Paper - Equivalent ages in mouse and human embryos
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Otis EM and Brent R. Equivalent ages in mouse and human embryos. (1954) Anat Rec. 120(1):33-63. PMID 13207763
| Online Editor | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This historic 1954 paper by Otis and Brent compares the timeline of human development with two common laboratory model of mouse development.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Equivalent Ages in Mouse and Human Embryos
Eileen M. Otis and Robert Brent
University of Rochester, Atomic Energy Project, Rochester, New York
Twenty-Nine Figures (1954)
- Work performed under USAEC Contract Number W-7401-eng-49.
Introduction
In the course of investigating some genetic effects of radiation on mice, the question of the applicability of some of the conclusions to human material arose. The mortality rates of a particular genetic defect which is a prenatal lethal in mice and rats and presumably in other mammals had been determined and a prediction of the probable time of death for human embryos in similar genetic situations was desirable. Should, for example, a death time in the mouse centering about the 7th day of a 20-day -gestation period be considered comparable to the third month of human pregnancy or to some earlier period? It would be possible to use the stage of development of a single organ such as the eye or heart to equate two mammals. A single organ, however, might be precocious or lag in relation to the whole embryo, so that it seemed preferable to determine equivalent stages in the mouse and human on the basis of the development of as many embryological structures as could be clearly timed in the mouse and found in the literature on the human embryo.
Materials and Methods
The mouse embryos used were the offspring of Bagg albino or Carworth Farm CFCW strain by the genetically normal siblings of lines under investigation for semi-sterility. These lines were originally constructed from crosses of 057 females by irradiated or non-irradiated control dba males. Succeeding generations were, for genetic purposes, maintained by outcrossing to Bagg albino or Carworth Farm C-FCVV until the present study was begun. Embryos used in determining the equivalency table were intended to be comparable to embryos recovered in semi-sterility studies and were not maximally hybridized. They are }’C>unger by about 12-18 hours than those described by Snell ( ’41) and older by at least 36 hours than those described by Melissinos (’07). At 7 days and 12 hours they correspond fairly closely to the 8 day specimens of Sobotta (’11).
The total gestation time of the strain was 19 days, 7.5 hours i 8 hours. Gestation time was determined by timing 86 pregnancies from the hour of mating to the first hour of delivery.
When embryos of a particular age were to be collected, males were placed with three females each at about 10: 30 ]?.M. and left for one hour. At the end of this time females were examined and those with mating plugs were isolated for autopsy at a subsequent appropriate period of gestation. Individual ovulation and fertilization times were unascertainable so that the age of the embryo is determined by the mating time plus or minus 30 minutes. The time for mating was selected as the most favorable time for fertilization if ovulation occurred a.s early as 11: 30 P.M. (Snell et al., ’40). Mating will presumably take place after the onset of estrus, but ovulation is said to occur at various times during estrus. Brambell (’28) states that ovulation takes place during late pro—estrus or very early estrus. Allen (’22) found evidence that ovulation occurred at the end of estrus. Thus, it was possible that two embryos of the same mating age but from different pregnancies might vary markedly in fertilization age. To minimize variations of timing from this source, samples of several pregnancies were taken. To the 12th day of gestation, all the living embryos from a. single pregnancy were sectioned and two embryos from each of two other pregnancies of the same mating time. If, in comparing embryos from separate pregnancies, differences in development were found to be greater than the differences between embryos from the same uterus, a new partial sample was obtained from three more pregnancies of the same duration. In 31 pregnancies to the end of the 12th. day thus timed, a series at 11 days 0 hours gave embryos 12 hours younger than others of its mating age indicating relatively late ovulation or aipoor uterine environment. After the 12th day of gestation differences between embryos from the same uterus were less apparent and only two embryos were taken from each of three pregnancies. If no marked discrepancies were observed, all were admitted for study. A satisfactory distinction was never drawn between embryos of 13 days 0 hour-s and 13 days 12 hours. The oldest members of the 13 day 0 hour group and the youngest of the 13 day 12 hour group overlapped despite repeated sampling of pregnancies of both mating times. In view of the comparative regularity of samples from pregnancies of a shorter duration where differences were more pronounced, it seemed unlikely that all of the 13 day 12 hour autopsies were of late ovulating females. It appeared that morphogenetic changes were either slower or less conspicuous; therefore, autopsies at 12 hour intervals were abandoned after the 14th day.
Embryos damaged in preparation or poorly oriented were used as far as possible and then eliminated from detailed study. Embryos admitted to study are listed in table 1.
In the preparation of serial sections, embryos were dissected out a-s rapidly as possible in warm Locke’s solution. The operation ordinarily took about 15 minutes in pregnancies of more than 9 days 12 hours duration and slightly longer in earlier pregnancies. Except when taking crown-rump measurements they were fixed immediately in Bouin’s for 48 hours or more and then washed in alcohol, cleared in tertiary butyl alcohol and paraffin embedded. In order to avoid damage, no incisions were made in the embryos for fixing. Most of the embryos were cut at 12 u. A. few older embryos were sectioned at 15 p, and at least one at each age level except for the 17th day was sectioned at 10 u. With very small embryos (less than 10 days) orientation in the paraflin block proved unsatisfactory. These were oriented in agar blocks immediately after clearing and then paraffin embedded. The sections were stained in Harris’s hemotoxylin and eosin B or eosin Y. Embryos of less than 9 days were sectioned in utero where the plane of the section could not be controlled. One sagittal series was obtained for each 24 hour time interval after 9 days and 12 hours. All other embryos were transverse sectioned.
Table 1
| Table 1 - Age and Measurements of mouse embryos admitted to study |
|---|
| Table to be formatted. (see template discussion page) |
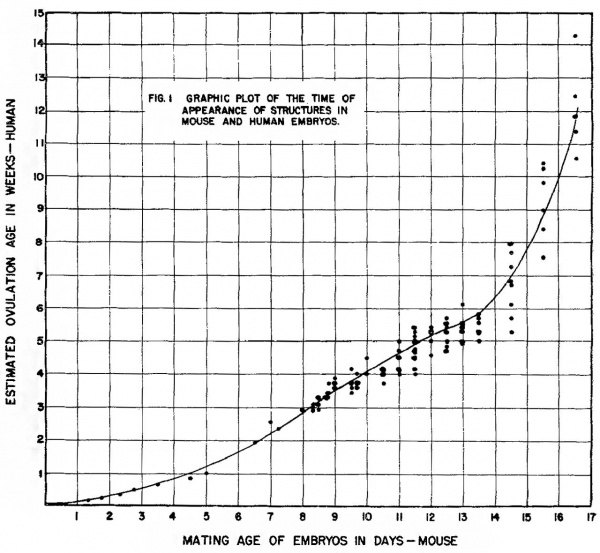
Assignment to the equivalency table and graph. Each developing structure identified in both mouse and human embryos was listed in table 3 and entered on the graph (fig. 1) as a point against the earliest time at which it is said to appear in the human and the time at which is was clearly present in any mouse embryo of a particular age level.
Numbers of the abscissa of the graph indicate the end of a day of mouse gestation. Numbers on the ordinate indicate the end of a week of human gestation. Thus, a structure arising at 10 -days, 0 hours, or 10 completed days in the mouse and at 28 days or the end of the 4th week in the human is a coordinate at 10,4 respectively.
In referring to mouse gestation time i11 the text, a morphogenetic change occurring, for example, at 13 days, 0 hours is said to occur at the end of the 13th day; one occurring at 13 days, 12 hours, is said to occur on or during the 14th day.
Age assignment in the mouse. Many of the structures were present in all mouse embryos of a given age group and not in the preceding age group. These must have arisen at some time between the preceding period and the age to which they were assigned. The points representing these structures have been assigned to the appropriate 12 hour age level rather than to a position between successive age levels. Assignment in the mouse thus remains comparable to human assignment in which a structure can only be timed as arising in the youngest embryo in which it has as yet been seen.
From 8 days 0 hours until dedifferentiation makes the somite count uncertain, somite formation in the mouse assists in narrowing the assignment error. A structure appearing first in a 6 somite embryo of 8 days and 12 hours mating time has clearly arisen later than one already present in a two somite embryo of the same mating Tl1e somite period has been arbitrarily divided in constructing the table and gra.ph as though somites were formed in the mouse at regular intervals although the rate is known to Vary somewhat in the rat (Butcher, ’29). Table 1 gives the distribution of mouse embroys in this series by somite count and the age assigned to embryos of a particular somite count for graphic presentation.
Variations in mouse embryos of the same mating age. The variation in embryos of a particular mating age is most apparent during the period of somite formation. While crown—rump measurements also provide an objective approach to differences between embryos of the same age, they do not always bear a direct relation to developmental age as do the somites. At 9 days and 0 hours mating age, for example, the somite count for all embryos of that mating age varies between 9 and 16 or 17. Within one uterus, embryos of 13, 14, 15, 16, and 17 somites were present. Progressive changes such as the increasing closure of the anterior neuropore, the broadening of the optic sulcus, and the deepening of the otic invagination varied directly with the «somite count.
In the pre-somite period, variations are at least as great as in the period of somite formation, but are less easily measured. At 7 days 22 hours, 5 of the embryos from one uterus were in the head fold stage with foreguts varying from about 30 to about 100 p in length. Two others without foregut each had a short allantoic outgrowth; one was still in the early primitive streak stage. Similar variation has been recorded by Allen and MacDoWell ( ’40).
From the 11th to 17th day crown-rump measurements were taken before fixation (table 1). Of the 6 sectioned embryos at 15 days and 12 hours, the smallest, 12.8 mm in length, was not demonstrably younger than the largest, 14.2 mm in length. Variations in the nucleated red blood cells were not greater than differences i11 successive samples from the same embryos. The primary ossification center of the fetal humerus appeared to be slightly longer proportionately in the larger embryo, extending through about 40% of the developing shaft as compared with about 30-35% in the smaller embryo.
While it appears that variation between surviving embryos of the sa.me mating age decreases in the later days of gestation, the appearance may only reflect the fact that in older embryos, suitably objective measurements are difficult to obtain.
Estimated age in Human embryos. In the literature on human embryology, various methods of describing the age of an embryo are used. pre-somite embryos and those in the process of forming the first somites are usually timed from menstrual history to an estimated ovulation day or period of two or three days. In the latter event, a mean day was assumed for assignment to the graph. Occasionally the initial age estimate of an early embryo has been subsequently revised by the same or another investigator. Any structure described in the initial investigation is assigned against the revised age.
Embryos in the Carnegie collection from 13 somites to about 20 mm in crown-rump length have been timed by Streeter (’42, ’45, ’48, ’49) using macaque embryos of known ovulation age for comparison. Age estimates are at a mean day plus or minus one day. Assignment of structures seen in these embryos to the graph presented here depends on the classification of the embryos as a younger, older or middle member of the group.
Age estimates beyond the 6th week of gestation are sometimes given only in weeks or portions of weeks. For the graph, these have been translated into an appropriate day. For example, a structure seen first in embryos estimated to be at the 13th Week of gestation appears at 87 days, at the end of the 9th Week, 62 days.
In still older embryos, age estimates are frequently given in months together with crown-rump measurements. Crown-rump measurements have been converted to days using principally the‘ tables of Streeter (’20) with appropriate corrections for fertilization rather than menstrual age. Comparison of the Streeter tables with those of Mall ( ’18) shows a difference of about 10 days in menstrual age for embryos up to about 80 mm in length. The Streeter tables were used because the transition from estimates based on comparison with the macaque to those based on crown—rump length gave a smooth progression without imposing a sudden shift in coordinates which would give the appearance of an abrupt change in the rate of development of the mouse embryo with respect to the human. Conversion values used in this work are listed in table 2. The use of an exact day is for convenience in graphic presentation and in no way implies that the figure chosen is a true mean with a measurable variance.
Table 2
| Table 2 - Ages assumed for human embryos |
|---|
| Table to be formatted. (see template discussion page) |
Results and Discussion
Results of the investigation are presented in table 3 and figure 1.
Some selection of material has been made in order to avoid a comparison drawn largely from the development of one system to the exclusion of others. The number of comparisons between mouse and human embryos is further limited by the lack of adequate time estimates for the development of some structures otherwise well described in the human. No structure is listed in the table where the equivalence of stages de-scribed in the human with those seen in mouse embryos was doubtful. Some uncertainty existed that late histological changes in the skin, retina, and cerebral cortex and the development of certain portions of the nervous system were exactly comparable in b-otl1 organisms so that they also have been omitted. In consequence the material presented for the mouse constitutes only a very limited time table.
Table 4 lists for three systems the mean equivalent human age for each successive mating age in the mouse. Since the variance of the mean ages based on all structures is unknown, no test can be made of the differences in the mean equivalent ages based on a single system. By inspection, however, no one system, measured against human equivalence, appears to lag with respect to others. The digestive and circulatory systems are perhaps temporarily slower in differentiation than the nervous system on the 13th, 14th and 15th days. The nervous system is still undergoing architectural changes at this time while the main features of the circulatory and digestive systems are already established. Subsequent advances are histological, and the digestive system and its «derivatives appear to enter a period of rapid differentiation after the 16th day.
The curve in figure 1 representing the relation between mouse embryos of a given mating age and human embryos at a given estimated ovulation age, is based on matching stages in the development of various tissues and organs. It has been put in by eye to pass close to the mean age of origin in human embryos of all the tabulated structures arising a.t a selected time in the mouse. The points representing individual matched structures are fixed by mating age or somite count on the mouse axis but vary about the mean estimated ovulation age of the human axis. Each structure has, in both organisms, a mean time of origin with an associated variance neither of which is measured in thisinvestigation. A true mean point of origin and its variance would require repeated sampling at fixed time intervals of very short duration for each structure, a condition not easily satisfied in a general survey even of tlie experimental animal for which time can be directly controlled.
Table 3
| Table 3 - Mouse Human Comparison |
|---|
| Table to be formatted. (see template discussion page) |
Table 4
| Table 4 - Comparison of mouse-human equivalent ages by systems |
|---|
| Table to be formatted. (see template discussion page) |
The individual points are essentially single samples of a universe, the mean and variance of which are unknown or, as in the mouse measurements, suppress-ed. In estimating the age relations between mouse and human embryos, the only Variance retained is that expressing the timing difference of individual structures from the mean age of human embryos equivalent to a particular mouse age.
The curve in figure 1 appears to have an abrupt change in slope between the 14th and 15th days. Various mathematical transformations, including semilogarithmic and logarithmic on both scales, showed that the rates of development in the two organisms are not related as any simple continuous function.
Figure 1 Graphic plot of the times of appearance of structures in mouse and human embryos.
All transformations tried showed discontinuity between the entries before and after the 14th day.
The validity of the relationship read directly from the curves is sufficient if the errors inherent in the measurements are borne in mind. A genetic lethal, for example, operating to kill mouse embryos at about 10 days and 12 hours of gestation, would, if it occurred in human chromosomes, be expected to be lethal at the 28th or 29th day of human gestation. If, as in the present data, at 12 hour time interval existed between inspections, the reservation must be maintained that in the mouse the lethal might operate at any time after 10 days and 0 hours, introducing a measurement error of one to 11 hours. On the human axis, errors in estimated ovulation age certainly increase with age. Despite careful medical histories and the use of various means of comparison, deviations are probably never less than plus or minus three or 4 days even at the third week. Older embryos can be expected to deviate by as much as plus or minus 7 to 12 days from the mean (Mall, ’18).
The curves do provide a better estimate of equivalency than a direct comparison by per cent of elapsed pregnancy or equivalence based on the study of very young stages. At 16 days and 15 hours about 87% of total pregnancy (19 days and 7 hours) has elapsed in the mouse. This age level is about equivalent to the 84th or 85th day of human gestation, or about 30% of human gestation. This difference in the per cent of pregnancy remaining until term suggests that the mouse may be born in a relatively immature state compared to the human or that human gestation is prolonged beyond a “finishing stage.” It is also possible that a change in developmental rate with a very rapid differentiation of «specializing tissues takes place in the last days of mouse gestation. The data presented here appear to indicate such a change after the 14th day but the inadequacies in measuring dispersion from the means prevent its clear statistical recognition. The deliberate adoption of the Streeter tables for age conversion from human crownrump length prevents an artificial increase in human age in this transitional period. Certainly the respiratory system shows a. marked advance between the 16th and 17th days shifting from a dense mesenchymal bed penetrated by thickly lined bronchial branches of the third and fourth order to a more mesh like tissue with extensive thin walled developing alveoli. It is to be expected that the tissues of those parts of the respiratory, digestive, and excretory systems which must be functional at term will, if in a relatively primitive state at the beginning of the last quarter of -gestation, go through rapid differentiation.
Assuming that the shift in developmental rate of the mouse after the 14th day is real, it is of interest that it occurs at about the period when the establishment of essential organs i-s complete and only histological differentiation and increase in the volume of tissues remain to be finished. The major architectural changes recorded after 14 days and 12 hours in this study are the completion of valves in the heart and the fusion of certain areas of the brain.
The day of origin of some structures in the mouse embryos described in this investigation does not always agree with the timing established in other investigations. The divergence of our embryos from those of Snell a.nd Melissinos was discussed earlier. It should be noted that hybrid and inbred strains may differ in developmental level by as much as 24 hours or more. If the equivalency table is to be used by other investigators a comparison should always be made of embryos, preferably in the middle period of somite formation. Our data on the origin of certain structures agree well with those of Snell or Chase and Chase (’41) when compared by somite count and less Well in mating age. Some of the differencemay be due to the use of an earlier mating period associated with the same mean ovulation time. The extent of such a difference would depend on the length of time sperm remains capable of fertilization. The more important differences, however, are likely to be encountered through the use of different strains.
Summary
A table and graph for estimating equivalent ages of mouse and human embryos are presented. The determination was made by matching stages of embryonic structures in both organisms. Each structure appears graphically as a coordinate of the time at which it was observed in mouse embryos of known mating age and the time at which it was reported to appear in human embryos.
The rate of development of the mouse with respect to the human increases with increasing age, particularly after the 14th day. Equivalency cannot be based on per cent of elapsed pregnancy or the comparison of stages other than the one for which an equivalent estimate is needed.
Mouse strains may difler by as much as 24 hours in their developmental rates so that application of the equivalency graph to data obtained from strains other than the one used in
this investigation requires a comparison of one or more embryos with the time table.
The authors wish to thank Mr. Wesley Grabrio for determin ing some equivalent stages in the eye and digestive system and Mr. James Cotanche for the preparation of slides.
Literature Cited
ALLEN, E. 1922 The oestrous cycle in the mouse. Am. J. Anat., 30: 297-371.
ALLEN, E., AND E. MACDOWELL 1940 Variation in mouse embryos of 8 days gestation. Anat. Rec., 7?’ : 165-173.
Atwell WJ. The development of the hypophysis cerebri in man, with special reference to the pars tuberalis. (1926) Amer. J Anat. 37: 139-193.
Atwell WJ. A human embryo with seventeen pairs of somites. (1930) Contrib. Embryol., Carnegie Inst. Wash. Publ. 407, 21: 1-24.
BACH, L., AND R. SEEFELDER 1911-1912 Atlas zur Entwicklungsgeschichte des menschlichen Auges. Engelmann, Leipzig.
BENDER, K. 1925 Tiber die Entwicklung der Lungen. Zeitschr. f. Anat. und Entwicklungsgechichte, 7.5 : 639-704.
BRAMBELL, F. 1928 The development and morphology of the gonads of the mouse. Part III. The growth of the follicles. Proc. R-. Soc. London, Series B, 103: 258-272.
BUTCHER, E. 0. 1929 The development of the somites in the white rat (Mus norvegicus albinu) and the fate of the myotomes, neural tube, and gut in the tail. Am. J. Anat., 44: 381-439.
CHASE, H., AND E. CHASE 1941 Studies on an anophthalmic strain of mice. I. Embryology of the eye region. J. Morph., 68: 279-301.
Congdon ED. Transformation of the aortic-arch system during the development of the human embryo. (1922) Contrib. Embryol., Carnegie Inst. Wash. Publ 277, 14:47-110.
Corner GW. A case of true lateral hermaphroditism in a pig with functional ovary. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. , : 137-142.
Davis CL. Description of a human embryo having twenty paired somites. (1923) Carnegie Instn. Wash. Publ. 332, Contrib. Embryol., 15: 1-51.
Gillman J. The development of the gonads in man, with a consideration of the role of fetal endocrines and the histogenesis of ovarian tumors. (1948) Carnegie Contrib. to Embryol. 32: 81-131.
GIRGIS, A. 1926 Description of a human embryo of twenty—two paired somites. J. Anat., 60: 382-410.
Hertig AT. and Rock J. Two human ova of the pre-villous stage, having a developmental age of about seven and nine days respectively. (1945) Contrib. Embryol., Carnegie Inst. Wash. Publ. 557, 31: 65-84.
— 1949 A series of potentially abortive ova recovered from fertile women prior to the first missed menstrua1 period. Am. J. Obs. and Gyn., 58: 968-993.
Heuser CH. A human embryo with 14 pairs of somites. (1930) Carnegie Instn. Wash. Publ. 414, Contrib. Embryol., Carnegie Inst. Wash. 22:135-153.
1932 A pre-somite human embryo with a definite chorda canal. Carnegie Contrib. to Embry01., 2.3: 251-267.
Heuser CH. Rock J. and Hertig AT. Two human embryos showing early stages of the definitive yolk sac. (1945) Contrib. Embryol., Carnegie Inst. Wash. Publ. 557, 31: 85-99.
HEUSER, C., J. ROCK AND A. HERTIG 1945 Two human embryos showing early stages of the definitive yolk sac. Carnegie Contrib. to Embi-yol., 31 : 85-99.
HEWER, E. 1935 The development of nerve endings in the human foetus. J. Anat., 69: 369-379.
HOCHSTETTER, F. 1929 Beitriige zur Entwicklungsgeschiehte des menschlichen Gehirns. II. Teil. Die Entwicklung des Mittel und R-autenhirns. Deuticke, ‘Wien.
HOGG, I. 1941 Sensory nerves and associated structures in the skin of human fetuses of 8 to 14 weeks of menstrual age correlated with functional capability. J. Comp. Neur., 75: 371-410.
Ingalls NW. A human embryo at the beginning of segmentation, with special reference to the vascular system. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. 274, 11: 61-90.
Johnson FP. The development of the mucous membrane of the oesophagus, stomach and small intestine in the human embryo. (1910) Amer. J Anat., 10: 521-559.
JOHNSON, F. 1910 The development of the mucous membrane of the oesophagus, stomach and small intestine in the human embryo. Am. J. Anat., 10: 521—561.
KRAMER, T. 1942 The partitioning of the truncus and conus and the formation of the membranous portion of the interventricular septum in the human heart. Am. J. Anat., 71: 343-370.
LEWIS, W., AND C. HARTMAN 1933 Early cleavage stages of the egg of the monkey (Macacus rhesus). Carnegie Contrib. to Embryol., 24: 187-201.
LEWIS, W., AND E. WRIGHT 1935 On the early development of the mouse egg. Carnegie Contrib. to Embryol., 25 : 113-144.
LUDWIG, E. 1928 fiber einen operativ gewonnenen menschlichen Embryo mit einem U1-segmente (Embryo Dal). Morph. J ahrb., 59: 41-104.
McClure CFW. and Butler EG. The development of the vena cava inferior in man. (1925) Amer. J Anat. 35(3): 331-383.
MCCLURE, C., AND E. BUTLER 1925 The development of the vena cava inferior in man. Am. J. Anat., 35: 331-38-4.
Mall FP. On the age of human embryos. (1918) Amer. J Anat. 23: 397-422.
MANN, I. 1928 The development of the human eye. Cambridge Univ. Press, London.
Msnrssnvos, K. 1907 Die Entwicklung des Eies der Manse von den ersten Furchungs-Phiinomenen bis zur Festsetzung der Allantois an der Ectoplacentarplatte. Arch. mikr. Anat., ?'0.' 577-628.
NoBAoK, C., AND G. ROBERTSON 1951 Sequences of appearance of ossification centers in the human skeleton during the first five prenatal months. Am. J. Anat., 8.9: 1-28.
Odgers PNB. The formation of the venous valves, the foramen secundum and the septum secundum in the human heart. (1935) J. Anat., 69: 412-422. PMID 17104548
PATTEN, B. 1946 Human embryology. Blakiston and Company, Philadelphia.
Payne F. General description of a 7-somite human embryo. (1925) Contrib. Embryol., Carnegie Inst. Wash. Publ. 361, 16: 115-124.
Pearson AA. The development of the olfactory nerve in man. (1941) J Comp. Neurol. 199-217.
RICHARDSON, K. 1937 (In Franklin, K., A monograph on veins.) Chapt. III. The embryology of veins. Thomas, Baltimore.
SENSENIG, E. 1949 The early development of the human vertebral column. Carnegi-e Contrib. to Embryo1., 3-3: 21-41.
SNELL, G. 1941 Biology of the laboratory mouse. Blakiston, Philadelphia.
SNELL, G., E. FEKETE, K. HUMMEL AND L. Law 1940 The relation of mating, ovulation and the estrous smear in the house mouse to time of day. Anat. Rec., 7'6 : 39-54.
Sosorrn, J. 1911 Die Entwicklung des Eies der Maus vom ersten Auftreten de Mesoderms an bis zur Ausbildung der Embryonalanlage und dem Auf— tretcn der Allantois. Arch. mikr. Anat., '78: 271-352.
STERNBERG, H. 1927 Beschreibung eines menschlichen Embryos mit vier Ursegmentpaaren, nebst Bemerkungen fiber die Anlage und friiheste Entwicklung einiger Organe beim Menschen. Zeitschr. f. Anat. u. Entwick., 8:2: 142--240.
Streeter GL. On the development of the membranous labyrinth and the acoustic and facial nerves in the human embryo. (1906) Amer. J Anat. 6:139-165.
- 1917a The development of the scala tympani, scala vestibuli and perioticular cistern in the human embryo. Am. J. Anat., 21: 299-320.
- 1917b The factors involved in the excavation of the cavities in the cartilaginous capsule of the ear in the human embryo. Am. J. Anat., 2.2: 1-25.
- 1918 The histogenesis and growth of the otic capsule and its con-tained periotic tissuespaces in the human embryo. Carnegie Contrib. to Embryoh, 7 : 5-54.
- 1920 Weight, sitting height, head size, foot length, and menstrual age of the human embryo. Carnegie Contrib. to Embryol., 11 : 143-170.
- 1942 Developmental horizons in human embryos. Description of agegroup XI, 13 to 20 somites, and age group XII, 21 to 29 somites. Carnegie Contrib. to Embryol., 30: 211-245.
- 1945 Developmental horizons in human embryos. Description of age group XIII, embryos about 4 or 5 millimeters long, and age group XIV, period of indentation of the lens vesicle. Carnegie Contrib. to Embry0l.,. .31: 27-63.
- 1948 Developmental horizons in human embryos. Description of agegroups XV, XVI, XVII, and XVIII, being the third issue of a survey of the Carnegie collection. Carnegie Contrib. to Embryol., 3.2: 133-203.
STREETER, G. 1949 Developmental horizons i11 human embryos (fourth issue) : A review of t.he histogenesis of cartilage and bone. Carnegie Contrib. to Emb1'y01., 3.3.‘ 149-167.
Thompson E. Time and rate of loss of nuclei by the red blood cells of human embryos. (1951) Anat. Rec., 111: 317-325.
WEED, L. 1917 The development of the cerebro~spina.1 spaces in pig and in man. Carnegie Contrib. to Embryo1., 5 : 116 pages.
WELLER, G. 1933 Development of the thyroid, parathyroid and thymus glands in man. Carnegie Contrib. to Embryol., 24: 93-139.
WES'1‘, G. 1937 A human embryo of twenty—five somites. J. Anat., 71: 169-200.
WILson, J. 1945 Embryology of the human heart. Ward’s Natural Science Est, Inc., Rochester, New York.
Explanation of Figures
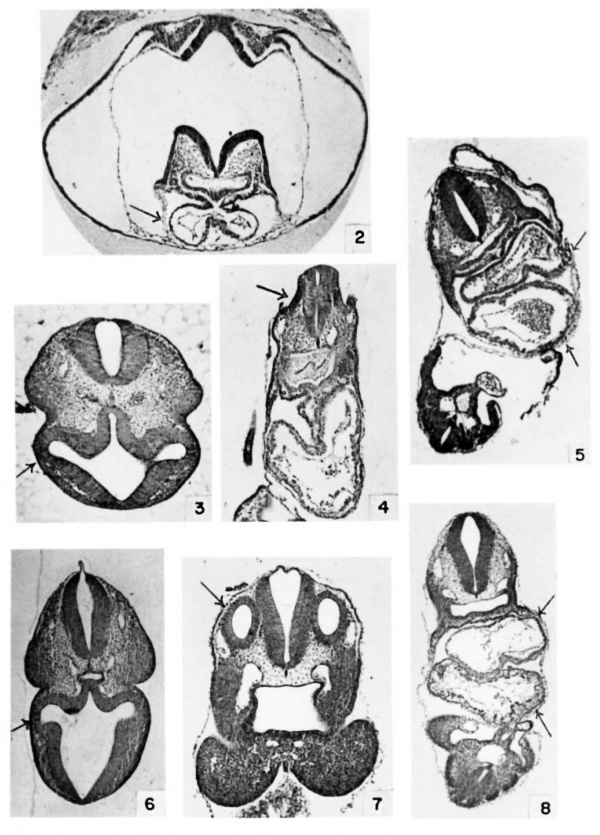
The illustrations were chosen primarily as an aid in matching embryos of other strains to the strain used in this investigation. Only a few of the structures in table 3 can be illustrated. The figures, therefore, present a partial syndrome of structures characteristic of successive mating ages. Arrows direct attention to structures to be compared in successive mating ages.
Sections were stained in Harris ’s hematoxylin and eosin Y or eosin B.
Plate 1
2 Heart at 8 days, 12 hours, (E8.5) 6 somites. Cf. also table 3, no. 11 and no. 17. (X 35.)
3 Eye at 8 days, 22 hours, (E9.0) 16 somites. (X 29.)
4 Ear at 8 days, 18 hours, (E9.0) 13 somites. (X 29.)
5 Heart at 8 days, 21 hours, (E9.0) 15 somites. Cf. also table 3, no. 28. (X 35.)
6 Eye at 9 days, 9 hours, (E9.5) 23 somites. Cf. also table 3, no. 38. (X 13.)
7 Ear at 9 days, 9 hours, (E9.5) 23 somites. (X 26.)
8 Heart at 9 days, 9 hours, (E9.5) 23 somites. (X 18.)
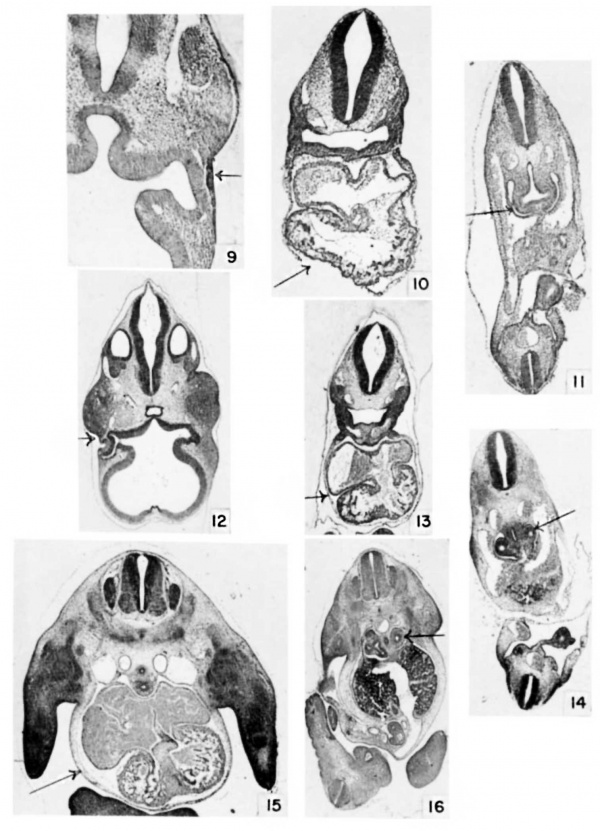
Plate 2
9 Eye at 9 days, 18 hours, E9.5) 26 somites. (X 34.)
10 Heart at 9 days, 18 hours, E9.5) 26 somites. (X 20.)
11 Lung at 9 days, 18 hours, E9.5) 26 somites. Cf. also table 3, no. 43 and 44. (X 17.)
12 Eye at 10 days, 12 hours. E10.5) (X 14.)
13 Heart at 10 days, 12 hours. E10.5) (X .11.)
14 Lung at 10 days, 12 hours. E10.5) (X 11.)
15 Heart at 11 days, 12 hours. E11.5) Cf. also table 3, no. 74 (X 10.)
16 Lung at 11 days, 12 hours. E11.5) (x 8.)
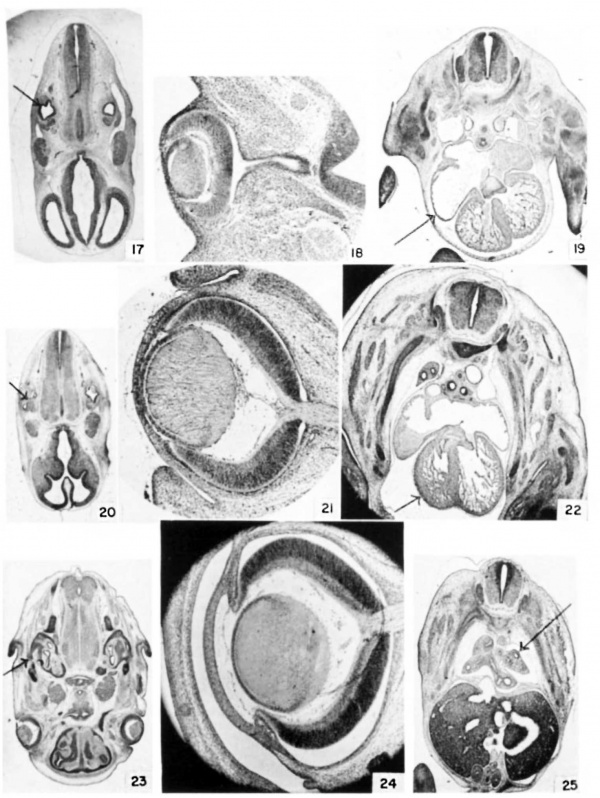
Plate 3
17 Ear at 11 days, 12 hours. (E11.5) Cf. also table 3, no. 78. (X 5.)
18 Eye at 12 days, 12 hours. (E12.5) Cf. also table 3, no. 105. (X 26.)
19 Heart at 12 days, 12 hours. (E12.5) (x 8.)
20 Ear at 12 days, 12 hours. (E12.5) (X 4.7.)
21 Eye at 14 days, 12 hours. (E14.5) (X 26.)
22 Heart at 13 days, 12 hours. (E13.5) (X 8.)
23 Ear at 14 days, 12 hours. (E14.5) Cf. also table 3, no. 130. (X 4.7.)
24 Eye at 16 days, 12 hours. (E16.5) (X 26.)
25 Lung at 13 days, 0 hours. (E13) Cf. also table 3, no. 117. (X 7.)
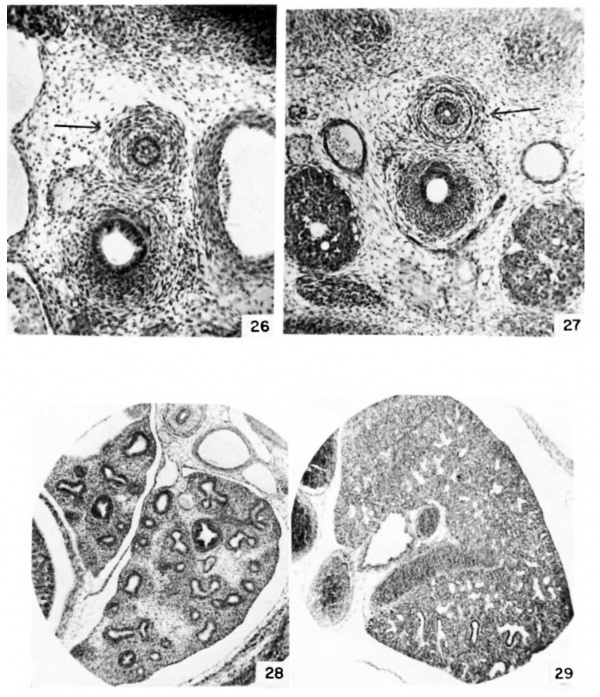
Plate 4
26 Detail of oesophagus and trachea at 13 days, 0 hours. {E13) (X 40.)
27 Detail of oesophagus and trachea at 13 days, 12 hours. {E13.5) (X 27.)
28 Detail of lung at 14 days, 12 hours. {E12.5) (X 27.)
39 Detail of lung at 16 days, 12 hours. {E16.5) (x 27.)
- ↑ Otis EM and Brent R. Equivalent ages in mouse and human embryos. (1954) Anat Rec. 120(1):33-63. PMID 13207763