the forms seen in a 30cm. embryo (fig. 7, cell a). At this stage (7.5) most of the small cells have open nuclei——none being present that have differentiated into true small lymphocytes.
By comparing the cells around the artery with those of the surrounding reticulum, one is soon convinced that both are parts of a general splenic mesenchyme that has begun to differentiate along two quite distinct lines. 1) That immediately around the arterial wall has drawn in its processes until the cells lie close together and are connected by short delicate cytoplasmic strands. lvlany of the nuclei are small, round, deeply staining structures, with a distinct nuclear membrane and one or two coarse chromatin granules. 2) The mesenchyme surrounding the more compact area has become converted into a loose spongy network, the meshes of which are crowded with fully differentiated erythrocytes, which have, without doubt, been brought in by the blood—strcam, as there are but few isolated regions of erythropoietic activity in the spleen at this stage. By a careful study of the two areas, all possible stages between the cells around the arterial wall and those of the general reticulum can be identified.
The above findings are closely related to the observations on the development of the lymph nodes in the pig as reported by Sabin. The latter found the first evidence of the formation of lymphatic nodes in embryos 3 cm. long. In these early nodes the capillaries penetrating the connective tissue are surrounded by clumps of nuclei which lie “within the syncytium and belong to the connective tissue.” They are distinguished from those of the remaining portion of the connective tissue by the fact that they are situated in clumps and that some show mitotic figures, while others are smaller and take the deep stain of a newly divided nucleus. Up to an 8—cm. pig, the node has none of the characteristics of the adult structure. T here are no lymph cords, nor germ centers, no lymphocytes and no sinuses. In an 8-cm. embryo the artery is seen lying parallel with the vein and extending into the core of the node. Associated with the development of the artery is the formation of the first lymphoid elements of the node. Surrounding the arterial capillaries are groups of small cells that can readily be separated from the adjoining connective tissue cells. The latter possess large, faintly staining and oval nuclei and their protoplasm is in the form of a definite network, whereas the smaller cells have deeply staining nuclei and a distinct nuclear membrane. The nuclear network and the chromatin granules are coarser and there are one or more nucleoli. Moreover, the protoplasm makes a narrow but definite rim around the nucleus.
Sabin reports that “between the connective tissue cells and the lymphocytes one can see every possible transition.” Yet the presence of all intermediate stages is not held as sufficient evidence to prove their local origin, for in seine cases the clumps of free cells appeared as though they had filtered through the vessel wall. Sabin is of the opinion that such evidence is not sufficient to prove either the hematogenous or connective-tissue origin of the lymphocytes.
The group of small cells around the capillaries in the nodes, as described by Sabin, remind one of the small mesenchyme cells surrounding the early arteries of the spleen. Sabin states that in early stages the small cells are distinctly a part of the connective tissue. The same is true in the spleen, although many cells can be found in the process of rounding off to form free lymphoid cells.
Danchakofi’ sees the arteries grow into the chick spleen at about the middle of embryonic life. The mesenchyme around the arteries proliferates and forms lymphoid hemocytoblasts by the rounding—ofi' and isolation of its cells, but their differentiation into granulocytes is no longer evident. Thus the arteries and their branches become surrounded by areas of mesenchyme cells which soon appear as islands of mesenchymal tissue which occupy the interstices between the arteries and the early pulp of the organ. These islands constitute the rudiments of the follicles. The lymphoid hemocytoblasts that are in the islands show intense proliferative activity, resulting in daughter cells having the character of ‘dwarf hemocytoblasts’ which are further differentiated into true small lymphocytes. The small lymphocytes are present both in the red and the white pulp and are morphologically identical in both regions.
According to the above description, ‘dwarf hemocytoblasts’ are the essential precursors of the true small lymphocytes; they are produced by rapid proliferation of lymphoid hemoeytoblasts, the daughter cells of which never reach the large lymphocyte stage.
The early lymphoid arterial sheaths of the pig contain Very few large lymphocytes (hemocytoblasts) and mitotic figures are S0122; ce. The great majority of the small lymphocytes are, therefore, cut off from the mesenchyme without passing through the hemocytoblast stage. Additional evidence for this View is furnished by the large number of intermediate forms which lead from the typical small lymphocyte to the fixed mesenchyme cell (fig. 7).
Van der Stricht sees the small lymphocytes formed during the later part of embryonic life and attributes their increase in number to cell division, and not to a direct transformation of reticular cells.
Jolly, in a study of the development of the spleen of the white rat, found the small lymphocytes arising from the ‘primitive spleen cells’ which he describes as large cells with a slightly basophilic cytoplasm and large clear nuclei. ‘Lymphocytes’ were not seen, however, until near the time of birth. In developing lymph nodes he saw the same type of large lymphoid cells formed by transformation of the mesenchyme tissue. Maximow reports the formation of small lymphocytes from fixed cells in developing lymphatic nodules. These fixed cells are Very small cells which round off and become ameboid, when they appear either as small or medium-sized lymphocytes or histogenous Wandering cells which become transformed into small and large lymphocytes. Some may also form granulocytes. Sabin also believes in the possibility of the derivation of small lymphocytes directly from the mesenchyme in developing; lymph nodes, although she admits that the evidence for this is not very convincing.
The "writers find, however, that in the embryonic spleen of the pig evidence for the derivation of free lymphoid cells from the fixed cells is by no means lacking. figures 6 a.n.d 7 show reticular cells at Widely separated stages of embryonic development, in both of which numerous small lymphoid cells are being cut off from the local reticulum, many of them being transformed into typical small lymphocytes without the intervention of the large lymphocyte stage. Not many of these smaller lymphocytes can be proliferative products of large hemocytoblasts, for the latter are rarely observed in the early follicular rudiments.
According to Weidenreich and Downey (pp. 367—369), lymphocytes of various types, including the typical small lymphocyte, and large mononuclears or macrophages can be cut off directly from the reticulum of the lymph nodes of adult animals. Their figures 2, 3, and 4 give the details of the process as seen in the interfollicular tissue of guinea-pig lymph node an.d in a germ center of a follicle of cat node. Froin this it is evident that the process of isolation of free lymphoid cells from the fixed tissue which was seen in the early spleen persists in the hematopoietic organs of adult mammals. In the adult animal all types of lymphoid cells may be derived from the fixed tissue of the same region of the organ, while in the early spleen large lymphoid cells are derived from the mesenchyme of the pulp and small lymphocytes from that of the future lymphoid sheaths of the arteries, while the large mononuclears do not appear until later.
The fact that the earliest small lymphocytes exhibit marked ameboid properties has led different Workers to believe that they reached the primary follicular rudiments by migration from the pulp portion of the organ. With this interpretation in mind, a careful study was made of various spleens in which the first small lymphocytes were evident. In all cases the ameboid activity is very pronounced, but it is limited to the regions surrounding the arteries. No indications were found of cells migrating toward the follicles. On the other hand, migration from the follicles into neighboring sinuses is quite frequently observed. This is especially true where a large venous sinus extends parallel with the lymphoid sheath of the artery. Here the small cells enter the slow blood—current of the sinus and continue their differentiation before being carried into the general circulation. Small lymphocytes were not observed in the pulp previous to the formation of lymphoid arterial sheaths. The first small lymphocytes of the follicles could, therefore, not be derived from the pulp.
According to Schridde and Turk, lymphocytes are derived from the endothelium of lymph—vessels and myeloid cells from the walls of blood-vessels. The embryonic spleen is an ideal organ for testing this theory. It has been shown that the mesenchyme bordering the earliest sinuses is capable of giving off free cells, most of which differentiate into erythrocytes. But it has also been shown that this mesenchyme differs in no way from that in other regions of the organ, and it can, therefore, not be regarded as being more highly differentiated or specialized than is the latter. The liberation of free cells is by no means confined to the sinus rnesenchyme, and hence the process has no value as support for the theory of Schridde and Turk. Most of the free lymphoid cells derived from the mesenchyme of all parts of the organ, before the lymphoid arterial sheaths are established, differentiate into erythrocytes. A few granulocytes also differentiate from similar lymphoid cells, but the latter never show any relationship to the endothelium of blood-vessels. The preparations show very clearly that the first cells to differentiate granules in their protoplasm are cut off from the mcsenchyme, and that they are usually located at some distance from the nearest blo0d—vessels or sinuses.
For the lymphocytes of the follicles the case is still clearer. The follicles are expanded portions of a continuous lymphoid sheath the cells of which are derived from the condensed mesen— chyme surrounding the arteries. Lymphocytes of all types, but especially the smaller ones, are liberated from this mesenchyme, the endothelium of the vessel taking no part in the process. It might, of course, be assumed that the dense mesenchyrne surrounding the artery constitutes a part of the vessel wall, and in that case the free lymphocytes liberated by the mesenchyme would be derived from cells of the vessel W’8.ll—‘SChI'lClde’S ‘Blutgefasswandzellen.’ However, according to the theory, lymphocytes would come only from the walls of lymph—vessels, while the blood-vessel-wall cells would furnish only myeloblasts and erythrocytes. In the developing spleen it is very clear that the follicles and lymphoid sheaths are in no way associated with lymph-vessels. They are developing about the arteries, and lymph-vessels are not present: hence the facts do not conform to the theory, even though we count the condensed mesenchyme about the arteries as a part of the vessel Wall.
The endothelium of well-established vessels of the spleen apparently plays no part in the formation of fixed or free cells, or of ‘adventitial’ cells, about the vessel wall, and hence Herzog’s claim that the adventitial cells derived from the endothelium of growing capillaries in experimental material give rise to numerous small lymphocytes with dark nuclei does not apply to the early spleen.
Relations Between Pulp and Follicular Tissue
The relationship of the spleen pulp to the malphigian bodies is a question over which there has been much discussion among hematologists. Because. the early spleen is composed entirely of ‘pulp,’ and the small lymphocytes are never numerous until they appear later in the adventitia of the arteries, a region which continues to furnish most of the small lymphocytes of the organ, and because myeloid metaplasia is confined to the pulp, many authors have believed that pulp and follicles are independent in origin and function and that they are more or less antagonistic to one another.
Since the question is discussed in detail in the paper by Weidenreich and Downey, there is no necessity for reviewing the extensive literature here. HoWeveij there are a few points regarding the origin of pulp and follicles in the mammalian embryo which need further consideration. As shown by the writers and others, the splenic rudiment soon becomes converted into a loose, spongy mesenchymatous organ with many wide blood sinuses, and numerous free cells held in place by the strands of fixed cells remaining from the original mesenchyme. At this time, the great majority of the free cells are either erythroblasts or hemocytoblasts which will differentiate into erythroblasts at a later period. There are no follicles or arteries with lymphoid sheaths at this stage. The structure of the entire organ corresponds to that of the pulp of late embryonic or adult spleens, excepting that in the adult spleen erythropoiesis is nearly or completely suppressed. The frequently expressed statement that the early embryonic spleen consists entirely of ‘pulp’ seems, therefore, to be essentially correct.
Because the so—called lymphoid tissue of the spleen, i.e., the tissue of the lymphoid sheaths of the arteries and of the malpighian bodies or follicles, appears later in the development of the organ, and because during myeloid metaplasia in the adult the erythroblasts and myelocytes appear almost exclusively in the pulp, it has been assumed that the follicles and lymphoid sheaths consist of tissue which is foreign to the original spleen in its early embryonic stages.
Even the lymphoid cells of the two regions are supposedly different in origin and function; those of the follicles being true ‘lymph—adenoid’ lymphocytes and those of the pulp histogenous, myelopotent, myeloblastic, or leukoblastic lymphoid cells, ordinarily remaining dormant and undifferentiated, but difierentiating into myelocytes or erythroblasts when under the influence of the proper irritant, or those of the pulp belong to a special race of ‘splenocytes’ (Naegeli, Tiirk, Banti) which are exclusively splenoid and not included in either the myeloid or lymphoid tissues} Dominici, Downey, and Weidenreich and others have shown, however, that every type of lymphoid cell found in the pulp can be duplicated in the follicles, and they have also brought sufficient evidence to show abundant emigration of true lymphocytes from follicles to pulp. In so far as cell structure alone is 1 For literature on this question see the papers by Downey and Weidenreich, Hertz, Werzberg, H. fischer, Ziegler, and Klein concerned, there is no evidence whatever for the view that the lymphoid cells of the two regions of the adult spleen are different.
The question of the origin of the erythroblasts, myelocytes, and megakaryocytes in the pulp of the embryonic and myeloid metaplastic adult spleen still remains to be disposed of. Several theories have beenvproposed by the dualists. They believe that the cells in question may be differentiated in loco from myelopo— tent leukoblastic pulp cells (Paremusoff) which are normally a part of the pulp parenchyme, or that they are of extraparenchymatous origin, either from undifferentiated embryonic connective tissue or adventitial cells (H. fischer), from cells of the vascular endothelium (Schridde), or from the bone-marrow by way of the blood-stream (Ziegler).
According to the monophyletic view, the lymphocyte is not a highly specialized cell. It is therefore capable of differentiating into a granulocyte or erythroblast in response to definite changes in environment brought about‘ by the liberation of toxic irritants in myeloleukemic conditions Which, on account of the arrangement of the circulation, affect the pulp first. With extensive and prolonged metaplasia the peripheral regions of the follicles may also become affected (Dominici, Weidenreich). In the e1nbryonic spleen‘ unknown conditions favor the continued differentiation of lymphocytes of the pulp (hemocytoblasts, large lymphocytes) along myeloid lines through late embryonic or postnatal stages, While similar lymphocytes which are confined to the territory of the follicles remain lymphoid.
It is evident that exact knowledge of the history of the origin and development of both pulp and follicles in the spleen must bring the final decision in this question. .
According to Danchakoff, the follicular tissue of the bird spleen is established by the condensation of the mesenchyme about the arteries which grow into the organ at a time when it consists entirely of ‘pulp.’ ' This mesenchymatous arterial sheath is later transformed into a lymphoid sheath with follicles by the liberation of free cells from the mesenchymal syncytium. Danchakoff states that the first free cells derived from the condensed mesenchyme about the arteries are of the type of large hemoeytoblasts identical with those of the pulp portion of the organ. Instead of differentiating into erythroblasts or granulocytes, as do most of the hemocytoblasts of the pulp, these hemocytoblasts proliferate rapidly and give rise to numerous daughter ‘dwarf— hemocytoblasts’ which in turn differentiate into the small lymphocytes so characteristic of the follicular regions.
According to Danchakoff, the differences in t.he'dift'erential products of the two regions (small lymphocytes in the follicles, erythroblasts and granulocytes in the pulp) are due entirely to Variations in the environmental. conditions. The arterial sheaths consist of Very dense tissue in which the blood is confined entirely to closed channels, while the pulp is of very loose structure with a slow and ‘open’ circulation. The latter type of circulation favors erythroblast and granulocyte differentiation, or it at least permits any substance contained in the blood which might influence cell differentiation to come in direct contact with the cells of the pulp, while it could only reach those of the follicles by slow diffusion.
Such an explanation, while satisfactory to those of the mono phyletic school, would not satisfy the dualists, for they can always claim that the ingrowing arteries bring ‘adventitial’ cells with them which furnish the condensed tissue from Which the lymphocytes of the follicles are eventually derived. Herzog claims that the endothelium of growing; capillaries of the omentum stiniulatedy by implantation of foreign bodies gives rise to numerous fixed and free adventitial cells from which numerous small lymphocytes with dark nuclei are derived. While DaI1chakoff’s Work seems to show clearly that the condensed tissue about the arteries is derived from the mesenchyme of the original splenic rudiment, still the participation, of the endothelium of ingrowing arteries is not absolutely ruled out.
Conditions are somewhat; less complicated in the spleens of pig embryos, for here the arteries do not grow into the organ at a comparatively late stage as in the chick, but are developed from small Vessels which already exist in the primitive rudiment, i.e., they are derived from vessels which already exist in the mesenchyme of the region in which the splenic rudiment is established. These vessels can usually not be recognized as arteries until the condensation of the mesenchyme surrounding them has begun. There is no special activity on the part of the endothelium of the arteries, and it is very evident that the syncytium of condensed tissue surrounding them has been derived from the general mesenchyme of the rudiment. Since there are no ingrowing vessels, there is no tissue to be brought into the organ after it has been established. An occasional lymphocyte proliferating in the tissue after migration from the vessels (Gulland) is of no importance, since the evidence for the derivation of the great bulk of free cells from the fixed mesenchyme is complete.
Since the free cells in all parts of the organ can be traced back to the mesenchyme of the rudiment, it follows that environmental factors, as claimed by Danchakoff in a series of recent papers, must be of chief importance in determining the line of differentiation which the cells will follow. That the ‘environment’ in the neighborhood of the arteries is different from that of the pulp is evident.
The Writers find that the specialization of cell types begins earlier than was noted by Danchakoff in the chick. In the pig most of the cells liberated from the condensed mesenchyme about the arteries at the time the lymphoid sheaths are being established are small, and many of them have dark nuclei with abundant chromatin, although the amount and arrangement of this chromatin does not usually correspond exactly to that of the typical small lymphocyte which seems to require a certain period of ‘ageing’ (Pappenheim) before acquiring all of its characteristic features. Small dark nuclei also appear in many of the mesenchyme cells of this region, which seems to indicate that the differentiation along small lymphocyte lines may begin béfore the cell is entirely free. There is a noticeable difference in this respect in the pulp of these same sections. Here all of the lymphoid cells are of the large ‘hemocytoblast’ type, while all of the free small cells belong to the erythrocyte series. The first small lymphocytes are, therefore, derived from the mesenchyme surrounding the arteries. Those believing in the ‘predestination’ theory can, of course, use these facts in favor of their view. Then they must also assume that the mesenchyme which enters into the formation of the lymphoid arterial sheaths is ‘predistined’ to form both large and small lymphocytes, while that of the pulp region can form only large lymphocytes during the early stages. The fact that the lymphocytes of the sheaths remain lymphoid, while the majority of those in the pulp differentiate into erythrocytes, is then due entirely to ‘predestination.’ Those who believe that the lymphatic and myeloid tissues are absolutely separate and independent can also use the above facts in support of their views. Because the ‘lymphoid’ tissue of the arterial sheaths and follicles appears later than the ‘myeloid’ tissue of the pulp, its cells must be of different origin.
This conclusion is not justified, however, when all factors are taken into consideration. It has been shown that the mesen— chyme of the arterial sheaths is derived from that of the original splenic rudiment and that it remains continuous with that of the pulp portion of the organ. The development of certain vessels into arteries causes, or is at least associated with, rapid proliferation of neighboring mesenchyme cells, resulting in marked eondensation of the tissue surrounding the arteries (figs. 6, 8). This mesenchyme then resembles that of the original splenic rudiment before it contains many free cells. It soon assumes a looser texture, owing to the liberation of many of its cells. The fixed cells remaining form a supporting‘ network which is directly continuous with and is derived from the mesenchyme of other parts. of the organ. ' Up to this point the history of the original rudiment is repeated by the mesenchyme surrounding the arteries. The chief difference appears when it isiseen that the continued production of lymphoid cells in the regions of the future follicles does not lead to differentiation of those cells along myeloid lines, as is the case with the lymphocytes of the pulp. Since no foreign tissue has entered the organ, it seems most logical to assume, with Weidenreich, Danchakoff, and others, that the arrangement of the vascular system in the two parts of the organ is not only responsible for the condensation of mesenchyme about the arteries, but also for the products derived from the mesenchyme of the two regions.
Cells of the ‘myeloid’ series are usually not found in the follicles or lymphoid sheaths, but we are not justified in stating that they never occur in these regions. In a 17-cm. pig embryo the writers noted a few myelocytes in the peripheral portion of an arterial sheath. The surrounding portion of the pulp contained none of these granulocytes. At this stage, and up to 30 em., the latter are usually differentiated from hemocytoblasts immediately after they are cut off from the condensed mesenchyme in the extreme marginal portions of the organ, far removed from either arteries or venous sinuses. The occasional occurrence of myeloeytes Within the arterial sheaths, therefore, seems to indicate that they are differentiated from lymphocytes within the sheath, i.e., within the lymphoid part of the spleen.
Attention may be called to the fact that Hertz, working with experimental myeloid metaplasia, saw accumulations of large lymphocytes around arteries of the pulp. These were interpreted as the beginnings of new follicles, and since he saw promyclocytes and myelocytes in these collections he believes that myelocytes can be found in the follicles. Werzberg, on the other hand, working with similar material, never found myelocytes in the follicles. Dominici states that the myeloid metaplasia of the spleen caused by repeated hemorrhages in rabbit may also involve the periphery of the follicles. In his experiments small lymphocytes seemed to be the mother cells of the other forms of lymphocytes. Weidenreich (’11) also states that during myeloid metaplasia the peripheral portion of the follicles may be involved, and that to the extent that the large lymphocytes eom.e under the special conditions of the pulp they may differentiate into myelocytes in place of forming small lymphocytes.
All of the facts enumerated above seem to indicate clearly that the lymphoid portion of the spleen is not composed of tissue which is fundamentally different from that of the pulp, but that the special conditions or ‘environment’ of the pulp cause or permit differentiation along myeloid lines of cells which are identical with and derived from the same source the cells of the follicular portions of the organ. The myeloid reaction of the pulp in the embryo and during myeloid metaplasia of the adult is the result of factors which do not usually exist in the normal adult. In the spleen of the latter. excepting in those animals in which myelocytes can be found in the spleen pulp throughout life, no types of cells are found in the pulp which are not also seen in the follicles, as was shown especially by Dominici and by Weidenreich and Downey.
In their figure 10 Downey and Weidenreich have shown that in postnatal animals lymphocytes, both large and small, which are identical with those of the normal pulp and follicles may differentiate granules in their cytoplasm. That the typical small lymphocyte can differentiate granules is of special importance, since it shows that the genuine, fully cliffei'entiat-ecl lymphocyte (Ehrlich) is capable of differentiating into a granulocyte. It is therefore, quite unnecessary to assume that the myeloid reaction depends on the existence of a special line of ‘micrornyeloblasts’ or ‘microlymphoidocytes’ (Pappenheim), or ‘myeloblasts’ of Schridde and Nacgeli of either local or foreign origin.
The development of the follicles or malpighian bodies from the arterial sheaths of the spleen is of special interest. Downey and Weidenreich have shown that in adult animals rapid increase in the number of lymphocytes of the follicles of both lymph nodes and spleen is associated with hypertrophy of the reticulum of the follicle. The. hypertrophied reticulum consists of large, pale cells which contain few or no fibers in their cytoplasm. Usu_ally this hypertrophied reticulum forms a pale mass in the center of the follicle, the so—called ‘germ-center.’ The accumulation of lymphocytes around this nucleus of large reticular cells forms the largest part of the follicle.
In some animals the arrangement of cells is frequently just the reverse of what has just been described. The hypertrophied reticulum forms a peripheral ring about the follicle and the accumulation of lymphocytes in the center. In such cases the large lymphocytes are also near the periphery, while in the usual type of follicle the majority of them are to be found in a zone immediately surrounding the central nucleus of hypertrophied reticulum. In both cases the large lymphocytes are more or less closely associated with the large reticular cells. Weidenreich and Downey have shown that the explanation of this is to be found in the fact that many of the large lymphocytes are cut off directly from the reticulum in the same way that they are derived from the mesenchyme of the splenic rudiment.
This same hypertrophy and condensation of mesenchyme is noted when the arterial sheaths are formed in the embryo, and it is seen again when the follicles are formed from the sheaths. The condensed mesenchyme about the arteries is soon converted into a lymphoid sheath by the process of isolation and liberation of many of its cells, which continues until the fixed tissue remaining is small in quantity. A follicle originates within such a sheath by the hypertrophy and proliferation of some of the fixed cells within a limited area. This hypertrophied mesenchyme, or reticulum as we may call it at this stage, then gives rise to numerous largo lymphocytes which by proliferation form numerous small lymphocytes. It is only after the first follicles have been formed that many large lymphocytes are encountered in the lymphoid portion of the organ, for the early lymphoid sheaths contain very few of them, as has already been pointed out (fig. 7).
Development of Follicles in Post-Natal Animals
The follicles do not develop until after birth. As newborn pigs were not available, the development of follicles was followed in a series of one - to twenty-one-day old rabbits. In the one-day rabbit some of the lymphoid arterial sheaths are similar to those of the late pig embryos, i.e., they are composed of a mass of small lymphocytes and a network of reticulum consisting of fine strands and small cells. Large lymphocytes are rare. In other cases an arterial sheath similar to the one described above is partially or almost completely surrounded by a broad zone of hypertrophied reticulum, the cells of which are very large and very numerous. Numerous large and medium-sized lymphocytes are included in this zone. Their cytoplasm shows varying degrees of basophilia, and the structure of their nuclei varies from that which is typical for the large lymphocyte to that of the nuclei of the reticular cells of this zone. On the other hand, the cytoplasm of many of the reticular cells is more basophilic than is usually the case and their nuclei contain more than the usual amount of chromatin. Such cells are more or less isolated from the general syncy— tium and some of them are entirely free. There are numerous transition stages between these cells and typical large and medium—sized lymphocytes. In other Words, it is not difficult to trace the large and medium—sized lymphocytes directly to the fixed cells of the reticulum.
In some cases a more or less circumscribed area of hypertrophied reticulum occurs in the marginal zone of the lymphoid sheath. The reticular cells of these regions are large, protoplasmic, rounded cells whose cytoplasm is of varying degrees of basophilia. Among them, but more often surrounding them, are large and medium—sized lymphocytes, many of them in mitosis. The nuclear and cytoplasmic characters of some of these cells indicate that they have been cut off from the reticulum. Small lymphocytes are numerous in the peripheral regions of these areas and there are numerous transitions between them and the larger cells.
It is evident that this entire group of cells represents an early follicle with a germ center composed of large reticular cells from which some of the lymphocytes have been derived. This is the typical structure of the follicles of the adult rabbit at one stage of their activity. They are developed in the marginal zone of the lymphoid sheath a.t some distance from the artery, and many of them remain in this position in the adult. The lymphoid arterial sheaths remain prominent in the rabbit, and the follicles are merely enlarged or condensed portions of the sheaths, usually associated with hypertrophied reticulum.
The appearance of large lymphocytes i.n the lymphoid portion of the spleen of postnatal animals is associated With marked activity on the part of the reticulum characterized morphologically by hypertrophy and increased basophilia of its cells. In the embryo the condensed and hypertrophied mesenchyme about the arteries gives rise to small lymphocytes only, while in the pulp of the embryo the first lymphoid cells cut off from the condensed mesenchyme have the general characters of large lymphocytes. Not all of the large lymphocytes of the follicles and lymphoid arterial sheaths of the young rabbits are derived from the fixed tissue. Some of them are derived from small lymphocytes which enlarge and gradually assume the characters of large lymphocytes as they pass from the central to the peripheral regions of the follicles or sheaths.
This transformation of small to large lymphocytes takes place at the same time that other large and medium-sized lymphocytes are being cut off from the fixed tissue. Special activity on the part of the fixed tissue is, therefore, associated not only with the liberation of lymphocytes from the reticulum, but also with the transformation of small to large lymphocytes.
The derivation of lymphocytes from the fixed tissue in postnatal animals -is a well-known process which has been discussed in detail by Weidenreieh and Downey, by Tsehaschin, and by Mollier C13). It proves that the reticulum of the lymphoid organs, the fibroblastic tissue of the omentum and possibly also that of the loose connective tissue retains the capacity of the embryonic mesenchyme to liberate free lymphoid cells. The germ centers of the follicles of spleen and lymph nodes are regions in which the reticulum is especially active, as is manifest by the hypertrophy, increase in number, isolation, and transformation of its cells. This special activity on the part of the reticulum results in the formation of lymphocytes from it, and it is also associated in some way with the proliferation of the lymphocytes surrounding the g1'er.vn center. ;\ccording to Sabin and others, the developinent of the follicle preceded by a special arrangement of the vascular capillary network. It is possible that the hypertrophy of the reticulum in the follicles of the postnatal animals is due to the formation of a dense capillary network which is absent in the lymphoid sheaths of the embryo. However, the proof for this is lacking.
Summary
1. The spleen of the White rat, pig, and gopher is first seen as a dense mass of D1eSG1lCl1_VTllal,O1lS tissue in the left dorsolateral portion of the mesentery. In the first stages of its development it is impossible to make a sharp distinction between the coelomic epithelium and the mesenchyme of the mesogastrium. Proliferative activity on the part of the epithelial cells may result in the crowding of some of the daughter cells into the underlying mesenchyme. After the splenic rudiment is established (15—mm. pig), the peritoneal cells are sharply separated from the mesenchyme by a distinct limiting membrane.
2. The earliest splenic vessels are branches of the mesenteric artery. These continue to bifurcate and form a network which supplies the entire mesenteric tissue. '1.‘ his primitive capillary system communicates with both arteries and veins. In 6- to 7—cm. pig embryos there is communication between the Vascular capillary network and the primitive splcnic sinuses, and the open circulation that is characteristic of the adult spleen is established.
3. In the pig the blood—forming activity of the spleen begins in embryos of from 3 to 4 cm. in length. Cells of the mesenchyme which bear no definite relation to vessels or sinuses become more basophilic and lose their connection with the mesenchymal syncytium. When free these cells become the large lymphocytes which are typical of the early stages in the development of all hematopoietic organs.
4. Erythropoietic activity in the spleen is recognized first in 4- to 6-cm. pig embryos. Erythroblasts are seen in small groups in lacunae that were formed by the first free cells as they were cut off from the mesenchymal syncytiunn. The first groups of erythroblasts are usually at some distance from the sinuses, which are produced as splits in the mesenchyme independent of the process of hemocytoblast formation. At a, later stage, however, a few hemocytoblasts which differentiate into erythrocytes may be formed from the margins of sinuses. Erythropoiesis, which is largely eXtra—vascular, becomes very active in 15- to 17-cm. pig embryos, converting the greater part of the organ into a pulplike structure.
5. The extensive extravascular erythropoietic activity may be influenced by environmental factors that are introduced by the early establishment of an ‘open circulation’ in the embryonic spleen.
6. Granulopoiesis is very limited in the pigs spleen at any sta.ge of its development. A few mononuelear granulocytes are developed in the marginal regions of the organ from cells that are cut off from the mesenchyme.. There is no evidence that granulocytes are derived from ‘myeloblasts’ which have their origin in the endothelium.
7. The differentiation of ‘White pulp’ in the spleen is initiated at the time of the formation of distinct arteries (6-cm. pig). A lymphoid sheath is developed around the arteries. This arterial sheath is at first composed of very dense mesenchyme which later is resolved into a loose network, the meshes of which are packed with small lymphocytes. The great majority of these lymphocytes are cut off from the mesenchyme, and they differentiate into typical small lymphocytes without passing through the hemocytoblast stage.
8. Although the lymphoid or follicular portion of the spleen appears comparatively late in the development of the organ and is confined to the region of the arteries, it cannot be regarded as a tissue’ which is foreign to and of different origin from that of the red pulp, for its mesenchyme is derived from and is continuous with that of the pulp, and its free cells migrate into the pulp.
9. Large lymphocytes are rare in the lymphoid portion of the spleens of embryos. Their appearance in postnatal animals is associated with hypertrophy of the reticulum in the marginal portions of the lymphoid arterial sheaths or in the germ centers of developing follicles. Most of the first large lymphocytes are derived from this hypertrophied reticulum, but others are the result of growth of small lymphocytes. Large lymphocytes soon appear in all parts of the follicles and lymphoid sheaths and many of them migrate into the pulp.
Literature Cited
BAUM UNI) HILLE 1908 Die Keimcentren in den Lymphdriisen Von Rind, Schwein, Pferd und Hund, und ihre Abhéingigkeit vom Lebenselter der Tiere. Anat. Anz., Bd. 32.
BILLROTH, THEOD. 1862 Neuc Beitréige zur vergleichenden Anatomie der Milz. Zeitschr. f. wissensch. Zool., Bd. 11.
Bxscnorr, ,T. L. 1842 Entwickelungsgeschichte dcr Saugetiere und des Menschen. Leipzig, 1842.
BREMER, JOHN 1914 The earliest blood vessels of man. Am. Jour. An2.t., vol. 16.
BUTTERfiELD AND STILLMAN 1917 The broader aspects of hematological diagnosis. Am. Jour. of Med. Science, vol. 154.
CH0 RONSHITZKY, B. 1900 Die Entstehung der Milz, Leber, Gallenblase, u. s. W. bei den vcrschiedenen Abteilungen der Wirbeltiere. Anat. Hefte, Bd. 13.
DAIBER, MARIE 1906 Zur Frage nach der Entstehung und Regenerati0ns— féihigkeit der Milz. Jena. Zeitsch. f. Naturwiss, Bd. 42.
DANCHAKOFF, V. 1908 Die erste Entstehung der Blutzellen beim Hi'1hnerembryo und der Dottersaclc £1.15 blutbildendos Organ. Anat. Hefte, Bd. 37.
1908 Untersuchungen fiber die Entwicklung V011 Blut und Bindegewebe bei Vogeln. Arch. f. mikr. Anat., Bd. 73.
1916 a Uber die Entwicklung des Blutes in den Blutbildungsorganen bei Tropidonotus natrix. Arch. 1°. mikr. Anat., Bd. 78.
Danchakoff V. The origin of blood cells. (1916) Anat. Rec. 10(5): 397-414.
Danchakoff V. The equivalence of different homatopoietic anlages by method of stimulation of the different stem cells I. (1916) Amer. J Anat. 20:
1918 Cell potentialities and differential factors, considered in relation to erythropoiesis. Am. Jour. Ana.t., vol. 24.
DOWNEY, HAL 1913 The socalled ‘cndothelioid’ cells. Anat. Rec,-.., Vol. 9.
DOWNEY, HAL, TIND WEIDENREICH F. 1912 Tiber die Bildung der Lymphocyten in Lymphdriisen u. Nlilz. Arch. f. rnikr. Ana.t., Bd. 80.
V. EBNER, J. 1899 Uber die Wand der Oapillaren Milzvenen. Anat. Anz., Bd. 15.
ELIASBERG-, M. 1903 Blutbildung in der Milz. Inaug.-Diss. Dorpat.
EMMEL, V. E. 1914 Concerning certain cytological characteristics of the erythroblasts in the pig embryo, and the origin of the non-nucleated erythryoytes by a. process of cytoplasmic constriction. Am. Jour. Ai1at., vol. 16.
EVANS, FRANK 1916 The reaction of the spleen in acute infections. The Johns Hopkins‘ Hospital Bu1l., vol. 27.
FISCHER, H. 1909 Myeloische Metaplasie u. fétale Blutbildung u.. deren Histogenese. Berlin, J . Springer, 1909.
Fosrnn UND BALFOUR 1876 Grundziigc der Entwicklungsgeschichte der Tiere. Leipzig, 1876.
FREYER, J. 1872 Uber die Betoilung der Milz bei der Entwiclilung der roten Blutkorperchen. Inaug.-Diss. K('iI1i.g5b01‘g. Ems, V. 1872 Die Milz als Blulbildendcs organ. lnzmg.-Diss. Dorpat, 1872. G'('>'rTF., G. 1175 Entwicklungsgeschichtc der Unke. Leipzig, 1875.
GULLAND, G. L. 1894 The development of the lymplmtic glands. Jour. of Path. and Basin, Vol. 23. GtI"rIu,
KARL 1987 Ein Beitrag zur Morphologie des Schweincblutes. Aral]. f. milu". Aunt-., Bd. 70.
HELLY, F. 1992 Die Blutbzihneil der Milz und deren funlztionclle Bedeutung. Arch. f. mikr. Anat, Bd. 61. HEm‘\\~iL:, O. 1906 Entwittklung der Milz. 1-Izmdb. d. vergl. u. exp. Entxv. der Wi1'belt.iere, Bd. 3, Jena, G. fisclier.
HERTZ, R. 1910 Zur Fragc. der expcrimentellen Inyeloischen Milzinetaplasie. Zeitsch. J". klin. Mcdizin, Bd. 71. I-1Ev.z0G, G. 1915 Experiineiitclle Untersuchungen libel‘ die Einheilung VOI1 1*“1'e1ridkf‘)rpc1"11. Zieg1e,1"s Beitr. z. pa-fl]. Anat. u. allgem. Path., Bd. 61.
HOYHR, H. Zur Histologie der capillaren Vcnen in der Milz. Anat. Anz., Bd. 17. JANo§u<, J. 1895 Le pancreas et la rate. Bib. Anatoinique, 1895.
JOLLY, J. 1905 Sui‘ Vévolution des globules rouges dans la sang (les embryons de mammiféres. C. R. Soc. Biul. P:1ris., T. 58.
JORDAN, H. E. 1915 Hiiemopoiesis in the yulk sac of the pig embryo. Anat. Rec., vol. 9.
JOST, J. 1982 Beitriige zur Lehrc. Von der Blutent-wie.1:.l1mg des embryonalcn Rindcs und Sehales. Arch. f. mikr. Analz, Bd. 61.
KERVILY, M. 1994 Sur la presence do inegacaryocytes dans la rate des plusieurs mamrriifires adultos normaux. U. R. Soc. Biol. Paris, T. 72.
KLEIN, S. 1914 Die Myelogonie. Berlin, J. Springer, 1914.
KOLLIKER, A. 1879 Entwicklung des Menschen und der h('jherei1 Thiere. Leipzig, W’. Engelmann. 1902 Milz. Handb. d. Geweblellr. des Mcnschen, Leipzig.
KOLLMAN, JUL. 1872 Entwicklung der Milz bei den Allen uud den Menschcn. Verli. Schweiz. Natur Gcs., Bd. 83. Korcx, H. 1881 Uber die Beteiligung der Milz und Knoclienmarkes an der Bildung roten Blutki'srpe.rchon bei Viigcln. Virc.how’s Archiv, Bd. 86.
KOSTANJ-.'cKI, V. Die embryonale Leber in ihrcr Beziehung zur Blutbildung. ' An:-it. 1-iefte, Bd. 3.
KRAATZ, -V. 1897 Zur Efltistohung der Milz. Inaug.-Diss. .\/'19.rburg. KUBORN, I’. 1890 Du développement des vaisseaux et du sang dans le foie embryon. Anat. Anz., Bd. 5.
KULTSCHITZKY, N. 1895 Zur Frage fiber den Bau der Milz. Arch. f. mikr. Ana-t-., Bd. 46.
KUPFFER, V. 1892 Uber die Entwicklung der Milz und Pancreas. Milncli. med. WOChe1lSC]’l1‘., Bd. 28. KY1s‘i:R, J. 1870 Uber die Milz dos Menschen und einigcr Séiiugetiere. Arch. f. mikr. An2a.t., Bd. 6.
LAGUESSE, E. 1890 Recherches sur Ie développcment de la rate choz Ies poissons. Jour. de l’Anat. et do la. phys., T. 26. 1891 Lo tissu splenique et son développemcnt. Anat. Anz., Bd. 6. 1894 L9. ratie est elle d’origine ontodermique ou mesodermique? Bib. Anatomique, 1894.
LEWIS, TH. 1904 Further observations on the function of the spleen and other hemolymph glands. Jour. Anat. and Phys., vol. 38.
LOBENHOFFEH, W. 1908 Uber extravaskuléire Erythropoiése in der Leber unter pzi’r.hr>10gischen und norm a.len Verliéiltnissen. Ziegler’s Beitr. zur path. Anat. u. allg. Pa.th., Bd. 43.
MALL, F. 1902 The circulation of the blood in the r.log’s spleen. Am. Jnur. A11at., vol. 2.
M.«xURI<:e, F. 1890 Die erstre Anlage der Milz und «ins erste Auftreten von lymp‘noi<iisnhen Zcllen bei Amphibien. Morph. Jahrb., Bd. 16.
MAXIMOW, A. 1908 llbcr embryonale Entwicklung der Blut— und Bindegewebezellen bei den Siiugetieren. Verhandl. der Anat. Gesells(':h., 22. Vers., Berlin, 1908.
1998 Untersmeliungen iibcr Blut und Bindegjewebc. I. Die friihesten Entwicklxingstaclicn der Blut— und Tiimlegewebezellen beim Séiugetier Embryo, bis zum Aufgang; der Blutbildung in der Leber. Arch. f. mikr. Ana/c., Bd. 73.
1910 Ifntersuchungen iiber Blut und Binclegewebe. LII. Die embryonale Histogenese des Knochonmarks der S5'u1getie1'e. Arch. f. mikr. An2.t.., Bd. 76.
lVllETENS, H. 1900 Entstehung der Weisson Blutlzorperchen und der Milz hei Bufo vulgaris. Jenaismhe Zoitschr. f. Naturwise, Bd. 46.
MINOT, CH. 1892 Human embryology. New York, Wm. Wood & Co.
MOLLIER, S. 1909 Die Blutbildung in der embryonalen Lebcr des Mensa-hen und der W'ir1'>e1t-ierc. Arch. f. mikr. Ana.t., Bd. 74. 1911 Tiber den Bau der capillaren Milzvenen. Arch. f. mikr. Ana/c., Bd. 78. 1913 Die Lymphoepitlielialen Organe. {$‘etvzungsbe1'. der Gesellsch. f. Morphologie u. Physiologie in Mfmchen, Bd. 29.
M'L"LLE1=., W. 1871 Milz. Stricke:r’s Handbuch, vol. 2, Leipzig.
NZALGELI, O. 1908 Bllimiiiwersuchungeli und B1utdi2ign<.>stik. Leipzig, 1908.
NE}LTA4ANN, E. 1874 Neue Beitriige zur Kenntnis der Blutbildung. A1-(eh. f. Heilkunde, Bd. 15.
NICOLAS, A. 1903 Recherches sur‘ le développement du pancreas, du foie ct de 12. rate, chez les St-ex-let. Arch. do Biol, T. 20.
PAPPENHEIM, A., UNI) ST. Szmcsi 1912 Hiimozytologisc,-lie Beobachtungen bei experimenteller Saponinvergiftung; der Kaninchen. Folia I-Iae1nat., Bd. 13.
PEREMUSOFF, J. 1911 Zur Kenntnis der Zellen der Milzpulpa. Folia 1-1a.cmat., Archiv, Bd. 12.
PE-REMESCHKO, J. 1867 Tiber die Entwicklung der Milz. Sitz. d. Akad. d. Wise. in Wien, Mat. Nat. Kla.sse., Bd. 56, Apt. II.
PIPER, H. 1902 Die Entwicklung Von Leber, Pankreas, und Milz bei den Vertebraten. Inaug.-Diss., Freiburg, 1902.
RADFORD MARION 1938 The development of the spleen. Jour. Aunt. and Phys.. Vol. -'-12.
REAGAN, F. 1917 Experimental studies on the origin of vascular endothelium and of erythrocytes. Am. Jour. Anat., V01. 21.
REMAK, ROHHR1‘ 1850 Untersuchungen iiber die Entwicklung des Wirbeltierreichs. Leipzig.
RICHTER, J. 1902 Vergleicliende Untersuchungcn fiber den mikioskopischen Bau der Lymphdrfisoii V011 Pferd, Rind, Schwein, und Hund. Arch. f. mikr. A11-at., Bd. 60.
RI1\'1)}«'LE1scH, M. 1872 Ulner die Wandungen der capillaren Milzvcnon. Berl. klin. Woclieiisolm, Bd. 45.
ROBERTSON, J. 1885 A contribution to splenic histology. Jour. of Anal). and Phys., vol. 20.
SABIN, FLORENCE 1994 The development of the lymphatic nodes in the pig and their relation to the lymph liezirts. Am. Jour. Anat., vol. 4. 1911 The development of the spleen. Handb. ml. Entwgsch. d. Menschen. Keibcl u. Mall, Bd. 11, Leipzig, 1911. 1916 The method of growth of the lyn1pl1a3r,ic system. Science, Vol. M.
SAXER, FR. 1896 fiber die Entwioklung und den Ban normaler Lymphdrfisen und die Entstehung der roten und der weisscn Blutkorperchen. Anat. Hefte, vol. 13. _ SCHHNK, S. 1873 Lehrbueh der Ernbryologie des Menschen und der Wirbel— thiere. Leipzig, 1873.
SCHMIDT, M. B. 1892 Ubcr Blutzellenhildiuig in Leber und Milz unter I.lOI‘Ifld-l.8I1 und p2'L1‘,l1()l0g1SCl1e1’1 Vo1'h:'i.l‘missen. Zieglci-’s Beitrfige zur path. Anat. 11. allg. I’atli., Bd. 11.
SCHRIDDE, H. 1909 Die enibryonale Blutbildung. Erwiderung an Uerrn Prof. A. Maxiinow. Tentralbl. f. allgein. 1’zitli. u. path. Anal“, Bd. 20. 197,18 Uher Regeneration dos Blutes unt-er normalen L1. kmnkhafteii Verhi/iltnissen. Con’r,ra,lbl. f. allgem. Path. 11. path. Anat., Bd. 19, N0. 21.
SCHWEIUGER-SEIDEL 1863 Unfersuchungen iiber die Milz. Virch. Arohiv, Bd. 27, Abt. II.
SOBOTTA, J. 1914 Anatomie der Milz. Jena, 1914.
STOCKALH), CHAS. 1915 The origin of blood and vascular endothclium in embryos Without a circulation of the blood and in the normal embryo. Am. Jour. Anat., Vol. 18.
1915 The development of wandering mesenchyme cells. Am. Jour. Anat., vol. 18.
1915 An experimental study of the origin of blood and vascular endothelium in tlie teleost embryo. Anat. Rec., vol. 9.
THOMA, 11. 189.5 Ubor die Blutgeff-i.sse. der Milz. \V'<-,rliandl. (1. Ana-t». Gcsellsch. in Basel, 1895.
TONKOFF, WM. 1990 Die Entwicklung der Milz bei den Ainnioten. Arch. f. inilu‘. A11-.:.t,., Bd. 56.
’J.‘0L1>'r, CARL 1879 Buu und Wacl1stumve1'éindcrungen der Clékréso ‘u. S. w. Denkschr. d. mat. iiziturw. Klasse d. Akad. (1. Wise. zu Wien.
TSCHASCHIN, S. 1913 llber die ‘ruhenden W:-1nde1~zel1en' und ihre Beziehungen zu den ancleren Zollforinen des Bindogewebus und zu den LVmphoz_y1.en. Folia, Ha.emat.ol., Arcliiv, lid. 17.
T1'.'faK, W. 1908 1,.‘ her Regcneratio11 dos Blutes unter nornmaleii und krainklii-iften Ve1'h£i.]tnissen. Centralbl. f. allgcm. Path. u. ].):1.tl'i. AmLt., Bd. 19. 191.2 Vorlesungen fiber klinischc 1‘.la(-31Il‘rIl1.()l(_)glG. ‘Z. Tcil. Wien u. Leipzig, W’. Bmiiiniiller, 1912.
VAN DER STRICIIT, O. 1890 Le développement du sang dans le foie embryonnaire. Arch. de Biologie, T. 11. 1892 Nouvelle reeherehes sur la genése des globules rouges et des glubules blames du sang. Arch. de Biologie, T. 12.
VENZLAFF, W. 1911 llbcr Genesis und Morphologie der roten Blutl<<':'arpe1'ehen der Vogel. Arch. f. mikr. Anat., Bd. 77.
VON SCHULTE 1914 Early stages of Va.seulogenesis in the cat, With special reference to the mesenchymal origin of the endothelium. Mem. of Wis— tar Inst. of Aunt. and Biol, 110. 3.
WEIDENREICH, F. 1903414 Die rotcn Blutkorperehen. Ergebn. der Anat. u. Entwieklu11gsg;esch., Bd. 13 u. 14. 1901 Das Gofiissystem dcr IIlGI1SCl1ll(3l1CI1 Milz. Arch. f. mikr. Anat., Bd. 58.
1910 Die Morphologic der Blutzellen und ihre Beziehung zu Ein— ander. Anat. Rec., Vol. 4.
1911 Die Leueocyten u. verwandte Zellformen. Wiesba.den, J. F. Bergmann.
WEIL, H. 1914 Uber die Bildung Von Leucozyten in der mensehliehen und tierisehen Thymus des e1'w:L(:l1se11en Organismus. Arch. 1'. mikr. Ana.t., Bd. 83, Abt. l.
W1«;Rzm:rm, A. 1911 Neue experim<:n'1elle Beitréige zur Fmge der myeloiden l\'le,1.a.|)lasie. Vireh. Areh., Bd. 204.
W IIITING, H. 1897 On the comparative histology and physiology of the spleen. Trans. R. Soc. in Edenborough, 1897.
WOIT, O. 1897 Zur Entwicklung der Milz. Anat. Ilefte, Bd. 9.
Z11«1(1~m:R, K. 1906 Experimentelle Ll. kliniselie Uiitmsuuliuiigen 11bCl' die Histo;3_f{C1]CsC der myeloidcn Leukéimie. Jena, G. fiselier, 1906.
Plates
Plate 1
1 Section of the peritoneal epithelium and the underlying mesenchyme of the mesentery, in the region offthe primary splcnic rudiment of a 7.5-mm. pig embryo. The cell at E is being crowded into the mescnchyme.
2 Section of the peritoneal epithelium and underlying splenic tissue of a 15-mm. pig embryo. The two tissues are distinctly separated and show dificrcnt cytological features.
3 Section through a sinus in the spleen of a 6-cm. pig embryo. Processes of the mesenchyme cell, mes.c., extend into the early sinus.
Plate 2
4 Section of zi venous sinus in the spleen of a, 24-min. pig embryo. Some of the mesenchyme cells lining the sinus are elongated into typical reticulo-endothelial struutiircs. The cell (encZ0.c.) on the right wall shows strongly basophilic eyt-oplasin and a nucleus similar to that of :1 large lymphocyte. The cell is being cut off from the mesenchyme.
5 A group of hemocytoblasts in the rnesenchyinc of the spleen of a 4—cm. pig embryo. The structure of the nuclei of cells A and B is intermediate between tliat of the mesenchyme cell, n7,c.~'.r:,, and the lymphocytoblast C.
6 A slightly oblique section of an artery and surrounding mesenchyme in the spleen of at 735-0110. pig embryo. The presence of numerous small and mediumsized dark nuclei indicates the beginning of the differentiation of small and medium-sized lymphocytes from the niesenchyme.
Plate 3
7 Section of an artery and its lymphoid sheath in the spleen of a. 30-cm. pig embryo, showing intermediate stages in the formation of small lymphocytes from the reticulum. Cells a, b, c, d, successive steps in the line of differentiation: cells 6, f, g, h, free lymphoid cells very similar in nuclear structure to cells c and d; l and 10, large lymphocytes.
8 Cross-section of an artery in the spleen of a, 6-cm. pig.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The development of the mammalian spleen, with special reference to its hematopoietic activity (1921). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_development_of_the_mammalian_spleen,_with_special_reference_to_its_hematopoietic_activity_(1921)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G