Paper - Normal development of the trachea and esophagus in man
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Smith EI. The early development of the trachea and esophagus in relation to atresia of the esophagus and tracheoesophageal fistula. (1957) Contributions To Embryology, No. 245
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Early Development of the Trachea and Esophagus in relation to Atresia of the Esophagus and Tracheoesophageal Fistula
E. Ide Smith
Department Of Embryology, Carnegie Institution Of Washington, Baltimore, Maryland
With Four Plates And Eleven Text figures
- This investigation was begun during the author's tenure of the Halsted Fellowship of the Department of Surgery, Johns Hopkins University, under the direction of Dr. Alfred Blalock, Director of the Department of Surgery, and with the co-operation and invaluable assistance of Dr. George W. Corner and Dr. Chester H. Heuser of the Department of Embryology of the Carnegie Institution of Washington.
Introduction
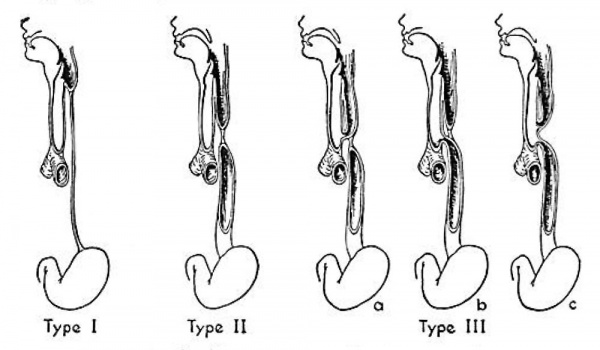
Among the important congenital abnormalities now amenable to surgical correction is atresia of the esophagus, with or without tracheoesophageal fistula. Estimates of the frequency of the condition vary from lngalls and Prindle's (19.19) report of 6 cases in 30,497 live births at the Boston Lying-In Hospital to the statement by Belsey and Donnison (1950) that it occurs as often as I in 800 pregnancies. The common variations of this abnormality have been classified by Vogt (I929) as shown in figure I. The most frequently seen type, more than 90 per cent of all cases, is that designated as IIIb, in which the upper esophageal pouch ends blindly and a fistulous tract leads from the trachea to the lower esophageal segment.
Fig. 1. Vogt’s classification of atresia of the esophagus. Type I, atresia of entire esophagus. Type ll, atresia of a segment of the esophagus with defined upper and lower esophageal pouches. Type III, atresia of the esophagus with tracheoesophageal fistula: rz, fistula between the upper esophageal segment and the trachea; b, fistula between the lower esophageal segment and the trachea; c, fistulae between both esophageal segments and the trachea. From Leven ct al., Ann. Surg., vol. 136, p. 704, 1952.
Esophageal atresia with tracheoesophageal fistula was first described in 1696 by Thomas Gibson, whose interesting account is quoted by Ingalls and Prindle (I949). The condition has always aroused considerable interest, and reviews of the subject have been published by Keith (igio), Plass (1919), Rosenthal (1931), Gage and Ochsner (1936), and Lanman (1940). The problem of tracheoesophageal fistula without esophageal atresia has been extensively reviewed by Ferguson (1951).
The possibility of definitive treatment by division of the fistula and simultaneous primary end-to-end anastomosis of the esophageal pouches, originally suggested by the anatomist Sir Arthur Keith, in 1910, set a goal toward which surgeons struggled through the next thirty years. In 1939, working independently, Leven (1941) and then Ladd (1944) had the first successes of any sort with infants with a type IIIb atresia of the esophagus. Both infants were handled by multiple-stage procedures: primary gastrostomy to permit feeding, ligation of the tracheoesophageal fistula in the chest, and exteriorization of the upper pouch in the neck. Keith's idea of a primary ligation of the fistula and simultaneous esophageal anastomosis was pioneered by Lanman (1940) and by Shaw (1939), but the first success was attained in 1941 by Haight and Towsley (1943). Since that time various improvements have raised the survival rate in recent years in some clinics above 60 per cent.
While the surgery of congenital abnormalities has been advancing rapidly in the past twenty-five years, our knowledge of the basic processes leading to the production of these same abnormalities has not kept pace. ]ust as investigations into the basic physiology, pathology, and bacteriology of infectious-disease processes have furthered the prevention and therapy of these diseases, so may an understanding of the basic processes in the causation of congenital abnormalities be expected ultimately to indicate the means of treating them or preventing their occurrence.
The once widely accepted view of Mall (1908) that all anomalies of human development result from faulty implantation of the embryo due to maternal disease was succeeded by a general tendency to assume that all congenital anomalies result from genetic causes inherent in the fertilized ovum. This view, as E. S. J. King (1943) has commented, hindered research by perpetuating the belief that congenital anomalies must be accepted as accomplished facts, and that their causes and the path of their development can never be traced.
In recent years, the hope that abnormalities of development may ultimately be explainable iti terms of developmental physiology has been encouraged by several lines of investigation, including the analysis of differentiation, as exemplified, for instance, by the organizer theory of Spemann (1928) and the production of embryonic anomalies by various experimental means. It is now clear that the immediate cause of any anomaly of development is a disturbance of growth at a critical time in the differentiation of the embryonic tissues. Such disturbance may result from the action of unfavorable genes; from environmental causes such as trauma, the action of toxins, certain viruses, nutritional deficiencies, and various other harmful agents; or from combinations of genetic and environmental factors.
Hypotheses concerning the causes of abnormal growth must be consistent with the principles of physiological embryology now being developed from experimental studies, and they must rest upon the morphological features of normal development. In the study of any particular anomaly, the path of normal development must be clearly defined and, as far as material is available, must be compared with that of embryos which exhibit the anomaly. In this way, the time of first departure from the normal may be ascertained, and the investigator may be able to discover whether the abnormality results from deficiency or cessation of development, or from a local excess in the growth of tissues such as has been suggested by Patten (1953) as a possible explanation of embryonic abnormalities. At present, human embryological material is available in quantities and in excellence of preparation hitherto not known, and so a reevaluation of the clinically accepted concepts of abnormal development becomes possible. Such a re-evaluation of the nature of tracheoesophageal anomalies is the aim of the present paper.
Normal Development of the Trachea and Esophagus in Man
The development of the human trachea and esophagus has been described by Lewis (1912), by Grosser (1912), and by Puiggr6s—Sala (I937). The development of the respiratory system in the pig was investigated by Flint (r907). A monograph by I-Ijortsjti (1945) on the development of the respiratory system in the cat has been published. The later phases of pulmonary development have been described by Wells and Boyden (I954). ]ohns (1952) has described the development of the esophageal epithelium.
The rich material of the Carnegie Collection of human embryos now permits a more precise account of the early development of the trachea and esophagus and a much more accurate statement of the relation of the stages of this process to the general development and age of the embryo than have previously been possible. The following account is based on a survey of U2 embryos in horizons x to xv, from which 58 specimens were selected for detailed study. Streeter’s comments in his Horizon articles (1942, 1945, and 1948) on various phases of development, and his previously published descriptions of the individual embryos as cited,have been freely drawn upon.
Horizon X 21 to 23 Days, 4 to 12 Somites
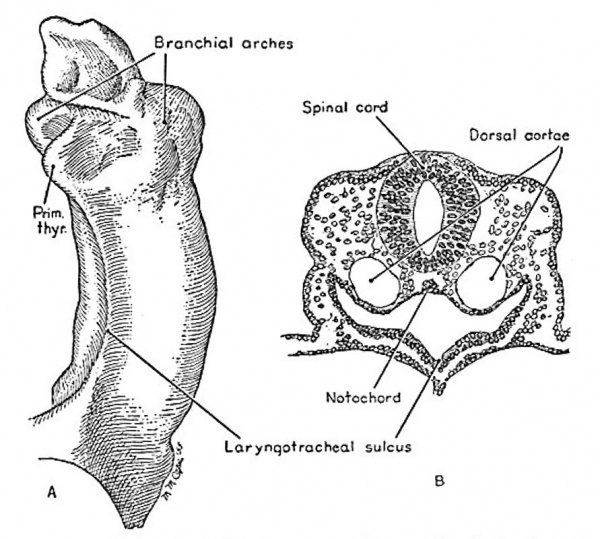
At horizon x, the first primordium of the respiratory system is seen in the mid-line of the ventral wall of the foregut between the thyroid primordium eranially and the anterior intestinal portal caudally. A cellular proliferation produces a ridge on the external surface of the foregut and a corresponding mid-line groove, the laryngotracheal sulcus, on the internal surface. The ridge widens as it extends caudally. A slight furrow is seen in this region in the 8-somite Dandy (I910) embryo, no. 391. In the Io—somite Corner (1929) embryo, no. 5074 (fig. 2), this laryngotracheal sulcus is well defined. Vi/hereas the lining cells of the foregut are single-layered and in some areas almost squamous, in the laryngotracheal sulcus the lining consists of 2- to 3-cell layers, and the cells appear transitional or almost columnar with a striated border suggesting possible ciliation. They are surrounded by undifferentiated mesenchyme.
Fig. 2. A, the ventrolateral aspect of a model of the foregut of embryo no. 5074, horizon 3:, showing the earliest pulmonary primordium, the laryngotracheal sulcus, between the thyroid and the anterior intestinal portal. Drawing by Mary M. Cope. X75. B, section 2-2-3, same embryo, at the level of the laryngotracheal sulcus. The notochord forms a portion of the thin dorsal wall of the foregut. The differentiation of the cells of the ventral wall of the fort-gut is noted. X 100.
Horizon XI 24 to 26 Days, (13 to 20 Somites)
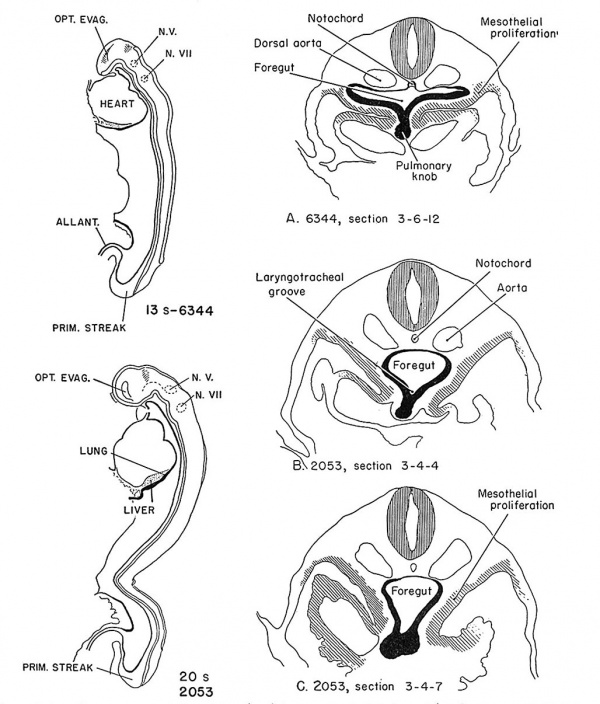
The next stage of development is well illustrated by two embryos of horizon xi, no. 6344 and no. 2053 (Davis, 1923). In figure 3, the foregut has increased in length as the embryo has grown. The laryngotracheal groove has elongated. A definite thickening of the ventral wall of the foregut is present cephalad to the liver primordium. Number 6344 has a laryngotracheal groove 168 microns long, which begins just caudal to the thyroid primordium as a shallow furrow and ends with a knoblike thickening of the endodermal wall. The groove lies directly in the dorsal mcsocardium and laterally is in contact with the coelomic cavities. The ventral and lateral walls of the foregut are 3 to 4 cells in depth; in contrast, the dorsal wall is only I cell thick. The nuclei of the cells of the future respiratory system have grouped themselves ventrally, and numerous mitoses are seen.
In a later example of horizon xi, the Davis embryo (no. 2053) of 20 somites, a well marked laryngotracheal groove extends from the contracted caudal end of the pharynx to a point just cephalad to the liver diverticulum, and ends in a bilobular swelling separated from the liver by a shallow transverse sulcus. The groove is separated from the dorsal digestive tract by :1 longitudinal sulcus running from the cephalic portion of the laryngotracheal groove. The respiratory primordium lies cranial to the liver, caudal to the thyroid, dorsal to the sinus venosus, and ventral to the descending aortae, spinal cord, and notochord (sections A, B, C of fig. 3). The microscopic appearance is little changed from that of no. 63.14.
Horizon XII, 25 to 27 Days (21 to 29 Somites)
Figure 4 shows the characteristic state of the endodermal~tr-aet of embryos of horizon xii. In no. 5056, an early representative of the period, there is a knoblike lung bud adjacent to the sinus venosus. On transverse section, the notochord, previously an integral part of the dorsal wall of the foregut, has moved farther dorsalward between the paired aortae. The long axis of the growing foregut has become oriented in a dorsoventral direction. Separation of the respiratory and digestive tracts, as described below, is beginning. Small sprouts of vessels can be seen forming the pulmonary vasculature. In no. 5923, the lung bud has become more prominent and separation has progressed. The ventral wall of the foregut reaches a thickness of 7 to 8 cells, whereas the dorsal wall is only 3 or 4 cells thick. Small vascular twigs are present. Below the point of separation of the trachea and esophagus, the wall of the esophagus thickens and the caliber of the lumen diminishes.
The early formation of the tracheoesophageal septum was studied carefully, for it seemed crucial in the development of a tracheoesophageal fistula. Although the septum is often pictured as resulting from the “pinchingofi" of the lung bud from the foregut, this explanation does not appear to be correct. Ridges of endodermal cells develop from the lateral walls at the caudal end of the lung-bud region of the foregut. The union of these proliferating ridges, within the lumen of the foregut, divides it into a ventral respiratory and a dorsal digestive portion. At this point there is seen no evidence of external pressure upon the foregut, no condensation of mesenchyme, no indentation of the basement membrane indicative of a “pinching—o ." (See fig. 1.}, pl. t; figs. 16, 17, pl. 2.) This interpretation of the manner of separation is in keeping with modern concepts of embryonic organization. I-ljortsjii (1945) has described a similar tracheoesophageal division in the cat. After division by the cellular ridges, the innermost cells in the core of the septum apparently undergo necrosis, to form coalescent vacuoles (fig. I7, pl. 2); finally the basement membrane collapses, and mesenchyme passively fills the space so vacated.
A second feature of this horizon and the next, which was subjected to a careful investigation, is the presence of lateral esophageal grooves as described by Lewis (tgxz) and Forssner (1907). figure 5 shows the caudal and dorsal course described for these grooves running from just dorsal to the traehcoesophageal ridges to the dorsal wall of the esophagus. The internal ridges thus produced are similar in histological structure to the ridges forming the tracheoesophageal septum. Their importance lies in the fact that overgrowth could well result in a type III!) abnormality as illustrated on the right in figure 5. In the light of Patten’s suggestion (1953) that excessive development, as well as deficient growth, may precipitate abnormalities, these ridges are of considerable interest. Definite evidence of them was found in at least 6 embryos of horizon xii studied (nos. 1062, 4245, 4736, 4759, 7724, 5035). This second ridge of endodermal cells can be seen in figures 13 and 15 (pl. 1). A sagittal section from no. 7724 (fig. 15, pl. 1) shows evidence of a well developed lateral esophageal groove. It is of interest to note the manner in which the protruding mass of this groove occludes the lumen of the esophagus dorsally, while a communication between the respiratory tree and the lower esophagus still exists. A similarity can be seen between this section and that of the Harvard embryo shown in figure 23 (pl. 4).
Horizon XIII, 27 to 29 Days (4 to 5 mm) Long
Figure 6 illustrates the continued development of the lung, trachea, esophagus, and stomach. In transverse sections, the trachea and primary bronchi can be distinguished. In the older embryos of this hori7.on, that part of the respiratory tract cephalad to the bifurcation of the bronchi certainly seems to represent the future trachea, and not the lung bud as claimed by some authors whose descriptions imply that the trachea appears at a much later time.
Embryos of horizon xiii indicate clearly the semiindependence of the various growth phases involved: branching of the bronchial tree, formation of the tracheoesophageal septum, and lengthening of the trachea and esophagus. figure 7 exemplifies this point. Three lung buds are shown in which the cranial extent of tracheoesophageal separation has been marked. Despite a comparable degree of bronchial development, three different levels of tracheoesophageal separation are seen.
A marked diminution in the diameter of the esophagus just caudal to the tracheoesophageal separation is noted. This finding has also been reported by Johns (1952) as occurring in all embryos between 5 mm. and 23 mm. It would appear that the elongation of the esophagus causes a reduction in its diameter. In no. 836, the esophagus narrows at one point to such an extent that no lumen is visible in the sections. Microscopic evidences of the endodermal formation of the tracheoesophageal septum, however, continue to be found. Numerous cells are seen to be migrating from the lining of the coelomic cavity to contribute to the supporting structures of the trachea and esophagus (fig. 6).
Horizon XIV, 29 to 31 Days (6 to 7 mm) Long
Figure 8 shows the endodermal tract of horizon xiv. In front of the esophagus, a definite trachea with two primary bronchi is present. The bronchi begin to curve backward. As Streeter (1945) has pointed out, the right bronchus tends to be longer and directed downward, the left bronchus being more horizontal and shorter. Further, the growing ends of the bronchi are bulbous, as is characteristic of centers of active cell proliferation. In the older members of the series, secondary foci of proliferation can be seen which have become free of the dominance of the growing tip. They mark sites where secondary bronchi are to branch ofl. Such scc— scribed by Streeter in the Horizons series, from which secondary foci do not occur in the trachea, which, it is the following paragraphs are abstracted.
Fig. 6. Left, profile reconstructions (X35) of the gut tracts in two embryos from horizon xii (no. 5056, 25 somites; no. 5923. 28 somites). /-I is :1 transverse section (X50) of the respiratory region in an early embryo of this horizon. B, C, D are transverse sections (X50) at three levels to show the shape of the lung bud in a later embryo in the same horizon. There is an increase in mesenchymal activity in the cells adjacent to the coelomic cavity. The epithelial nature of the division of the respiratory and digestive tubes is shown clearly in the last three transverse sections. Profile reconstruction from Streeter, I942, figure 2, xii.
Fig. 7.Three embryos of horizon xiii, showing the disparity of bronchial branching and trachcoesophagcal separation. + marks the level at which the trachea and esophagus have a common lumen. X65. From Streeter, I945, figure I, xiii.
Fig. 3. Left, drawing of the foregut of a 4-mm. human cm- Inner eplthelml tube Surrounded by 3‘ nchly vascular’
bryo in the region of the developing lung bud, showing the lo- izcd mC5CnCh)'m3l 20113: Which ll‘ tun‘ is partly en‘ cation of the lateral esophageal groove (L. E. G.) in relation to closed by mesothelium facing the coelomic spaces. The the tracheoesophageal septum (T. E. S.) and the lumen of the lumen of thc esophagus remains small_ The begin mldlm (T) fmd of the. esophagus (El OVml°l'Cl°p'“°'“ Of ning pulmonary blood supply is more clearly defined. the internal ridges of this groove would occlude the esophagus along the line of the groove. Maintciiance of :1 communication The Small “Renal lwlgs descend from the 5‘-“h 30”“ between the trachea and the esophagus, as indicated in the draw- arch with irregular bilaterzil channels. A small com ing by the region outlined by a broken line, would result in a mo“ pulmonary vein drains into the left atrium-1_ (See picture similar to a type lllb atresia of the esophagus with a no. 721, fig‘ 9, -m horizon xv.)
tracheoesophageal fistula as illustrated on the right. From Keibel and Mall, Mamml of Hmmm Embryology, volume 2, The rcmammg Em" Stages: honzons XV to x"l“!
figures 237 and 238. were not studied in detail, having been sufliciently deA. section 6-2-4 B. sectio 6-2-6
Fig. 6. Left, profile reconstruction (X30) of the gut tract in an embryo of horizon xiii. no. 836. .-1. B, C, D are drawings (X 30) of transverse sections of this embryo at the level of the respiratory primordium. From the surrounding inesenchyme the supporting connective tissue and muscle layers begin differentiation. The profile reconstruction is taken from Streeter, 1945, figure 1, xiii.
evident, is already specialized in its own way. Likewise, the early steps in specialization of the larynx can be seen.
figure 9 illustrates the microscopic appearance of figure 10, xv, shows the developing trachea, bronchi, the lung and esophagus at this stage. There is an and esophagus in this horizon. Definite secondary
‘bronchi are present. figure 9 and figure 18 (pl. 3)
show that the supporting frameworks of the trachea and the esophagus are distinct, one from the other. As noted in the previous horizon, pulmonary vessels can be recognized, and a plexus of capillaries surrounds the primary bronchi.
Horizon XVI, 32 to 34 Days, Embryos (about 8 to 11 mm) Long
Figure 10, xvi, illustrates the continued differentiation and elongation of the trachea and the oesophagus. Separation of the two components is almost complete. figure 19 (pl. 3) shows further differentiation of the supporting structures.
Horizon XVII, 34 to 36 Days, Embryos about 11 to 14 mm Long
The endodermal tract of this embryo is shown in figure IO, xvii. Separation of lung and trachea from the foregut is essentially complete. A definite larynx is seen. Differentiation of the lung into five lobes has begun. figure 20 (pl. 3) exhibits the microscopic structure. Elsewhere condensations of mesenchyme foreshadowing the tracheal cartilages can be identified.
Horizon XVIII, 36 to 38 Days, Embryos about 14 to 16 mm Long
Figure 21 (pl. 3) illustrates the microscopic structure of the trachea and the esophagus at horizon xviii. Mesenchymal condensation about the trachea is marked. Submucosal and muscular layers can be identified about the esophagus. The aorta, pulmonary arteries, and vagus nerves are also visible.
From these studies of normal embryos, the following conclusions have been reached concerning the development of the trachea and the esophagus. The epithelium, termed by Streeter “the dictator tissue of the lung,” is truly the initiator and governor of the formation of the tracheoesophageal septum. The mesenchyme throughout plays only a passive role. Angiogenesis occurs in response to epithelial growth. The original laryngotracheal groove, which is seen as early as the Io-somite stage, is probably the direct primordium of the pulmonary system. The critical period of development with respect to the occurrence of atresia and fistula probably begins at this stage or perhaps earlier.
Fig. 8. Left, profile reconstruction of the gut tract and developing vasculature from an embryo of horizon xiv, no. I380. X35.
A, B, C, D are drawings of frontal sections showing the trachea, primary bronchi, and esophagus. X20. Profile reconstruction from Streeter, I945, figure I, xiv.
Through horizons xii to xiv, three crucial phases of development occur concomitantly: rapid elongation of the trachea and the esophagus, division of the trachea In horizon xv, definite separation has occurred, and from the esophagus, and bronchial budding. That these the supporting tissues of the trachea and esophagus are somewhat independent processes may be competitive has been suggested.
present.
Fig. 9. Drawings of sections taken through the respiratory and esophageal regions in horizons xiii, xiv, and xv to show the difierentiation of the supporting tissues of the lung and esophagus. In horizon xiii is still seen the proliferating coeloniic epithelium which is the source of the supporting tissues of the pulmonary and esophageal regions. In hori'/.on xiv, further organization of the mesenchyme occurs, and angiogenesis is seen about the esophagus. Continued vascular development is seen in horizon xv with the appearance of a common pulmonary vein. Embryo no. 721 is X50; the remaining sections are X 100. Drawings by I. F. Didusch from Streeter, 1945, figure 4, xv.
Description of Abnormal Embryos
Five early human embryos with esophageal atresia the right common carotid artery arose directly from the have been reported. We have fortunately been able to arch of the aorta. Other malformations included absence study the microscopic sections of three of them. of the posterior nares, abnormal development of the oc 1. Gladstone (1935) demonstrated an embryo of cipitocervical region of the skull and vertebral column, 19-mm. crown—rump length to the Anatomical Society and an abnormal development of the brain. Gladstone of Great Britain and Ireland. From his description, this reported evidence of impaired nutrition and a diseased specimen belongs to horizon xix (39-:1 days). The cm— condition of the tissues, affecting particularly the embryo had a blind cranial esophageal pouch, and from the lar and endothelial walls of the heart and blood vessels. dorsal aspect of the trachea, just above the bifurcation, a He believed that the atresia of the esophagus and the tracheoesophageal fistula led to the patent end of a distal tracheoesophageal fistula could not be explained simply segment of esophagus continuous with the stomach. The as due to the development of a retroesophageal subclavian anomaly is, therefore. type lllb. The size of the cranial artery. In View of the multiplicity and extent of the deesophagus diminished as it terminated in imperfectly vclopmental defects exhibited by this embryo, Gladstone developed epithelial and muscular coats. Among other suggested that “the evidence appeared to be in favour abnormalities present was defective development of the of the anomalies being primarily due to some general vascular system with persistence of the right dorsal aorta cause, e.g., a diseased condition of the chorion and emgiving rise to a retroesophageal subclavian artery, while bryo interfering with the normal development, rather than to a local cause such as pressure of a persistent right dorsal aorta on the oesophagus and trachea.” Our efforts to locate this embryo in order to borrow it for study were not successful.
2. Ysander (1925), in an inaugural dissertation on double monsters, described an 8—mm double monster embryo, lent by Dr. Robert Meyer. Efforts to trace the present whereabouts of this embryo were not successful. Through the kindness of Dr. John Naeslund of Uppsala, Sweden, we were able to obtain the original monograph. Notes on the specimen are filed in the Carnegie Collection (no. 9334). This embryo, whose endodermal tract is shown in figure 22 (pl. 4), has two esophagi, both of which become atretic at the level of the atria. Beginning buds, the presence of pigment about the lens, and the development of secondary bronchi would place it in horizon xvi (33 days, 8 to II mm.). The presence of a foramen ovale secundum suggests an even greater age. The detailed description of the esophagus is as follows (with minor verbal emendations):
6504 We
at the level of the carinae of both tracheae, there are fistulae continuous with what appear to be caudal esophageal segments opening into a large common stomach.
Although the total length is 8 mm., the embryo is obviously stunted, because other features place it in an older age group. The form of the upper and lower lung
Fig. 10. Profile reconstructions of the respiratory system, esophagus, and stomach in embryos of horizons xv (no. 6504), xvi (no. 6510), and xvii (no. 6520). There is Continued branching of the bronchial tree with dorsal inclination of the bronchi. The larynx is differentiated. The narrowness of the esophagus in horizon xvi is to be noted. X45. Profile reconstructions from Streeter, 1945, figure 5, xv; figure 5, xvi; figure G, xvii.
At first the two oesophagi run in the ordinary way immediately behind their respective tracheae, and appear to be normal, both as regards the structure of tlie walls and the width of the lumen. 25 mm. below the commencement of the trachea in the case of the left component, and 20 mm. in the case of the right component (measured on the model), the oesophagi suddenly come to an end. Without any demonstrable dilatation the epithelial tube ends blindly, and is continued downwards as a strand of mesenchyme, which soon becomes lost in the thin posterior wall of the peri-tracheal mesenchyme. . . . This, however, is not the last trace of the oesophagi, for out of the tracheal bifurcation of each component a tube projects, which is lined with epithelium. Further down, this tube curves downwards and inwards toward the centre of the double monster, where, at a distance of 23 mm. in case of the left component, and 19 mm. in case of the right component, from the bifurcation (measured on the model), it opens up into a large common ventriculus, thus proving itself to be the true continuation of the oesophagus, the course of which was interrupted by the obliteration.
In anticipation of a discussion of the causes of tracheoesophageal fistulae, several observations about this monster are of interest. The lungs are normally developed for the corresponding stage of development. The heart is enlarged, although the points of esophageal obliteration lie cranial to the heart. The upper segment of the esophagus is continuous with the trachea through the mesenchymal condensation. The fistulae do not exhibit the narrowing seen in other specimens. Ysander made no mention of any epithelial occlusion. In relation to this specimen, a report by Panse and Gierl_ich (1949) is of interest. In each of a pair of identical twins, one a stillborn anencephalic monster and the other an externally normal newborn who lived only a few hours, a Vogt type Illb esophageal atresia was found at postmortem examination.
3. The late Dr. F. T. Lewis described (1912) an I8.t—mm. embryo in the Harvard Collection which gives evidence of a Vogt type Illb atresia of the esophagus and also a retroesophageal right subclavian vessel. This embryo was first described by Minot (1910) and was estimated to be about 40 days of age; it is classified as horizon xix. Although otherwise normal, the embryo exhibits epithelial occlusion in the duodenum and anus. The lungs appear to have developed to a stage consistent with the length and age of the embryo. A model, figure 23 (pl. 4), clearly shows the dilatation of the caudal esophagus compared with the extreme narrowness of the fistulous tract shortly after it leaves the trachea. Through the kindness of Dr. Lewis, the sections of this embryo were made available for examination. Memoranda and notes are filed in the Carnegie Collection (no. 9335).
4. Gruenwald (1940) reported a 9—mm. human embryo with a type Illb atresia of the esophagus. On the basis of development, length, and estimated age, this embryo would be classified as belonging to horizon xvi. The lung buds appear to be somewhat retarded in growth for an embryo of that horizon. The case is remarkable in that the lower segment of the esophagus arises from the upper third of the trachea, 630 microns above the bifurcation. The upper segment is only 140 microns long. The embryo shows agenesis of both ureteric buds and hypoplasia of the right umbilical artery. As in the preceding specimen, the esophagus is extremely narrow in the section closest to the trachea.
Fluss and Poppen (1951) have suggested that the upper pouch of the esophagus in this embryo represents a pharyngeal diverticulum, the connection between esophagus and trachea, described by Gruenwald as the fistula, being simply the common foregut normal at this stage. They suggest that the trachea may not be differentiated to the same extent as the lung bud and larynx. Such an interpretation is not in accord with the descriptions based on adequate material, as outlined above in the section on normal development, which show that in horizons xv to xvi a definite trachea, separated significantly from the esophagus, exists and will shortly be followed by development of the larynx.
5. Another embryo with an atresia of the esophagus was reported by Professor M. Yamasaki of Tohoku University, Sendai, Japan, in 1933. We are greatly indebted to Professor Yamasaki for generously lending the specimen for study, and to Colonel Carl F. Tessmer of the Medical Corps, U. S. Army (attached to the Atomic Bomb Casualty Commission), for facilitating its temporary transfer to Baltimore. Models and other records are preserved in the Carnegie Collection (no. 873x). The embryo, measuring 8.5 mm., had abnormalities of other regions, including the nervous system, the face, and the spinal column. The embryo is classified as belonging to horizon xvi because of pigmentation of the eye vesicle, incipient phalangeal rays on the hand plate, separation of the lens vesicle, and the presence of primordia of the cochlear duct and of the pancreas. The head is small; the cervical curvature is slight; and the heart does not appear to be enlarged. The trachea and lungs seem somewhat retarded in comparison with other embryos of horizon xvi.
On examination, an upper esophageal pouch is seen to end blindly (figs. 24, 25, pl. 4). The larynx begins just below the thyroid pouch. At this point, the epithelial lining is of fairly uniform thickness, approximately three cell layers. Few mitoses are present. Beyond the separation of the trachea and esophagus, there is a definite zone of condensation about the cranial portion of the trachea which is well differentiated from the condensation about the dorsal gut. The cells about the trachea and in the more cranial portion have elongated nuclei, in contrast with the rounded nuclei of the cells of the wall of the gut. The wall of the cranial esophagus is formed of two layers of oblong cells. The trachea, at the point of bifurcation, has a finger-like projection of epithelium and surrounding connective tissue which is directed toward a more caudal blind stump of esophagus that enters the stomach (fig. 26, pl. 4). Yamasaki postulates that this embryo with a type II atresia of the esophagus developed along the same pathway as an embryo with a type III!) atresia of the esophagus with a tracheoesophageal fistula and differs only in the development of an atretic segment in the mid-portion of the fistula. Thinning of the fistulous tract in this same region has been‘ noted both in the Harvard embryo and in the Gruenwald embryo which were described above.
The embryo also shows an overgrowth of the duodenal mucosa. The pneumatoenteric recess is present, but does not seem large enough to cause changes by pressure. Both 4th aortic arches are present, but without exaggeration of size.
Hypothetical Explanations of the Anomaly
The many hypotheses suggested to explain the origin of atresia of the esophagus and tracheoesophageal fistula reflect the basic lack of understanding of the origin of all congenital defects. The hypotheses can be divided into the following groups.
Epithelial Occlusion
Kreuter (1905) suggested that a physiological occlusion of the lumen of the esophagus, which had been described in the 19- to 2o-mm. stage, might lead to atresia if recanalization did not occur. Subsequent work has led to some doubt whether complete occlusion of the lumen occurs. The present investigation has revealed little evidence of epithelial occlusion in horizons x to xv, although narrowing of the esophagus in the region just caudal to the bifurcation of the trachea was noted, reducing the caliber of the esophageal lumen. (See horizon xiii, no. 836.) Johns (1952) has noted vacuolization in the esophageal epithelium in the 13- to I6-mm. stage (horizons xvii to xviii). Neither of these findings, however, appears to correspond to a “solid” stage of esophageal development suggested by Kreuter. The occurrence of esophageal atresia in embryos considerably younger than the 19- to 20-mm. group, in which epithelial occlusion is stated to occur, points to some earlier cause. Epithelial occlusion also fails to explain the frequent accompaniment of a tracheoesophageal fistula.
Intraembryonic Pressure
Prcmzrc of the heart. It has been suggested by Schmitz (1923) that enlargement of the embryonic heart combined with marked dorsal curvature in the cervical region may displace the esophagus and trachea dorsally, the esophagus being pinched off against the vertebral column. As the embryo subsequently enlarges, the cranial segment is pulled free. Although several of our specimens had signs of cardiac enlargement, esophageal atresia and tracheoesophageal fistula have been known to occur in acardiac or microcardiac monsters. This fact, combined with general objections to hypotheses dependent upon pressure, leads the present author to question this general explanation.
Pressure from abnormal uesrclr. The frequent association of a retroesophageal right subclavian artery with tracheoesophageal fistulae and esophageal atresia has led to the speculation by Fluss and Poppen (1951) and others that the abnormally persistent caudal portion of the right dorsal aorta, passing dorsal to the esophagus, may press upon it and cause an atresia. Against this hypothesis is the finding of esophageal atresia in two embryos at an early stage of development when the vessel in question appears too small to exert significant pressure. The presence of an abnormal vessel, though frequent and of considerable surgical importance, is not a constant feature of esophageal atresia. The presence of a retroesophageal artery in the Gladstone and Harvard embryos would seem to represent the simultaneous occurrence of a related, but not causative, anomaly.
Pressure by the pncmnatoenteric recesses. Broman (1904) suggested pressure from the pneumatoenteric recesses as the cause of the abnormality. These recesses are connected with the pericardial cavity and with the chorionic cavity at one stage of development. The presence of excess fluid, accentuated by the movements of the heart, was thought to cause pressure against the dorsal portion of the foregut with pinching-off of the digestive tube. These recesses, however, develop somewhat later than the stages indicated as critical by this study, that is, horizons xi to xv.
Despite the continued arguments for the causation of defective growth by abnormal pressures on developing structures, considerable doubt exists as to the importance of mechanical pressure. The disturbance of spatial relations thus produced might influence growth by altering the concentration or locus of action of “organizers," but mechanical pressure per se seems to be an unlikely cause of malformations. The extremely gelatinous nature of the embryo in these early stages would appear to permit considerable displacement of developing structures.
Differential Growth
Hypotheses of the third group are based on biochemical and physical alterations, caused by unknown factors, of the complex integration of growth necessary to normal development. Yamasaki (1933) has stated that “according to my interpretation the dysfunction of active cellular proliferation is to be looked upon as the primary and fundamental reason for the abnormal development of the lung buds while other factors, such as the abnormal heart size, play only a contributory role in the process.”
He called attention to the difference between the thin dorsal layer of cells and the rather profuse epithelium on the ventral side of the primitive foregut. Clinical experience showing esophageal atresia to be far more common than tracheal atresia, which is in fact exceedingly rare, can be correlated with this finding. Yamasaki went on to postulate that the rapid growth of the trachea and pulmonary primordium uses up so much of the “growth potential," as expressed in available cells, that the posterior digestive segment cannot provide enough cellular material to complete the esophagus.
Flg. It. (I, lung bud and trachea ventrally (shaded) and esophageal tissue dorsally (stippled). 1:, normal development of the trachea (I) and the esophagus caudal to the pharynx (p), showing synchr0ni'/.ed growth and continuity of the esophagus. 5, rapid elongation of the trachea, resulting in a thinning-out of the segment of the esophagus along the dorsal wall so that a coinmunication or fistula is left. :1’, with elongation of the trachea the fistula comes to occupy a position near the bifurcation. From Gruenwald (I940), figure 3.
Gruenwald (1940) suggested a similar chain of events in that a delay in separation accompanying a rapid elongation of the trachea may carry the developing digestive tube caudalward so rapidly that it loses the ability to (liflcrentiatc into a separate and normal esophagus over the distance which the trachea has grown (fig. I I). \Vith a decrease in the rate of growth of the trachea, the cranial segment of the digestive tract may begin to differentiate, and may even make up the growth (lifference and overlap the lower segment. He points to the presence of esophageal tissue on the dorsal wall of the trachea, or of muscular bands passing from the upper segment of the esophagus to the trachea, as evidence that esophageal tissue stretched thin by rapid elongation of the trachea is present on the dorsal wall of the respiratory tree. Our study has substantiated the semi—independent nature of the processes of elongation and tracheoesophageal division.
Rosenthal (1931), whose views reflect the experienced judgment of G. L. Streeter, with whom he discussed his cases, believed the defect to be caused by faulty union of the epithelial ridges which divide the foregut internally. He pointed out, without attempting a detailed explanation, that some alteration from a truly craniocaudal division, causing the growth to be directed rlorsalward, might divide the foregut in such a way as to produce the abnormality under discussion. The possibility that epithelial ridges of the lateral esophageal groove play this role was not mentioned.
Discussion
It has been the purpose of this investigation to elucidate the early development of the pulmonary and esophageal portions of the foregut in the early human embryo, and to attempt to clarify the abnormal processes which lead to esophageal atresia and tracheocsophageal fistula. A complete solution to these problems is still lacking, but the morphological analysis presented above makes it possible to question and possibly discard some of the hypotheses and to suggest lines of evidence from which an explanation may ultimately be attained.
As illustrated by the specimens brought together in this study, abnormalities in the differentiation of the trachea and esophagus are well established as early as hot‘i'/.on xvi or xvii (fifth week of embryonic life). The disturbances of embryonic development which cause the anomalies must, therefore, be looked for at earlier stages. The observations on normal embryos reported above show that the tracheopulmonary primordium is distinguishable as early as 21 days after fertilization. At least as early as this, possibly earlier, the tissues are sufficiently differentiated to respond to specific organizer actions.
Anatomical defects of the type we are considering must have their beginnings at this and the immediately preceding stages. Disturbances acting much earlier, for example before hori7.on x or xi (fourth week), would in all probability result in more widespread defects. A later disturbance, after horizon xviii_. say. would require deterioration of structures already well developed. At such later stages, the specific developmental phases of the trachea and esophagus have passed their period of greatest vulnerability to disturbance. Explanations of esophageal atrcsia caused by disturbances after this critical period call for a high degree of coincidence to account for the rather constant anatomical appearance of the abnormality.
Among the morphological points brought out by this investigation which suggest in what direction we must look for the site of the primary organizational defect. the most crucial is the apparent importance of the epithelium of the foregut as the determining factor in the separation of the trachea from the esophagus. The mesenchyme seems to have only a passive role in this process. The possibility of epithelial imbalance results from the apparent independence in timing shown by elongation of the trachea and esophagus, bronchial branching, and tracheoesophageal separation. The defects in question thus appear to arise from disturbances in the differentiation and growth pattern of the epithelial lining of the foregut at the time when separation of the trachea and esophagus is impending or is in its earliest stages.
The hypothesis that the abnormality is caused by abnormal intraembryonic pressures has very little morphological support. Many investigators have stressed that the embryonic tissues appear most susceptible to injury, and consequent abnormality, when they are undergoing the most rapid and complex development. During horizons x to xv, three rapid and complex major changes are occurring: elongation and growth; separation of the trachea from the foregut; and branching and growth of the bronchial tree. At this time, abnormalities may come about either as the result of retardation of growth or as the result of excessive growth in certain portions of the developing structure; in fact, probably both are involved. The concept of “tissue availability," suggested by Gruenwald and Yamasalti, seems useful in analyzing the sequence of events. The present investigation suggests that the development of both esophageal atresia and tracheoesophageal fistula may involve hyperplasia of the lateral esophageal ridges. The atresia would seem to result from the too rapid elongation of the trachea, combined with diminution in esophageal substance such that the esophagus “cannot make ends meet,” as suggested by Gruenwald. The thickened tissue of the wall of the lateral esophageal groove provides cellular material for the continuation of the esophagus, at this point, with the development of a fistula. Lesions of type II may arise, as suggested by Yamasaki, from a failure of the fistula to persist or to develop. The tenuous nature of the fistula was noted in the Harvard embryo. Where no suggestion of a listulous tract exists, we would assume only the factor of too rapid a respiratory elongation without abnormal development of lateral esophageal grooves. fistulae without atresia and defects of type III: would appear to be due to localized or limited failure of the developing tracheoesophageal ridges, perhaps caused by imbalance between tracheoesophageal division and elongation.
The similarities between esophageal atresia and imperforate anus. and between tracheoesophageal and urogenital iistulae, are striking. Atresia of the ventral and secondarily developing system is rare in both the former regions; and the urogenital septum resembles the tracheoesophageal septum in many respects. In a brief study, the mechanism of separation appeared to be similar, that is, epithelial division rather than pressure from the ingrowth of mesenchymal bars. The frequent oc currence of a fistula from the blind “inner" segment to the ventral respiratory or urogenital system follows much the same pattern.
Although the time of onset of the disturbance can be determined within close limits, and its morphological stages can at least be subjected to reasonable conjecture, the causal factors that actually operate in individual human cases, by interfering with the growth process at the critical time, remain to be determined by clinical study.
Evidence for genetic causation of these defects in man is scanty in spite of their relative frequency in newborn infants. Three cases have been reported in which the abnormality occurred in more than one pregnancy in the same family, in each instance in the same generation (Mackenzie, 1884; Grieve and McDermott, 1939; Lanman, 1940). In the first of these reports, three children
Table 1
lV‘l:\TF.RNr\L PR!-Z.\':\TAL FACTORS IN CASES OF ESOPHAGE.-\L ATRESIA \V|TH 'I'RACHEOESOPHr\GEa\L fiSTL'L:\
Acute infections or metabolic disturbances in first trimester. . 6
Chronic disease . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. I2 Hydramnios . . . , , . . . . . . . . . . , . . . . . . . . . . . . . . . . . . . . , . . . . . .. 20 Antepartum bleeding . , . . . . . . . , . . . . . . . . . , _ . . . . . . . . . . . . . .. 6
From cases of Children's Hospital, Boston, and Boston Lying-In Hospital. lngalls and Prindle: Esophageal atresia with lracheoesophagcal fistula. of the same father but by different mothers had the abnormality. In another case mentioned previously (Panse and Gierlich, 1949), both of a pair of twins were affected; and Ysander (1925) described the anomaly in both portions of a double monster. Ingalls and Prindle (1949), in reviewing 107 cases of esophageal atresia at the B05ton Children’s Hospital, found no evidence of a genetic element in their origin. As yet, no geneticist has observed a strain of laboratory animals carrying these defects, such as are now well known in connection with many other anomalies.
Possible environmental causes of malformations can be studied in two ways: beginning with malformed infants and studying the pregnancy of the mothers; or beginning with mothers who have a suspicious complication in pregnancy and determining the outcome, as has been done in studies on German measles. Table 1 gives prenatal data from a group of 87 cases of the Boston Lying-In Hospital and the Boston Children's Hospital. The rather high proportion of various disturbances of pregnancy, coupled with a high incidence of prematurity, suggests that the embryonic abnormalities were part of a picture of generally inadequate gestation. Ingalls and Prindle also remarked on the frequent incidence of early bleeding from the uterus in the prenatal history of the cases. Such inadequacy, however, could result either from genetic weakness of the embryo or from a disturbance of gestation due to causes operating through the maternal environment. That this is a generalized factor is suggested by the frequent occurrence of associated abnormalities as shown in table 2.
Experimental evidence for the causation of these particular defects by environmental agencies is almost entirely lacking. Warkany at al. (1948) have reported one rat fetus in a group born to a vitamin-A-deficient mother in which a traeheoesophageal fistula was present. In view of the repeated production of various other com— mon defects, such as spina bifida, cleft palate, and cardiac lesions, by a wide variety of traumatic and toxic agents, it is to be expected that, sooner or later, carefully timed experiments will result in the production of tracheoesophageal defects.
In the present state of knowledge, we can only suppose that tracheoesophageal defects of the human embryo may result from either genetic or environmental causes or from a combination of both, such as a maternal infection or nutritional deficiency acting upon a genetically weak embryonic tissue.
As stated above, the time at which any injurious stimulus or agent is acting upon the embryo seems to be of more importance than the specific nature or quantitative amount of the noxious agent. For example, defective genes, X rays, cortisone treatment, and vitamin deficiency might all produce the same abnormality if applied at the same period of development. It is to be hoped that the present study, by defining the time at which the human embryo becomes susceptible to defects of organization of the trachea and the lower esophagus, may assist clinicians in studying prenatal histories in the search for their specific environmental causes.
Table 2
Associated ABNORMALITIES 1-'ou.\'n IN 233 BABIES won HAD ESOPHAGEAI. ATRESIA
Congenital heart disease . . . . . . . . . . . . , . . . . . . . . . . . . . . . . . . .. 24
Malformation of anus or rectum . . . . . . . . . . . . . . . . . . . . . . . . .. 23
Meckel’s diverticulum . . . . . . . . . . . . . . . , , . . . . . . . . . . . . . . . . .. I0
Atresia or stenosis of small intestine . . . . . . . . . . . . . . . . . , . . .. 7
Malrotation of colon and intestines . _ . . . . . . . , . . . . . . . . . . . .. 4
Coarctation of aorta . . . . . . . . . . . . . . . . . . . , . . . . . . . . . . . . . . . . . 4
Vertebral anomalies . . . . . . . . . , . . . . . . . . . . . . . . . . . . . . . . . . . .. 4
Stenosis of ureter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 4
Annular pancreas . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Mongolism . . . . . . . . . . . . . . . . . . . . . . . . . . . , . . . . . . . . . . . . . . . . . 2
Pyloric stenosis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 2
Duplication of stomach . . . . . . . . . . . . . , . . . . . . . . . . . . . . . . . . .. 2
Absence of kidney . . . . . . . . . . . . . . . . . . . . . . . . . . . . . , . . . . . . .. 2
Horseshoe kidney . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Harelip and cleft palate. . . ._ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Hypospadias . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
I-lypoplasia of kidneys . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Diaphragmatic hernia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. ]
Agenesis of lung . . . . . . . . . . . . . . . . . . . . . . . . . . . , . . . . . . . . . . . . 1
Stenosis of bronchi . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 1
Septate vagina and bicornuate uterus . . . . . . . . . . . . , . . . . . . . .. 1
Pseudohermaphroditism . . . . . . . . . . . . . . . . . . . . . , . . . . . . . . . . . . 1
Club foot . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . , . . . . . . . 1
Various minor anomalies . , . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
From Cross: The surgery of infancy and childhood.
Conclusions
1. A review of the normal development of the trachea and esophagus from horizon x to horizon xviii (23 to 38 days after ovulation) shows that the critical period at which congenital atresia of the esophagus and tracheoesophageal fistula must begin to develop is in horizons x to xvi.
2. The earliest observed embryonic cases are in horizon xvi or xvii.
3. Study of live embryonic specimens showing these defects, three of which have been examined by the pres ent author, suggests that the mechanism of their production is a growth defect of the esophagus and trachea, coupled with overgrowth of the epithelium which bulges into the forcgut over the lateral esophageal ridges.
4. In the present state of knowledge, the causation of these defects must tentatively be ascribed to genetic defects or disturbances of the maternal environment (infectious diseases, toxic conditions, or nutritional disturbances during pregnancy) acting at a critical period in the organization of the trachea and esophagus.
Literature Cited
B1-:1.s1=.s', R. H. R., and C. P. DoNN1so.\1. 1950. Congenital atresia of the oesophagus. Brit. Med. Iour., vol. 2, pp. 324-328.
Baosnxx, I. 1904. Die Ent\\-‘ickelungsgeschichte der Bursa omentalis und Eihnlicher Rezessbildungen bei den Wirbeltieren.
611 pp. Wiesbaden.
Cormrsa, G. W. 1929. A well-preserved human embryo of 10 somites. Carnegie Inst. Wash. Pub. 394, Contrib. to Emhryol., vol. 20, pp. 81-101.
D.-ixtw, \V. F.. 1910. A human embryo with seven pairs of somites measuring about 2 mm. in length. Amer. Iour. 1\nat., vol. 10, pp. 85-108.
D1\VlS, C. L. 1923. Description of a human embryo having twenty paired somites. Carnegie Inst. ‘\Vash. Pub. 33:, Contrib. to Embryol., vol. 15, pp. 1-51.
fizxetrsox, C. F. 1951. Congenital tracheoesophageal fistula not associated with atresia of the esophagus. Laryngoscope, vol. 61, pp. 713-766.
Fuxr, I. M. 1907. The development of the lungs. Amer. Iour. Anat., vol. 6, pp. 1-165.
FLUSS, 72., and K. I. Poppen. 1951. Embryogcnesis of tracheoesophageal fistula and esophageal atresi:1. Arch. Pathol., vol. 52, pp. 168-181.
FORSSNER, H. 1907. Die angeborenen Darm- und Osophagusatresien. Anat. Hcfte, vol. 34, pp. 1-163.
G,-u:r., .\I., and A. Ocnsxza. 1936. The surgical treatment of congenital tracheo-esophageal fistula in the newborn. Ann. Surg., vol. 103, pp. 725—,37.
GLADSTONE, R. I. 1935. Exhibit of lantern slides of 19 mm. embryo. In Proceedings of the Anatomical Society of Great Britain and Ireland. Iour. Anat., vol. 70, pp. 198-199.
GRIEVE, I. G., and I. G. MCD1-:1z.\1or'r. 1939. Congenital atresia of the oesophagus in two brothers. Canadian Med. Assoc. Iour., vol. 41, pp. 185-186.
GROSS, R. E. 1953. The surgery of infancy and childhood: its principles and techniques. 1000 pp. Philadelphia.
Gnossen, O. 1912. The development of the respiratory apparatus. Irz Keibel and Mall (eds.), Manual of human embryology, vol. 2, chap. 17, pp. 473-493. Philadelphia.
GRUI-1.\‘WALD, P. 1940. A case of atresia of the esophagus combined with tracheo-esophageal fistula in a 9-mm. human embryo, and its embryological explanation. Anat. Rec., vol. 78, pp. 293-302.
Harem“, C., and H. A. Towsmzv. 1943. Congenital atresia of the esophagus with tracheoesophageal fistula. Extrapleural ligation of fistula and end-to-end anastomosis of esophageal segments. Surg., Gynecol. and Obstet., vol. 76, pp. 672-688.
HIORTSI5, C. H. 1945. De epiteliala lunganlagens tidiga morfogenes hos F:-Ii: cam: L. Monograph. 184 pp. Lund.
INGALLS, T. H., and R. A. PRINDLE. 1949. Esophageal atrcsia with tracheoesophageal fistula. Epidemiologic and teratologic implications. New England Iour. Metl., vol. 240, pp. 987995
Ionxs, B. A. E. 1952. Developmental changes in the oesophageal epithelium in man. Iour. Anat., vol. 86, pp. 431-442.
KEITH, A. 1910. A demonstration on constrictions and occlusions of the alimentary tract of congenital or obscure origin. Brit.
Med. Iour., vol. 1, pp. 301-305.
KING, E. S. I. 1948. Lecture l: Significance of modern embryology in pathology. Med. Iour. Australia, vol. 2, pp. 705710.
KLEBS, E. 1869. Handbuch der pathologischen Anatomic. Vol. 1, p. 164. Berlin.
KREUTER, E. 1905. Die angeborenen Verschliessungen und Verengcrungen des Darmkanals im Lichte der Entwicklungsgeschichte. Deut. Ztschr. f. Chir., vol. 79, pp. 1-89.
Lana, W. E. 1944. The surgical treatment of esophageal atresia and tracheoesophageal fistulas. New England Iour. Med., vol. 230, pp. 625-637.
L/lNMA.\', T. H. 1940. Congenital atresia of the esophagus. Arch. Surg., vol. 41, pp. 1060-1083.
Levers, N. L. 1941. Congenital atresia of the esophagus with trachcoesophageal fistula. Iour. Thor. Surg., vol. 10, pp. 643-657.
Liawrs, F. T. 1912. The development of the oesophagus. In Keibel and Mall (eds.), Manual of human embryology, vol. 2, chap. 17, pp. 355-368. Philadelphia.
hil.-\CKENZlE, M. 1884. A manual of diseases of the throat and nose. Vol. 2. Diseases of the oesophagus, nose, and nasopharynx. 400 pp. New York.
Mall FP. Normal plates of the development of vertebrates. Anat. Rec, (1908).
M.\1_1_, F. P. 1908. A study of the causes underlying the origin of human monsters. (Third contribution to the study of the pathology of human embryos.) Iour. Morphol., vol. 19, pp. 3-368.
Minot CS. A Laboratory Text-Book Of Embryology. (1903) Philadelphia:P. Blakiston's Son & Co. 402 pp
PANSE, F., and I. GIERLICH. 1949. Zur Pathogenese der Aneuccphalie. Virchow’s Arch. f. pathol. Anat. u. Physiol., vol. 316, PP- 135-148
PATTI-ZN, B. M. 1953. Embryological stages in the establishing of myeloschisis with spina bifida. Amer. Iour. Anat., vol. 93, pp- 365-396
P1..»\ss, E. D. 1919. Congenital atresia of the esophagus with tracheoesopliageal fistula: associated with fused kidney. A case report and survey of the literature on congenital anomalies of the esophagus. Iohns Hopkins Hosp. Reports, vol. 18, pp. 259-286.
PUIGGR6S—SALA, I. 1937. Ueber die Entwicklung der Lungenanlage des Menschen. Ztschr. f. Anat. u. Entwicklungsgesch., vol. 106, pp. 209-225.
ROSENTHAL, A. H. 1931. Congenital atresia of the esophagus with tracheo-esophageal fistula. Arch. Pathol., vol. 12, pp. 756-772.
Sc11:\111'z, I. A. 1923. Ueber die formale Genese der Oesophagusmissbildungen. Virchow’s Arch. f. pathol. Anat. u. Physiol., vol. 247, pp. 278-293.
SCHRIDDE, H. 1908. Ueber die Epithelproliferationen in der embryonalen menschlichen Speiscrohrc. Virchow’s Arch. f. pathol. Anat. u. PhysioI., vol. 191, pp. 178-192.
SHAW, R. 1939. Surgical correction of congenital atresia of the esophagus with tracheo-esophageal fistula. Case Report. Iour. Thor. Surg., vol. 9, pp. 213-219.
S1=1s.\mNN, H. 1928. Croonian Lecture of 1927: Organizers in animal development. Proc. Roy. Soc. London, B, vol. 102, pt» 177-137
STREETER, G. L. 1942. Developmental horizons in human embryos. Description of age group xi, 13 to 20 somites, and age group xii, 21 to 29 somites. Carnegie Inst. Wash. Pub. 541, Contrib. to Embryol., vol. 30, pp. 211-245.
1945. Developmental horizons in human embryos. Description of age group xiii, embryos about 4 or 5 millimeters long, and age group xiv, period of indentation of the lens vesicle. Carnegie lnst. Wash. Pub. 557, Contrib. to Embryol., vol. 31, pp. 27-63.
1948. Developmental horizons in human embryos. Description of age groups xv, xvi, xvii, xviii, being the third issue of a survey of the Carnegie Collection. Carnegie Inst. Wash. Pub. 575, Contrib. to Embryol., vol. 32, pp. 133-203.
V061‘, C. 1929. Congenital esophageal atresia. Amer. Iour. Roent., vol. 22, pp. 463-465.
WARKANY, I., C. B. Rom, and I. G. W1LsoN. 1948. Multiple congenital malformations: a consideration of etiologic factors. Pediatrics, vol. 1, pp. 462-471.
W1:1.1_s, L. I., and E. A. BOYDEN. 1954. The development of the bronchopulmonary segments in human embryos of horizons xvii—xix. Amer. Iour. Anat., vol. 95, pp. 163-202.
YAMASAKI, M. 1933. Ein menschlicher Embryo von 8.5 mm Scheitel-Steissliinge mit fehlcrhaften und ungewohnlichen Bildungen. Arb. aus elem Anat. Inst. 1]. Kaiserlich-Iapanischen Uni\'ersiti1't 7.. Sendai, vol. 15, pp. 27-59.
Ys.mnr.R, F. 1925. Human diplo-terata. inaugural dissertation. Uppsala.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - Normal development of the trachea and esophagus in man. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Normal_development_of_the_trachea_and_esophagus_in_man
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G